Abstract
The Rapoport–Luebering glycolytic bypass comprises evolutionarily conserved reactions that generate and dephosphorylate 2,3-bisphosphoglycerate (2,3-BPG). For >30 years, these reactions have been considered the responsibility of a single enzyme, the 2,3-BPG synthase/2-phosphatase (BPGM). Here, we show that Dictyostelium, birds, and mammals contain an additional 2,3-BPG phosphatase that, unlike BPGM, removes the 3-phosphate. This discovery reveals that the glycolytic pathway can bypass the formation of 3-phosphoglycerate, which is a precursor for serine biosynthesis and an activator of AMP-activated protein kinase. Our 2,3-BPG phosphatase activity is encoded by the previously identified gene for multiple inositol polyphosphate phosphatase (MIPP1), which we now show to have dual substrate specificity. By genetically manipulating Mipp1 expression in Dictyostelium, we demonstrated that this enzyme provides physiologically relevant regulation of cellular 2,3-BPG content. Mammalian erythrocytes possess the highest content of 2,3-BPG, which controls oxygen binding to hemoglobin. We determined that total MIPP1 activity in erythrocytes at 37°C is 0.6 mmol 2,3-BPG hydrolyzed per liter of cells per h, matching previously published estimates of the phosphatase activity of BPGM. MIPP1 is active at 4°C, revealing a clinically significant contribution to 2,3-BPG loss during the storage of erythrocytes for transfusion. Hydrolysis of 2,3-BPG by human MIPP1 is sensitive to physiologic alkalosis; activity decreases 50% when pH rises from 7.0 to 7.4. This phenomenon provides a homeostatic mechanism for elevating 2,3-BPG levels, thereby enhancing oxygen release to tissues. Our data indicate greater biological significance of the Rapoport–Luebering shunt than previously considered.
Keywords: 2,3-BPG; erythrocyte; glycolysis
The Rapoport–Luebering glycolytic shunt generates and dephosphorylates 2,3-bisphosphoglycerate (2,3-BPG). These reactions have been considered to be catalyzed by a single 2,3-BPG synthase/2-phosphatase (BPGM) (1, 2). This enzyme has mutase activity that converts the glycolytic intermediate, 1,3-BPG, to 2,3-BPG. BPGM also acts as a phosphatase, converting 2,3-BPG to 3-phosphoglycerate (3-PG), which then reenters the main glycolytic pathway. This has been the textbook perception of this metabolic pathway for >25 years (3).
Metabolic flux through the Rapoport–Luebering shunt carries an energetic cost for the cell because it bypasses the ATP-generating phosphoglycerate kinase. Nevertheless, in vertebrates and lower eukaryotes, 2,3-BPG fulfills an essential role in glycolysis by priming the phosphoglycerate mutase reaction that converts 3-PG to 2-PG (4). More recently, experiments in Dictyostelium have suggested that 2,3-BPG, through an unknown mechanism, provides a molecular link between the turnover of phosphorylated inositol derivatives and glycolytic flux (5). Nonetheless, the most well studied function for 2,3-BPG is its regulation of whole-body oxygen homeostasis; 2,3-BPG executes this role because it is the main allosteric effector of hemoglobin in the majority of mammals. By preferentially binding to deoxyhemoglobin, 2,3-BPG facilitates oxygen release from the erythrocyte to the surrounding tissues (6, 7). Thus, the regulation of erythrocyte 2,3-BPG levels is key to efficiently meeting tissue oxygen demands while also providing an important physiological adaptation to oxygen deprivation (8), including that which occurs at high altitude (9) or during postoperative anemia (10).
Despite the importance of carefully regulating 2,3-BPG turnover, little is known about how this might be achieved. Some attention has focused on the observation that physiologically relevant alkalinization of erythrocytes increases levels of 2,3-BPG, but the mechanism behind this effect is not clearly established, despite nearly four decades of research into erythrocyte cell biology (11). Another long-standing puzzle in this field is that the turnover of 2,3-BPG in erythrocytes is considerably in excess of that expected from the in vitro kinetic parameters of BPGM (11, 12).
The current study fills in these important gaps in our understanding of 2,3-BPG metabolism by identifying a second enzyme component of the Rapoport–Luebering shunt, namely, a separate 2,3-BPG phosphatase activity catalyzed by an evolutionarily conserved multiple inositol polyphosphate phosphatase (MIPP1). Using Dictyostelium as a model lower eukaryote, we show how changes in MIPP1 expression have a significant effect on cellular 2,3-BPG concentration. Our data additionally reveal a mechanism to link the turnover of phosphorylated inositol derivatives with changes in glycolytic flux. We show how the acute pH sensitivity of human MIPP1 offers a means to regulate hemoglobin oxygen affinity. Our determination that MIPP1 converts 2,3-BPG to 2-PG reveals how glycolysis can completely bypass 3-PG, which activates the AMPK cascade (13) and also functions as a precursor for serine biosynthesis (14). Overall, our data show that the Rapoport–Leubering shunt not only includes an additional catalytic reaction but also should be considered an important regulatory system with several roles in cell physiology.
Results and Discussion
Dictyostelium Mipp1 Shows 2,3-BPG Phosphatase Activity in Vitro and in Vivo.
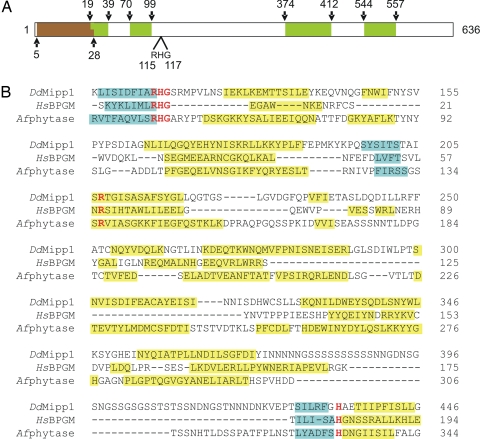
Previous experiments in Dictyostelium and other eukaryotic microorganisms have suggested that there may be a molecular link between glycolytic flux and the turnover of phosphorylated inositol derivatives (5). To investigate this idea, we used a bioinformatic approach to examine the enzymes involved in inositol phosphate metabolism for candidate overlapping functions associated with glycolysis. Dictyostelium contains a membrane-associated enzyme that has previously been characterized as a DdMipp1 (15). We threaded the DdMipp1 sequence through the PHYRE server (www.sbg.bio.ic.ac.uk/phyre) after first omitting the Ser/Asn-rice repeat sequences [Fig. 1 and supporting information (SI) Fig. S1], which are found in many Dictyostelium proteins, but are generally believed to have little structural or functional significance (16, 17).
Fig. 1.
Alignment of DdMipp1, HsBPGM, and Afphytase. (A) Schematic of DdMipp1. The predicted transmembrane domain (according to Peptool, v2) is shown in brown; Ser/Asn-rich, simple-sequence repeats are shown in green. (B) D. discoideum (Dd) Mipp1A (DDB0186447; www.dictybase.org), human (Hs) BPGM (PDB 1T8P), and Aspergillus ficuum (Af) phytase (PDB 1IHP). Catalytically important residues (26, 40, 41) that are conserved in all three proteins are shown in red type. The PHYRE server provided the predicted secondary structural elements of DdMipp1 and the actual secondary structures of BPGM and phytase (blue, beta-sheet; yellow, helix; the graphic does not distinguish 310 helices from alpha-helices).
Molecular threading predicted that the DdMipp1 structure is not only similar to that of phytases but also shares similarity to human BPGM (HsBPGM). Fig. 1 illustrates the predicted conservation of α-helix and β-sheet secondary structures in HsBPGM and DdMipp1. This predicted structural similarity led us to investigate whether these two enzymes might share the capacity to hydrolyze 2,3-BPG.
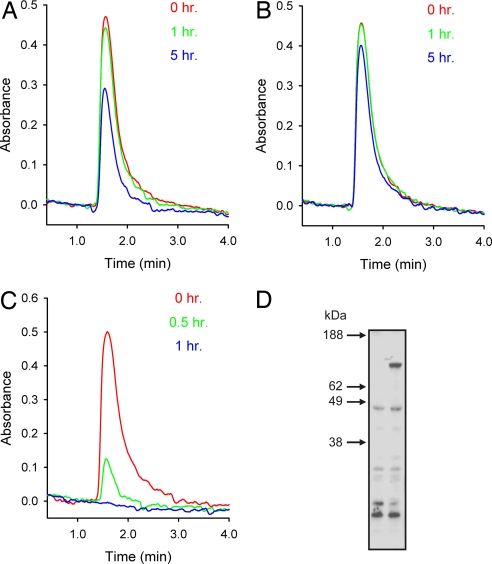
To study the catalytic activity of DdMipp1, we attempted to express recombinant, epitope-tagged protein in either Escherichia coli or Pichia pastoris. However, these preparations were not catalytically active. Instead, we measured 2,3-BPG hydrolysis by cell extracts prepared from strains of Dictyostelium in which endogenous DdMipp1 expression was genetically manipulated. Extracts from mipp-null cells hydrolyzed exogenous 2,3-BPG ≈2.5-fold more slowly than did wild-type cell extracts (Fig. 2 A and B). Conversely, in extracts that were made from cells in which untagged DdMipp1 was overexpressed (Fig. 2 D), the rate of 2,3-BPG hydrolysis was increased >20-fold (Fig. 2 A and C). These experiments show that DdMipp1 can metabolize 2,3-BPG.
Fig. 2.
The 2,3-BPG phosphatase activity of DdMipp1. (A–C) The particulate fraction of extracts from wild-type (A), mipp1-null (B), and Mipp1-overexpressing (C) cells were incubated with 100 μM 2,3-BPG. Aliquots (100 μl) were assayed for 2,3-BPG at the indicated times by MDD-HPLC. Reaction rates (nmol/mg/h) were as follows: wild-type, 17.5; mipp1-null, 7.7; and DdMipp1 overexpressing, 331. (D) Western analysis of lysates from 2 × 105 cells (left lane, wild-type; right lane, overexpressing Mipp1; predicted mass, 70 kDa) suspended in LDS loading buffer (Invitrogen) and run on 4–12% precast polyacrylamide gels (Invitrogen). After transfer to nitrocellulose membrane, samples were probed with an anti-Mipp1 antibody (Eurogentec) raised against the CSFKPTKFDSRSPLIQ sequence at the C terminus. This antibody was insufficiently sensitive to detect Mipp1 in wild-type cells (D, left lane).
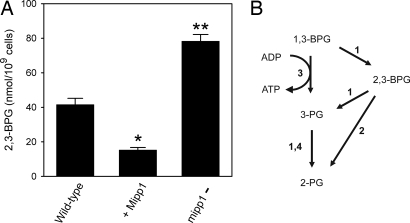
We next investigated whether DdMipp1 has the capacity to regulate cellular levels of 2,3-BPG in vivo. We considered this question to be intriguing because cellular levels of 2,3-BPG are only 1% of the total cellular content of InsP6 (5, 18), one of DdMipp1's alternate canonical substrates (15). The levels of 2,3-BPG in Dictyostelium have been estimated at ≈6 μM (5), which is too low for our MDD-HPLC system to accurately resolve from other organic phosphates. Instead, we purified 2,3-BPG from Dictyostelium by anion-exchange chromatography and then quantified it enzymatically (see Materials and Methods). We found that the overexpression of DdMipp1 reduced cellular levels of 2,3-BPG to 37% of those in wild-type cells (Fig. 3A). Conversely, cells in which DdMipp1 was eliminated had 90% higher levels of 2,3-BPG compared with wild-type cells (Fig. 3A). These data show that, in vivo, DdMipp1 is a physiologically relevant regulator of the metabolic flux of 2,3-BPG through the Rapoport–Luebering shunt (Fig. 3B).
Fig. 3.
Cellular levels of 2,3-BPG in Dictyostelium respond to genetic manipulations of expression. (A) Levels of 2,3-BPG in wild-type Dictyostelium, in cells in which the mipp1 gene was disrupted, and in cells in which DdMipp1 was overexpressed. Data (means and SE from three to four experiments) are corrected for recovery of 2,3-BPG through the extraction procedure (76%) (see Materials and Methods). *, P < 0.002; **, P < 0.0005 (compared with wild-type cells; unpaired t test). (B) These data extend the Rapoport–Luebering shunt. Enzymes: 1, BPGM; 2, MIPP1; 3, phosphoglycerate kinase; 4, phosphoglycerate mutase.
Human MIPP1 Has Biologically Significant 2,3-BPG Phosphatase Activity.
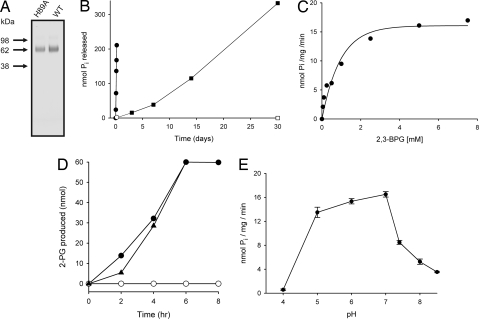
Is this 2,3-BPG phosphatase activity of DdMipp1 conserved in higher organisms? To answer this question, we prepared recombinant HsMIPP1 with a C-terminal myc-poly(His) epitope tag by using the P. pastoris expression system (Fig. 4A). After purification by using Ni-Sepharose, the protein migrated as a single diffuse band with an apparent size of 70 kDa, rather higher than the predicted size of 54 kDa (Fig. 4A). This disparity was due to glycosylation; after treatment of recombinant HsMIPP1 with endoglycosidase, the protein migrated with a lower apparent size of 55 kDa (data not shown). We found that HsMIPP1 (Fig. 4B) shares with DdMipp1 (Figs. 2 and 3) the ability to dephosphorylate 2,3-BPG. Deglycosylation of HsMIPP1 elicited only a small (10% ± 1%; n = 3) decrease in phosphatase activity. Kinetic parameters (Vmax = 15.8 ± 0.2 nmol/mg of protein per min; Km = 0.61 ± 0.02 mM; n = 3) were derived from substrate-saturation plots (Fig. 4C). We also prepared a catalytically compromised His89Ala mutant of HsMIPP1 (19) that showed only 1% of the 2,3-DPG phosphatase activity of the wild-type enzyme (0.18 nmol/mg of protein per min) (Fig. 4 A and B).
Fig. 4.
The 2,3-BPG 3-phosphatase activity of HsMIPP1. (A) SDS/PAGE of recombinant wild-type (WT) and His89Ala HsMIPP1 was carried out by using an Xcell II MiniCell and 4–12% NuPage Bis-Tris gels (Invitrogen) according to the manufacturer's recommendations. Proteins were visualized with SimplyBlueSafeStain (Invitrogen). (B) Pi release was recorded when 40 μg of either wild-type HsMIPP1 (filled circles) or a His89Ala catalytically impaired mutant (open circles) was incubated with 5 mM 2,3-BPG at 37°C for up to 5 h (as described in Materials and Methods). Additionally, 40 μg of wild-type MIPP1 was incubated at 4°C for up to 30 days with 5 mM 2,3-BPG (filled squares). “No enzyme” controls also were performed (open squares). (C) HsMIPP1 (40 μg) was incubated with various concentrations of 2,3-BPG to derive kinetic parameters. (D) Briefly, 60 nmol of either 2,3-BPG (filled circles), 3-BPG (open circles), or both together (filled triangles) were incubated with MIPP1. The 2-PG was assayed by coupling its metabolism to NADH oxidation (see Materials and Methods). (E) The effect of pH on enzyme activity of HsMIPP1 was determined with 2,3-BPG as substrate by using the following buffers: 50 mM sodium acetate (pH 4–5), 50 mM Bis-Tris·HCl (pH 6–7), and 50 mM Tris·HCl (pH 7.4–8.5). All graphs are representative experiments (triplicate data points) except E, which plots means and SEMs from three independent experiments.
In most mammalian cells, MIPP1 is restricted to the interior of the endoplasmic reticulum (20, 21). MIPP1 can still access cytosolic substrates in some cell types (19), but apparently not in others (22). It may be a profitable research direction to determine in which of these cells MIPP1 may regulate 2,3-BPG levels, but first it is necessary to develop an assay with sufficient sensitivity to accurately measure changes in the cytosolic pools of 2,3-BPG, which can be as low as 0.5 μM (23).
However, the situation is very different for mammalian erythrocytes. These cells offer a unique paradigm for MIPP1 as a regulatory glycolytic enzyme. MIPP1 is located in the erythrocyte plasma membrane, and its active site faces into the cell (24, 25). Moreover, erythrocytes contain 6–7 mM 2,3-BPG (11), and there are no inositol phosphates to compete for the active site (indeed, MIPP1's role in erythrocytes has previously been a complete mystery). The activity of recombinant HsMIPP1 toward 1 mM 2,3-BPG was 9 nmol/mg of protein per min (Fig. 4C), which is almost identical to the previously reported activity of HsBPGM (7.3 nmol/mg of protein per min) (26). Of course, although the activities of BPGM and MIPP1 are similar, their relative levels of expression also will determine their individual contributions to 2,3-BPG metabolism in vivo. We studied this problem by determining the capacity of rat (Rn) MIPP1 in erythrocytes because its catalytic properties are near identical to those of HsMIPP1 (refs. 22 and 27 and data not shown). We used anion-exchange chromatography to concentrate RnMIPP1 from detergent-solubilized erythrocyte lysates (Fig. 5). Phosphate contamination and limitations of assay sensitivity prevented us from accurately assessing 2,3-BPG hydrolysis in these RnMIPP1 preparations. Instead, we measured their Ins(1,3,4,5)P4 3-phosphatase activities (47.5 ± 4 mmol/liter of cells per h) (Fig. 5). Next, by using recombinant RnMIPP1, we established that the Vmax for 2,3-BPG phosphatase activity was 80-fold lower than the Vmax for its Ins(1,3,4,5)P4 3-phosphatase activity (data not shown). We thereby indirectly estimated that the capacity of MIPP1 to hydrolyze 2,3-BPG in vivo is 0.6 ± 0.05 mmol/liter of cells per h (n = 4). In comparison, BPGM hydrolyzes 0.1–0.5 mmol 2,3-BPG/liter of cells per h (2, 28). Thus, our data reveal that MIPP1 is a major 2,3-BPG phosphatase on par with BPGM.
Fig. 5.
The capacity of Mipp1 in rat erythrocytes. A detergent-lysed extract from 4 ml of rat erythrocytes was fractionated by anion-exchange chromatography and assayed for 2,3-BPG synthase (filled squares) and Mipp1 [i.e., Mg2+-independent Ins(1,3,4,5)P4 3-phosphatase] (filled circles). Mean total activity from four preparations is shown.
During the weeks that erythrocytes are stored at 4°C before transfusion, 2,3-BPG becomes depleted, causing a temporary but clinically significant impairment of oxygen transport (29). We have found that HsMIPP1 hydrolyzes 2,3-BPG at 4°C (Fig. 4B), albeit considerably more slowly than at 37°C. Therefore, MIPP1 can contribute to the depletion of 2,3-BPG during erythrocyte storage.
The Phosphatase Activity of Avian MIPP1.
In birds, 2,3-BPG also is an important regulator of hemoglobin oxygen affinity during embryonic development. Levels of 2,3-BPG in erythrocytes increase dramatically during the last week of embryonic development and then disappear within a few days of hatching (30). The decline in 2,3-BPG levels is accompanied by an elevation in the levels of inositol phosphates, which then assume the role of regulating hemoglobin oxygen affinity (30). We found that recombinant chicken Mipp1 can actively hydrolyze both 2,3-BPG (815 ± 26 nmoles/mg of protein per min) and inositol phosphates (505 ± 6 nmoles/mg of protein per min for InsP6). Thus, although the nature of hemoglobin's regulatory ligand switches during development, Mipp1 metabolizes both and, thus, can contribute to regulating hemoglobin oxygen affinity throughout the life of chickens and other birds. A Thr27Gly mutant version of chicken Mipp1 showed >95% lower activities against both substrates (5.2 ± 0.01 and 22 ± 0.8 nmoles/mg of protein per min for 2,3-BPG and InsP6, respectively), indicating that a single active site is involved. Note that the specific activity of avian Mipp1 toward 2,3-BPG is 50-fold greater than that of human MIPP1 (Fig. 4C), reflecting phylogenetic differences in both erythrocyte physiology and the rate of 2,3-BPG turnover in vivo (30).
A Glycolytic Reaction: 2,3-BPG 3-Phosphatase.
We next investigated the positional specificity of HsMIPP1 toward 2,3-BPG. We used an assay in which 2-PG (but not 3-PG) is enzymatically coupled to NADH oxidation (see Materials and Methods). In these experiments, a 60-nmol aliquot of 2,3-BPG was completely hydrolyzed to 2-PG (Fig. 4D, filled circles). We conclude that MIPP1 selectively hydrolyzes the 3-phosphate from 2,3-BPG in contrast to HsBPGM, which specifically removes the 2-phosphate (Fig. 3B) (31). Thus, hydrolysis of 2,3-BPG to 2-PG is a glycolytic reaction that bypasses both phosphoglycerate kinase and monophosphoglycerate mutase in vivo (Fig. 3B).
We also investigated whether HsMIPP1 might have mutase activity. However, it was unable to form 2-PG from 60 nmol 3-PG (Fig. 4D, open circles). Note that HsBPGM needs catalytic amounts of 2,3-BPG as a cofactor to support the conversion of 3-PG to 2-PG (4, 32). When MIPP1 was incubated with 60 nmol 3-PG plus 60 nmol 2,3-BPG, only 60 nmol 2-PG were formed (from hydrolysis of 2,3-BPG, not by mutase activity on 3-PG) (Fig. 4D). Thus, MIPP1 does not display any phosphoglycerate mutase activity.
The Effect of pH on MIPP1 and Its Regulatory Significance.
As hemoglobin releases oxygen, its affinity for H+ increases, causing intracellular alkalinization (11). This elevated intracellular pH drives a positive feedback loop, increasing levels of 2,3-BPG, thereby facilitating more oxygen release (11). Hyperventilation-induced alkalosis at high altitudes is another situation in which an increased pH is associated with adaptive increases in erythrocyte 2,3-BPG levels (9). However, the mechanisms by which pH affects intracellular levels of 2,3-BPG are not fully understood (11). We now report that the dephosphorylation of 2,3-BPG by MIPP1 is especially sensitive to pH, with enzyme activity decreasing ≈50% upon elevating pH within the physiological range (33) of 7 to 7.4 (Fig. 4E). Thus, MIPP1 is well suited to the task of adjusting erythrocyte 2,3-BPG levels in response to changes in both pH and tissue oxygen demand.
Concluding Comments.
Glycolytic enzymes are among the most ancient and conserved of all proteins (4). However, in certain organisms and in some cell types, individual enzymes in this pathway take on ancillary, regulatory roles by controlling the turnover of specific metabolic intermediates that have additional functions. In the current study, the Rapoport–Luebering shunt is shown to have considerably more biological significance than has previously been appreciated. We have demonstrated that this glycolytic bypass includes an extra, and hitherto unrecognized, metabolic step that is conserved from Dictyostelium to mammals: a 2,3-BPG 3-phosphatase activity catalyzed by MIPP1 (Fig. 3B). There are several reasons for considering this phosphatase activity to have regulatory significance.
First, the addition of MIPP1 to the Rapoport–Luebering pathway offers an opportunity for the cell to separately regulate both the synthesis and degradation of 2,3-BPG in ways that are not possible if only a single enzyme, BPGM, were to be solely responsible for both reactions. The regulatory potential of Mipp1 is illustrated by our data showing substantial changes in 2,3-BPG levels in vivo when expression of the Dictyostelium phosphatase was genetically manipulated (Fig. 2). Moreover, in mammalian erythrocytes, we estimated that the capacity of MIPP1 to hydrolyze 2,3-BPG is on par with BPGM, which was previously thought to be the only 2,3-BPG phosphatase. Indeed, our data answer the long-standing puzzle in the field, whereby measurements of 2,3-BPG metabolism in vivo indicated it to be in excess of the capacity of BPGM (11, 12).
In Dictyostelium, and perhaps also in other eukaryotic cells, Mipp1 offers the ability to link inositol phosphate metabolism, glycolytic flux, and anaerobic ATP synthesis. The occurrence of such a link has been suggested previously (5), but evidence for a mechanism was lacking. The 2,3-BPG 3-phosphatase activity of MIPP1 also is of regulatory importance because it provides a means to bypass both PGM, a p53-dependent control point in glycolysis (34), and 3-PG, a glycolytic intermediate (Fig. 3B). The latter activates the AMPK signaling cascade (13) and also functions as a precursor for serine biosynthesis (14).
The sensitivity of MIPP1 to physiologically relevant increases in cellular pH (Fig. 4E) provides a mechanistic explanation for the pH-dependent regulation of 2,3-BPG levels in erythrocytes. As explained in the previous section, this observation considerably improves our understanding of the homeostatic mechanisms that control tissue oxygen delivery. These results are especially significant in that they solve a long-standing puzzle as to the function for human erythrocyte MIPP1 in cells that do not contain any inositol phosphates.
The fact that BPGM both synthesizes and metabolizes 2,3-BPG at the same active site has made its phosphatase activity difficult to specifically target by pharmacological means. However, mammalian erythrocyte MIPP1 offers a potential alternative, specific target. A drug that inhibits MIPP1 may be therapeutically useful by acutely increasing cellular 2,3-BPG levels and improving oxygen delivery to tissues in a number of clinical situations in which oxygen deprivation is a problem. Examples include respiratory distress, cardiovascular failure, stroke, or during cardiopulmonary bypass surgery. Our demonstration that HsMIPP1 is active as a 2,3-BPG phosphatase even at 4°C (Fig. 4B) suggests that its inhibition might preserve 2,3-BPG levels in erythrocytes stored before transfusion. Genetic-based therapies also are on the horizon after recent advances that make it possible to produce erythrocytes from hematopoietic stem cells ex vivo (35).
Materials and Methods
Dictyostelium Strains and Culture.
All Dictyostelium discoideum strains were generated in the Ax2 wild-type background. Mipp1 (the single active homologue of HsMIPP1; DDB0186447) was disrupted by using standard techniques to generate the mipp1-null strain. Cells overexpressing full-length, untagged Mipp1 were generated by electroporation of Ax2 cells with the plasmid pJSK166, generated by cloning the full coding sequence into the Dictyostelium extrachromosomal expression vector pRHI8. Complete details of these constructs and strains were described previously (36).
Cells were grown on SM agar plates (10 g/liter proteose peptone, 1 g/liter yeast extract, 10 g/liter glucose, 1.9 g/liter KH2PO4, 1.3 g/liter K2HPO4.3H2O, 0.49 g/liter MgSO4, and 17 g/liter agar) on a lawn of Klebsiella aerogenes by standard methods. Cells were harvested when most of the bacteria were consumed. Residual bacteria were removed by repeated washing with buffer comprising 16.5 mM KH2PO4 and 3.8 mM K2HPO4 (pH 6.2). Cell pellets were then frozen on dry ice before analysis.
2,3-BPG Hydrolysis by Dictyostelium Cell Extracts.
Particulate fractions from Dictyostelium cell lysates (15) were incubated (0.5 mg of protein per ml) at 22°C in buffer containing 20 mM triethanolamine (pH 6.5), 5.9 mM EGTA, 0.5 mM EDTA, and 0.1 mM 2,3-BPG. The 100-μl aliquots were quenched in 1 vol 20% trichloroacetic acid/2 mM EDTA. Protein was removed by centrifugation, and acid was removed by four extractions with 2 vol diethyl ether. Final pH was adjusted to 7.0 with 1 M triethanolamine (pH 8.0). The 2,3-BPG levels were then measured by MDD-HPLC (37, 38) by using Tricorn Mini Q 4.6/50 PE columns (GE Healthcare). The column was eluted at a flow rate of 1 ml/min, with a gradient generated by mixing buffer A (21 μM YCl3) and buffer B (0.8M HCl and 25 μM YCl3). A linear gradient of 5–20% B over 7.5 min was used. The postcolumn detection reagent (0.4 ml/min) was 2.13 M triethanolamine (Merck) containing 500 μM 4-(2-pyridylazo)resorcinol (pH 9.75) (Merck). Absorbance data (520 nm) were recorded with an inline detector.
Assay of Cellular 2,3-BPG Levels.
Cells (109) were extracted with 1.5 ml of 2 M PCA and then neutralized with 1.95 ml of 1 M KOH. The 2,3-BPG was then purified by a modification of a procedure published previously (32). Samples were diluted to 50 ml with water and were applied to 4 × 0.8-cm AG 1-X8 anion-exchange columns (Cl− form; Bio-Rad). The column was washed with 5 ml of water, followed by 5 ml of 0.1 M HCl; then the 2,3-BPG was eluted with 5 ml of 0.2 M HCl, which was lyophilized to dryness under vacuum. Control experiments indicated a 76% recovery of 2,3-BPG through these procedures. The 2,3-BPG was measured by coupling its hydrolysis to NADH oxidation (measured at 340 nm) by lactate dehydrogenase (39). These assays (1 ml) contained 16 μmol Tris·HCl (pH 7.5), 3 μmol KCl, 4 μmol MgCl2, 1 μmol ADP, 0.1 μmol NADH, 1 μmol 2-phosphoglycolic acid, 12.5 units phosphoglycerate mutase, 4 units enolase, 4 units pyruvate kinase, and 5.5 units lactate dehydrogenase.
Quantification of the Mipp1 Activity of Rat Erythrocytes.
Briefly, ≈4 ml of rat blood was drawn into a syringe containing 1 ml of ice-cold 2% disodium citrate (freshly prepared by adjusting 2% trisodium citrate to pH 5.0 with HCl) plus 0.2 ml 15% glucose. The erythrocytes were sedimented by centrifugation (2,500 × g for 5 min), the plasma and white cells were aspirated, and the erythrocytes were washed three times with 3 ml of 0.154 M NaCl and 1.5 mM Hepes (pH 7.2). The erythrocytes were then solubilized for 1 h at 4°C in an equal volume of buffer A comprising 10 mM Bis-Tris (pH 7.4), 1 mM EDTA, 4% (wt/vol) CHAPS, 1 μg/ml leupeptin, and 0.1 μg/ml aprotinin. The preparation was loaded onto a MonoQ 10/10 anion-exchange column, which was eluted with a gradient generated by mixing buffer A with buffer B (buffer A plus 1 M KCl) as follows: 0–5 min, 0% B; 5–33 min, B increased from 0–30%. The flow rate was 1 ml/min, with 1-ml fractions collected. The Ins(1,3,4,5)P4 3-phosphatase activity of rat MIPP1 was assayed at 37°C in the following buffer: 100 mM KCl, 25 mM Hepes (pH 7.2), 0.02% (wt/vol) CHAPS, 1 mM EDTA, and 5 μM [3H]Ins(1,3,4,5)P4 (10,000 dpm per assay). The absence of [Mg2+] eliminates Ins(1,3,4,5)P4 5-phosphatase. Reactions were analyzed by gravity-fed anion-exchange columns (27).
Enzymes and Other Assays.
The His89Ala mutant of human MIPP1 was created by site-directed mutagenesis (QuikChange; Stratagene) by using MIPP1 plasmid as a template with the following sense primer: 5′-CTGGTCGCCCTCATTCGCGCCACCCGCTACCCCACG-3′ (mutagenic bases are underlined) and its complementary antisense primer. Recombinant avian, human, and rat MIPP1 proteins were each prepared as described previously (22, 27). Deglycosylation of the purified HsMIPP1 (13.5 μg) was performed by using 12 μg (0.3 units) of endoglycosidase Hf (New England Biolabs) for 2 h at 37°C according to the manufacturer's instructions. A Pi release assay (22) was used to study the dephosphorylation of 1 mM InsP6 and 5 mM 2,3-BPG (22) in (unless otherwise stated) 50 mM Tris·HCl (pH 7.4). Kinetic parameters were determined by nonlinear regression analysis from substrate-saturation plots by using SigmaPlot. Bisphosphoglycerate synthase was assayed as described previously (32).
Supplementary Material
Acknowledgments.
We thank Drs. Andrew Craxton, James Caffrey, and Martina Sumner for assistance. This work was supported by a Wellcome Trust Program Grant (to A.J.H.), a Biotechnology and Biological Sciences Research Council studentship (to J.S.K.), and the Intramural Research Program of the National Institutes of Health/National Institute of Environmental Health Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710980105/DCSupplemental.
References
- 1.Kauffman KJ, Pajerowski JD, Jamshidi N, Palsson BO, Edwards JS. Description and analysis of metabolic connectivity and dynamics in the human red blood cell. Biophys J. 2002;83:646–662. doi: 10.1016/S0006-3495(02)75198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rapoport I, Berger H, Elsner R, Rapoport S. pH-dependent changes of 2,3-bisphosphoglycerate in human red cells during transitional and steady states in vitro. Eur J Biochem. 1977;73:421–427. doi: 10.1111/j.1432-1033.1977.tb11333.x. [DOI] [PubMed] [Google Scholar]
- 3.Stryer L. Biochemistry. 2nd Ed. San Francisco: Freeman; 1980. pp. 276–278. [Google Scholar]
- 4.Fothergill-Gilmore LA, Michels PA. Evolution of glycolysis. Prog Biophys Mol Biol. 1993;59:105–235. doi: 10.1016/0079-6107(93)90001-z. [DOI] [PubMed] [Google Scholar]
- 5.Fischbach A, Adelt S, Muller A, Vogel G. Disruption of inositol biosynthesis through targeted mutagenesis in Dictyostelium discoideum: Generation and characterization of inositol-auxotrophic mutants. Biochem J. 2006;397:509–518. doi: 10.1042/BJ20060277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benesch R, Benesch RE. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967;26:162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- 7.Benesch R, Benesch RE, Yu CI. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci USA. 1968;59:526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duhm J, Gerlach E. On the mechanisms of the hypoxia-induced increase of 2,3-diphosphoglycerate in erythrocytes. Studies on rat erythrocytes in vivo and on human erythrocytes in vitro. Pflugers Arch. 1971;326:254–269. doi: 10.1007/BF00592506. [DOI] [PubMed] [Google Scholar]
- 9.Samaja M, Brenna L, Allibardi S, Cerretelli P. Human red blood cell aging at 5,050-m altitude: A role during adaptation to hypoxia. J Appl Physiol. 1993;75:1696–1701. doi: 10.1152/jappl.1993.75.4.1696. [DOI] [PubMed] [Google Scholar]
- 10.Wallis JP, Wells AW, Whitehead S, Brewster N. Recovery from post-operative anaemia. Transfus Med. 2005;15:413–418. doi: 10.1111/j.1365-3148.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 11.Mulquiney PJ, Kuchel PW. Model of 2,3-bisphosphoglycerate metabolism in the human erythrocyte based on detailed enzyme kinetic equations: Computer simulation and metabolic control analysis. Biochem J. 1999;342(Pt 3):597–604. [PMC free article] [PubMed] [Google Scholar]
- 12.Mulquiney PJ, Bubb WA, Kuchel PW. Model of 2,3-bisphosphoglycerate metabolism in the human erythrocyte based on detailed enzyme kinetic equations: in vivo kinetic characterization of 2,3-bisphosphoglycerate synthase/phosphatase using 13C and 31P NMR. Biochem J. 1999;342(Pt 3):567–580. [PMC free article] [PubMed] [Google Scholar]
- 13.Ellingson WJ, Chesser DG, Winder WW. Effects of 3-phosphoglycerate and other metabolites on the activation of AMP-activated protein kinase by LKB1-STRAD-MO25. Am J Physiol Endocrinol Metab. 2007;292:E400–E407. doi: 10.1152/ajpendo.00322.2006. [DOI] [PubMed] [Google Scholar]
- 14.Albers E, Laize V, Blomberg A, Hohmann S, Gustafsson L. Ser3p (Yer081wp) and Ser33p (Yil074cp) are phosphoglycerate dehydrogenases in Saccharomyces cerevisiae. J Biol Chem. 2003;278:10264–10272. doi: 10.1074/jbc.M211692200. [DOI] [PubMed] [Google Scholar]
- 15.Van Dijken P, et al. A novel, phospholipase C-independent pathway of inositol 1,4,5-trisphosphate formation in Dictyostelium and rat liver. J Biol Chem. 1995;270:29724–29731. doi: 10.1074/jbc.270.50.29724. [DOI] [PubMed] [Google Scholar]
- 16.Eichinger L, et al. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katti MV, Sami-Subbu R, Ranjekar PK, Gupta VS. Amino acid repeat patterns in protein sequences: Their diversity and structural-functional implications. Protein Sci. 2000;9:1203–1209. doi: 10.1110/ps.9.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephens LR, Irvine RF. Stepwise phosphorylation of myo-inositol leading to myo-inositol hexakisphosphate in dictyostelium. Nature. 1990;346:580–583. doi: 10.1038/346580a0. [DOI] [PubMed] [Google Scholar]
- 19.Chi H, et al. Targeted deletion of Minpp1 provides new insight into the activity of multiple inositol polyphosphate phosphatase in vivo. Mol Cell Biol. 2000;20:6496–6507. doi: 10.1128/mcb.20.17.6496-6507.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali N, Craxton A, Shears SB. Hepatic Ins(1,3,4,5)P4 3-phosphatase is compartmentalized inside endoplasmic reticulum. J Biol Chem. 1993;268:6161–6167. [PubMed] [Google Scholar]
- 21.Caffrey JJ, Hidaka K, Matsuda M, Hirata M, Shears SB. The human and rat forms of multiple inositol polyphosphate phosphatase: Functional homology with a histidine acid phosphatase up-regulated during endochondral ossification. FEBS Lett. 1999;442:99–104. doi: 10.1016/s0014-5793(98)01636-6. [DOI] [PubMed] [Google Scholar]
- 22.Cho J, et al. Avian multiple inositol polyphosphate phosphatase is an active phytase that can be engineered to help ameliorate the planet's “phosphate crisis.”. J Biotechnol. 2006;126:248–259. doi: 10.1016/j.jbiotec.2006.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tauler A, Gil J, Bartrons R, Carreras J. Levels of glycerate 2,3-P2, 2,3-bisphosphoglycerate synthase and 2,3-bisphosphoglycerate phosphatase activities in rat tissues. A method to quantify blood contamination of tissue extracts. Comp Biochem Physiol B. 1987;86:11–13. doi: 10.1016/0305-0491(87)90167-2. [DOI] [PubMed] [Google Scholar]
- 24.Craxton A, Ali N, Shears SB. Comparison of the activities of a multiple inositol phosphate phosphatase from several sources: A search for heterogeneity in this enzyme. Biochem J. 1995;305:491–498. doi: 10.1042/bj3050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estrada-Garcia T, Craxton A, Kirk CJ, Michell RH. A salt-activated inositol 1,3,4,5-tetrakisphosphate 3-phosphatase at the inner surface of the human erythrocyte membrane. Proc R Soc London. 1991;244:63–68. doi: 10.1098/rspb.1991.0052. [DOI] [PubMed] [Google Scholar]
- 26.Garel M-C, et al. Amino acid residues involved in the catalytic site of human erythrocyte bisphosphoglycerate mutase. Functional consequences of substitutions of His10, His187 and Arg89. Eur J Biochem. 1993;213:493–500. doi: 10.1111/j.1432-1033.1993.tb17786.x. [DOI] [PubMed] [Google Scholar]
- 27.Craxton A, Caffrey JJ, Burkhart W, Safrany ST, Shears SB. Cloning and expression of rat hepatic multiple inositol polyphosphate phosphatase. Biochem J. 1997;328:75–81. doi: 10.1042/bj3280075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Momsen G, Vestergaard-Bogind B. Human erythrocyte 2,3-diphosphoglycerate metabolism. Influence of 1,3-diphosphoglycerate and Pi. In vitro studies at low pH with computer simulations. Arch Biochem Biophys. 1978;190:67–84. doi: 10.1016/0003-9861(78)90254-0. [DOI] [PubMed] [Google Scholar]
- 29.Hogman CF, Lof H, Meryman HT. Storage of red blood cells with improved maintenance of 2,3-bisphosphoglycerate. Transfusion. 2006;46:1543–1552. doi: 10.1111/j.1537-2995.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 30.Isaacks RE, Lai LL, Goldman PH, Kim CY. Studies on avian erythrocyte metabolism. XVI. Accumulation of 2,3-bisphosphoglycerate with shifts in oxygen affinity of chicken erythrocytes. Arch Biochem Biophys. 1987;257:177–185. doi: 10.1016/0003-9861(87)90556-x. [DOI] [PubMed] [Google Scholar]
- 31.Rose ZB, Liebowitz J. 2,3-diphosphoglycerate phosphatase from human erythrocytes. General properties and activation by anions. J Biol Chem. 1970;245:3232–3241. [PubMed] [Google Scholar]
- 32.Ravel P, Garel MC, Toullec D. New procedures to measure synthase and phosphatase activities of bisphosphoglycerate mutase. Interest for development of therapeutic drugs. C R Acad Sci III. 1997;320:27–33. doi: 10.1016/s0764-4469(99)80083-3. [DOI] [PubMed] [Google Scholar]
- 33.Jensen FB. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol Scand. 2004;182:215–227. doi: 10.1111/j.1365-201X.2004.01361.x. [DOI] [PubMed] [Google Scholar]
- 34.Ma W, Sung HJ, Park JY, Matoba S, Hwang PM. A pivotal role for p53: Balancing aerobic respiration and glycolysis. J Bioenerg Biomembr. 2007;39:243–246. doi: 10.1007/s10863-007-9083-0. [DOI] [PubMed] [Google Scholar]
- 35.Douay L, Andreu G. Ex vivo production of human red blood cells from hematopoietic stem cells: What is the future in transfusion? Transfus Med Rev. 2007;21:91–100. doi: 10.1016/j.tmrv.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 36.King JS. Cardiff, UK: Cardiff University; 2007. The effects of inositol metabolism on lithium sensitivity and chemotaxis in Dictyostelium discoideum. PhD thesis. [Google Scholar]
- 37.Mayr GW. A novel metal-dye detection system permits picomolar-range h. p.l.c. analysis of inositol polyphosphates from non-radioactively labelled cell or tissue specimens. Biochem J. 1988;254:585–591. doi: 10.1042/bj2540585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casals I, Villar JL, Riera-Codina M. A straightforward method for analysis of highly phosphorylated inositols in blood cells by high-performance liquid chromatography. Anal Biochem. 2002;300:69–76. doi: 10.1006/abio.2001.5449. [DOI] [PubMed] [Google Scholar]
- 39.Rosa R, Prehu MO, Beuzard Y, Rosa J. The first case of a complete deficiency of diphosphoglycerate mutase in human erythrocytes. J Clin Invest. 1978;62:907–915. doi: 10.1172/JCI109218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jedrzejas MJ. Structure, function, and evolution of phosphoglycerate mutases: comparison with fructose-2,6-bisphosphatase, acid phosphatase, and alkaline phosphatase. Prog Biophys Mol Biol. 2000;73:263–287. doi: 10.1016/s0079-6107(00)00007-9. [DOI] [PubMed] [Google Scholar]
- 41.Garel MC, et al. A recombinant bisphosphoglycerate mutase variant with acid phosphatase homology degrades 2,3-diphosphoglycerate. Proc Natl Acad Sci USA. 1994;91:3593–3597. doi: 10.1073/pnas.91.9.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.