Abstract
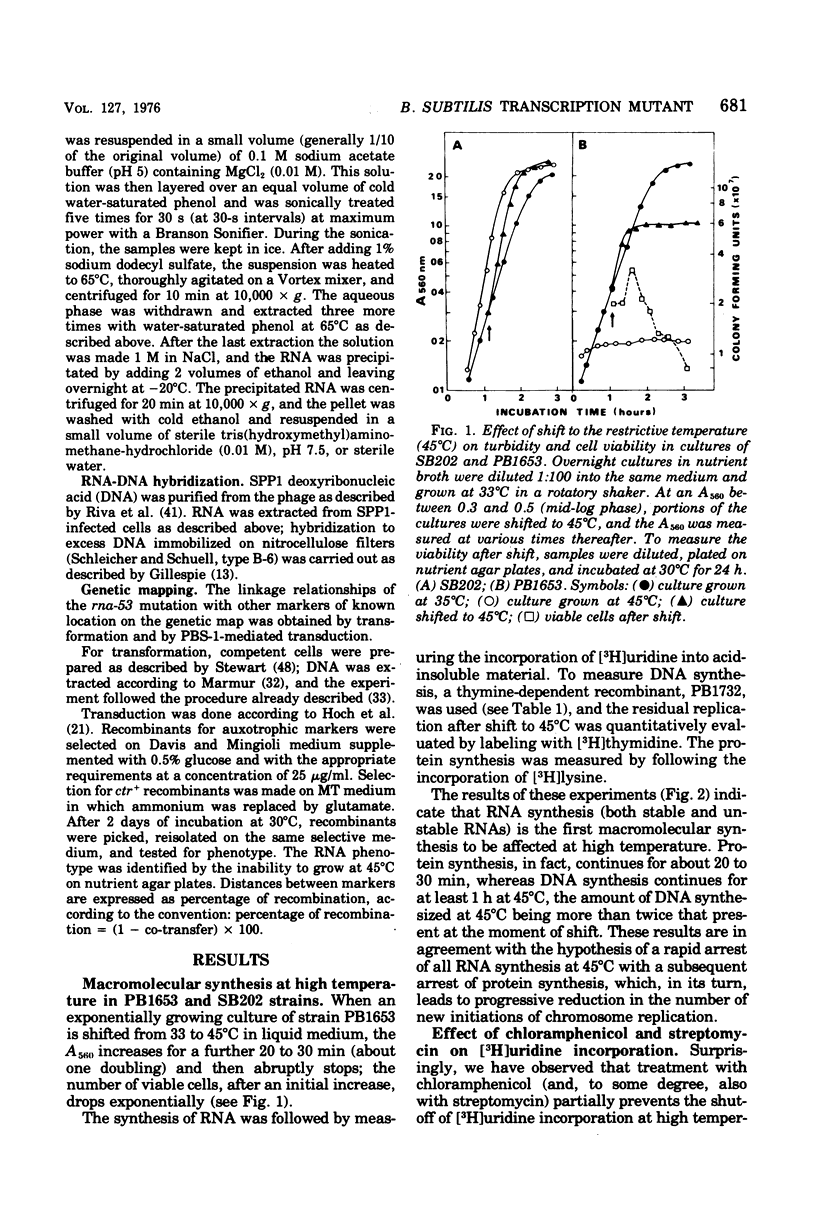
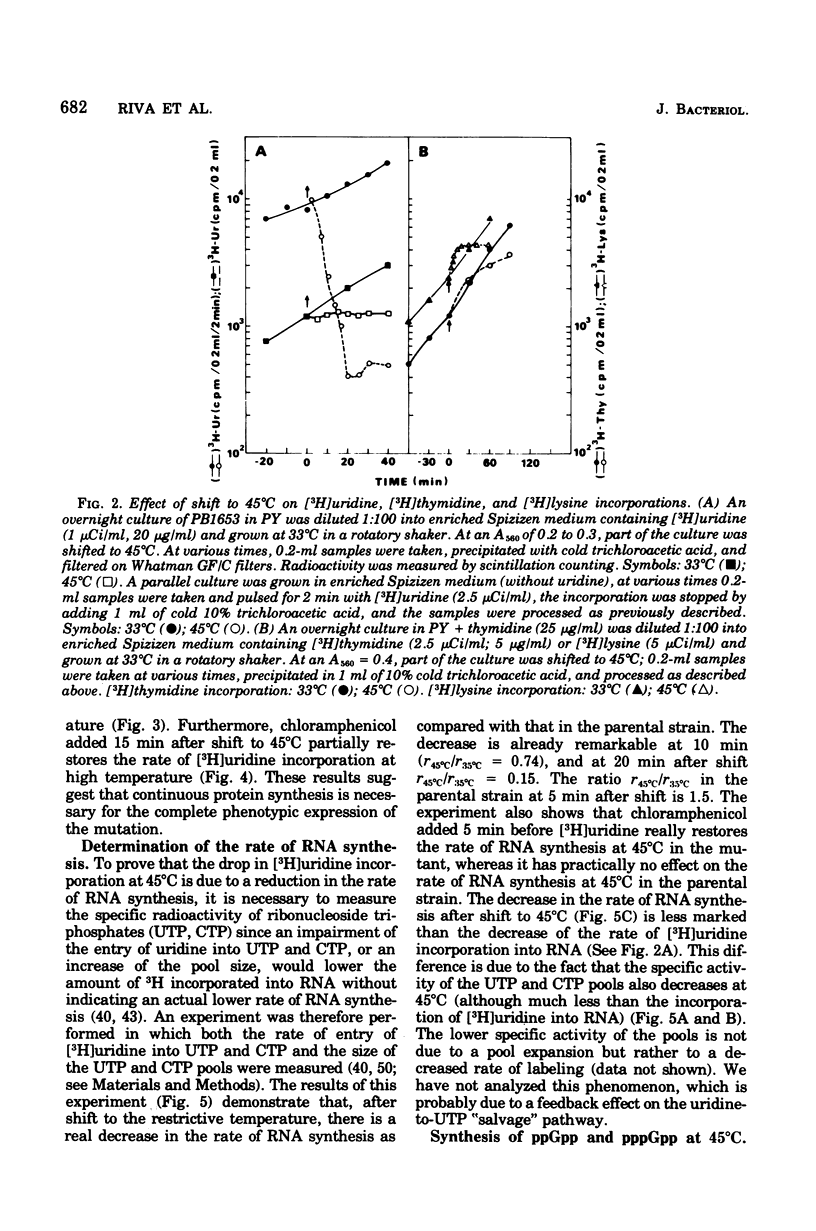
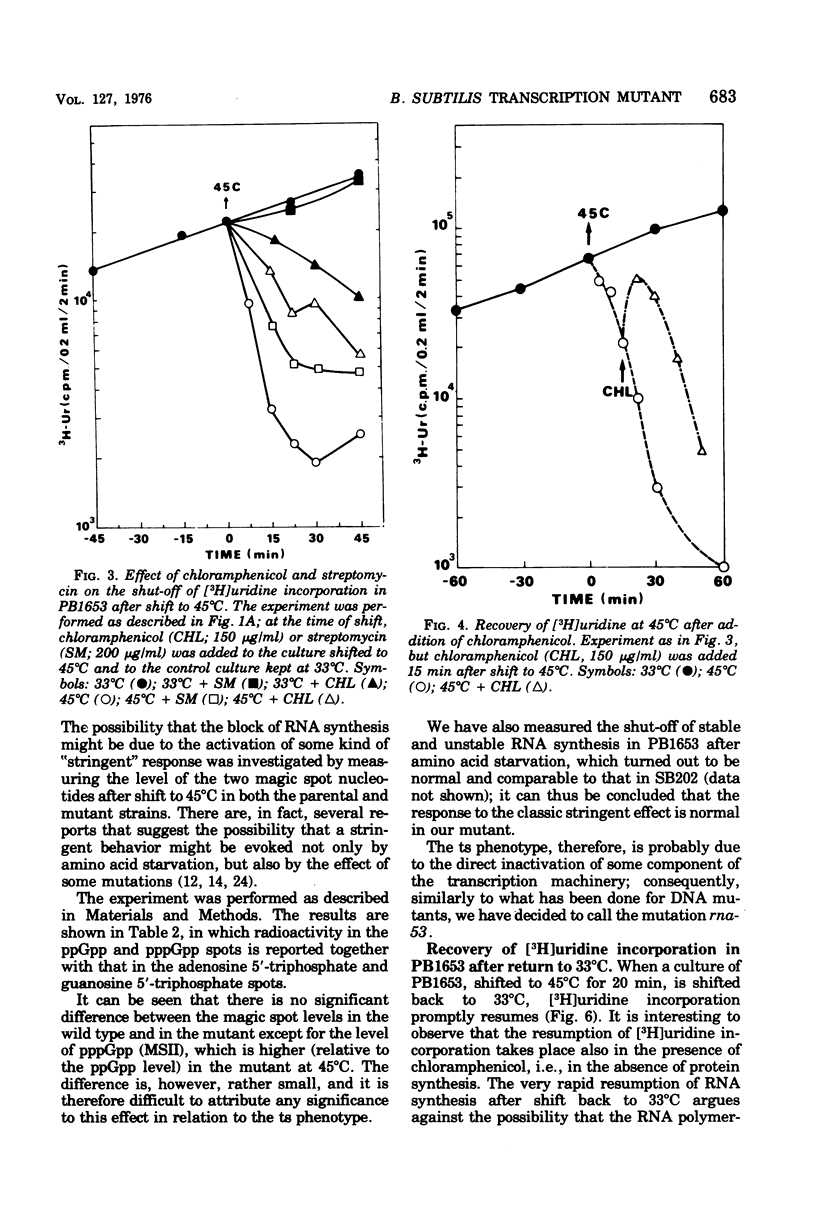
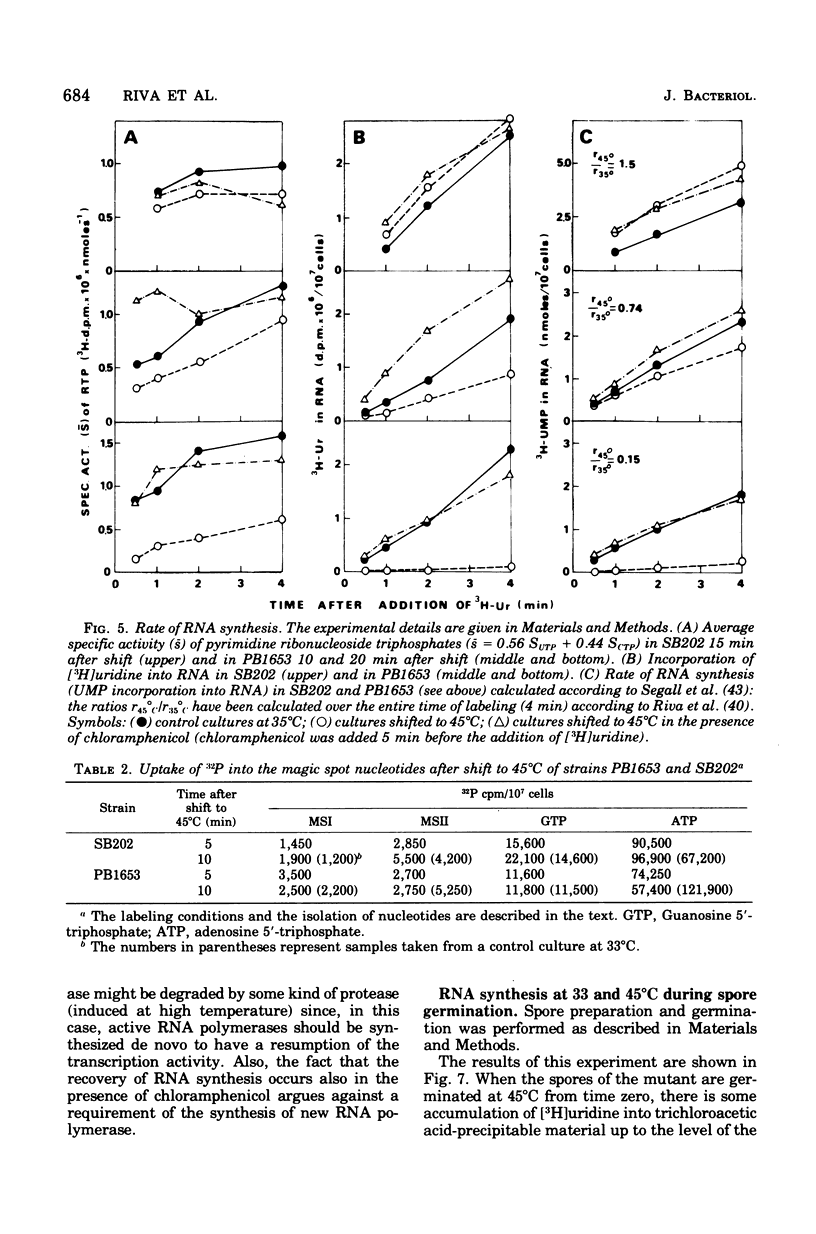
A Bacillus subtilis temperature-sensitive mutant (PB1653) has been isolated in which the rate of ribonucleic acid (RNA) synthesis sharply decreases after shift to 45 degrees C. Both stable and unstable RNAs are affected by the mutation. The possibility that the block of transcription at high temperature could be due to a "stringent" effect, mediated by an increase in the concentration of "magic spot" nucleotides, has been ruled out. Treatment with chloramphenicol (or streptomycin) rapidly restores the rate of RNA synthesis at 45 degrees C. The synthesis of RNA in the mutant during the early phases of spore germination is not temperature sensitive. The phage-specific transcription during infection with SPP1 phage, at high temperature, is less affected than that of the bacterial chromosome. In vitro experiments indicate that, in the mutant at high temperature, RNA polymerase undergoes a change in template specificity. The rna-53 mutation has been located on the B. subtilis genetic map near the hisA locus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. L., Sueoka N. Phase transitions in ribonucleic acid synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1968 Jan;59(1):153–160. doi: 10.1073/pnas.59.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal N., Kleinschmidt A. K., Spatz H. C., Trautner T. A. Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet. 1967;100(1):39–55. doi: 10.1007/BF00425774. [DOI] [PubMed] [Google Scholar]
- Brevet J. Direct assay for sigma factor activity and demonstration of the loss of this activity during sporulation in Bacillus subtilis. Mol Gen Genet. 1974 Feb 6;128(3):223–221. doi: 10.1007/BF00267111. [DOI] [PubMed] [Google Scholar]
- Buu A., Sonenshein A. L. Nucleic acid synthesis and ribonucleic acid polymerase specificity in germinating and outgrowing spores of Bacillus subtilis. J Bacteriol. 1975 Oct;124(1):190–200. doi: 10.1128/jb.124.1.190-200.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Duffy J. J., Geiduschek E. P. RNA polymerase from phage SP01-infected and uninfected Bacillus subtilis. J Biol Chem. 1975 Jun 25;250(12):4530–4541. [PubMed] [Google Scholar]
- Duffy J. J., Petrusek R. L., Geiduschek E. P. Conversion of Bacillus subtilis RNA polymerase activity in vitro by a protein induced by phage SP01. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2366–2370. doi: 10.1073/pnas.72.6.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esche H. Gene expression of bacteriophage SPP1. II. Regulatory aspects. Mol Gen Genet. 1975 Dec 23;142(1):57–66. doi: 10.1007/BF00268755. [DOI] [PubMed] [Google Scholar]
- Esche H., Schweiger M., Trautner T. A. Gene expression of bacteriophage SPPI. I. Phage directed protein synthesis. Mol Gen Genet. 1975 Dec 23;142(1):45–55. [PubMed] [Google Scholar]
- Friesen J. D., Fiil N. P., Parker J. M., Haseltine W. A. A new relaxed mutant of Escherichia coli with an altered 50S ribosomal subunit. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3465–3469. doi: 10.1073/pnas.71.9.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier K., Schlessinger D. Magic spot metabolism in an Escherichia coli mutant temperature sensitive in elongation factor Ts. J Bacteriol. 1974 Mar;117(3):1195–1200. doi: 10.1128/jb.117.3.1195-1200.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf A. L., Linn T. G., Losick R. Isolation of a new RNA polymerase-binding protein from sporulating Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):490–494. doi: 10.1073/pnas.70.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Hansen J. N., Spiegelman G., Halvorson H. O. Bacterial spore outgrowth: its regulation. Science. 1970 Jun 12;168(3937):1291–1298. doi: 10.1126/science.168.3937.1291. [DOI] [PubMed] [Google Scholar]
- Harford N. Bidirectional chromosome replication in Bacillus subtilis 168. J Bacteriol. 1975 Mar;121(3):835–847. doi: 10.1128/jb.121.3.835-847.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford N., Sueoka N. Chromosomal location of antibiotic resistance markers in Bacillus subtilis. J Mol Biol. 1970 Jul 28;51(2):267–286. doi: 10.1016/0022-2836(70)90142-7. [DOI] [PubMed] [Google Scholar]
- Haworth S. R., Brown L. R. Genetic analysis of ribonucleic acid polymerase mutants of Bacillus subtilis. J Bacteriol. 1973 Apr;114(1):103–113. doi: 10.1128/jb.114.1.103-113.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey C., Losick R., Sonenshein A. L. Ribosomal RNA synthesis is turned off during sporulation of Bacillus subtilis. J Mol Biol. 1971 Apr 14;57(1):59–70. doi: 10.1016/0022-2836(71)90119-7. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Magasanik B. Mechanism of beta-galactosidase induction in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):475–494. doi: 10.1016/0022-2836(67)90053-8. [DOI] [PubMed] [Google Scholar]
- Kimura A., Muto A., Osawa S. Control of stable RNA synthesis in a temperature-sensitive mutant of elongation factor G of Bacillus subtilis. Mol Gen Genet. 1974 May 31;130(3):203–214. doi: 10.1007/BF00268800. [DOI] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Leighton T. J. An RNA polymerase mutation causing temperature-sensitive sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1179–1183. doi: 10.1073/pnas.70.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesant-Kejzlarová J., Lepesant J. A., Walle J., Billault A., Dedonder R. Revision of the linkage map of Bacillus subtilis 168: indications for circularity of the chromosome. J Bacteriol. 1975 Mar;121(3):823–834. doi: 10.1128/jb.121.3.823-834.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. G., Greenleaf A. L., Shorenstein R. G., Losick R. Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1865–1869. doi: 10.1073/pnas.70.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T., Losick R., Sonenshein A. L. Rifampin resistance mutation of Bacillus subtilis altering the electrophoretic mobility of the beta subunit of ribonucleic acid polymerase. J Bacteriol. 1975 Jun;122(3):1387–1390. doi: 10.1128/jb.122.3.1387-1390.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Shorenstein R. G., Sonenshein A. L. Structural alteration of RNA polymerase during sporulation. Nature. 1970 Aug 29;227(5261):910–913. doi: 10.1038/227910a0. [DOI] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Mazza G., Eisenstark H. M., Serra M. C., Polsinelli M. Effect of caffeine on the recombination process of Bacillus subtilis. Mol Gen Genet. 1972;115(1):73–79. doi: 10.1007/BF00272219. [DOI] [PubMed] [Google Scholar]
- Milanesi G., Brevet J. Template specificity of transcription during sporulation of Bacillus subtilis. Nucleic Acids Res. 1974 Mar;1(3):397–412. doi: 10.1093/nar/1.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanesi G., Cassani G. Transcription after bacteriophage SPP1 infection in Bacillus subtilis. J Virol. 1972 Aug;10(2):187–192. doi: 10.1128/jvi.10.2.187-192.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanesi G., Melgara F. In vivo and in vitro transcription of SPP1 DNA by host RNA polymerase. J Virol. 1974 Dec;14(6):1613–1614. doi: 10.1128/jvi.14.6.1613-1614.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J., Kerjan P., Aubert J. P., Szulmajster J. Proteolytic conversion in vitro of B. subtilis vegetative RNA polymerase into the homologous spore enzyme. FEBS Lett. 1972 Jun 1;23(1):47–50. doi: 10.1016/0014-5793(72)80281-3. [DOI] [PubMed] [Google Scholar]
- Pero J., Nelson J., Fox T. D. Highly asymmetric transcription by RNA polymerase containing phage-SP01-induced polypeptides and a new host protein. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1589–1593. doi: 10.1073/pnas.72.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pero J., Tjian R., Nelson J., Losick R. In vitro transcription of a late class of phage SP01 genes. Nature. 1975 Sep 18;257(5523):248–251. doi: 10.1038/257248a0. [DOI] [PubMed] [Google Scholar]
- Riva S., Cascino A., Geiduschek E. P. Coupling of late transcription to viral replication in bacteriophage T4 development. J Mol Biol. 1970 Nov 28;54(1):85–102. doi: 10.1016/0022-2836(70)90447-x. [DOI] [PubMed] [Google Scholar]
- Riva S., Polsinelli M., Falaschi A. A new phage of Bacillus subtilis with infectious DNA having separable strands. J Mol Biol. 1968 Jul 28;35(2):347–356. doi: 10.1016/s0022-2836(68)80029-4. [DOI] [PubMed] [Google Scholar]
- Riva S., van Sluis C., Mastromei G., Attolini C., Mazza G., Polsinelli M., Falaschi A. A new mutant of Bacillus subtilis altered in the initiation of chromosome replication. Mol Gen Genet. 1975;137(3):185–202. doi: 10.1007/BF00333015. [DOI] [PubMed] [Google Scholar]
- Segall J., Tjian R., Pero J., Losick R. Chloramphenicol restores sigma factor activity to sporulating Bacillus subtilis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4860–4863. doi: 10.1073/pnas.71.12.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L., Cami B., Brevet J., Cote R. Isolation and characterization of rifampin-resistant and streptolydigin-resistant mutants of Bacillus subtilis with altered sporulation properties. J Bacteriol. 1974 Oct;120(1):253–265. doi: 10.1128/jb.120.1.253-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman G. B., Whiteley H. R. Purification of ribonucleic acid polymerase from SP82-infected Bacillus subtilis. J Biol Chem. 1974 Mar 10;249(5):1476–1482. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R. Physical heterogeneity among Bacillus subtilis deoxyribonucleic acid molecules carrying particular genetic markers. J Bacteriol. 1969 Jun;98(3):1239–1247. doi: 10.1128/jb.98.3.1239-1247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Losick R. An immunological assay for the sigma subunit of RNA polymerase in extracts of vegetative and sporulating Bacillus subtilis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2872–2876. doi: 10.1073/pnas.71.7.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow R. M., Lazzarini R. A. The rates of synthesis and chain elongation of ribonucleic acid in Escherichia coli. J Biol Chem. 1969 Mar 10;244(5):1128–1136. [PubMed] [Google Scholar]
- Yamagishi H., Takahashi I. Transducing particles of PBS 1. Virology. 1968 Dec;36(4):639–645. doi: 10.1016/0042-6822(68)90194-3. [DOI] [PubMed] [Google Scholar]


