Introduction
The structural and morphological aspects of heart development have been well-described over the past years. Continuing research is performed on the genetic pathways, transcription factors and growth factors important for determination and specification of the various components and segments of the cardiovascular system during embryogenesis. It is necessary to realize that the intricate expression patterns and cellular and molecular interactions take place in a growing embryo with an increasing oxygen and fuel consumption. This is accompanied by an expanding cardiovascular system that is continuously adapting to the changing needs of the embryo. As the cardiovascular system is the first to function in an embryo we have to incorporate in our scientific thinking the forces generated by the heart with the local effects on the various components and segments as early as the heart is present. The cyclic activity of the heart generates both pressure and flow, both dampened and transmitted by the physico-chemical characteristics of the wall of the system and of the blood, incorporating their changing composition during development.
Here, we will concentrate on the influence of the blood flow and the ensuing shear stress [5] in the rapidly changing geometry of the developing cardiovascular system. The forces exerted on the wall will activate intracellular signaling pathways inducing alterations in gene expression patterns that are at the basis of differentiation steps. As a consequence, abnormal flow patterns and the origin of congenital malformations are closely interlinked. The same holds for disease processes such as plaque formation after birth and during aging.
Geometrical changes during development
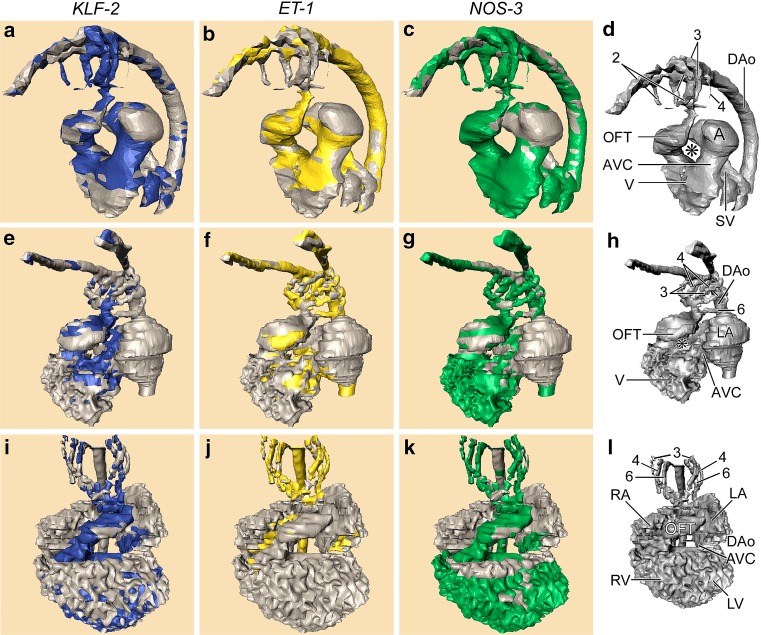
At the time of the first contractions, the heart is an almost straight tube with only a light curvature. The blood becomes pumped regularly from the caudally located venous pole towards the cranially located arterial pole. Here, the heart is connected to the aortic sac that divides in a bilateral set of pharyngeal (or branchial) arteries. The latter expands from only one left and right artery towards as many as five sets in a tightly controlled pattern of, e.g., adding the fourth set, whereas the first set disappears. The pharyngeal arch arterial system in whatever constellation in an early embryo, will aggregate towards the partly bilateral dorsal aorta (Fig. 1). Finally, the pharyngeal arterial system and dorsal aorta will become asymmetric because several segments disappear on one side, but persist on the contralateral side. Remnants of the disappearing vessels, however, can still be found as they remain connected to microcirculatory networks in, e.g., the craniofacial region. Flow dividers and complex 3D curves are inherent to the changing geometry of the arterial system and are, therefore, players in the shear stress theatre.
Fig. 1.
Computerized reconstructions after serial histological sections of embryos represent three subsequent stages of the developing chick heart. First row (a–d), left lateral view of Hamburger Hamilton (HH) stage18. Second row (e–h), left ventrolateral view HH24. Third row (i–l), ventral view HH27. The cardiac and vascular compartments are annotated in d, h and l, respectively. Note the disappearance of the second pharyngeal arch artery PAA (compare d, h) and the emergence of the sixth one in h. The asterisk denotes the inner curvature of the looping heart that becomes tighter during development. The outflow tract (OFT) is connected to the pharyngeal arch arteries (PAA). The OFT is located right sided in HH18, subsequently it becomes wedged between both atria and centrally localized in the cardiac contour in HH27. The stained parts in the columns represent the expression of the respective shear stress-induced genes, KLF2 (blue), ET1 (yellow) and NOS3 (green). Note that KLF2 is restricted to the narrow zones (AVC, OFT, PAA 2, 3, 4), whereas ET1 is preferentially localized in the wider segments. NOS3 is mainly absent from the wider atrial segments. Abbreviations: 2/3/4/6th 2nd, 3rd, 4th and 6th pharyngeal arch arteries, * inner curvature of the heart, A primitive Atrium, AVC Atrioventricular canal, DAo Dorsal aorta, LA left atrium, LV left ventricle, OFT Outflow tract, RA right atrium, RV right ventricle, SV sinus venosus, V primitive ventricle. Figure modified after [12
]
Meanwhile, the heart tube is looping three-dimensionally, while keeping the arterial and venous poles close together. The complex loop results in a tight inner curve and a wider outer curve (Fig. 1) with foreseeable differences in flow and shear profiles. The next step will be local expansion of the outer curve resulting in chamber formation, including atrium and ventricles. Separation of these chambers by septation and valve formation is very complex and beyond the scope of this paper. Nevertheless, this is important to understand the changes in flow in relatively late phases of cardiac development.
The rendering of correct and accurate 3D images of the growing and remodeling cardio-vascular system is far from trivial. Several approaches have been used to ascertain lumen boundaries and wall dimensions including optical [24, 54], echo-Doppler ultrasound [6, 24, 35] and even micro-MRI [21, 22, 42]. Casting techniques have provided solid lumen models of specific stages of development [39] that have been confirmed by computational fluid dynamics [7]. The combination of these approaches is needed to assess the reciprocal effects of wall movement by contraction and of fluid flow to generate frictional forces. The ensuing shear stress on the endothelial and endocardial inner lining of the vascular system will be transmitted either intracellularly to result in gene expression changes, e.g., Krüppel-like factor-2 [8], intra-epithelially as, e.g., calcium fluxes [36], or even trans-epithelially as signals towards underlying mesenchyme or myocardium [14].
The venous system connects to the sinus venosus and drains the various parts of the embryo and the extra-embryonic membranes, including the yolk sac and in mammals also the placenta. Although most of the blood is stored in the extra-embryonic membranes, forces are generally low here, and this part of the circulation is mostly ignored. Experimentally, however, it is used as an easily accessible gateway to change the inflow of the blood by, e.g., ligating yolk sac vessels in chicken embryos [13, 20].
Flow patterns in the embryo
It is evident that flow patterns and wall geometry are intricately linked. A linear heart tube with sequential chambers as in fish [24, 31] will show similar patterns as the embryonic heart tube in mammals and birds [12, 40]. It is challenging to model and quantify 3D changes during further development of cardiac looping. This step is essential to combine computational fluid dynamics [7, 24] and (micro-) particle velocimetry [23, 26, 54] with vascular wall characteristics, even serving the construction of artificial valves [48] in adult life.
Not only flow patterns are important but also the type of flow, being laminar, oscillatory or pulsatile. Furthermore, in vitro experiments demonstrate that KLF2 expression is time dependent [55]. In a continuously growing and remodeling embryonic vascular bed we have to realize that these aspects lead to temporal profiles of changes in shear and concurrent cellular reactions. For this reason reactions evoked under steady flow conditions in vitro are not easily transferable to in embryo situations.
Shear stress and gene expression
As blood flow is an aspect of the vascular system, which is lined with endothelium and endocardium, these cells are mostly influenced by shear stress. In isolated HUVEC an estimated 3% of the investigated genes are up- or down regulated by laminar shear stress [37] whereas in intact umbilical vein segments even 17% of the genes is affected by mechanical forces, nearly half of these being shear-related [1]. Most of the shear related genes, approximately 900 [1], are not changed by stretch from which it is concluded that endothelial cells are able to discriminate between shear and stretch forces.
Laminar shear stress activates integrins and thereby induces changes in gene expression such as MMP9, includes metabolic steps, such as MAPK phosphorylation and results in increased NFκB DNA binding [47]. NFκB is indeed an important intermediary between shear stress and changes in cellular function [16, 38], although the pathways may start differently, e.g., by activating integrins [47], TNFα [38], KLF2 [10, 29], TGFβ-SMAD2 [11], E-selectin [9] and many others. Many of these studies have been performed in vitro and in adult atherosclerosis settings [41]. Vascular remodeling has been linked to hemodynamic forces as well [32, 33], whereas the control of arterial branching has been ascribed to flow-driven regulation of genes such as VEGF and ephrin-B2 [28], The differentiation of the early vascular bed into arterial and venous conduits is likewise flow dependent.
Shear stress in the embryo
In embryonic stages few reports have demonstrated the involvement of laminar flow on changing gene expression patterns [12, 13, 29]. It is becoming increasingly important to discriminate between various types of flow as it has been demonstrated in adult vessels that laminar, oscillatory and pulsatile flow patterns exert different reactions with respect to the emergence of atherosclerotic events [4, 8, 52, 53]. Patterns of flow responsive genes such as ET1, KLF2 and NOS3 have been analyzed [12] in the early chicken embryo during looping of the cardiac tube (Fig. 1). These genes are differentially expressed in the endocardium and confine to areas with low (ET1) and high shear (KLF2 and NOS3). Low shear areas are found in the wide parts of the heart, whereas high shear is found in the narrow parts, i.e. the atrioventricular canal and the outflow tract. This is nicely illustrated when a computational fluid dynamical model [54] is superimposed on the geometry of the looping heart tube [13, 17] demonstrating low shear in the inner curve and high shear in the outer curve [18], co-localizing with the mentioned gene products.
Shear stress sensing
Endothelial cells are able to discriminate between cyclic stretch and shear stress [1]. The sensing mechanisms involves, e.g., integrins and caveolae, but also cell junctions and adhesion molecules [30, 49, 56]. The primary cilium also referred to as the cell’s antenna [34, 43] can be added as they are also present on endothelial and endocardial cells [25, 36, 53], even during embryonic development [52]. These cilia dissociate under high and under laminar flow showing their sensitivity to changing flow conditions. Central to these diverse “receptors” is the cytoskeleton, integrating both the microtubular and microfilamentous elements with the primary cilium (unpublished). Cytoskeletal deformation by blood flow, amplified by the primary cilium, activates the membrane linked effector molecules, which then signal to the nucleus to activate gene expression. After induced breakdown of the microtubules or the microfilaments in cells under various flow conditions, the expression of shear dependent genes changes, demonstrating the direct link between flow changes, cytoskeleton and differentiation. Essential in immediate primary cilium-mediated response appears to be a calcium signal as demonstrated in polaris-deficient mice [3] and in polycystic kidney disease, a mutant lacking cilia [36].
Changing hemodynamics results in cardiovascular malformations
About a decade ago, the venous clip model was introduced to analyze the effects of venous return to the heart [19, 20] by temporal or permanent ligation of a yolk sac vein, forcing the blood to make a deviation and return to the sinus venosus via a different route. Marking with minute amounts of dyes the return paths (or streamlines) from various veins, they showed that the complete venous flow pattern through the heart was changed. This situation was maintained over prolonged time. It was also evident that the various streamlines hardly mixed, even so in the contracting heart, and remained separate from each other after fusion of small veins into larger collecting vessels. After survival many of these embryos presented with major cardiovascular defects, including ventricular septal defects and outflow tract anomalies [2, 20]. From these results the conclusion seemed warranted that hemodynamic alterations are at the base of congenital cardiovascular defects [18]. The next step was to investigate gene expression patterns in the venous clipped embryos and they changed accordingly [13] providing a direct link between altered hemodynamics, changed gene expression patterns and congenital malformations.
Cardiac function has been assayed in these embryos [51] using Doppler-frequency detection methods. Combined with the heart rate, various parameters could be calculated including peak systolic, time averaged, and end diastolic velocities, mean blood flow and stroke volume. The increase of these parameters during normal development is used as golden standard for the interpretation of results after clipping [45, 50]. A combination with pressure-volume loop assessment [44] confirmed an increase in stiffness of the cardiac tube and reduced contractility by the changed mechanical load [46].
The pathways involved are also under investigation with pharmacological approaches using, e.g., epinephrin [27]. This causes a significant increase in heart rate, peak and mean velocities, peak and mean blood flows, stroke volume and aortic diameter. Endothelin receptor blockade resulted in diminished endothelin signaling that is different for the embryonic and the extra-embryonic part of the circulation. This is mainly due to the absence of the ETA receptor in the vitelline vascular system, while both the ETA and ETB receptors are present and apparently functioning in the embryo proper [15].
Summary
It is evident that hemodynamic factors have a dominant function already during early cardiogenesis. Flow and ensuing shear stress are sensed by endothelial cells by, ciliary modified, cytoskeletal deformation which then activates a number of subcellular structures and molecules. Shear stress dependent changes mostly converge towards NFκB signaling and DNA binding, thereby altering metabolic paths and influencing differentiation of the cells. Geometry of the vascular system heavily affects the flow and shear patterns, as is the case in the adult vasculature where atheroprone areas nicely coincide with the frequency of the primary cilium as shear stress sensor.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Andersson M, Karlsson L, Svensson PA, Ulfhammer E, Ekman M, Jernas M, Carlsson LM, Jern S. Differential global gene expression response patterns of human endothelium exposed to shear stress and intraluminal pressure. J Vasc Res. 2005;42:441–452. doi: 10.1159/000087983. [DOI] [PubMed] [Google Scholar]
- 2.Broekhuizen MLA, Hogers B, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC, Wladimiroff JW. Altered hemodynamics in chick embryos after extraembryonic venous obstruction. Ultrasound Obstet Gynecol. 1999;13:437–445. doi: 10.1046/j.1469-0705.1999.13060437.x. [DOI] [PubMed] [Google Scholar]
- 3.Cano DA, Murcia NS, Pazour GJ, Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development. 2004;131:3457–3467. doi: 10.1242/dev.01189. [DOI] [PubMed] [Google Scholar]
- 4.Cheng C, Helderman F, Tempel D, Segers D, Hierck BP, Poelmann RE, van Tol A, Duncker DJ, Robbers-Visser D, Ursem NT, van Haperen R, Wentzel JJ, Gijsen F, van der Steen AF, de Crom R, Krams R. Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis. 2007;195:225–235. doi: 10.1016/j.atherosclerosis.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Davies PF, Spaan JA, Krams R. Shear stress biology of the endothelium. Ann Biomed Eng. 2005;33:1714–1718. doi: 10.1007/s10439-005-8774-0. [DOI] [PubMed] [Google Scholar]
- 6.Dealmeida A, McQuinn T, Sedmera D. Increased ventricular preload is compensated by myocyte proliferation in normal and hypoplastic fetal chick left ventricle. Circ Res. 2007;100:1363–1370. doi: 10.1161/01.RES.0000266606.88463.cb. [DOI] [PubMed] [Google Scholar]
- 7.DeGroff CG, Thornburg BL, Pentecost JO, Thornburg KL, Gharib M, Sahn DJ, Baptista A. Flow in the early embryonic human heart: a numerical study. Pediatr Cardiol. 2003;24:375–380. doi: 10.1007/s00246-002-0343-9. [DOI] [PubMed] [Google Scholar]
- 8.Dekker RJ, van Thienen JV, Elderkamp YW, Seppen J, de Vries CJM, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJG. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular-tone regulating genes. Am J Pathol. 2005;167:609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dichiara MR, Kiely JM, Gimbrone MA, Jr, Lee ME, Perrella MA, Topper JN. Inhibition of E-selectin gene expression by transforming growth factor beta in endothelial cells involves coactivator integration of Smad and nuclear factor kappaB-mediated signals. J Exp Med. 2000;192:695–704. doi: 10.1084/jem.192.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fledderus JO, van Thienen JV, Boon RA, Dekker RJ, Rohlena J, Volger OL, Bijnens APJJ, Daemen MJAP, Kuiper J, van Berkel TJC, Pannekoek H, Horrevoets AJG. Prolonged shear stress and KLF2 suppress constitutive pro-inflammatory transcription through inhibition of ATF2. Blood. 2007;109:4249–4257. doi: 10.1182/blood-2006-07-036020. [DOI] [PubMed] [Google Scholar]
- 11.Gittenberger-de Groot AC, Azhar M, Molin DG. Transforming growth factor beta-SMAD2 signaling and aortic arch development. Trends Cardiovasc Med. 2006;16:1–6. doi: 10.1016/j.tcm.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Groenendijk BCW, Hierck BP, Gittenberger-de Groot AC, Poelmann RE. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev Dyn. 2004;230:57–68. doi: 10.1002/dvdy.20029. [DOI] [PubMed] [Google Scholar]
- 13.Groenendijk BCW, Hierck BP, Vrolijk J, Baiker M, Pourquie MJBM, Gittenberger-de-Groot AC, Poelmann RE. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ Res. 2005;96:1291–1298. doi: 10.1161/01.RES.0000171901.40952.0d. [DOI] [PubMed] [Google Scholar]
- 14.Groenendijk BCW, Van der Heiden K, Hierck BP, Poelmann RE. The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model. Physiology. 2007;22:380–389. doi: 10.1152/physiol.00023.2007. [DOI] [PubMed] [Google Scholar]
- 15.Groenendijk BCW, Vennemann P, Stekelenburg-de Vos S, Wladimiroff JW, Nieuwstadt FTM, Westerweel J, Hierck BP, Ursem NTC, Poelmann RE. The endothelin-1 pathway and the development of cardiovascular defects in the hemodynamically challenged chicken embryo. J Vasc Res. 2008;45:54–68. doi: 10.1159/000109077. [DOI] [PubMed] [Google Scholar]
- 16.Helderman F, Segers D, de Crom R, Hierck BP, Poelmann RE, Evans PC, Krams R. Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol. 2007;18:527–533. doi: 10.1097/MOL.0b013e3282ef7716. [DOI] [PubMed] [Google Scholar]
- 17.Hierck BP, Van der Heiden K, DeRuiter MC, Gittenberger-de-Groot AC, Poelmann RE. Fluid shear stress controls cardiovascular development. A functionomic approach. Wien Klin Wochenschr. 2007;119:10–13. [PubMed] [Google Scholar]
- 18.Hierck BP, Van der Heiden K, Poelmann RE (2008) Fluid shear stress and inner curve remodeling of the embryonic heart. Choosing the right lane! Scientific WorldJournal (in press) [DOI] [PMC free article] [PubMed]
- 19.Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circ Res. 1997;80:473–481. doi: 10.1161/01.RES.80.4.473. [DOI] [PubMed] [Google Scholar]
- 20.Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal. Cardiovasc Res. 1999;41:87–99. doi: 10.1016/S0008-6363(98)00218-1. [DOI] [PubMed] [Google Scholar]
- 21.Hogers B, Gross D, Lehmann V, DeGroot HJM, DeRoos A, Gittenberger-de Groot AC, Poelmann RE. Magnetic resonance microscopy at 17.6-Tesla on chicken embryos in vitro. J Magn Reson Imaging. 2001;14:83–86. doi: 10.1002/jmri.1155. [DOI] [PubMed] [Google Scholar]
- 22.Hogers B, Gross D, Lehmann V, Zick K, DeGroot HJM, Gittenberger-de Groot AC, Poelmann RE. Magnetic resonance microscopy of mouse embryos in utero. Anat Rec. 2000;260:373–377. doi: 10.1002/1097-0185(20001201)260:4<373::AID-AR60>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 23.Hove JR. Quantifying cardiovascular flow dynamics during early development. Pediatr Res. 2006;60:6–13. doi: 10.1203/01.pdr.0000219584.22454.92. [DOI] [PubMed] [Google Scholar]
- 24.Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 25.Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones EA, Baron MH, Fraser SE, Dickinson ME. Measuring hemodynamic changes during mammalian development. Am J Physiol Heart Circ Physiol. 2004;287:H1561–H1569. doi: 10.1152/ajpheart.00081.2004. [DOI] [PubMed] [Google Scholar]
- 27.Kroese JM, Broekhuizen ML, Poelmann RE, Mulder PG, Wladimiroff JW. Epinephrine affects hemodynamics of noninnervated normal and all-trans retinoic acid-treated embryonic chick hearts. Fetal Diagn Ther. 2004;19:431–439. doi: 10.1159/000078996. [DOI] [PubMed] [Google Scholar]
- 28.le Noble F, Fleury V, Pries A, Corvol P, Eichmann A, Reneman RS. Control of arterial branching morphogenesis in embryogenesis: go with the flow. Cardiovasc Res. 2005;65:619–628. doi: 10.1016/j.cardiores.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, Graf T, MacRae CA, Lepore JJ, Lo CW, Kahn ML. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell. 2006;11:845–857. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259:381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 31.Liebling M, Forouhar AS, Wolleschensky R, Zimmermann B, Ankerhold R, Fraser SE, Gharib M, Dickinson ME. Rapid three-dimensional imaging and analysis of the beating embryonic heart reveals functional changes during development. Dev Dyn. 2006;235:2940–2948. doi: 10.1002/dvdy.20926. [DOI] [PubMed] [Google Scholar]
- 32.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucitti JL, Tobita K, Keller BB. Arterial hemodynamics and mechanical properties after circulatory intervention in the chick embryo. J Exp Biol. 2005;208:1877–1885. doi: 10.1242/jeb.01574. [DOI] [PubMed] [Google Scholar]
- 34.Marshall WF, Nonaka S. Cilia: tuning in to the cell’s antenna. Curr Biol. 2006;16:R604–R614. doi: 10.1016/j.cub.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 35.McQuinn TC, Bratoeva M, Dealmeida A, Remond M, Thompson RP, Sedmera D. High-frequency ultrasonographic imaging of avian cardiovascular development. Dev Dyn. 2007;236:3503–3513. doi: 10.1002/dvdy.21357. [DOI] [PubMed] [Google Scholar]
- 36.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J (2008) Endothelial cilia are fluid-shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation (in press) [DOI] [PMC free article] [PubMed]
- 37.Ohura N, Yamamoto K, Ichioka S, Sokabe T, Nakatsuka H, Baba A, Shibata M, Nakatsuka T, Harii K, Wada Y, Kohro T, Kodama T, Ando J. Global analysis of shear stress-responsive genes in vascular endothelial cells. J Atheroscler Thromb. 2003;10:304–313. doi: 10.5551/jat.10.304. [DOI] [PubMed] [Google Scholar]
- 38.Partridge J, Carlsen H, Enesa K, Chaudhury H, Zakkar M, Luong L, Kinderlerer A, Johns M, Blomhoff R, Mason JC, Haskard DO, Evans PC. Laminar shear stress acts as a switch to regulate divergent functions of NF-{kappa}B in endothelial cells. FASEB J. 2007;21:3553–3561. doi: 10.1096/fj.06-8059com. [DOI] [PubMed] [Google Scholar]
- 39.Pentecost JO, Sahn DJ, Thornburg BL, Gharib M, Baptista A, Thornburg KL. Graphical and stereolithographic models of the developing human heart lumen. Comput Med Imaging Graph. 2001;25:459–463. doi: 10.1016/S0895-6111(01)00020-9. [DOI] [PubMed] [Google Scholar]
- 40.Reckova M, Rosengarten C, Dealmeida A, Stanley CP, Wessels A, Gourdie RG, Thompson RP, Sedmera D. Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circ Res. 2003;93:77–85. doi: 10.1161/01.RES.0000079488.91342.B7. [DOI] [PubMed] [Google Scholar]
- 41.Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LC, Wofovitz E. Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol. 2003;81:177–199. doi: 10.1016/S0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 42.Ruffins SW, Martin M, Keough L, Truong S, Fraser SE, Jacobs RE, Lansford R. Digital three-dimensional atlas of quail development using high-resolution MRI. Scientific WorldJournal. 2007;7:592–604. doi: 10.1100/tsw.2007.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 44.Stekelenburg-de Vos S, Steendijk P, Ursem NT, Wladimiroff JW, Delfos R, Poelmann RE. Systolic and diastolic ventricular function assessed by pressure–volume loops in the stage 21 venous clipped chick embryo. Pediatr Res. 2005;57:16–21. doi: 10.1203/01.PDR.0000147734.53277.75. [DOI] [PubMed] [Google Scholar]
- 45.Stekelenburg-de Vos S, Ursem NTC, Hop WCJ, Wladimiroff JW, Gittenberger-de Groot AC, Poelmann RE. Acutely altered hemodynamics following venous obstruction in the early chick embryo. J Exp Biol. 2003;206:1051–1057. doi: 10.1242/jeb.00216. [DOI] [PubMed] [Google Scholar]
- 46.Stekelenburg-de VS, Steendijk P, Ursem NT, Wladimiroff JW, Poelmann RE. Systolic and diastolic ventricular function in the normal and extra-embryonic venous clipped chicken embryo of stage 24: a pressure-volume loop assessment. Ultrasound Obstet Gynecol. 2007;30:325–331. doi: 10.1002/uog.5137. [DOI] [PubMed] [Google Scholar]
- 47.Sun HW, Li CJ, Chen HQ, Lin HL, Lv HX, Zhang Y, Zhang M. Involvement of integrins, MAPK, and NF-kappaB in regulation of the shear stress-induced MMP-9 expression in endothelial cells. Biochem Biophys Res Commun. 2007;353:152–158. doi: 10.1016/j.bbrc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Sutherland FWH, Perry TE, Yu Y, Sherwood MC, Rabkin E, Masuda Y, Garcia GA, McLellan DL, Engelmayr GC, Jr, Sacks MS, Schoen FJ, Mayer JE., Jr From stem cells to viable autologous semilunar heart valve. Circulation. 2005;111:2783–2791. doi: 10.1161/CIRCULATIONAHA.104.498378. [DOI] [PubMed] [Google Scholar]
- 49.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 50.Ursem NTC, Stekelenburg-de Vos S, Wladimiroff JW, Poelmann RE, Gittenberger-de Groot AC, Hu N, Clark EB. Ventricular diastolic filling characteristics in stage-24 chick embryos after extra-embryonic venous obstruction. J Exp Biol. 2004;207:1487–1490. doi: 10.1242/jeb.00902. [DOI] [PubMed] [Google Scholar]
- 51.Ursem NTC, Struijk PC, Poelmann RE, Gittenberger-de Groot AC, Wladimiroff JW. Dorsal aortic flow velocity in chick embryos of stage 16 to 28. Ultrasound Med Biol. 2001;27:919–924. doi: 10.1016/S0301-5629(01)00393-3. [DOI] [PubMed] [Google Scholar]
- 52.Van der Heiden K, Groenendijk BCW, Hierck BP, Hogers B, Koerten HK, Mommaas AM, Gittenberger-de-Groot AC, Poelmann RE. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev Dyn. 2006;235:19–28. doi: 10.1002/dvdy.20557. [DOI] [PubMed] [Google Scholar]
- 53.Van der Heiden K, Hierck BP, Krams R, de Crom R, Cheng C, Baiker M, Pourquie MJBM, Alkemade FE, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE (2007) Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis (in press) [DOI] [PubMed]
- 54.Vennemann P, Kiger KT, Lindken R, Groenendijk BCW, Stekelenburg-de Vos S, ten Hagen TLM, Ursem NTC, Poelmann RE, Westerweel J, Hierck BP. In vivo micro particle image velocimetry measurements of blood-plasma in the embryonic avian heart. J Biomech. 2006;39:1191–1200. doi: 10.1016/j.jbiomech.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Wang N, Miao H, Li YS, Zhang P, Haga JH, Hu YL, Young A, Yuan SL, Nguyen P, Wu CC, Chien S. Shear stress regulation of Kruppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun. 2006;341:1244–1251. doi: 10.1016/j.bbrc.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 56.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA. 2003;100:7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]