Abstract
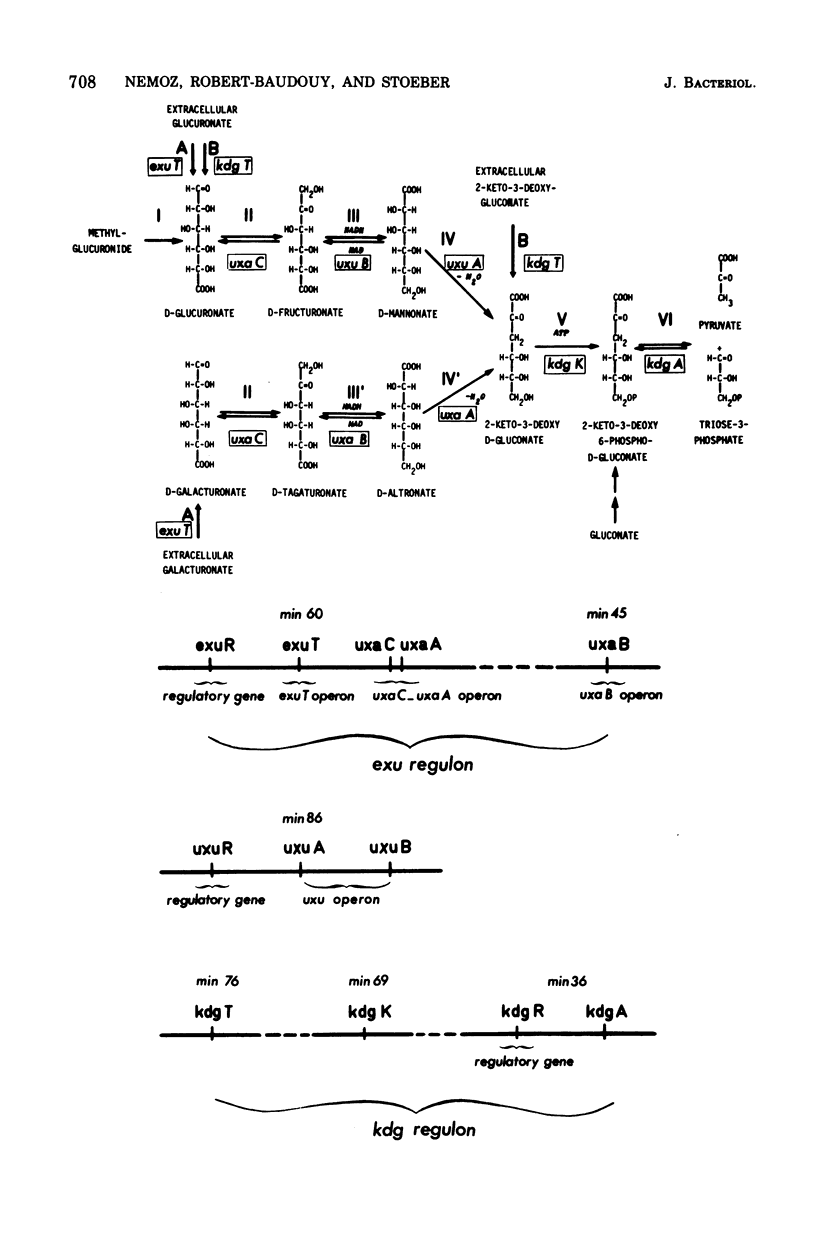
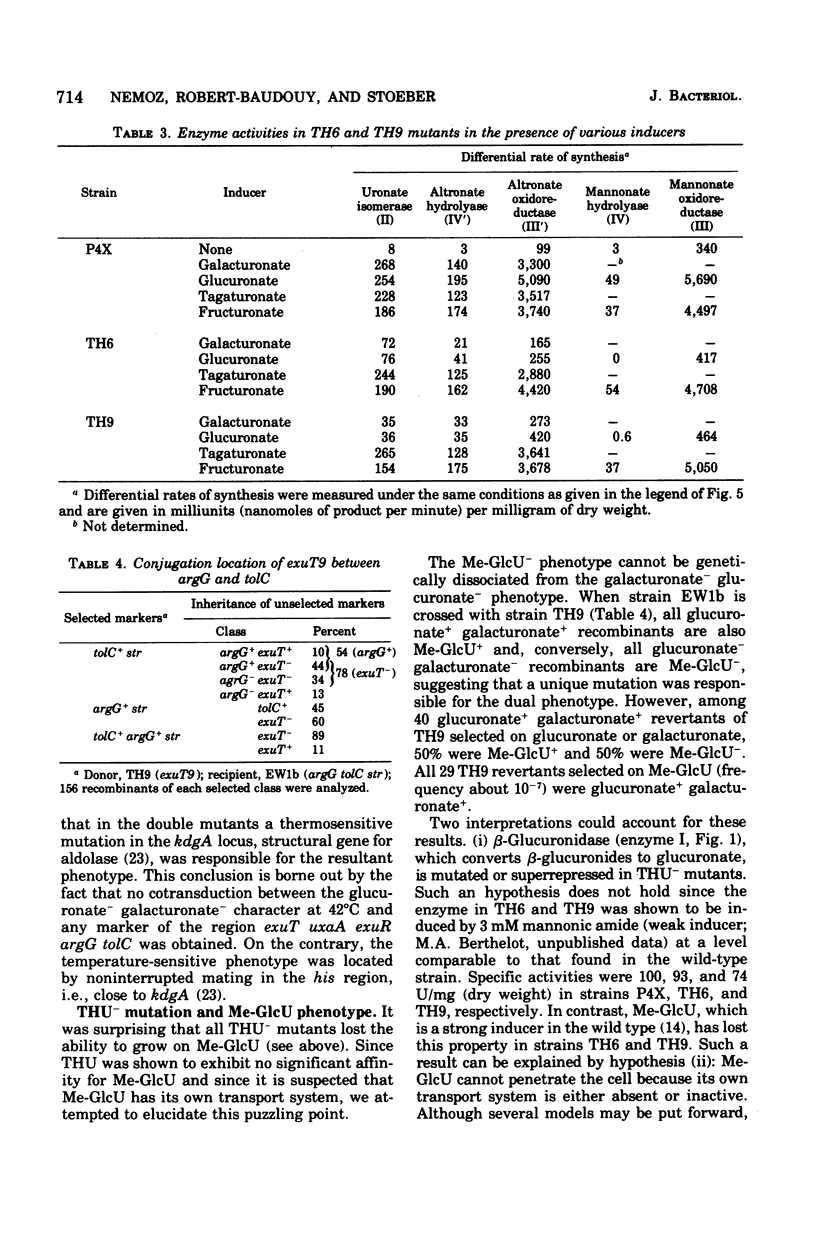
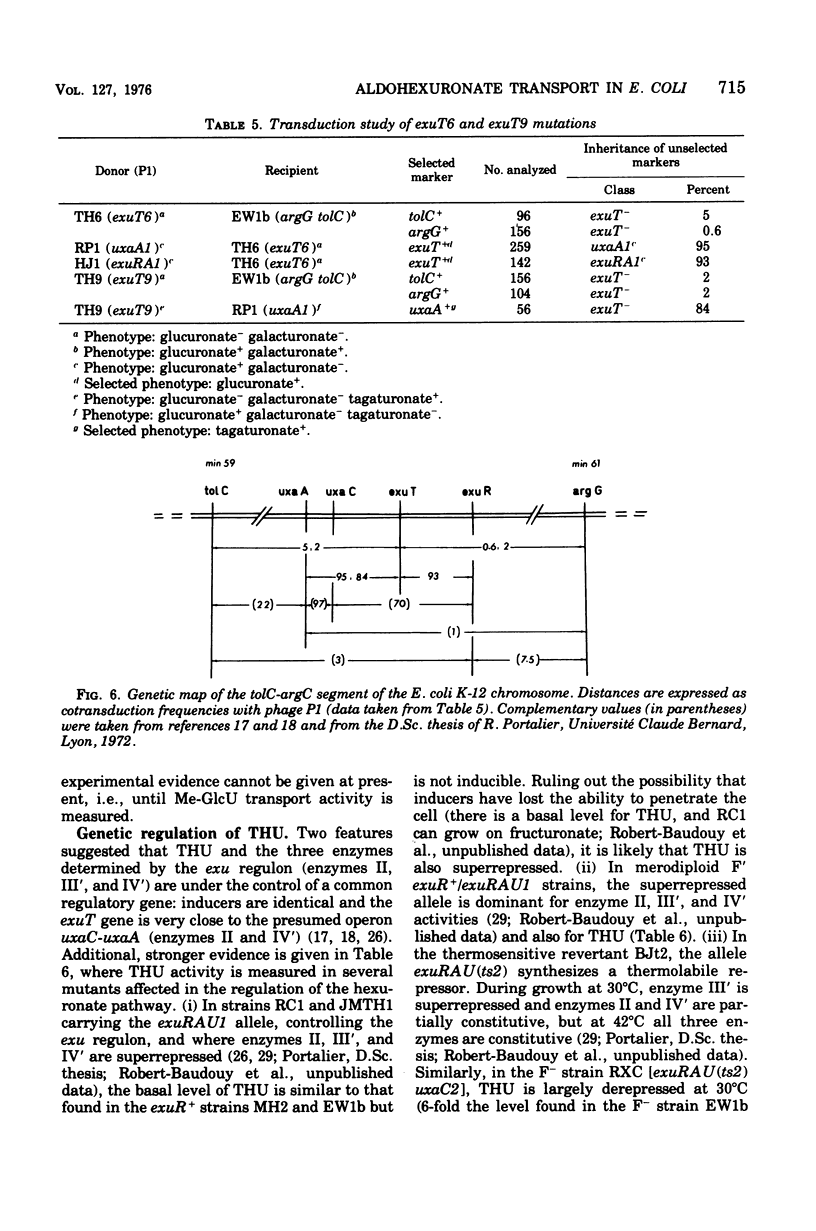
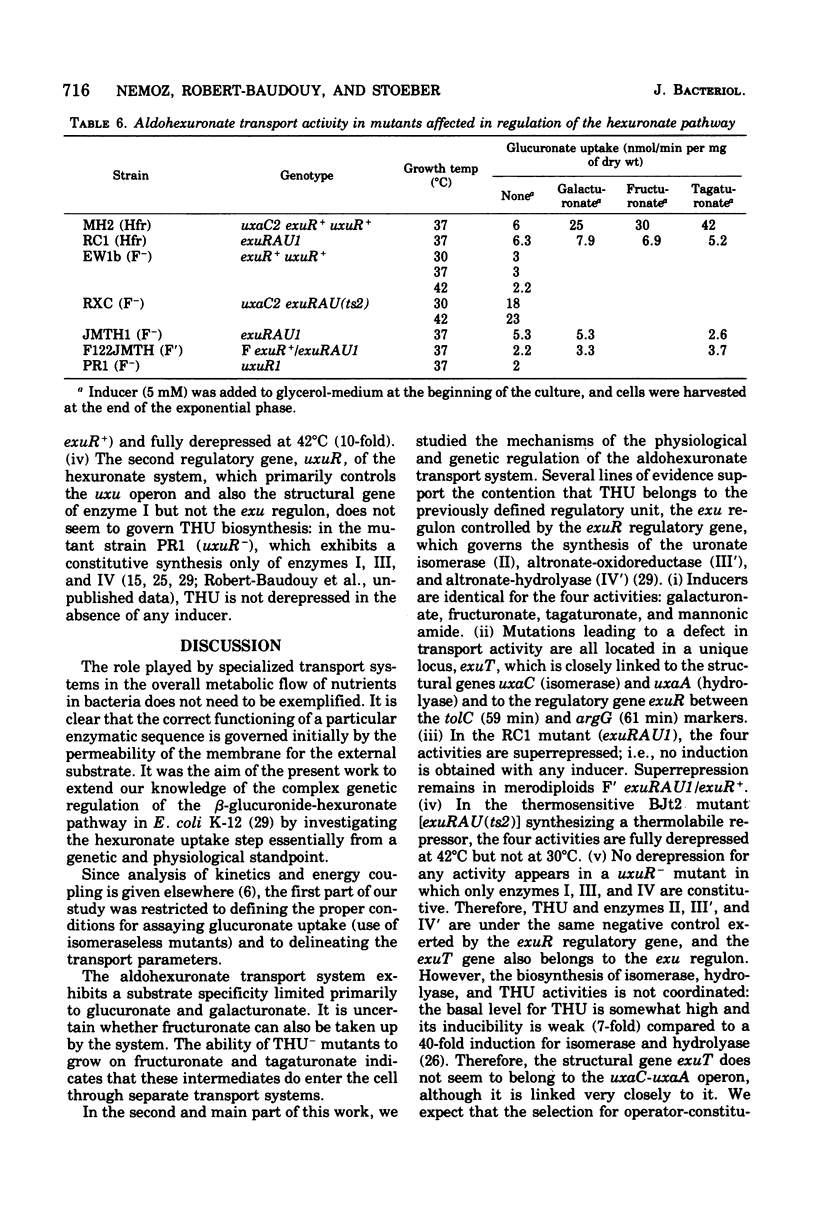
In Escherichia coli K-12, the specificity of the aldohexuronate transport system (THU) is restricted to glucuronate and galacturonate. There is a relatively high basal-level activity in uninduced wild-type or isomeraseless strains. Supplementary activity is obtained with the inducers mannonic amide (five-fold), galacturonate (fourfold), fructuronate (fivefold), and tagaturonate (sevenfold). Specific THU- mutants were selected as strains unable to grow on either aldohexuronate but able to grow on fructuronate or tagaturonate. The remaining transport activity in uninduced and induced THU- starins represents less than 20% of that found in the wild type. Conjugation and transduction experiments indicate that all of the THU- mutations are located in a unique locus, exuT, half-way between the tolC (59 min) and argG (61 min) markers. exuT is closely linked to the uxaC-uxaA operon (60 min) and to the regulatory gene exuR (60 min), which controls the above-mentioned operon and the uxaB operon (45 min). Growth on either aldohexuronate and transport activity are fully recovered when exuT mutants are allowed to revert to exuT+ on galacturonate or glucuronate. Reversion on glucuronate alone may lead to the mutational derepression of the 2-keto-3-deoxygluconate transport system, which is uninducible in the wild type, which also takes up glucuronate, and whose structural gene belongs to the kdg regulon. Such strains, which remain unable to grow on galacturonate, are exuT and kdgR (constitutive allele of the regulatory gene kdgR of the kdg regulon). THU activity is superrepressed in an exuR mutant in which the uxaC-uxaA operon and the uxaB operon are superrepressed; exuR+/exuR merodiploids are also superrepressed. In a thermosensitive exuR mutant in which the above-mentioned operons are constitutive at 42 degrees C, the THU activity is fully derepressed at this temperature. On the basis of these and other results, it is concluded that THU is coded for by the structural gene exuT, which is negatively controlled by the exuR gene product and which probably belongs to an operon distinct from the uxaA-uxaC operon.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abendano J. J., Kepes A. Sensitization of D-glucuronic acid transport system of E. coli to protein group reagents in presence of substrate or absence of energy source. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1342–1346. doi: 10.1016/0006-291x(73)91134-0. [DOI] [PubMed] [Google Scholar]
- Adler J., Hazelbauer G. L., Dahl M. M. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973 Sep;115(3):824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- LOVELESS A., HOWARTH S. Mutation of bacteria at high levels of survival by ethyl methane sulphonate. Nature. 1959 Dec 5;184:1780–1782. doi: 10.1038/1841780a0. [DOI] [PubMed] [Google Scholar]
- Lagarde A. E., Pouysségur J. M., Stoeber F. R. A transport system for 2-keto-3-deoxy-D-gluconate uptake in Escherichia coli K12. Biochemical and physiological studies in whole cells. Eur J Biochem. 1973 Jul 16;36(2):328–341. doi: 10.1111/j.1432-1033.1973.tb02917.x. [DOI] [PubMed] [Google Scholar]
- Lagarde A. E., Stoeber F. R. Transport of 2-keto-3-deoxy-D-gluconate in isolated membrane vesicles of Escherichia coli K12. Eur J Biochem. 1974 Mar 15;43(1):197–208. doi: 10.1111/j.1432-1033.1974.tb03400.x. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., PAPPENHEIMER A. M., Jr, COHEN-BAZIRE G. La cinétique de la biosynthèse de la beta-galactosidase chez E. coli considérée comme fonction de la croissance. Biochim Biophys Acta. 1952 Dec;9(6):648–660. doi: 10.1016/0006-3002(52)90227-8. [DOI] [PubMed] [Google Scholar]
- Novel G., Didier-Fichet M. L., Stoeber F. Inducibility of beta-glucuronidase in wild-type and hexuronate-negative mutants of Escherichia coli K-12. J Bacteriol. 1974 Oct;120(1):89–95. doi: 10.1128/jb.120.1.89-95.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel M., Novel G. Mutants d'Escherichia coli K-12, capables de croître sur méthyl-beta-D-galacturonide: mutants simples constitutifs pour la synthèse de la beta-glucuronidase et mutants doubles déréprimés aussi pour la synthèse de deux enzymes d'utilisation du glucuronate. C R Acad Sci Hebd Seances Acad Sci D. 1974 Aug 19;279(8):695–698. [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- Portalier R. C., Robert-Baudouy J. M., Némoz G. M. Etudes de mutations affectant les gènes de structure de l'isomerase uronique et de l'oxydoreductase altronique chez Escherichia coli K 12. Mol Gen Genet. 1974;128(4):301–319. doi: 10.1007/BF00268518. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Robert-Baudouy J. M., Stoeber F. R. Localisation génétique et caractérisation biochimique de mutations affectant le gène de structure de l'hydrolyase altronique chez Escherichia coli K 12. Mol Gen Genet. 1972;118(4):335–350. doi: 10.1007/BF00333569. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Stoeber F. R. Dosages colorimétriques des oxydoréductases aldoniques d'Escherichia coli K 12: applications. Biochim Biophys Acta. 1972 Nov 10;289(1):19–27. doi: 10.1016/0005-2744(72)90103-9. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Stoeber F. R. La D-altronate: NAD-oxydoréductase d'Escherichia coli K12. Purification, propriétés et individualité. Eur J Biochem. 1972 Mar 15;26(1):50–61. doi: 10.1111/j.1432-1033.1972.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Stoeber F. R. La D-mannonate: NAD-oxydoréductase d'Escherichia coli K12. Purification, propriétés et individualité. Eur J Biochem. 1972 Mar 27;26(2):290–300. doi: 10.1111/j.1432-1033.1972.tb01767.x. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Genetic control of the 2-keto-3-deoxy-d-gluconate metabolism in Escherichia coli K-12: kdg regulon. J Bacteriol. 1974 Feb;117(2):641–651. doi: 10.1128/jb.117.2.641-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J. M., Stoeber F. R. Rameau dégradatif commun des hexuronates chez Escherichia coli K12. Mécanisme d'induction des enzymes assurant le métabolisme du 2-céto-3-désoxy-gluconate. Eur J Biochem. 1972 Nov 7;30(3):479–494. doi: 10.1111/j.1432-1033.1972.tb02120.x. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Stoeber F. Mutations affectant le gène de structure de la 2-céto-3-désoxy-6-P-gluconate aldolase chez E. coli K 12. Mol Gen Genet. 1972;114(4):305–311. doi: 10.1007/BF00267499. [DOI] [PubMed] [Google Scholar]
- Robert-Baudouy J. M., Jimeno-Abendano J., Stoeber F. R. Individualité des hydrolyases mannonique et altronique chez Escherichia coli K 12. Biochimie. 1975;57(1):1–8. doi: 10.1016/s0300-9084(75)80103-9. [DOI] [PubMed] [Google Scholar]
- Robert-Baudouy J. M., Portalier R. C., Stoeber F. R. Régulation due métabolisme des hexuronates chez Escherichia coli K12. Modalités de l'induction des enzymes du système hexuronate. Eur J Biochem. 1974 Mar 15;43(1):1–15. doi: 10.1111/j.1432-1033.1974.tb03378.x. [DOI] [PubMed] [Google Scholar]
- Robert-Baudouy J. M., Stoeber F. R. Purification et propriétés de la D-mannonate hydrolyase d'Escherichia coli. Biochim Biophys Acta. 1973 Jun 6;309(2):473–485. doi: 10.1016/0005-2744(73)90045-4. [DOI] [PubMed] [Google Scholar]
- Stoeber F., Lagarde A., Nemoz G., Novel G., Novel M., Portalier R., Pouyssegur J., Robert-Baudouy J. Le métabolisme des hexuronides et des hexuronates chez Escherichia coli K 12: aspects physiologiques et et génétiques de sa régulation. Biochimie. 1974;56(2):199–213. doi: 10.1016/s0300-9084(74)80379-2. [DOI] [PubMed] [Google Scholar]
- WILLSON C., PERRIN D., COHN M., JACOB F., MONOD J. NON-INDUCIBLE MUTANTS OF THE REGULATOR GENE IN THE "LACTOSE" SYSTEM OF ESCHERICHIA COLI. J Mol Biol. 1964 Apr;8:582–592. doi: 10.1016/s0022-2836(64)80013-9. [DOI] [PubMed] [Google Scholar]
- Zwaig N., Kistler W. S., Lin E. C. Glycerol kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli. J Bacteriol. 1970 Jun;102(3):753–759. doi: 10.1128/jb.102.3.753-759.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



