Abstract
This paper estimates the prevalence of current injection drug users (IDUs) in 96 large U.S. metropolitan statistical areas (MSAs) annually from 1992 to 2002. Multiplier/allocation methods were used to estimate the prevalence of injectors because confidentiality restrictions precluded the use of other commonly used estimation methods, such as capture–recapture. We first estimated the number of IDUs in the U.S. each year from 1992 to 2002 and then apportioned these estimates to MSAs using multiplier methods. Four different types of data indicating drug injection were used to allocate national annual totals to MSAs, creating four distinct series of estimates of the number of injectors in each MSA. Each series was smoothed over time; and the mean value of the four component estimates was taken as the best estimate of IDUs for that MSA and year (with the range of component estimates indicating the degree of uncertainty in the estimates). Annual cross-sectional correlations of the MSA-level IDU estimates with measures of unemployment, hepatitis C mortality prevalence, and poisoning mortality prevalence were used to validate our estimates. MSA-level IDU estimates correlated moderately well with validators, demonstrating adequate convergence validity. Overall, the number of IDUs per 10,000 persons aged 15–64 years varied from 30 to 348 across MSAs (mean 126.9, standard deviation 65.3, median 106.6, interquartile range 78–162) in 1992 and from 37 to 336 across MSAs (mean 110.6, standard deviation 57.7, median 96.1, interquartile range 67–134) in 2002. A multilevel model showed that overall, across the 96 MSAs, the number of injectors declined each year until 2000, after which the IDU prevalence began to increase. Despite the variation in component estimates and methodological and component data set limitations, these local IDU prevalence estimates may be used to assess: (1) predictors of change in IDU prevalence; (2) differing IDU trends between localities; (3) the adequacy of service delivery to IDUs; and (4) infectious disease dynamics among IDUs across time.
Electronic supplementary material
The online version of this article (doi:10.1007/s11524-007-9248-5) contains supplementary material, which is available to authorized users.
Keywords: AIDS surveillance, Drug abuse treatment, Estimating injection drug use, Hierarchical linear models, HIV counseling and testing, Injection drug use (IDU), Multiplier methods, Prevalence
INTRODUCTION
Unsafe drug injection practices render users and at times contacts in their social, sexual, and injection networks at higher risk for contracting blood-borne infections, most notably HIV/AIDS and hepatitis B and C, but also other infectious diseases, such as hepatitis A.1,2,3 These practices also may lead to other negative health outcomes, e.g., cellulitis and other bacterial infections.4 Numerous epidemiologic studies have shown that these infections are spread through sharing contaminated drug injection equipment.5,6,7 Given the stigmatized nature of illicit and injection drug use, drug injection is often hidden.8,9 This concealment and fear of criminal action and social recrimination make it difficult to assess the actual number of injection drug users (IDUs) in a particular geographic area and time, as users are reluctant to report illicit drug and needle use.3,10,11 Although difficult, enumeration of IDUs by locality and time is important because this quantification aids in planning and assessing the need for, and scope and adequacy of local public health programs and policies for, drug users. Furthermore, evidence suggests that these IDU-related public health services and initiatives help to prevent wider infectious disease outbreaks, which could be transmitted to noninfected injectors and noninjecting populations.12–14
Research by Scott D. Holmberg and by Samuel R. Friedman et al., has estimated the prevalence of IDUs at the metropolitan statistical area (MSA) level for 19921 (median 76.7 per 10,000 population aged 15–64 years, range 25–231) and 1998 (median 60 per 10,000 population aged 15–64 years, range 20–173), respectively.15,16 MSAs are defined by the Office of Management and Budget (OMB) and have a central city with a population of 50,000 or greater. MSAs also include adjacent counties with a high level of social and economic integration with the central city.17 Both of these studies estimated the number of IDUs in the 96 U.S. MSAs that had the largest populations in 1992.15,16 MSAs were an appropriate unit of analysis because social, health, and economic data are more readily available for counties, from which MSA-level data can be derived, and because the social and economic integration, which characterizes MSAs, also corresponds with drug injection patterns.16,18 For example, many injection drug users live in the suburbs, but buy drugs in the city.16,19
Longitudinal estimates of IDU prevalence in MSAs are useful for both methodological and public health reasons. Methodologically speaking, longitudinal estimates are advantageous because repeated measures control for time-invariant characteristics within an MSA allow the study of change over time within MSAs, and the evaluation of differences between MSAs at anytime during the study period.20 In terms of public health, longitudinal quantification of IDU populations will allow future study of the patterns of change of IDU prevalence in relation to social, economic, and political predictors in metropolitan areas over the course of our study period. In addition, longitudinal IDU prevalence estimates can help researchers to forecast which MSAs may be at greater risk for experiencing outbreaks of drug injecting and infections associated with drug injection.
METHODS
Overview
Our analysis aims to estimate the number of injection drug users (those injecting in the prior year) in 96 large MSAs (those MSAs with populations larger than 500,000) for each year from 1992 to 2002. The June 30, 1993 OMB MSA definitions, which were based on the application of 1990 metropolitan area standards to 1990 census data, were used in these analyses, except in New England, where we instead used New England County Metropolitan Areas (NECMA) definitions.21,22
MSA-level estimates were computed by estimating national estimates of IDUs for each year, and allocating these to each MSA using methods detailed below. Multiplier methods and multiple data series have been widely used to estimate the number of problem drug users in the United Kingdom and elsewhere.23 National estimates were calculated using those from Holmberg15 and Friedman:16 two indicators of IDUs’ use of health services (HIV testing and drug treatment), and an estimate of IDUs’ encounters with law enforcement, derived from cocaine and heroin possession arrests. National estimates were attributed to MSAs using four data series based on different indicators of health service utilization and past estimates of IDU prevalence. These four data series were apportioned to the MSA by multiplier methods, and the resulting estimates were smoothed and then averaged to create an IDU estimate for each MSA and year. Potential biases introduced by these data series are discussed in the limitations section of the discussion. These MSA estimates were validated using yearly cross-sectional correlations with variables theoretically related to drug injection.
As in Friedman’s 2004 paper, we hypothesized that apportioning the national number of IDUs to the MSA using different data sources would help balance biases, as each data source has strengths and weaknesses. Each data source was chosen to help offset potential limitations of the others. For example, drug treatment data are subject to a service bias, so increases in treatment funding for IDUs, which increase the number of treatment slots available to IDUs, could make it appear as if the number of IDUs was increasing. By combining our series measures, some of which are either not subject to service bias or inversely related to services, we mitigate the effect of this service bias.
The steps used to create the longitudinal IDU prevalence estimates from 1992 to 2002 are described below and are outlined in Figure 1.
FIGURE 1.
Flowchart of methods for estimating IDUs in 96 MSAs from 1992 to 2002.
Step 1: Estimating the Number of IDUs in the United States Annually from 1992 to 2002
Overview. We employed three different data series (detailed below) to estimate the number of IDUs nationwide. Our first step was to create an index for each data series by dividing the number of IDUs in the U.S. for that year and the data series by the average number of IDUs over the study period for that series. For each year, index values were averaged across data sources to create an overall index for each year. The first set of national IDU estimates was the ratio of the overall index in a given year to that in 1992 multiplied by Holmberg’s 1992 estimate of the number of IDUs. The second set of estimates was the ratio of the overall index in a given year to that in 1998, multiplied by Friedman’s 1998 estimates. The final set of national estimates was created by averaging the first and second set of approximations (see Figure 2).
FIGURE 2.
National estimates of injection drug users by year in the U.S.
Component Data Series for Estimating the Number of IDUs in the U.S.
Treatment Episode Data Sets. The first data series uses the Substance Abuse and Mental Health Service Administration’s (SAMHSA) Treatment Episode Data Sets (TEDS) to determine the number of injectors entering treatment nationwide each year of the study period.24 These data sets identify the number and attributes of clients entering public or private substance abuse treatment programs that receive any state or federal funding,2 or facilities licensed by states to provide substance abuse treatment.24 The 33% of admissions known, but not reported to TEDS, may not be admissions at private facilities; and these admissions may not be injection admissions. As injectors are more likely to be poor and uninsured, it is likely that injectors may disproportionately utilize public treatment facilities.25 We calculated the national annual number of treatment episodes where admission documentation indicated that any substance was injected intravenously or intramuscularly.
HIV Counseling and Testing Services (CTS) Data. The second data series was obtained from the counseling and testing system of the Centers for Disease Control and Prevention (CDC). This system monitors the utilization of counseling and testing services (CTS) in CDC-supported sites.26 Specifically, we calculated the number of IDUs tested nationally for HIV for each year from 1992 to 2002.3 Clients are indicated as IDUs if they had injected a nonprescription drug since 1978.
Arrest and Offense Data. Finally, we used the Uniform Crime Reporting Program County-Level Detailed Arrest and Offense time series data, produced and distributed by Inter-university Consortium for Political and Social Research (ICPSR), to calculate the number of arrests between 1992 and 2002 for possession of opium, cocaine, and their derivatives (morphine, heroin, and codeine), henceforth referred to as hard-core drug arrests.27 Notably, these data include arrests of noninjecting users and of IDUs. To capture the number of IDUs arrested each year in this component series, we multiplied the national hard-core drug arrests by the percent of heroin or cocaine treatment admissions who indicated injection as a mode of drug administration. This percentage was calculated yearly using the TEDS data.
Combining Component Data Series Using Index Numbers
For each of these three time series, we created an index number, which serves as a way to compare values of a variable over time, by relating each value in a time series to a reference or standard value.28 The reference value for each component series is the average value for the 11 years of our study period. The index number was created by dividing the observed value for each year and series by the average for that series and multiplying this number by 100. The indices for the three series were then averaged for each year to create an overall index; this creates an index that is less sensitive to year-to-year fluctuation from individual data sources.
From the overall index, two sets of national estimates were created. The first set of national IDU estimates were based on Holmberg’s 1992 estimate of the number of IDUs in the 96 largest MSAs in the country. First, Holmberg’s 1992 IDU estimate for the 96 largest MSAs was inflated to represent the number of IDUs in the United States in 1992 as shown by Friedman and colleagues: 1,460,300 × 1.19 = 1,737,757.15,29 Then, to calculate national IDU estimates for each year of our study period, this national 1992 IDU estimate was multiplied by the ratio of the overall index for that year to the overall index in 1992.
The second set of national IDU estimates were based on the 1998 U.S. estimate (1,364,874) calculated by Friedman et al., 2004.16 For each year, this national estimate was multiplied by the ratio of the overall index for that year to the overall index in 1998.
Finally, we calculated our “best estimate” of the number of IDUs in the U.S. for each year by averaging the first and second sets of estimates described above. These national estimates were used to allocate IDUs to each MSA.30 These estimates appear in Figure 2.
Step 2: Calculating IDU Prevalence in Each MSA Over Time
Overview. To estimate the prevalence of IDUs in each MSA, we calculated the number of IDUs in each MSA and year from each of four data series to give: (1) number of IDUs in drug abuse treatment; (2) those receiving HIV CTS at publicly funded sites; (3) an interpolation and extrapolation based on Holmberg’s 1992 estimates and Friedman’s 1998 IDU estimates; and (4) those diagnosed with AIDS as adjusted for HIV prevalence. We will refer to these data series henceforth, as the “component series”; the number of IDUs from a given component series, MSA and year as “component series counts”; and MSA-level estimates derived from a given component series count and the national estimates as its “component estimate.” The process by which national estimates were allocated using each of the first three component series to create a component estimate for each MSA and year is described below. A discussion of the data sources used in previous studies, but not used in this paper, and other sources considered, may be found in Appendix A.As in Friedman et. al. (2004), we used multiplier techniques to allocate our national estimates of IDUs to each MSA.16 We used “Formula 1,” listed below, to apportion the national IDU estimate for each year to each MSA.
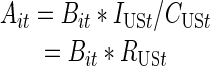 |
Formula 1 |
- Ait
Component estimate for an MSA i in year t
- Bit
Component series count4 for an MSA i in year t per 10,000 population 15–64 years5
- IUSt
Estimated number of injectors for the entire U.S. in year t
- CUSt
Component series count for the entire U.S. in year t
- RUSt
Ratio of the estimated number of U.S. injectors to the component series count for U.S. in year t
Component Data Series
Drug Treatment Data Series
SAMSHA’s Uniform Facility Data Set (UFDS) and TEDS Data. We used two data sources to create the component count from drug treatment data. First, to measure drug users in treatment, we used SAMSHA’s annual census of drug treatment centers initially called Uniform Facility Data Set (UFDS), since renamed the National Survey of Substance Abuse Treatment Services (N-SSATS). These data sets aim to measure client characteristics and use of privately and publicly funded substance abuse treatment programs in the U.S. on an average day.24,31–33 Second, to measure the proportion of drug users who inject, we used TEDS data, which capture initial entrance into substance abuse treatment at sites receiving state or federal funds.Using either the TEDS data or UFDS/N-SSATS data alone has particular limitations. For example, the TEDS data counts the number of treatment entries per year and many people have more than one. In addition, large fluctuations in year-to-year admissions, which were partially caused by shifts in funding levels, raised questions about the extent to which these counts were measuring the construct of interest. In the UFDS/N-SSATS data, however, information on injection drug use status was not collected after 1998. We, therefore, obtained the proportion of drug users who inject drugs in each MSA and year from TEDS and multiplied it by the total number of drug users in treatment documented in the UFDS/N-SSATS data. However, UFDS/N-SSATS data were unavailable for 1992, 1994, 1999, and 2001. As a result of this limited availability, the drug treatment component series estimates were only created for years where data were available and thus, our final IDU estimates only include treatment data for 1993, 1995, 1996–1998, 2000, and 2002.24,31,33Thus, using Formula 1, for each year, our national IDU estimates were allocated by taking the number of drug users (UFDS/N-SSATS) multiplied by the proportion of drug users entering treatment who injected in that MSA (TEDS) and multiplying this number by the ratio of our “best estimate” of the national number of IDUs to the number of drug users in treatment in the U.S. (UFDS/N-SSATS).
HIV testing series
CDC’s HIV CTS data We used Formula 1 to allocate the number of people tested for HIV who identified injection drug use as a risk factor in the CDC’s HIV CTS data.6 IDUs tested included IDUs cross-classified as men who have sex with men (MSM). Therefore, for each year we allocated our national IDU estimate by taking the number IDUs tested for HIV per population aged 15–64 years in the MSA times 10,000 and multiplying this number by the ratio of our “best estimate” of the national number of IDUs to the number of IDUs tested in the U.S.26,34–36
Previous estimate series
Continuation of Holmberg’s 1992 and Friedman’s 1998 IDU Estimates. Next, we created a series that was interpolated and extrapolated based on Holmberg’s15 and Friedman’s16 IDU estimates per population aged 15–64 years. As our time series estimates of IDUs are partially derived from these past studies, their methods are briefly described in the footnotes below.7,8,15In the present analyses, we interpolated yearly MSA-specific IDU estimates based on Holmberg’s 1992 and Friedman’s 1998 estimates. We then extrapolated the number of IDUs in each year from 1999 to 2002 by using the yearly change in IDUs between 1992 and 1998. To compute this interpolation and extrapolation, we first updated Friedman and Holmberg’s per capita estimates with revised population data.9 Friedman’s estimate was also adjusted by an inflation factor, which takes into account the current best estimate of the national number of IDUs. This process is described in more detail below.First, the inflation factor for Friedman’s 1998 estimates was the ratio of our current estimate of the number of IDUs in the country in 1998 divided by Friedman’s 1998 national estimate of the number of IDUs (1,575,494/1,364,874).16 The estimate of the number of IDUs in each MSA in 1998 was multiplied by this inflation factor and then divided by updated estimates of the population aged 15–64 years for each MSA in 1998. Holmberg’s estimates were also divided by updated estimates of the population aged 15–64 years for each MSA in 1992, but were not inflated based on the updated national estimates because Holmberg’s MSA-level IDU estimates were not created by allocating national IDU estimates to each MSA.As the proportion of IDUs in the population aged 15–64 years must fall between 0 and 1, we used the logs of Holmberg’s per capita 1992 estimates and log of Friedman’s inflated per capita 1998 estimate to create the interpolated and extrapolated estimates for 1992–2002. Interpolating and extrapolating on the log scale is desirable because after exponentiation IDU per capita estimates cannot be negative. After the per capita estimates were transformed, the 1998 number was subtracted from the 1992 number and then divided by 6, the difference between 1998 and 1992. The resulting number is the change in the log of the number of IDUs. This difference in logs was used for both interpolation and extrapolation and is equivalent to measuring the annual percent change between Holmberg and Friedman’s IDU estimates. The logs for each year were then exponentiated and multiplied by 10,000 to give an estimate of the number of IDUs per 10,000 for each MSA and year.
AIDS rates as Adjusted for HIV Prevalence
CDC’s Diagnoses of IDUs with HIV/AIDS. Finally, estimates of the number of IDUs for each MSA and year in our study period were created using data on the yearly number of incident AIDS case diagnoses with transmission categories noted as injection drug use or both male-to-male sexual contact and injection drug use. The CDC routinely adjusts case surveillance data on the number of AIDS diagnoses to account for the distribution of cases by transmission category for missing risk factor information. The CDC’s redistribution of cases with no identified risk factor to adjust for miscategorization may vary, considering that there is an increasing percentage of AIDS case data with unreported risk factor information over time.37 These AIDS case data were also adjusted for reporting delays, which may vary by state.38 AIDS case data were reported to the CDC using the uniform case definition and case surveillance report form, and all cases met the 1999 surveillance case definition.37–39These data were further adjusted for HIV prevalence in Formula 2a. Adapted from Friedman et al., Formula 2a (see below) assumes that each MSA’s share of the total number of injection-related AIDS cases diagnosed nationwide in year t equals that MSA’s share of the total number of injection-related HIV cases nationwide10 in year t.15,16 The proportion of injection-related HIV cases in the MSA (by definition) equals the seroprevalence of injection-related HIV in an MSA in 1992 multiplied by the number of IDUs in that MSA in a given year divided by the product of the seroprevalence of injection-related HIV in the US in 1992 with the IDUs in the U.S. in that year. This equation is then algebraically solved to find number of IDUs in an MSA in a given year (Formulas 2b and 2c).
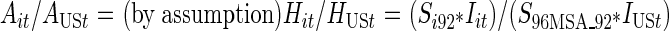 |
Formula 2a |
- Ait
Number of AIDS cases diagnosed in IDUs in the MSA i in year t
- AUSt
Number of AIDS cases diagnosed in IDUs in the U.S. in year t
- Hit
Number of HIV cases in IDUs in the MSA i in year t
- HUSt
Number of HIV cases in IDUs in the US in year t
- Si92
Seroprevalence of HIV/AIDS among IDUs in MSA i in year 1992
- Iit
Number of injection drug users in the MSA i in year t
- S96MSA_92
Seroprevalence of HIV/AIDS among IDUs in 1992
- IUSt
Number of injection drug users in the U.S. in year t
For each year we create a coefficient that is constant across MSAs:
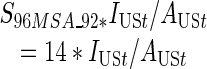 |
Formula 2b |
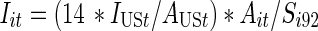 |
Formula 2c |
The assumptions inherent in Formula 2a warrant further discussion. In Formula 2a, it is assumed that the ratio of the number of AIDS cases diagnosed in an MSA and year to those in the U.S. in that year is equivalent to the ratio of HIV cases in an MSA and to those in the U.S. in 1992. (See Friedman et. al. 2004 and section on “Data Source Biases” below for a justification of this assumption.) Although HIV cases take time to develop into AIDS cases, Holmberg’s national and MSA-level HIV prevalence estimates for IDUs in 1992 were used in the calculation of component estimates from Formula 2a, regardless of the study period year.15 Using HIV prevalence data from 1992 allows for HIV cases to develop into AIDS cases. Ideally, we would use different HIV prevalence estimates for each year. For example, to estimate IDUs in 1992 we would use an HIV prevalence estimate from a time period before 1992, to allow time for HIV cases to progress into AIDS cases. However, 1992 was the earliest year for which we had access to HIV prevalence estimates for IDUs by MSA.11 Using national and MSA estimates of HIV seroprevalence among IDUs in 1992 assumes that the national and MSA-level HIV prevalence among IDUs did not change throughout our study period. The limitations of assumptions inherent in Formula 2a are further addressed in the “Discussion” section.
Data Source Biases.
These data sources were chosen so that biases of the different data sets would, in theory, cancel each other out. For instance, the CTS data and the TEDS data are reflective of health service provision: if health service funding for a service declines, so will the number of IDUs noted for that data set. However, the arrest data are not a function of health services. Cuts to health service funding will not affect arrest data, although arrest data are affected by changes in enforcement decisions. Similarly, a high component estimate based on IDUs being diagnosed with AIDS, in conjunction with low service-based component estimates, may reflect inadequate services for IDUs. A high component estimate resulting (after adjusting for the proportion of AIDS cases in an MSA is equal to the proportion of HIV cases there) from the AIDS case data in a given area may indicate IDUs having poor access to highly active antiretroviral treatment (HAART). It is important that our component estimates not all be subject to the same biases, so that when they are averaged, even if one component estimate declines because of funding cuts, another component estimate will be unaffected by this bias and will reflect what is truly occurring in the underlying IDU population.
Plausible Bounds, Outliers, Imputing Missing Values.
After creating each series of component estimates, we applied bounds to their values, to remove data values that were implausible. Component estimates were bounded between 13 and 660 per 10,000 population aged 15–64 years. These lower and upper bounds were set by taking 1/2 of the minimum and twice the maximum number of IDUs per 10,000 population aged 15–64 years from any MSA estimate from Holmberg’s 1992 and Friedman’s 1998 IDU estimates for MSAs. Values falling outside these bounds were set to missing and imputed (see below). The CTS, AIDS, and UFDS/N-SSATS component series had only minimal data that fell outside the plausible bounds; and no values from component estimate 3 (that interpolated and extrapolated from estimates for 1992 and 1998) fell outside the plausible range.
As it is very unlikely that any MSA would have a large increase in IDUs per capita in 1 year followed by an immediate decline the subsequent year, we explored removing such “outliers.”12 Only small differences were observed between data sets where outlier values were removed and imputed and those where outliers were not removed. Therefore, we decided not to remove outlier values from our final data set. Data points that were set to missing because data values fell outside plausible boundaries or missing in the raw data files were imputed. Single linear regression imputation was used to estimate the predicted value of these observations. For each component series, missing values were predicted by the other component series. (Years without UFDS data had one fewer component estimate). The missing values were replaced with these imputed predicted values for the MSA based on regression models of MSAs with nonmissing data.
Smoothing.
The values of each of the component estimates for each MSA across time were smoothed using loess regression, which fits curves to noisy data and smoothes data in a manner similar to computing a weighted moving average on time series data.40
Averaging to Create Final Estimates.
The predicted values of the component estimates resulting from the loess regressions for each MSA and year were averaged to create our final estimates13 (see Table 1 and online Appendix). SAS chose the smoothing coefficient, between .4 and 1 by intervals of .1, for each MSA based on the smallest AICC1 criterion (see Figure 1 for a flowchart of our study methods).41,42
TABLE 1.
Final estimates of IDUs per 10,000 population aged 15–64 in each of the 96 large U.S. MSAs
| MSA | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Akron, OH | 60 | 54 | 62 | 56 | 57 | 58 | 59 | 70 | 63 | 74 | 67 |
| Albany–Schenectady–Troy, NY | 67 | 59 | 60 | 55 | 54 | 52 | 53 | 50 | 57 | 50 | 62 |
| Albuquerque, NM | 239 | 219 | 224 | 206 | 199 | 194 | 191 | 195 | 187 | 191 | 183 |
| Allentown–Bethlehem–Easton, PA | 155 | 141 | 171 | 152 | 155 | 156 | 156 | 184 | 151 | 183 | 147 |
| Ann Arbor, MI | 38 | 36 | 36 | 36 | 35 | 35 | 36 | 35 | 36 | 36 | 37 |
| Atlanta, GA | 97 | 87 | 88 | 78 | 74 | 70 | 66 | 66 | 57 | 58 | 48 |
| Austin–San Marcos, TX | 242 | 211 | 239 | 202 | 191 | 174 | 157 | 163 | 128 | 129 | 101 |
| Bakersfield, CA | 315 | 289 | 306 | 284 | 280 | 277 | 269 | 288 | 256 | 273 | 240 |
| Baltimore, MD | 168 | 202 | 202 | 225 | 237 | 250 | 266 | 290 | 301 | 326 | 336 |
| Bergen–Passaic, NJ | 99 | 98 | 91 | 88 | 83 | 79 | 77 | 77 | 76 | 73 | 75 |
| Birmingham, AL | 56 | 49 | 57 | 48 | 48 | 48 | 48 | 59 | 49 | 59 | 49 |
| Boston, MA–NH | 76 | 104 | 83 | 116 | 122 | 130 | 137 | 101 | 149 | 107 | 161 |
| Buffalo–Niagara Falls, NY | 67 | 80 | 72 | 83 | 84 | 84 | 84 | 74 | 83 | 71 | 82 |
| Charleston–North Charleston, SC | 73 | 57 | 63 | 51 | 49 | 50 | 52 | 58 | 51 | 56 | 50 |
| Charlotte–Gastonia–Rock Hill, NC–SC | 88 | 72 | 81 | 69 | 67 | 65 | 63 | 70 | 60 | 69 | 56 |
| Chicago, IL | 72 | 62 | 70 | 60 | 59 | 57 | 57 | 66 | 56 | 67 | 57 |
| Cincinnati, OH–KY–IN | 82 | 67 | 77 | 63 | 61 | 60 | 60 | 73 | 63 | 76 | 65 |
| Cleveland–Lorain–Elyria, OH | 83 | 72 | 82 | 72 | 72 | 72 | 72 | 81 | 74 | 82 | 75 |
| Columbus, OH | 91 | 82 | 94 | 83 | 83 | 83 | 84 | 97 | 87 | 102 | 93 |
| Dallas, TX | 131 | 108 | 131 | 113 | 114 | 116 | 118 | 140 | 121 | 149 | 125 |
| Dayton–Springfield, OH | 65 | 56 | 60 | 52 | 51 | 50 | 51 | 59 | 59 | 69 | 68 |
| Denver, CO | 182 | 158 | 162 | 158 | 159 | 160 | 161 | 123 | 148 | 112 | 136 |
| Detroit, MI | 81 | 84 | 81 | 88 | 91 | 93 | 94 | 81 | 93 | 78 | 92 |
| El Paso, TX | 348 | 280 | 313 | 250 | 235 | 225 | 209 | 221 | 180 | 174 | 151 |
| Fort Lauderdale, FL | 109 | 87 | 101 | 81 | 79 | 77 | 75 | 87 | 71 | 83 | 67 |
| Fort Worth–Arlington, TX | 245 | 201 | 236 | 196 | 193 | 189 | 184 | 211 | 175 | 200 | 166 |
| Fresno, CA | 233 | 332 | 252 | 319 | 317 | 318 | 321 | 335 | 314 | 335 | 295 |
| Gary, IN | 116 | 98 | 120 | 112 | 118 | 124 | 129 | 127 | 130 | 130 | 132 |
| Grand Rapids–Muskegon–Holland, MI | 51 | 42 | 47 | 41 | 40 | 39 | 40 | 42 | 41 | 42 | 42 |
| Greensboro–Winston–Salem–High Point, NC | 91 | 72 | 83 | 66 | 63 | 62 | 61 | 74 | 58 | 69 | 54 |
| Greenville–Spartanburg–Anderson, SC | 53 | 41 | 48 | 39 | 38 | 38 | 38 | 43 | 40 | 45 | 42 |
| Harrisburg–Lebanon–Carlisle, PA | 88 | 81 | 100 | 92 | 102 | 118 | 132 | 162 | 128 | 137 | 109 |
| Hartford, CT | 154 | 153 | 157 | 154 | 153 | 150 | 146 | 141 | 138 | 133 | 131 |
| Honolulu, HI | 86 | 81 | 92 | 83 | 83 | 84 | 85 | 105 | 86 | 109 | 87 |
| Houston, TX | 235 | 178 | 217 | 165 | 159 | 154 | 146 | 172 | 130 | 152 | 114 |
| Indianapolis, IN | 101 | 92 | 95 | 83 | 78 | 74 | 73 | 84 | 75 | 87 | 79 |
| Jacksonville, FL | 163 | 119 | 146 | 112 | 109 | 107 | 107 | 127 | 107 | 129 | 107 |
| Jersey City, NJ | 238 | 191 | 204 | 162 | 147 | 132 | 124 | 135 | 128 | 138 | 139 |
| Kansas City, MO–KS | 79 | 70 | 77 | 67 | 66 | 65 | 62 | 65 | 55 | 57 | 48 |
| Knoxville, TN | 94 | 86 | 98 | 86 | 87 | 88 | 85 | 99 | 83 | 93 | 79 |
| Las Vegas, NV–AZ | 213 | 170 | 200 | 164 | 161 | 159 | 153 | 163 | 137 | 145 | 121 |
| Little Rock–North Little Rock, AR | 175 | 146 | 164 | 144 | 142 | 142 | 141 | 147 | 132 | 141 | 122 |
| Los Angeles–Long Beach, CA | 159 | 172 | 153 | 166 | 163 | 161 | 159 | 150 | 155 | 153 | 151 |
| Louisville, KY–IN | 122 | 105 | 142 | 123 | 130 | 136 | 139 | 160 | 141 | 168 | 146 |
| Memphis, TN–AR–MS | 75 | 74 | 72 | 67 | 64 | 63 | 64 | 78 | 70 | 84 | 76 |
| Miami, FL | 153 | 111 | 125 | 91 | 83 | 75 | 72 | 81 | 65 | 73 | 61 |
| Middlesex–Somerset–Hunterdon, NJ | 79 | 77 | 72 | 70 | 67 | 64 | 63 | 62 | 62 | 61 | 61 |
| Milwaukee–Waukesha, WI | 60 | 51 | 61 | 53 | 54 | 55 | 57 | 62 | 57 | 63 | 57 |
| Minneapolis–St. Paul, MN–WI | 57 | 46 | 53 | 43 | 42 | 43 | 44 | 54 | 46 | 58 | 49 |
| Monmouth–Ocean, NJ | 108 | 91 | 95 | 85 | 82 | 80 | 81 | 74 | 82 | 69 | 83 |
| Nashville, TN | 90 | 83 | 104 | 93 | 98 | 101 | 102 | 117 | 100 | 113 | 99 |
| Nassau–Suffolk, NY | 57 | 56 | 55 | 58 | 59 | 61 | 63 | 51 | 65 | 49 | 66 |
| New Haven–Meriden, CT | 155 | 144 | 149 | 144 | 143 | 142 | 139 | 131 | 130 | 122 | 120 |
| New Orleans, LA | 193 | 156 | 194 | 157 | 157 | 158 | 159 | 196 | 160 | 197 | 161 |
| New York, NY | 191 | 190 | 176 | 176 | 169 | 162 | 157 | 143 | 150 | 133 | 143 |
| Newark, NJ | 178 | 152 | 161 | 140 | 135 | 130 | 127 | 125 | 122 | 114 | 118 |
| Norfolk–Virginia Beach–Newport News, VA–NC | 81 | 79 | 92 | 85 | 88 | 91 | 92 | 108 | 94 | 108 | 95 |
| Oakland, CA | 161 | 183 | 153 | 173 | 169 | 166 | 161 | 146 | 148 | 138 | 133 |
| Oklahoma City, OK | 105 | 112 | 96 | 98 | 91 | 86 | 84 | 83 | 83 | 84 | 83 |
| Omaha, NE–IA | 57 | 60 | 58 | 58 | 57 | 55 | 56 | 63 | 62 | 69 | 68 |
| Orange County, CA | 137 | 145 | 121 | 127 | 121 | 117 | 114 | 100 | 105 | 87 | 96 |
| Orlando, FL | 78 | 70 | 83 | 73 | 75 | 77 | 79 | 97 | 86 | 103 | 93 |
| Philadelphia, PA–NJ | 151 | 145 | 160 | 150 | 151 | 151 | 153 | 173 | 161 | 187 | 173 |
| Phoenix–Mesa, AZ | 115 | 110 | 110 | 104 | 101 | 99 | 102 | 114 | 110 | 129 | 122 |
| Pittsburgh, PA | 75 | 69 | 80 | 73 | 75 | 76 | 79 | 92 | 86 | 96 | 94 |
| Portland–Vancouver, OR–WA | 197 | 210 | 181 | 201 | 197 | 195 | 196 | 170 | 195 | 176 | 194 |
| Providence–Fall River–Warwick, RI–MA | 84 | 98 | 78 | 107 | 111 | 115 | 119 | 67 | 119 | 67 | 120 |
| Raleigh–Durham–Chapel Hill, NC | 104 | 82 | 95 | 76 | 73 | 70 | 67 | 74 | 59 | 67 | 52 |
| Richmond–Petersburg, VA | 132 | 109 | 128 | 107 | 106 | 106 | 105 | 114 | 101 | 107 | 97 |
| Riverside–San Bernardino, CA | 169 | 181 | 142 | 155 | 147 | 144 | 138 | 120 | 117 | 101 | 91 |
| Rochester, NY | 78 | 70 | 78 | 71 | 70 | 69 | 69 | 74 | 74 | 81 | 82 |
| Sacramento, CA | 190 | 199 | 174 | 184 | 180 | 179 | 175 | 160 | 154 | 142 | 128 |
| St. Louis, MO–IL | 94 | 74 | 85 | 68 | 65 | 63 | 63 | 72 | 62 | 72 | 62 |
| Salt Lake City–Ogden, UT | 72 | 99 | 84 | 118 | 127 | 133 | 131 | 114 | 126 | 124 | 120 |
| San Antonio, TX | 254 | 216 | 220 | 187 | 174 | 162 | 158 | 158 | 145 | 146 | 135 |
| San Diego, CA | 142 | 163 | 147 | 160 | 158 | 157 | 152 | 147 | 143 | 139 | 133 |
| San Francisco, CA | 255 | 278 | 247 | 273 | 270 | 267 | 261 | 238 | 249 | 238 | 235 |
| San Jose, CA | 150 | 129 | 127 | 107 | 96 | 87 | 80 | 82 | 68 | 68 | 56 |
| San Juan–Bayamon, PR | 146 | 125 | 141 | 127 | 129 | 131 | 131 | 127 | 124 | 116 | 115 |
| Sarasota–Bradenton, FL | 98 | 91 | 108 | 99 | 103 | 107 | 111 | 134 | 121 | 146 | 130 |
| Scranton–Wilkes–Barre–Hazleton, PA | 43 | 43 | 43 | 45 | 47 | 48 | 48 | 41 | 51 | 44 | 55 |
| Seattle–Bellevue–Everett, WA | 156 | 197 | 168 | 195 | 192 | 187 | 183 | 165 | 173 | 156 | 164 |
| Springfield, MA | 141 | 155 | 150 | 173 | 183 | 193 | 203 | 171 | 213 | 180 | 224 |
| Stockton–Lodi, CA | 234 | 228 | 233 | 246 | 254 | 263 | 267 | 262 | 272 | 283 | 276 |
| Syracuse, NY | 64 | 53 | 59 | 50 | 48 | 47 | 45 | 48 | 44 | 47 | 43 |
| Tacoma, WA | 141 | 188 | 142 | 177 | 172 | 168 | 167 | 158 | 165 | 164 | 161 |
| Tampa–St. Petersburg–Clearwater, FL | 120 | 96 | 117 | 96 | 96 | 98 | 100 | 120 | 103 | 122 | 105 |
| Toledo, OH | 65 | 56 | 66 | 56 | 56 | 57 | 61 | 70 | 68 | 80 | 77 |
| Tucson, AZ | 223 | 224 | 229 | 229 | 232 | 235 | 235 | 238 | 233 | 237 | 230 |
| Tulsa, OK | 88 | 84 | 94 | 92 | 95 | 99 | 98 | 103 | 94 | 102 | 89 |
| Ventura, CA | 113 | 128 | 101 | 118 | 113 | 111 | 112 | 90 | 112 | 94 | 113 |
| Washington, DC–MD–VA–WV | 89 | 76 | 89 | 81 | 83 | 84 | 85 | 86 | 82 | 83 | 79 |
| West Palm Beach–Boca Raton, FL | 172 | 131 | 157 | 122 | 118 | 117 | 114 | 130 | 109 | 120 | 104 |
| Wichita, KS | 53 | 57 | 55 | 59 | 61 | 62 | 65 | 61 | 69 | 65 | 74 |
| Wilmington–Newark, DE–MD | 150 | 117 | 159 | 138 | 149 | 160 | 167 | 176 | 170 | 169 | 170 |
| Youngstown–Warren, OH | 30 | 32 | 38 | 38 | 41 | 44 | 48 | 61 | 56 | 71 | 63 |
Validity
To test the internal validity of our estimates, i.e., the extent to which our component estimates seem to be capturing the same underlying construct, we examined the correlation between each of our component estimates for each year. These correlations describe the extent to which our component estimates produce similar results. (Component estimates may not always produce similar estimates because each data source was selected to identify a different portion of the underlying IDU population. Therefore, we expect correlations to be less than 1.0.) In addition, to test the convergent validity (the extent to which our results are related to factors that theoretically reflect the actual population of IDUs in each MSA)43 we examined correlations between our IDU estimates and theoretically related constructs, such as unemployment, hepatitis C deaths, and poisoning deaths. Concordance between these constructs and our estimates lends credence to the validity of our estimates.43
Analysis
Longitudinal hierarchical linear models (HLM), which allow the study of how MSAs change over time and how changes differ across MSAs and take into account the correlation between years, were used to describe the average trend of the number of IDUs per 10,000 adults for our study period.44 With these models, questions regarding within-MSA and between-MSA change in the number of IDU per 10,000 may be addressed.44 To create these models, we explored several possible covariance structures, specifically, first-order autoregressive (AR1), toeplitz, and heterogeneous first-order autoregressive.44 In addition, we examined modeling both linear and quadratic expressions of time, operationalized as the number of years since 1992. The optimal model to describe the number of IDUs per 10,000 persons aged 15–64 years used an AR1 covariance structure, as well as linear and quadratic terms for time. Data were processed and analyzed using the SAS system version 9.1.41
RESULTS
Temporal Trends
Figure 2 displays three estimates of the number of IDUs in the U.S. from 1992 to 2002. These estimates appear relatively stable over time.
The online Appendix shows the estimates of the number of IDUs, number of IDUs per 10,000 population aged 15–64 years, and the range of the component estimates per 10,000 population aged 15–64 years for each of the 96 largest MSAs in the U.S. for each year from 1992 to 2002. The number of IDUs per 10,000 persons aged 15–64 years varied (across MSAs) from 30 to 348 (mean 126.9; standard deviation 65.3) in 1992 and from 37 to 336 (mean 110.6, standard deviation 57.7) in 2002. Overall, across the 96 MSAs the mean IDU prevalence mostly decreased during our study period, as did the dispersion of estimates over time.
Considering both the impact of linear (−2.27, p = 0.004) and quadratic (0.13, p = 0.02) specification for time (study year) in our multilevel model, we see that at the beginning of the time series mean injecting prevalence declined by 2.27 injectors per 10,000 people aged 15–64 years, but by the end of the year 1 (1993) the mean injecting prevalence only declined 2.14 injectors per 10,000 people aged 15–64 years. Thus, the overall decline in injecting prevalence was not constant for each year in our study period. The rate of decline decreased for each subsequent year in the study period until the inflection point was reached and then an increase in the prevalence of injectors was seen (See Figure 3). Consequently, the average IDU prevalence trajectory has a convex shape, although this curvature is not extreme because of the location of the inflection point and the magnitude of the quadratic estimate of time.44 Using standard techniques for quadratic equations, we find that the average prevalence of injecting reached its minimum (114 per 10,000 people aged 15–64 years) in year 9 (2000), and thereupon began to increase.44 In addition, MSAs that had higher prevalence in 1992 tended to have faster rates of decline (covariance = −126.8). MSAs where the prevalence declined more quickly also had rates of decline that slowed more quickly (covariance = −2.94).45
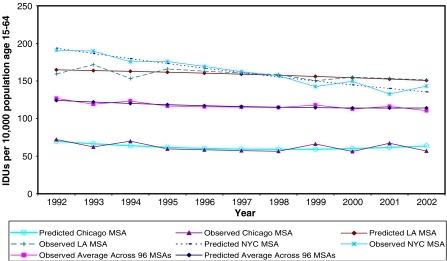
FIGURE 3.
Predicted and observed IDUs per 10,000 population aged 15–64 for selected MSAs and across all MSAs for 1992–2002.
There is considerable variation from the average trend in the number of injectors per 10,000 population aged 15–64 years across MSAs during our study period. That is, the average trajectory of IDUs across all 96 MSAs is not necessarily indicative of the trend of IDUs in a given MSA. This is demonstrated by the presence of statistically significant variation in the prevalence of injection in 1992 (variance = 4482), in the instantaneous rate of change (linear expression of time; variance = 54.9), and in the curvature (quadratic expression of time; variance = 0.23) of the trajectories between MSAs. The predicted average trajectory of IDU prevalence across all MSAs from 1992 to 2002, and the observed and predicted trends of IDU prevalence in those MSAs with the largest population in 2002 (Los Angeles–Long Beach, CA, New York, and Chicago MSAs) are shown in Figure 3. This figure shows that the predicted trends in IDU prevalence within these MSAs fit the observed IDU prevalence curves relatively well. This figure also shows that the IDU prevalence trend within MSAs vary from the trajectory of the overall average IDU prevalence across the 96 MSAs.
Validity
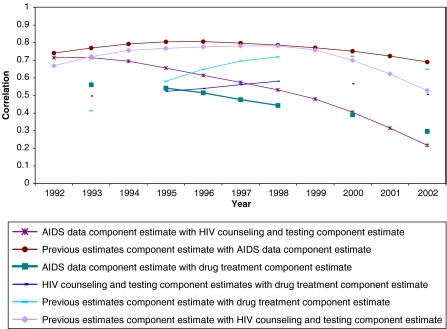
Figure 4 shows the correlations across MSAs of component estimates by year. In general, the intercorrelations were reasonably high and stable over time, which is expected because the component estimates aim to measure the same underlying construct. Moderately strong intercorrelations between component estimates over time are found with one exception: correlations between the MSA-level component estimates based on diagnosed AIDS cases and CTS data decreased over time. These results suggest that either the overall formula that relates HIV cases to AIDS cases, Formula 2, may become weaker over time, or that changes in service-provision factors over time may have weakened the value of CTS data as a marker for the number of IDUs.
FIGURE 4.
Correlations of MSA-level component estimates and final with one another by year.
We examined the yearly correlations of the unemployment rate, hepatitis C deaths (1999–2002 only), and poisoning deaths caused by drugs, medicinal substances, and biologicals (ICD-9 codes E850–E858, which include accidental drug overdose, wrong drug given or taken in error, and drug taken inadvertently) with our final IDU estimates for each year in our study period. These results are shown in Table 2. These results show moderate to high correlations over time, but that somewhat decreased in the later years of our study period for the poisoning mortality prevalence and unemployment validators. With the change to ICD-10 codes, hepatitis C deaths could be identified for the first time, and correlations with our IDU prevalence estimates were high. We also examined IDU prevalence (5-year lagged) with mortality from hepatitis C, i.e., IDU prevalence in 1994 was correlated with hepatitis C deaths in 1999 (r = .54) (not shown). Correlations of IDU prevalence with hepatitis C deaths in subsequent years ranged from .51 to .58.
TABLE 2.
Correlations of IDU prevalence estimates and theoretically related constructs over time
| Year | Unemployment | 2-year Lagged unemployment | Hepatitis C deaths* | Poisoning deaths+ |
|---|---|---|---|---|
| Overall | 0.444 | 0.435 | 0.424 | |
| 1992 | 0.425 | 0.437 | – | 0.606 |
| 1993 | 0.519 | 0.405 | – | 0.627 |
| 1994 | 0.482 | 0.419 | – | 0.585 |
| 1995 | 0.546 | 0.507 | – | 0.586 |
| 1996 | 0.532 | 0.511 | – | 0.622 |
| 1997 | 0.509 | 0.533 | – | 0.524 |
| 1998 | 0.504 | 0.513 | – | 0.503 |
| 1999 | 0.489 | 0.498 | 0.520 | 0.506 |
| 2000 | 0.470 | 0.490 | 0.490 | 0.337 |
| 2001 | 0.428 | 0.469 | 0.543 | 0.238 |
| 2002 | 0.385 | 0.435 | 0.510 | 0.244 |
*ICD-10 codes can more accurately capture hepatitis C deaths, but were not instituted until 1999. Therefore, hepatitis C correlations are only calculated from 1999 on.
+Mortality coding switched in 1999 from ICD-9 to ICD-10 codes.
Finally, correlations of the estimate of the prevalence of IDUs with theoretically related indicators, such as percent living in poverty in 1990 and 2000 and income inequality (ratio of income of households in the upper 10% to the income of the bottom 10%) were similar to those previously presented by Friedman and colleagues.16 Correlations between percent in poverty in 1990 and our IDU estimates per 10,000 population in 1992, the year closest to 1990; between poverty in 2000 and our IDU estimates per 10,000 population in 2000; and finally between income inequality 1990 and 2000 and our 1992 and 2000 IDU estimates were .334, .320, .263 and .198, respectively (each is significant at the .05 level). This suggests (for the given years at least) that our estimates were similarly accurate to the estimates presented in Friedman and colleagues’ paper. The magnitudes of the above correlations with our IDU estimates are expected to be lower than the magnitude of the correlations of our estimates with hepatitis C mortality prevalence because economic variables have a more complicated relationship with IDU prevalence.
DISCUSSION
Findings
Our national estimates of IDU prevalence suggest that IDU prevalence nationwide was relatively stable from 1992 to 2002. However, the results of the multilevel model show a decreasing average trend across the 96 MSAs, until 2000, after which there was a slight increase.
The finding that most MSAs experienced a decline in IDU prevalence merits discussion. The introduction of HAART had a considerable impact on the number of AIDS deaths. Pre-HAART, some declines in IDUs seen in MSAs may be the result of injection-related AIDS mortality. Injection-related AIDS mortality continues to a lesser extent, because of complex medication regimens and reluctance of providers to treat IDUs because of expectation of noncompliance and inexperience treating IDUs.37
Another explanation for the downward trend in IDU prevalence in metropolitan areas is that drug users have been employing alternative modes of drug administration. There are numerous reasons to choose modes of administration other than injection, including fear of HIV or hepatitis C. Researchers have suggested that because of increasing heroin purity, heroin users are delaying or not transitioning to drug injection because the high purity provides an efficient dose that is difficult to achieve through noninjection with more highly adulterated substances.4,46,47 Also, the rising use of methamphetamine and prescription drugs, which are often administered by means other than injection, may mark a shift away from injection drug use.48,49 It is also possible that increasing mortality caused by overdoses may have contributed to the decline of IDUs in MSAs.50 Future research using HLM methods should investigate whether other possible causes, such as changes in socioeconomic conditions, are associated with changes in IDU prevalence.
Although there was a decline in IDU prevalence overall IDU prevalence trends in individual MSAs deviate considerably from this average trend. Whereas both our predicted and final IDU prevalence estimates are not graphically represented for each MSA, Figure 3 gives an idea of how the predicted and observed estimates for individual MSAs differ from the predicted IDU prevalence across MSAs and how the predicted IDU prevalence for individual MSAs differs from the observed. Table 1 shows a general decrease over time in IDUs per 10,000 aged 15–64 years, but a few MSAs, most notably Baltimore, showed increases. In addition, MSAs with a sharp decrease in IDUs over time are noteworthy, specifically Austin, El Paso, Houston, Jersey City, San Antonio, and San Jose. Feedback from experts with knowledge of IDU trends in these localities was elicited and trends were reasoned to be plausible.
Validation
The results of our first validation test showed a relatively high correlation between each series of MSA-level component estimates across years, suggesting that the component estimates measure the same underlying construct and that our data series support each other (Figure 4). However, we also found a declining correlation between the counseling and testing and AIDS-based component estimates, which may suggest that the performance of the formula (Formula 2a) that relates HIV prevalence to AIDS cases deteriorates over time, or that our estimates are affected by service bias to a greater extent over time. Alternatively, the declining correlation between the AIDS case formula and the testing data could be caused by the fact that in Formula 2a we divide by Holmberg’s HIV seroprevalence for 1992, and as time since 1992 increases, there is more opportunity for HIV seroprevalence to change and we expect the correlation between the HIV seroprevalence in 1992 and the current year to decrease.
Finally, we considered the performance of our construct validity tests. Further, the results in Table 2 show a relatively high correlation between our IDU estimates and unemployment, hepatitis C deaths and deaths caused by poisoning. The correlations of unemployment and poisoning deaths with our final estimates decrease somewhat in later years. These decreasing correlations over time may imply that our estimates of IDU prevalence are less accurate in later years, or that there is dispersion in these theoretically related constructs over time. A plot of MSA-level poisoning deaths caused by drugs, medicinal substances, and biologicals over time showed such a pattern of increasing dispersion (plot not shown), signifying that a change occurred in poisoning deaths or the ascertainment of these deaths over time. The correlations between IDU prevalence and poisoning deaths may be affected by the switch from ICD-9 to ICD-10 codes in 1999 and this dispersion across MSAs may be the result of ICD-10 codes being more flexible than ICD-9 codes and physicians changing their coding practices as they gained more experience with ICD-10 coding. In addition, there is a lag between unemployment and IDU prevalence; that is, unemployment 2 years ago may relate to IDU prevalence today. The correlations with lagged unemployment were more consistent over time.
The results of our estimate validation using cross-sectional correlations of our IDU estimates with theoretically related variables over time are promising and give weight to the credibility of our estimates. Nevertheless, these estimates are not without bias and we strongly advise that researchers and policy makers consider our MSA-specific IDU prevalence estimates in conjunction with other MSA-specific research and data on IDUs. Moreover, several important limitations, including methodological and data availability, should be kept in mind when considering the validity of these estimates.
Limitations
Methodology.
Our findings must be considered in light of their limitations. First, there is no gold standard by which we can measure the performance of the methods used to produce our IDU estimates: there is a dearth of high-quality data on the number of IDUs across localities and time, in part because this population is hidden and marginalized. In addition to our limited ability to evaluate the accuracy of our estimates, there are also concerns about the multiplier methods used to produce them, although multiplier methods are a commonly used method, with a rich history in drug use research.21,51–53
Other methods, such as capture–recapture, exist for measuring hidden populations, but privacy restrictions in the U.S. prohibit the release of personal identifiers on national publicly available data sources, precluding us from using capture–recapture methods to estimate IDU prevalence.53–55 In addition, the use of capture–recapture methods was impractical as obtaining permission to procure data on IDUs would be unfeasible, given the vast number of state and municipal jurisdictions that the 96 MSAs studied cover.54 Further, the relative rarity of IDUs and their propensity to hide injection behaviors make household surveys ill-suited for the study of IDU prevalence.51,56 Many researchers advocate the use of multiplier methods in conjunction with other estimation techniques because the use of multiple estimation techniques will improve the level of certainty in the estimates.51 In the absence of alternative methods to estimate IDUs over many geographic areas in the U.S.52, our estimates are valuable approximations, which speak to the definitive need for improved methods to measure hidden populations.
Data Series.
The validity of our nationwide and MSA-level estimates of IDUs for 1992–2002 is affected in part by the limitations of each of our component data sources. Limitations of each time series data source, which may introduce systematic bias to our estimates, are discussed below.
Yearly fluctuations were observed in many data series. Fluctuations at the national level in the counseling and testing, FBI, and TEDS data were adjusted using indices. MSA-level fluctuations in the counseling and testing and UFDS/N-SSATS data were somewhat mitigated by loess smoothing of trends.
UFDS/N-SSATS data sets were not available for 1992, 1994, 1999, and 2001. A sensitivity analysis imputing the UFDS/N-SSATS data for these missing years showed that the yearly means of the overall MSA-level IDU prevalence estimates did not appreciably change.14
Two potential limitations arose with the arrest data. First, partial reporting of arrests was adjusted by ICPSR to help mitigate the underreporting with the assumption that the number of arrests for a 3-month period will be representative of yearly arrests, and that arrests do not fluctuate seasonally by reporting agency.27 Additional adjustments were made to account for changes in imputation procedures by the data provider and large fluctuations in hard-core drug arrests within states between years.
The second limitation of the arrest data is that we cannot discern if those arrested for heroin or cocaine possession were injectors. We assumed that the proportion of IDUs arrested for using hard-core drugs is similar to the proportion of IDUs using hard-core drugs that were entering treatment centers. A literature search did not find any comparison of the characteristics of heroin and cocaine users in the treatment and arrest populations. Past studies have shown a higher IDU prevalence in the general population for black adults than for white adults,57 which could mean that there is a higher proportion of IDUs arrested than entering treatment. Theoretically, it may be that a higher percentage of the population of hard-core drug users arrested for possession is black than the treatment population.58 However, considering the overwhelming and disproportionately high drug offense incarceration rates for black adults,59 it may be that black IDUs are disproportionately represented in arrest data compared to treatment data. Conversely, black hard-core drug users may be more likely to be (non-injecting) crack users,60 challenging the hypothesis that those arrested for hard-core drug possession are more likely to be IDUs.
Another possible difference between hard-core drug users in treatment and those arrested is that the population arrested may be disproportionately poorer than the drug treatment population. It may be argued that poor drug users are more likely to inject drugs because it is a more cost-effective route of drug administration.61 This would mean that the proportion of heroin- and cocaine-using IDUs in treatment would underestimate the proportion of IDUs arrested for heroin and cocaine possession. This underestimation actually may be counterbalanced by the fact that those entering drug treatment struggle with drug addiction and those addicted have been found more likely to be drug injectors,62–64 which would point to a greater proportion of hard-drug users who inject in treatment than arrested. Lastly, it is conceivable that the proportion of IDUs in private treatment varies across MSAs, which could bias our estimates across MSAs, such that an MSA with high proportion of IDUs in privately funded drug treatment programs would underestimate the number of IDUs.
The counseling and testing data series has several limitations. First, data only exist where there are publicly funded HIV counseling and testing sites. Thus, HIV testing of IDUs occurring at a health maintenance organization or at a blood donation center is not accounted for in these data and may lead to an underestimation of the number of IDUs. Second, reluctance to identify and report illicit and otherwise stigmatized behaviors may lead to misclassification and underreporting of IDUs in the counseling and testing data. Finally, the counseling and testing data classify injectors as anyone injecting since 1978, although definitions used to classify injectors may vary by health department, e.g., Massachusetts classifies injectors as those who have injected drugs in the last 3 years or since their last HIV test.35 This means we may not be capturing recent injectors in this IDU prevalence measure and that comparability across health department jurisdictions may be limited.
The component estimate derived from Formula 2a, based on the AIDS case data, contains spatial and temporal assumptions that merit consideration. First, this formula assumes that IDUs in all MSAs have equal access to HAART, which would mean time as HIV infection was similar across MSAs. In MSAs with good access to HAART, the time to AIDS would be greater, and after 1998 our AIDS case-based component estimate would underestimate IDU prevalence. Conversely, as the AIDS case-based component estimate is intended to counterbalance possible service biases in the drug treatment and HIV counseling and testing component estimates, differential access to HAART and other antiretroviral therapy and changes in the availability of these treatments over time may not be problematic. The rationale is as follows: we expect to capture IDUs who are receiving services, e.g., drug treatment and HIV testing component estimates. However, those receiving services are more likely to be receiving antiretroviral care.65 IDUs not receiving services are less likely to be receiving treatment for HIV and, therefore, more likely to develop AIDS. Thus, the AIDS case-based component counterbalances the service biases inherent in the drug treatment and counseling and testing component estimates.
Second, Formula 2a assumes HAART use did not change across our study period. Since HAART was first introduced in 1996 and later became the standard of care, access to HAART clearly changed over our study period.65,66 As HAART became more widely used, we would expect the time between HIV infection and AIDS to increase. Fewer HIV cases progressed to AIDS, which attenuated the relationship between HIV and AIDS. Mathematically, after HAART, AIDS case data became sparser and the results of Formula 2a became more varied and erratic because of smaller cell sizes. Greater variation in the AIDS case-based component estimate would explain why the correlations between AIDS case-based and counseling testing component estimates declined over time.
In contrast, although we expect time for AIDS to increase post-HAART, this does not mean that IDUs are necessarily benefiting from this increase. HIV surveillance data indicate that from 1994 to 1999, across 25 states, 43% of those who were diagnosed with HIV infection were diagnosed with AIDS within a year of their HIV diagnoses.67 These data also show that IDUs and MSM-IDUs are at greater risk for late diagnosis.67 More recently, a CDC survey conducted in 16 states from 2000 to 2003 found approximately half of those with AIDS had first tested positive for HIV within a year of being diagnosed with AIDS. Whereas this survey did not find IDUs and MSM-IDUs to be at greater risk for late reporting, others studies have.68
Third, MSA-level HIV prevalence among IDUs was only available for 1992 and 1998. The unavailability of annual MSA-level HIV prevalence data among IDUs may have affected the precision of our IDU estimates. The use of HIV prevalence data from 1992 may help explain declining correlations between the AIDS case-based and counseling and testing component estimates.
Finally, it is necessary to consider the component series based on the interpolation and extrapolation of Holmberg and Friedman’s estimates. This estimation assumes a linear change on the log scale for each MSA. The number of IDUs changed may not have changed linearly (on the log scale) over time in each MSA. Further, these estimates are subject to all the limitations, biases, and weaknesses that Holmberg and Friedman highlighted in their papers. These include the underreporting of injection, wide inclusion criteria ranges in the former and imperfect estimators, which may be subject to service bias in the latter study.15
CONCLUSION
Estimates of IDUs are essential for evidence-based policy-making. However, there are many challenges in calculating longitudinal IDU estimates for metropolitan areas in the U.S. There is neither a methodological gold standard, nor an accepted reference to compare our longitudinal estimates; as such, we attempted to expand on Holmberg and Friedman and colleagues’ previous work, acknowledging the imperfection of our methods and estimates. Nevertheless, tests of validity lend credence to our estimates, and suggest that our estimates will help policy makers characterize how and where IDU prevalence is changing over time. This characterization, in turn, may inform public health officials about population vulnerability to future epidemics. In addition, these estimates may be used in research into how socioeconomic conditions, public health programs, policies, and other factors affect the prevalence of IDUs in a population.
Electronic supplementary material
Component estimates and final estimates of the number of IDU in each of the 96 large US MSAs.
ESM (RTF 374 kb)
Acknowledgements
The research described in this paper is supported by the National Institute of Drug Abuse grant # R01 DA13336. We would like to thank the Centers for Disease Control and Prevention, specifically Dr. Tonji Durant and Andrew Mitsch at the National Center for HIV, National Center for HIV, Viral Hepatitis, STD, and TB Prevention and the Coordinating Center for Infectious Diseases for their useful comments on the manuscript and for providing data from the national AIDS surveillance and the HIV counseling and testing databases. In addition, we would also like to thank Dr. Scott Holmberg of RTI International for providing the IDU and IDU-HIV estimates, Mr. Spencer Lieb of the Florida Department of Health for his assistance in obtaining the AIDS surveillance database, Dr. Jane Maxwell for her time and feedback on the manuscript and our estimates, Mr. Bob Baxter, Dr. Carl Latkin, Dr. John Newmeyer, Dr. Shruti Mehta, and Dr. Bill Zule for their feedback on our estimates, and Mr. Michael Fanning, Dr. David Hurst, and Dr. Renee R. Stein for their assistance with the HIV counseling and testing data request.
Appendix A
Data Series Considerations
While creating national IDU estimates for 1992–2002, we considered implementing the methods and using the data sources utilized by Holmberg and Friedman and colleagues.15,16 Holmberg used data that we did not include, such as data from Ryan White programs, DAWN hospital data and local estimates from ethnographers, and data from treatment providers, law enforcement agencies, and heath departments, whereas Friedman and colleagues also used the National Household Survey on Drug Abuse (NHSDA). In some instances, our approach mirrored the methods used in these prior studies of IDU prevalence. Unfortunately, it was not always possible to use the same data sets because data set availability changed over time.15 In the creation of our longitudinal estimates of IDUs, we also found that information available in data sets changed over time, e.g., UFDS/N-SSATS data were only available: in 1993, 1995, 1996–1998, 2000, and 2002. Injection drug use information was not collected after 1998.
In addition, we considered using the ever- and past-year needle use variables in NHSDA to create our national IDU estimates for our study period. Statisticians at SAMSHA noted the following threats to the validity of the NHDSA data: 1. Underreporting of IDU behavior from in-person interviews, and 2. lack of data on marginalized populations known to inject drugs, such as the homeless and prison populations.15,29 The first threat to validity is inconsistent over time as NHDSA switched to computer-assisted technology to help mitigate known reporting biases of stigmatized behaviors, such as injection drug use. In some instances, adjustments to the data may be made to account for these different sampling strategies. However, when investigating time trends we found different sampling strategies over time made the data noncomparable.69–71 In some instances, the NHDSA proposed adjustments to offset differences caused by varied sampling methodology, incentives, interviewer experience, survey administration methods, imputation procedures, and questionnaires. However, when the proposed adjustments were made very large and inconsistent fluctuations in past-year and ever needle use still persisted. Whether the aforementioned changes in NHDSA methodology, or changing levels of stigmatization or fear of needle use disclosure on the part of those respondents who inject drugs, resulted in these irregularities, these fluctuations made time-series analysis invalid for the needle-use data. Our decision regarding trend analysis of the needle use data is consistent with the NHDSA recommendations against using longitudinal trend analysis for our study period.69–71 In addition, the needle use variables are not core variables in the NHDSA survey, so they are less precise estimates. Therefore, we used alternative data sources in the creation of our national IDU estimates.
In our search to identify data sources for our national estimates, we also investigated alternative data sources and time series. We examined using unintentional poisonings deaths caused by heroin, cocaine, and psychostimulants from the National Center for Vital Statistics Multiple Cause of Death data sets and injection drug-related endocarditis hospitalizations in the National Center for Health Statistics’ National Hospital Discharge Survey from 1992 to 2002.72,73 However, large increases in overdose deaths were observed over our study period. An underlying cause, other than increasing numbers of IDUs, seems to be driving increases in accidental poisoning deaths and injection-related endocarditis hospitalizations over time. Therefore, these indicators are not reflective of national injection drug use trends. The overdose data series is not indicative of trends in IDU drug use because this series includes drug users who have overdosed by other routes of administration (i.e., IDU is not coded in death data). In addition, increases in overdose deaths do not appear to be related to increasing drug injection, but rather changes in coding practice, aging drug users, polydrug mixing, increase in prescription narcotics, and drug strength and availability.50,74–77
Similarly, national injection-related endocarditis trends seem to be inconsistent with national injection drug use patterns over time. Injection-related endocarditis trends may be a function of the increase in methamphetamine use and/or increasing injection frequency among heroin injectors.78
Footnotes
Holmberg does not explicitly state the year to which his estimates apply, although data used to calculate these estimates are from 1990 to 1993. In previous papers, Holmberg’s estimates have been referred to as applying to 1992 and 1993. In this paper and henceforth, we will refer to these estimates as applying to 1992.
Treatment providers receiving state agency funding, including the federal block grant monies, are obligated to provide data on all clients admitted to treatment, regardless of the source of funding for individual clients. In 1997, TEDS was estimated to include 83% of admissions receiving state funding and 67% of all known admissions.64
Annual data on the national number of tests for the IDU risk exposure group for 1992–1994 were not feasibly available. We estimated the number of IDUs tested for HIV nationally for 1992–1994 by multiplying the number of IDUs tested in the 96 MSAs by the ratio of the number of IDUs tested nationally to those tested in the largest 96 MSAs averaged over 1995–2002. This ratio was essentially constant at 1.70 from 1995 to 2002. Inflating the IDUs tested in the 96 metros to create an estimate of the IDUs tested nationally in 1992–1994 assumes that the ratio number of IDUs tested for HIV in the nation to the 96 metros remains constant over our study period.
Component count refers to the number of IDUs ascertained for a data source in each MSA and year. The population aged 15–64 years was used because this age group is the population at risk.
The component series count based on Holmberg and Friedman data is per capita, not per 10,000. A linear trend for interpolation and extrapolation would allow the number of IDUs to be less than 0. IDU per capita values fall between 0 and 1 and were log transformed. Formula 1 was applied to the log of the per capita component series count. The final component estimates for this series were exponentiated and are shown per 10,000 and are on the same scale as our other component estimates.
In the Sarasota FL, Scranton PA, Seattle WA and Springfield MA MSAs CTS data were acquired from state health departments rather than the CDC. These data were requested when a separate analysis found substantial missing data for IDUs testing HIV positive. State-level data were used when they were judged to have more complete reporting.
Holmberg estimated the prevalence of injecting in 1992 for each of the 96 largest MSAs using a components model, which divides the population into risk groups and then calculates risk group size and seroprevalence.80 MSA-level data from a literature review of estimates created by researchers at federal, state, and local agencies, universities, and drug treatment programs were used to estimate IDU risk group size. Estimates that fell outside the prespecified range of plausible values were omitted. Final estimates for MSAs were calculated by averaging data series estimates.15
Friedman and colleagues estimated the number of current IDUs in the U.S. in 1998 based on Holmberg’s IDU estimates for 1992 and the National Household Survey on Drug Abuse (NHSDA). Then, for each of the 96 largest MSAs, IDU estimates that reflected service coverage were created based on drug treatment data, CTS data, AIDS case data adjusted for HIV prevalence, and Holmberg’s IDU estimates. These four component estimates were calculated based on multiplier methods and then averaged to create the final estimate for each MSA in 1998.16
The U.S. Census Bureau revised the estimate of population size for 1992 and 1998 based on Census 2000 data, which were not available when the previous papers were submitted for publication.
This formula uses seroprevalence estimates for 1992 as calculated by Holmberg: 14%, which refers to the HIV seroprevalence of IDUs in 1992 for the largest 96 MSAs. This 1992 seroprevalence was used as a proxy for the country as a whole.
HIV prevalence estimates for IDUs at the MSA-level for use in Formula 2a could have been created using the CTS data. However, we chose not to because we only had data for 1992–2002, and lack of prevalence estimates before 1992 would not have allowed for any lag time between HIV infection and the development of AIDS. Alternatively, we could have used the HIV prevalence estimates for 1998 put forth by Friedman and colleagues, but we decided that a longer lag time would allow more AIDS cases to develop and would more accurately describe the relationship between HIV and AIDS.16 In the absence of a better HIV seroprevalence proxy, we used Holmberg’s 1992 HIV seroprevalence estimates for IDUs.15
Data values were determined to be outliers if they differed from the previous and subsequent year by a factor of 2 or more. Component estimate values for initial and terminal years were considered outliers when the following conditions were true: for initial year values when the value in the subsequent year differed by a factor of 3 and for terminal year values when the preceding year value differed by a factor of 3 or more. Data points that met these criteria were set to missing. Final estimates were examined with and without removing outliers. (When outliers were removed, values set to missing were replaced with imputed values, which were computed using single regression imputation.)
The data were smoothed and then averaged, rather than averaged and then smoothed, because the former method is more intuitive. When we smooth each component estimate, we know what we are smoothing, as opposed to the average of the estimates, where it is not clear what noise is being smoothed. Also, in future analyses, we may wish to omit a component estimate from our final estimate and smoothing and then averaging allows this to be done more easily. Further, we compared estimates prepared using both the former and the latter methods and there was not much difference.
We did not impute UFDS/N-SSATS for the years 1992, 1994, 1999, and 2001 because we did not believe there was a need to estimate the variance of the individual series, as the treatment component estimate would be smoothed and averaged with the other component estimates. Results are shown for our final estimates calculate without using UFDS/N-SSATS data in the above years and using UFDS/N-SSATS, respectively: in 1992—126.9 and 121.8; in 1994—123.3 and 118.3; in 1999—118.0 and 113.9; in 2001—116.2 and 111.4.
Electronic supplementary material
The online version of this article (doi:10.1007/s11524-007-9248-5) contains supplementary material, which is available to authorized users.
References
- 1.Novick DM, Haverkos HW, Teller DW. The medically ill substance abuser. In: Lowinson JL, Ruiz P, Millman RB, Landgrod JG, eds. Substance Abuse: a Comprehensive Textbook. Third ed. Baltimore: Williams & Wilkins; 1997:534–550.
- 2.Trends in injection drug use among persons entering addiction treatment—New Jersey, 1992–1999. MMWR Morb Mortal Wkly Rep. 2001;50:378–381. [PubMed]
- 3.Rhodes T, Singer M, Bourgois P, Friedman S, Strathdee SA. The social and structural production of HIV risk among injecting drug users. Soc Sci Med. 2005;61:1026–1044. [DOI] [PubMed]
- 4.Gordon R, Lowry F. Bacterial infections in drug users. N Engl J Med. 2005;353:1945–1954. [DOI] [PubMed]
- 5.Friedland GH, Harris C, Butkus-Small C, et al. Intravenous drug abusers and the acquired immunodeficiency syndrome (AIDS). Demographic, drug use, and needle-sharing patterns. Arch Intern Med. 1985;145(8):1413–1417. [DOI] [PubMed]
- 6.Des Jarlais DC, Friedman SR. HIV infection among intravenous drug users: epidemiology and risk reduction. AIDS. 1987;1:67–76. [PubMed]
- 7.Vlahov D, Muñoz A, Anthony JC, Cohn S, Celentano DD, Nelson KE. Association of drug injection patterns with antibody to human immunodeficiency virus type 1 among intravenous drug users in Baltimore, Maryland. Am J Epidemiol. 1990;132:847–856. [DOI] [PubMed]
- 8.Bourgois P, Martinez A., Kral A, Edlin B, Schonberg J, Ciccarone D. Reinterpreting ethnic patterns among White and African American men who inject heroin: a social science of medicine approach. PLoS Med. 2006;3:1805–1815. [DOI] [PMC free article] [PubMed]
- 9.Needle RH, Coyle SL, Normand J, Lambert E, Cesari H. HIV prevention with drug-using population—current status and future prospects: introduction and overview. Public Health Rep. 1998;113(suppl 1):4–18. [PMC free article] [PubMed]
- 10.Mieczkowski T. Geeking up and throwing down: heroin street life in Detroit. Criminology. 1986;24(4):645-666. [DOI]
- 11.Turner CF, Lessler J, Devore J. Effects of mode of administration and wording on reporting of drug use. In: Turner CF, Lessler JT, Gfroerer JD, eds. Survey Measurement of Drug Use: Methodological Issues. Government Printing Office; Washington, D.C.;1992. DHHS Publication No. AMD 92-1929.
- 12.Semaan S, Des Jarlais DC, Sogolow E, et al. A meta-analysis of the effect of HIV prevention interventions on the sex behaviors of drug users in the United States. JAIDS. 2002;30(suppl 1):S73–S93. [PubMed]
- 13.Lurie P, Reingold AL, Bowser B, Eds. The Public Health Impact of Needle-Exchange Programs in the United States and Abroad, Volume 1. Rockville, MD: CDC National AIDS Clearinghouse; 1993.
- 14.Syed N, Hearing S, Shaw I, et al. Outbreak of Hepatitis A in the injecting drug user and homeless populations in Bristol: control by a targeted vaccination programme and possible parenteral transmission. Eur J Gastroenterol Hepatol. 2005;15:901–906. [DOI] [PubMed]
- 15.Holmberg SD. The estimated prevalence and incidence of HIV in 96 large U.S. metropolitan areas. Am J Public Health. 1996;86:642–654. [DOI] [PMC free article] [PubMed]
- 16.Friedman SR, Tempalski B, Cooper H, et al. Estimating numbers of injecting drug users in metropolitan areas for structural analyses of community vulnerability and for assessing relative degrees of service provision for injecting drug users. J Urban Health. 2004;81:377–400. [DOI] [PMC free article] [PubMed]
- 17.Office of Management and Budget. Standards for defining metropolitan and micropolitan statistical areas. Federal Register. 2000;65:8228–8238.
- 18.Wallace R, Wallace D. Community marginalisation and the diffusion of disease and disorder in the United States. BMJ. 1997;314:1341–1345. [DOI] [PMC free article] [PubMed]
- 19.Pierce TG. Gen-X junkie: Ethnographic research with young White heroin users in Washington, DC. Substance Use Misuse. 1999;34:2095–2114. [DOI] [PubMed]
- 20.Hedeker D, Gibbons R. Longitudinal Data Analysis. Hoboken, NJ: John Wiley & Sons, Inc.; 2006.
- 21.Office of Management and Budget. Metropolitan Areas And Components, 1993, With Fips Codes. September, 1996; Web Page. Available at: http://www.census.gov/population/estimates/metro-city/93mfips.txt. Accessed on January 24, 2007.
- 22.U.S. Census Bureau, Population Division Population Distribution Branch. Historical Metropolitan Area Definitions. March 17, 2005; Web Page. Available at: http://www.census.gov/population/www/estimates/pastmetro.html. Accessed on January 24, 2007.
- 23.Frischer M, Hickman M, Kraus L, Nariani F, Wiessing L. A comparison of different methods for estimating prevalence of problematic drug misuse in Great Britain. Addiction. 2001;96:1465–1476. [DOI] [PubMed]
- 24.DHHS SAMHSA Office of Applied Studies. Treatment Episode Data Set (TEDS) 1992–2002 [Computer file], ver. ICPSR04431-v2. Ann Arbor, MI: Synectics for Management Decisions, Incorporated. Inter-university Consortium for Political and Social Research.; 2006.
- 25.Friedman SR, Perlis T, Des Jarlais DC. Laws prohibiting over-the-counter syringe sales to injection drug users: relations to population density, HIV prevalence, and HIV incidence. Am J Public Health. 2001;91(5):791–793. [DOI] [PMC free article] [PubMed]
- 26.HIV Counseling and Testing in Publicly Funded Sites: 1995 Summary Report. Atlanta: DHHS. Centers for Disease Control and Prevention.; 1997.Available at: http://www.cdc.gov/hiv/topics/testing/resources/reports/pdf/ct97_doc.pdf. Accessed on November 21, 2007.
- 27.Department of Justice. FBI. Uniform Crime Reporting Program Data [United States]: County-Level Detailed Arrest and Offense Data, 1992–2002. [Computer file], ver. 3rd ICPSR ed. Ann Arbor, MI. Inter-university Consortium for Political and Social Research; 2004.
- 28.Columbia University Press. Web Page. Available at: http://www.answers.com/topic/index-number. Accessed on January 23, 2007.
- 29.Wright D, Gfroerer J, Epstein J. The use of external data sources and ratio estimation to improve estimates of hardcore drug use from the NHSDA. NIDA Research Monograph. 1997;167:477–497. [PubMed]
- 30.Committee on the Ryan White CARE Act: Data for Resource Allocation, Planning and Evaluation. Measuring What Matters: Allocation, Planning, and Quality Assessment for the Ryan White CARE Act; 2004. Available at: http://books.nap.edu/openbook.php?isbn=0309091152. Accessed on November 21, 2007.
- 31.DHHS SAMHSA Office of Applied Studies. Uniform Facility Data Set (UFDS): 1997–1998—Data on substance abuse treatment facilities. 2nd ICPSR edn. Ann Arbor, MI: Inter-university Consortium for Political and Social Research; 2004; National Survey of Substance Abuse Treatment Services (N-SSATS) Series.
- 32.DHHS SAMHSA Office of Applied Studies. Uniform Facility Data Set (UFDS): Data on Substance Abuse Treatment Facilities, 1993, 1995. [Computer File.].
- 33.DHHS SAMHSA Office of Applied Studies. National Survey of Substance Abuse Treatment Services (N-SSATS), 2000, 2002: (United States) [Computer file], 3rd ICPSR version. Ann Arbor, MI: Inter-university Consortium for Political and Social Research.
- 34.DHHS CDC. HIV Counseling and Testing in Publicly Funded Sites: 1996 Annual Report. Atlanta: 1998. Available at: http://www.cdc.gov/hiv/topics/testing/resources/reports/pdf/cts96.pdf. Accessed on November 21, 2007.
- 35.DHHS CDC HIV Counseling and Testing at CDC-Supported Sites-United States, 1999-2004. Atlanta; GA 2006. Available at: http://www.cdc.gov/hiv/topics/testing/resources/reports/pdf/ctr04. Accessed on November 21, 2007.
- 36.DHHS CDC HIV Counseling and Testing in Publicly Funded Sites, Annual Report 1997 and 1998. Atlanta, GA; 2001. Available at: http://www.cdc.gov/hiv/topics/testing/resources/reports/pdf/cts98.pdf. Accessed on November 21, 2007.
- 37.McDavid K, McKenna MT. HIV/AIDS Risk Factor Ascertainment: A Critical Challenge. AIDS Patient Care STDs. 2006;20:285–291. [DOI] [PubMed]
- 38.Green TA. Using Surveillance data to monitor trends in the AIDS epidemic. Stat Med. 1998;17:143–154. [DOI] [PubMed]
- 39.DHHS CDC. Cases of HIV Infection and AIDS in the United States and Dependent Areas, 2005: Technical Notes. Atlanta: ; 2006. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2005report/technicalnotes.htm. Accessed on November 21, 2007.
- 40.Cleveland WS. Visualizing Data. Summitt, NJ: Hobart Press; 1993.
- 41.SAS Institute. SAS/STAT® 9.1 User’s Guide. Cary, NC: SAS Institute; 2004.
- 42.Clifford M. Hurvich JSSC-LT. Smoothing parameter selection in nonparametric regression using an improved Akaike information criterion. J R Stat Soc. Ser B Stat Methodol. 1998;60.
- 43.Pedhazur E, Liora Pedhazur Schmelkin. Measurement, Design and Analysis: An Integrated Approach. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers; 1991:73–76.
- 44.Singer JD, Willet JB. Applied Longitudinal Data Analysis. New York, NY: Oxford University Press; 2003.
- 45.Bryk A, Raudenbush S. Heirarchical Linear Models: Applications and Data Analysis Methods, Second Edition. Thousand Oaks, CA: Sage Publications; 2002.
- 46.Hamid A, Curtis R, McCoy K, et al. The heroin epidemic in New York City: current status and prognoses. J Psychoact Drugs. 1997;29(4):375–391. [DOI] [PubMed]
- 47.Vlahov D, Fuller C, Ompad D, Galea S, Des Jarlais D. Updating the Infection Risk Reduction Hierarchy: Preventing Transition to Injection. J Urban Health. 2004;81:14–19. [DOI] [PMC free article] [PubMed]
- 48.Hunt D, Kuck S, Truitt L. Methamphetamine Use: Lessons Learned. Cambridge MA: Abt Associates Inc. 2006. Available at: http://www.ncjrs.gov/pdffiles1/nij/grants/209730.pdf. Accessed on November 21, 2007.
- 49.DHHS SAMHSA Office of Applied Studies. Primary Methamphetamine/Amphetamine Treatment Admissions: 1992–2002; 2004. Available at: http://oas.samhsa.gov/2k4/methTX/methTX.htm. Accessed on November 21, 2007.
- 50.CDC. Unintentional and undetermined poisoning deaths—11 States, 1990–2001. MMWR Morb Mortal Wkly Rep. 2004;53:233–238. [PubMed]
- 51.Archibald C, Jayaraman G, Major C, Patrick D, Houston S, Sutherland D. Estimating the size of hard-to-reach populations: a novel method using HIV testing data compared to other methods. AIDS. 2001;15:s41–s48. [DOI] [PubMed]
- 52.Kraus L, Augustin R, Frischer M, Kummler P, Uhl A, Wiessing L. Estimating prevalence of problem drug use at national level in countries of the European Union and Norway. Addict. 2003;98:471–485. [DOI] [PubMed]
- 53.Hickman M, Cox S, Harvey J, et al. Estimating the prevalence of problem drug use in inner London: A discussion of three capture–recapture studies. Addict. 1999;94:1653–1662. [DOI] [PubMed]
- 54.Cox S, Shipley M. Counting the uncatchable? An epidemiological method for counting drug misusers. Soc Psychiatry Psychiatr Epidemiol. 1997;32:19–23. [DOI] [PubMed]
- 55.Larson A, Bammer G. Why? Who? How? Estimating numbers of illicit drug users: lessons from a case study from the Australian Capital Territory. Aust N Z J Public Health. 1996;20:493–499. [DOI] [PubMed]
- 56.Hickman M, Seaman S, de Angelis D. Estimating the relative incidence of heroin use: application of a method for adjusting observed reports of first visits to specialized drug treatment agencies. Am J Epidemiol. 2001;153:632–641. [DOI] [PubMed]
- 57.Cooper H, Friedman SR, Tempalski B, Friedman R, Keem M. Racial/ethnic disparities in injection drug use in 94 U.S. metropolitan statistical areas. Ann Epidemiol. 2005;15:326–334. [DOI] [PubMed]
- 58.Day, Dawn. Drug Arrests: Are Blacks Being Targeted? April, 1995. Web Page. Available at: http://www.ndsn.org/april95/guest.html. Accessed on June 12, 2007.
- 59.Human Rights Watch. Punishment and Prejudice: Racial Disparities in the War on Drugs. A Human Rights Watch Report. 2000;12. Available at: http://www.hrw.org/reports/2000/usa/. Accessed on November 21, 2007.
- 60.Kang SY, Goldstein MF, Deren S. Health Care Utilization and Risk Behaviors among HIV Positive Minority Drug Users. Journal of Health Care for the Poor and Underserved. 2006;17:265–275. [DOI] [PubMed]
- 61.Deany, Paul. HIV and Injecting Drug Use: A New Challenge to Sustainable Human Development. December, 2000. Web Page. Available at: http://www.undp.org/hiv/publications/deany.htm . Accessed on June 12, 2007.
- 62.Irwin KL, Edlin BR, Faruque S, et al. Crack cocaine smokers who turn to drug injection: characteristics, factors associated with injection, and implications for HIV transmission. Drug Alcohol Depend. 1996;42:85–92. [DOI] [PubMed]
- 63.Dinwiddie S, Cottler L, Compton W, Abdallah AB. Psychopathology and HIV risk behaviors among injection drug users in and out of treatment. Drug Alcohol Depend. 1996;43:1–11. [DOI] [PubMed]
- 64.Barrio G, De La Fuente L, Lew C, Royuela L, Bravo M, Torrens M. Differences in severity of heroin dependence by route of administration: the importance of length of heroin use. Drug Alcohol Depend. 2001;63:169–177. [DOI] [PubMed]
- 65.Celentano D, Galai N, Sethi AK, et al. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15:1707–1715. [DOI] [PubMed]
- 66.Centers for Disease Control and Prevention. Epidemiology of HIV/AIDS—United States, 1981–2005. MMWR Morb Mortal Wkly Rep. 2006;55:589–592. [PubMed]
- 67.Neal J, Fleming PL. Frequency and predictors of late HIV diagnosis in the United States, 1994 through 1999. [Abstract]. Presented at the 9th Conference on Retrovisuses and Opportunistic Infections: Washington State Convention Trade Center.
- 68.Hu D, Byers R, Fleming P, Ward J. Characteristics of persons with late AIDS diagnosis in the United States. Am J Prev Med. 1995;11:114–119. [PubMed]
- 69.DHHS SAMHSA. Summary of Findings from the 1998 National Household Survey on Drug Abuse: Revised Methodology and Adjustment of 1979–93 Estimates. Web Page. Available at: http://www.oas.samhsa.gov/nhsda/98SummHtml/NHSDA98Summ-02.htm#P172_13001. Accessed on February 27, 2007.
- 70.DHHS SAMHSA. National Household Survey on Drug Abuse: Main Findings 1997 Appendix A: Key Definitions, 1972–1997 Survey Years. Web Page. Available at: http://oas.samhsa.gov/nhsda/1997Main/nhsda1997mfWeb-121.htm#P24776_520354. Accessed on February 27, 2007.
- 71.DHHS SAMHSA. M-6: National Survey on Drug Use & Health: Summary of Methodological Studies, 1971–2005. Web Page. Available at: http://www.oas.samhsa.gov/methodsHY/NSmethods.pdf. Accessed on February 27, 2007.
- 72.DHHS NCHS. Multiple Cause of Death File, 1992–2002 [Computer file] U.S. Dept. of Health and Human Services, National Center for Health Statistics [producer].
- 73.DHHS NCHS. National Hospital Discharge Survey 1992–2002 [Computer File. ICPSR version]. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor].
- 74.Warner-Smith M, Darke S, Lynskey M, Hall W. Heroin overdose: causes and consequences. Addiction. 2001;1113. [DOI] [PubMed]
- 75.Dasgupta N, Brownstein JS, Jonsson-Funk M. Spatial Patterns in Opioid-related Mortality in the United States, 2002. Presented at: Association of American Geographers/NIDA Symposium, Chicago, IL. March 2006.
- 76.Connecticut Department of Public Health. Report to the General Assembly Public Act 03-159: A Report on Fatal and Non-Fatal Drug Overdoses in Connecticut: January 2004. Available at: http://digitalarchive.oclc.org/da/ViewObjectMain.jsp;jsessionid=84ae0c5f82406163900e6cd143b2aaf4c2102de2efba?fileid=0000062817:000004106541&reqid=34681. Accessed on November 21, 2007.
- 77.Sanford CK, N.C. Injury and Violence Prevention Unit. Deaths from Unintentional Drug Overdoses in North Carolina, 1997–2001: A DHHS Investigation into Unintentional Poisoning-Related Deaths. September 2002. Available at: http://www.communityhealth.dhhs.state.nc.us/Injury/unintentional%20poisonings%20report-9-02-final.pdf. Accessed on November 21, 2007.
- 78.Cooper HLF, Brady JE, Ciccarone D, Tempalski B, Gostnell K, Friedman SR. Nationwide increase in hospitalizations for illicit-injection-drug-related infective endocarditis. Clinical Infectious Diseases. 2007. 45(9):1200–1203 [DOI] [PMC free article] [PubMed]
- 79.DHHS SAMHSA. Treatment Episode Data Set (TEDS) Series. Web Page. Available at: http://webapp.icpsr.umich.edu/cocoon/SAMHDA-SERIES/00056.xml. Accessed on June 12, 2007.
- 80.Turner CF, Miller HG, Moses LEE, Eds. AIDS, Sexual Behavior and Intravenous Drug Use. Washington, DC: National Academy Press; 1989. National Academy of Sciences/Committee on AIDS Research. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Component estimates and final estimates of the number of IDU in each of the 96 large US MSAs.
ESM (RTF 374 kb)