Abstract
We sought to explore the role of tumor necrosis factor-alpha (TNF-α) in the pathogenesis of peripheral nerve ischemia-reperfusion (IR) injury. We established an ischemia-reperfusion model in wild type (WT) and TNF-α knockout (KO) mice. Electrophysiology, behavioral score and morphological indices (edema and ischemic fiber degeneration [IFD]) were examined to determine the influence of TNF-α on peripheral nerve structure and function following ischemia followed by reperfusion. TNF-α and nuclear factor-kappa B (NF-κB) expression were evaluated using immunohistochemistry. TNF-α KO mice, compared to WT had, in sciatic nerve, marked improvement in nerve pathology. This is a region subject to moderate ischemia-reperfusion injury. There was also significant improvement in electrophysiological and some behavioral indices. TNF-α and NF-κB expression were abundant in sciatic-tibial nerves of WT mice subjected to IR, but there was less, or complete lack of, expression in ischemic nerve of TNF-α KO mice. We conclude that TNF-α plays an essential role in the pathogenesis of peripheral nerve ischemia-reperfusion injury, possibly partly through the activation of NF-κB.
Keywords: ischemia-reperfusion (IR) injury, peripheral nerve, tumor necrosis factor-alpha (TNF-α), nuclear factor-kappa B (NF-κB)
INTRODUCTION
Ischemia to nerve results in ischemic fiber degeneration (IFD) [1]. Reperfusion following ischemia (IR) results in additional fiber degeneration, and mechanisms of reperfusion injury have been postulated to be due to the additional insult of inflammatory response [2] and oxidative injury [3]. The pro-inflammatory cytokines, especially tumor necrosis factor-alpha (TNF-α), is a major component of the inflammatory response following IR injury and is rapidly up-regulated from mRNA level to protein level [4–7]. TNF-α regulates inflammation, immune modulation, and apoptosis by activating nuclear factor-kappa B (NF-κB) and the caspase pathway [8, 9]. Studies support a key role of TNF-α in IR injury to the central nervous system [10]. The role of TNF-α in the pathogenesis of peripheral nerve damage is less well understood and the molecular mechanism of TNF-α in IR injury is still unclear.
In this study, we explore the role of TNF-α in IR-induced peripheral nerve injury using TNF-α knock-out (KO) mice. Using the IR model, we evaluate the role of TNF-α on peripheral nerve electrophysiology, structure and function. Using immunohistochemistry, we investigate the signal pathway of TNF-α in IR injury to peripheral nerve.
MATERIALS AND METHODS
Animals
Thirty-four male mice at 6 to 10 weeks of age were used in this experiment. B6,129SF2/J and TNF-α-deficient [TNF(-/-)] mice of strain B6,129S-Tnftm1Gkl (Jackson Laboratory, Bar Harbor, ME) were used as wild type (WT) control (n=20) and TNF-α KO control (n=14), respectively. The experimental protocol was approved by the Mayo Clinic Institutional Animal Care and Use Committee and conformed to NIH guidelines for the use of animals in research.
Ischemia-Reperfusion Model
The model was produced by ligating and then releasing 6-0 silk suture ties placed around the supplying arteries to the right sciatic-tibial nerve, as previously described [11]. After 2 hours of ischemia, while maintaining the right hind limb at 28°C, the ties were released using a slipknot technique ideal for ready release and rapid reperfusion. The limb temperature was monitored with an intramuscular thermistor probe (Bailey Instruments, Saddle Brook, NJ) and was maintained at 28°C during ischemia by using an infrared lamp. Animals were studied 7 days after reperfusion.
Electrophysiology
We used techniques that are standard for our laboratory [12]. The conduction velocity (CV) and amplitude of sensory nerve action potential (SNAP) of digital nerve and compound muscle action potential (CMAP) of sciatic-tibial nerve were measured using fine stainless steel near-nerve stimulating and recording electrodes in this study. SNAP was recorded from the ankle while stimulating the digital nerve at the tip of the digit. CMAP was recorded from the dorsum of the hind paw while stimulating at the level of the sciatic notch. Recordings were done at 35.0°C, amplified 1000-fold and stored on computer disk, and analyzed off-line using Nicolet digital oscilloscope (Nicolet Instruments, Madison, WI).
Behavioral Score
Evaluation of limb neural function was performed using our standard behavioral scoring system [4]. The function of the limb was scored with the observer blinded to the status of the mice. Scores ranged from 0 (no function) to 20 (normal function). The score was based on gait (0 = no function to 3 = normal), paw position (0 = no flexion to 3 = normal), grasp (0 = no grasp to 3 = normal), pinch sensitivity (0 = absent to 2 = present), discoloration (0 = blackened to 3 = normal), inflammation (0 = marked to 3 = normal), and self-mutilation (0 = foot completely removed to 3 = normal). Increasing function was indicated by a larger score.
Neuropathology: Edema and Ischemia Fiber Degeneration (IFD)
After anesthesia with pentobarbital, mice underwent intracardiac perfusion with 4% paraformaldehyde. Following fixation, the entire lengths of sciatic-tibial nerves were harvested. The nerves were divided into four segments: proximal sciatic, distal sciatic, mid tibial, and distal tibial. The distal tibial and distal sciatic nerves were osmicated, dehydrated, infiltrated, and embedded in Spurr’s resin. Transverse sections of 10 µm were stained with 1% toluidine blue. Under 400× magnification, these sections were graded for edema and IFD using previously described methods [13]. Fibers were considered to be undergoing IFD if axonal changes were visible. The axon may be swollen or shrunken, watery and light, or dark and shrunken. Secondary myelin changes were typically seen including attenuation, collapse, or breakdown. For each section the percent of fibers undergoing IFD was graded from 0 to 4 as follows: 0 ≤ 2%; 1 = 3–25%; 2 = 26–50%; 3 = 51–75%; 4 ≥75%. Edema was semiquantitatively graded from 0 to 4 as follows: 0 = normal; 1 = mild edema; 2 = moderate edema; 3 = severe edema; 4=severe and global edema. No distinction was made as to endoneurial, perivascular, or subperineurial edema.
Immunohistochemistry
After intracardiac perfusion with 4% paraformaldehyde, the proximal sciatic and mid tibial nerves were harvested and post-fixed in 2% paraformaldehyde for 24 hours, immersed in 30% sucrose for 24 hours, covered with OCT compound, frozen with liquid nitrogen, and then stored at −80°C. Frozen nerves were cut into 10 µm transverse sections in a freezing microtome (Microm Cryostat, HM 505 E, Carl Zeiss, Thornwood, NY) and thaw-mounted on gelatin-coated slides. Sections were stained with the following antibodies: rabbit anti- NF-κB antibody and goat anti-TNF-α antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). We used NF-kB p65 antibody, which binds to p65 subunit. A marked increase in staining occurs precisely because of NF-kB activation, such being the property of the antibody. Additionally, most of NF-kB staining was localized to Schwann cells, where staining was usually intense, suggesting a translocation of NF-kB p65 into nucleus in Schwann cell after ischemia/reperfusion injury. Filtered 3,3′-diaminobenzidine was used the chromogen, as previously described [14]. NF-κB positivity was graded according to the density (number/10000µm2): 0 = ≤5; 1 = 6–15; 2 = 16–25; 3 = 26–50; 4 = ≥51. Any visible brown staining in tissue of interest was considered positive. Negative controls were generated by omission of the primary antibody, and no staining was observed.
Statistics
All experimental data were expressed as mean ± SEM. For comparison of electrophysiological data, the unpaired t test was used; for comparison of grading data, Mann-Whitney U test was used.
RESULTS
Behavioral score and electrophysiology
The behavioral and electrophysiological abnormalities are shown in Table 1. Moderate functional deficits developed in the behavior of both WT and TNF-α KO groups. Behavioral scores showed no significant difference between groups. In electrophysiological evaluations, both CV and amplitude of right SNAP revealed no significant differences between wild and TNF-αKO groups. The mean CV of sciatic-tibial nerve for the TNF-α KO group was significantly higher than for the WT group (p<0.05).
Table. 1.
Behavioral Score and Electrophysiology in wild type and TNF-α knock out mice after ischemia-reperfusion injury
| Group | Side | BS | SNAP | Sciatic CMAP | ||
|---|---|---|---|---|---|---|
| CV (m/s) | Amp (µV) | CV (m/s) | Amp (mV) | |||
| Wild | Left | 20 | 27.42±0.48 | 29.38±2.76 | 19.00±0.62 | 10.08±0.23 |
| Right | 11.3±1.1 | 10.75±3.33 | 6.89±2.52 | 12.18±1.86 | 1.31±0.48 | |
| TNF-α -/- | Left | 20 | 27.77±0.84 | 22.82±2.22 | 17.37±0.52 | 9.92±0.70 |
| Right | 12.9±1.1 | 12.56±4.17 | 4.68±1.76 | 16.92±2.59 * | 1.89±1.04 | |
BS, Behavioral score; CV, Conduction velocity; Amp, Amplitude
p<0.05 (WT vs. TNF-α KO)
Edema and IFD
Edema and IFD grades were similar for WT and TNF-α KO right tibial nerve (the ischemic side) but were markedly different for right sciatic nerve. Edema grades of right tibial nerve were similar for WT and TNF-α KO (2.0 ± 0.3 and 1.8 ± 0.5, respectively). In contrast, right sciatic nerve edema was much greater for WT (1.7 ± 0.3) than TNF-α KO (0.3 ± 0.2; p<0.05). While marked edema occurred in both right tibial and sciatic nerves of WT, the sciatic nerve of the TNF-α KO group showed less edema (Fig. 1). IFD grades of right tibial nerve for WT and TNF-α KO groups were similar at 2.8 ± 0.5 and 2.0 ± 0.6, respectively (difference NS). In contrast, IFD for right sciatic nerve was 2.2 ± 0.4 for WT and 0.3 ± 0.3 for TNF-α KO (Fig. 2). While the majority of fibers degenerated in right tibial nerve of the WT group, only moderate degeneration occurred in right tibial nerve of the KO group (Fig. 3). The difference was significant compared to the sciatic nerve grade between wild and TNF-α KO groups (p<0.05).
Figure 1.
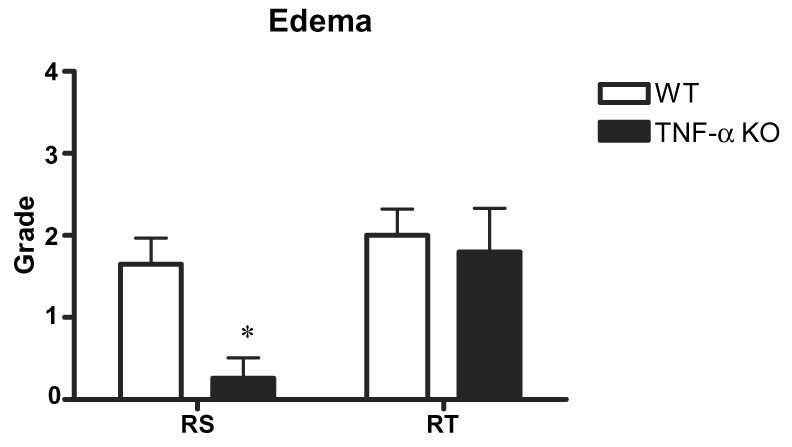
Edema grades of right tibial (RT) and sciatic (RS) nerves in WT (n=20) and TNF-α KO (n=14) mice. Edema grade of right sciatic nerve in the TNF-α KO group was significantly lower than in the WT group.
*p<0.05; WT group vs. KO group.
Figure 2.
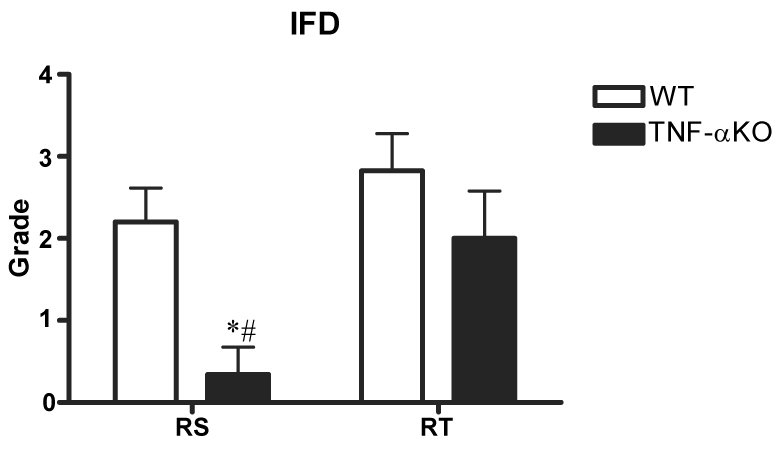
IFD grades of right tibial (RT) and sciatic (RS) nerves in WT (n=20) and TNF-α KO (n=14) mice. IFD grade for right sciatic nerve was significantly lower in the TNF-α KO mice than in the WT mice.
*p<0.05; WT group vs. KO group.
#p<0.05; Right tibial vs. Right sciatic
Figure 3.
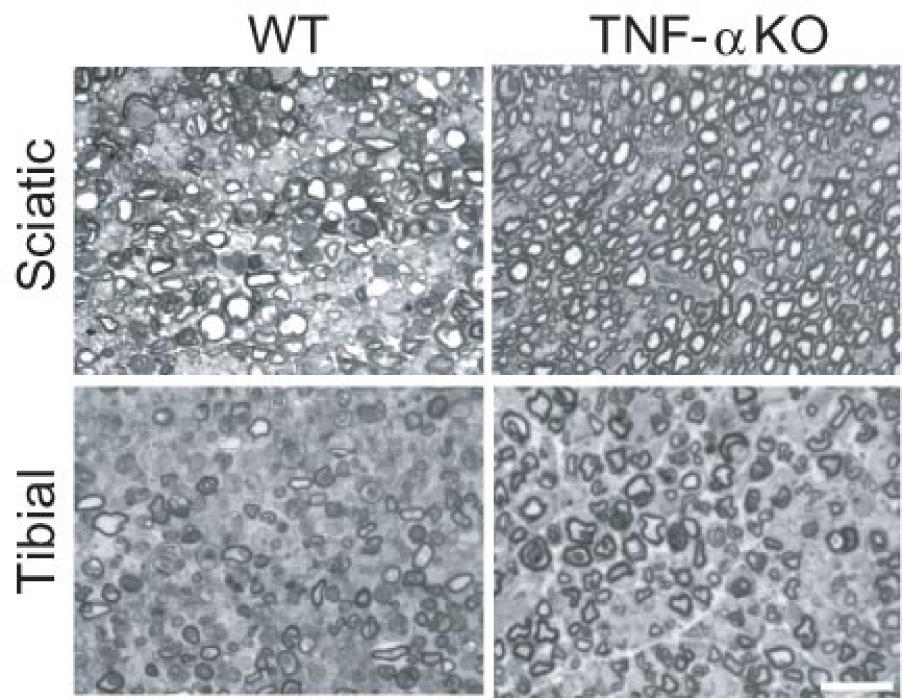
Microscopic findings of right tibial and sciatic nerves in WT and TNF-α KO groups. The majority of fibers degenerated in both tibial and sciatic nerves of the WT group and in tibial nerve of the TNF-α KO group. Few degenerated fibers were seen in right sciatic nerve of the TNF-α KO group. Bar=25µm
Immunohistochemistry
NF-κB
The grade for NF-κB in right tibial nerve was increased significantly to 2.1 ± 0.4 for the WT group, compared to 0.9 ± 0.5 for the TNF-α KO group (p<0.05) (Fig. 4). Staining for activated NF-κB was increased and was localized in Schwann cells with intense staining, suggesting a translocation of NF-kB p65 into the nucleus in Schwann cell after ischemia/reperfusion injury.
Figure 4.
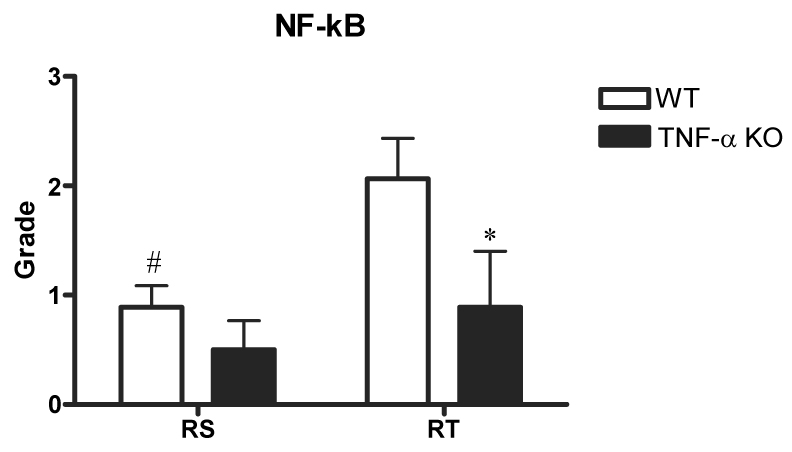
Grades of immunohistochemical staining with NF-κB in right tibial (RT) and sciatic (RS) nerves for WT (n=20) and TNF-α KO (n=14) groups. Tibial nerve of TNF-α KO group exhibited lower grades than that of WT group. NF-kB staining was localized in Schwann cells with intense staining, suggesting a translocation of NF-kB p65 into nucleus in Schwann cell after ischemia/reperfusion injury.
*p<0.05; WT group vs. KO group.
#p<0.05; Right tibial vs. Right sciatic
There was no significant difference in sciatic nerve between WT and TNF-α KO groups. Excessive positive staining for NF-κB was seen in right tibial nerve of the WT group, however, less positive staining was found in right tibial nerve of the TNF-α KO group (Fig. 5).
Figure 5.
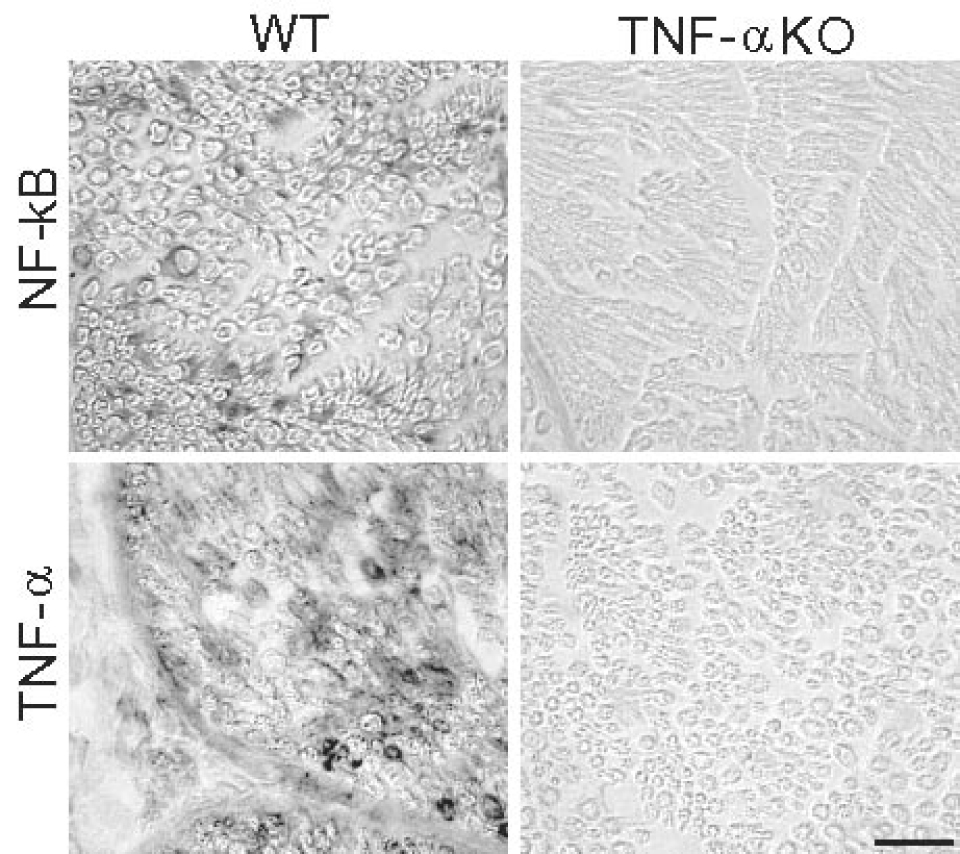
Microscopic findings of immunoreactivity for NF-κB and TNF-α in WT and TNF-α KO groups. Massive positive staining for NF-κB and TNF-α were seen in wild group (tibial nerve only). Minimal or negative staining was shown in TNF-α KO group. Bar=25µm
TNF-α
TNF-α expression was not observed in both right tibial and sciatic nerves of the TNF-α KO groups. In contrast, TNF-α positive cells were scattered in both right tibial and sciatic nerves of the WT group (Fig. 5). Minimal staining of TNF-α was seen in non-ischemic (left) nerves of the WT group.
DISCUSSION
There are two major findings in this study. First, mice lacking TNF-α are significantly protected from the effects of IR injury, supporting the hypothesis that TNF-α is implicated in the pathogenesis of fiber degeneration that follows reperfusion. Second, neuroprotection in TNF-α KO mice occurs provided that ischemia is not extreme. Marked neuroprotection is seen in the sciatic nerve, where ischemia is moderate, but not in tibial nerve (extreme ischemia).
Previous studies have demonstrated that ischemia to nerve can cause fiber degeneration, and reperfusion following ischemia causes additional fiber degeneration, mediated by an inflammatory response and oxidative stress [2, 3]. The current finding of less edema and fiber degeneration in sciatic but not tibial nerve is consonant with our previous studies that demonstrate that neuroprotection, whether with hypothermia or antioxidants, occurs when the ischemic insult is moderate (sciatic nerve) but fails when the insult is extreme [11, 12, 15, 16]. We have previously reported the development of an inflammatory response and oxidative stress with reperfusion, and have hypothesized that both of these mechanisms play an important role in the pathogenesis of fiber degeneration [13, 17]. In the inflammatory response, pro-inflammatory cytokines are believed to be key mediators of inflammation-induced IR injury; key mediators include TNF-α and IL-1 [6, 18].
Recent studies have focused on a specific role of TNF-α in peripheral nerve disease. Special emphasis has been placed on the relationship between fiber degeneration, TNF-α and painfulness [4, 19]. TNF-α KO mice were reported to have less axonal degeneration following nerve transaction [20]. Much less information is available on its role in IR injury to nerve. In other tissues, TNF-α has complex actions because of the multiple biological activities, so that its actions are sometimes described as representing a double edged sword in IR injury, with different effects, depending on time and conditions [21, 22]. Our current study is the first to demonstrate a neuroprotective role that a lack of TNF-α confers, using a model of TNF-α KO mouse. There is significant reduction in pathological changes (edema, and IFD) in the moderately ischemic sciatic nerve of TNF-α KO mouse. There is only modest neuroprotective function in the more severely ischemic tibial nerve, indicating that ischemia alone of sufficient severity will cause IFD. Severe axonal degeneration was found in tibial nerve of WT mice subjected to IR injury with shrunken, dark and collapsed axons and similar changes, slightly less severe in TNF-α KO mouse. We have previously described the time-course of TNF-α response to IR. TNF-α mRNA is rapidly elevated in the peripheral nerve in IR, reaching a peak after 24 hours reperfusion and maintaining a high level until 7 days of reperfusion [4]. Taken together, these studies support a role for TNF-α in IR-induced peripheral nerve damage, providing the ischemic insult is not extreme. Another reason why deletion of TNF-α provides incomplete neuroprotection relates to the fact that reperfusion injury is mediated by oxidative stress in addition to the inflammatory response. Oxidative injury results in apoptosis through mitochondrial dysfunction and activation of the caspase pathway. We have demonstrated marked oxidative injury (with 8-hydroxy-2′-deoxyguanosine, a widely used DNA oxidation marker) and apoptosis (with TUNEL staining) affecting peripheral nerve after IR injury [23, 24]
TNF-α binds to two cell surface receptors: TNFR1 (p55) and TNFR2 (p75) [25]. TNF-α activities are predominately mediated by TNFR1. An opposite regulation between TNFR1 and TNFR2 is reported in many models [26]. TNF-α binding to TNFR1 leads to the activation of NF-κB, and initiates the inflammatory cascade [8]. As expected, we found no TNF-α expression in TNF- KO mice, but high expression in WT mice. The current findings on NF-κB are not robust. The expression of NF-κB is dramatically elevated in ischemic tibial nerve of WT compared to KO mice, where neuroprotection is not found, and increased by 30% (difference not significant) in sciatic nerve of WT mice compared with TNF-α KO mice. These findings confirm that TNF-α exerts a neuroprotective effect, but the role of activation of NF-κB must await additional studies and especially its role in oxidative stress. Oxidative stress increases markedly in the reperfused ischemic nerve. Oxidative stress is known to induce the activation of NF-κB [27]. The increased NF-κB expression in WT tibial nerve but not in KO mice supports the notion that oxidative stress may induce NF-κB through TNF-α activation. We had previously demonstrated expression of NF-κB in diabetic Schwann cell when undergoing IR injury by using double-labeling with S-100 [23, 24].
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health (NS 22352 Peripheral Nerve Ischemia) and Mayo Funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McManis PG. Low PA. Factors affecting the relative viability of centrifascicular and subperineurial axons in acute peripheral nerve ischemia. Exp Neurol. 1988;99:84–95. doi: 10.1016/0014-4886(88)90129-x. [DOI] [PubMed] [Google Scholar]
- 2.Nukada H, Lynch CD, McMorran PD. Aggravated reperfusion injury in STZ-diabetic nerve. J Peripher Nerv Syst. 2002;7:37–43. doi: 10.1046/j.1529-8027.2002.02004.x. [DOI] [PubMed] [Google Scholar]
- 3.Nagamatsu M, Schmelzer JD, Zollman PJ, Smithson IL, Nickander KK, Low PA. Ischemic reperfusion causes lipid peroxidation and fiber degeneration. Muscle Nerve. 1996;19:37–47. doi: 10.1002/mus.880190103. [DOI] [PubMed] [Google Scholar]
- 4.Mitsui Y, Okamoto K, Martin DP, Schmelzer JD, Low PA. The expression of proinflammatory cytokine mRNA in the sciatic-tibial nerve of ischemia-reperfusion injury. Brain Res. 1999;844:192–195. doi: 10.1016/s0006-8993(99)01830-2. [DOI] [PubMed] [Google Scholar]
- 5.Shames BD, Barton HH, Reznikov LL, Cairns CB, Banerjee A, Harken AH, Meng X. Ischemia alone is sufficient to induce TNF-alpha mRNA and peptide in the myocardium. Shock. 2002;17:114–119. doi: 10.1097/00024382-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci. 2002;22:3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohtaki H, Yin L, Nakamachi T, Dohi K, Kudo Y, Makino R, Shioda S. Expression of tumor necrosis factor alpha in nerve fibers and oligodendrocytes after transient focal ischemia in mice. Neurosci Lett. 2004;368:162–166. doi: 10.1016/j.neulet.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 2003;66:1403–1408. doi: 10.1016/s0006-2952(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 10.Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–1368. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- 11.Mitsui Y, Schmelzer JD, Zollman PJ, Kihara M, Low PA. Hypothermic neuroprotection of peripheral nerve of rats from ischaemia-reperfusion injury. Brain. 1999;122:161–169. doi: 10.1093/brain/122.1.161. [DOI] [PubMed] [Google Scholar]
- 12.Mitsui Y, Schmelzer JD, Zollman PJ, Mitsui M, Tritschler HJ, Low PA. Alpha-lipoic acid provides neuroprotection from ischemia-reperfusion injury of peripheral nerve. J Neurol Sci. 1999;163:11–16. doi: 10.1016/s0022-510x(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 13.Iida H, Schmelzer JD, Schmeichel AM, Wang Y, Low PA. Peripheral nerve ischemia: reperfusion injury and fiber regeneration. Exp Neurol. 2003;184:997–1002. doi: 10.1016/S0014-4886(03)00385-6. [DOI] [PubMed] [Google Scholar]
- 14.Schmeichel AM, Schmelzer JD, Low PA. Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathy. Diabetes. 2003;52:165–171. doi: 10.2337/diabetes.52.1.165. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura N, Schmelzer JD, Wang Y, Schmeichel AM, Low PA. The therapeutic window of hypothermic neuroprotection in experimental ischemic neuropathy: protection in ischemic phase and potential deterioration in later reperfusion phase. Exp Neurol. 2005;195:305–312. doi: 10.1016/j.expneurol.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura N, Schmeichel AM, Wang Y, Schmelzer JD, Low PA. Multiple effects of hypothermia on inflammatory response following ischemia-reperfusion injury in experimental ischemic neuropathy. Exp Neurol. 2006;202:487–496. doi: 10.1016/j.expneurol.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Schmelzer JD, Schmeichel AM, Iida H, Low PA. Ischemia-reperfusion injury of peripheral nerve in experimental diabetic neuropathy. J Neurol Sci. 2004;227:101–107. doi: 10.1016/j.jns.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Donnahoo KK, Shames BD, Harken AH, Meldrum DR. Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999;162:196–203. doi: 10.1097/00005392-199907000-00068. [DOI] [PubMed] [Google Scholar]
- 19.Zelenka M, Schafers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116:257–263. doi: 10.1016/j.pain.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Siebert H, Bruck W. The role of cytokines and adhesion molecules in axon degeneration after peripheral nerve axotomy: a study in different knockout mice. Brain Res. 2003;960:152–156. doi: 10.1016/s0006-8993(02)03806-4. [DOI] [PubMed] [Google Scholar]
- 21.Teoh N, Field J, Sutton J, Farrell G. Dual role of tumor necrosis factor-alpha in hepatic ischemia-reperfusion injury: studies in tumor necrosis factor-alpha gene knockout mice. Hepatology. 2004;39:412–421. doi: 10.1002/hep.20035. [DOI] [PubMed] [Google Scholar]
- 22.Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87–113. doi: 10.1007/978-1-4615-0123-7_3. [DOI] [PubMed] [Google Scholar]
- 23.Iida H, Schmeichel AM, Wang Y, Schmelzer JD, Low PA. Schwann cell is a target in ischemia-reperfusion injury to peripheral nerve. Muscle Nerve. 2004;30:761–766. doi: 10.1002/mus.20159. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Schmeichel AM, Iida H, Schmelzer JD, Low PA. Ischemia-reperfusion injury causes oxidative stress and apoptosis of schwann cell in cute and chronic experimental diabetic neuropathy. Antidoxid Redox Signal. 2005;7:1513–1520. doi: 10.1089/ars.2005.7.1513. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 26.George A, Buehl A, Sommer C. Wallerian degeneration after crush injury of rat sciatic nerve increases endo- and epineurial tumor necrosis factor-alpha protein. Neurosci Lett. 2004;372:215–219. doi: 10.1016/j.neulet.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 27.Newman WH, Zunzunegui RG, Warejcka DJ, Dalton ML, Castresana MR. A reactive oxygen-generating system activates nuclear factor-kappaB and releases tumor necrosis factor-alpha in coronary smooth muscle cells. J Surg Res. 1999;85:142–147. doi: 10.1006/jsre.1999.5668. [DOI] [PubMed] [Google Scholar]


