Abstract
Objectives
Growth-factor based angiogenesis, with or without cell therapy, is a promising therapeutic modality for patients with coronary artery disease. We compared the relative efficacies of surgically delivered vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2) in a swine model of hypercholesterolemia-induced endothelial dysfunction which captures many of the pathophysiologic abnormalities of human coronary disease.
Methods
Yucatan mini-swine (20–30 kg), fed a high cholesterol diet (total 20 weeks), underwent circumflex ameroid placement to create chronic myocardial ischemia, followed three weeks later by perivascular administration of VEGF (2 μg; n=6), FGF-2 (100 μg; n=6), or placebo (n=7) in the ischemic territory. Normocholesterolemic animals (n=7) served as controls. Four weeks later, endothelial function, collateral-dependent perfusion, as well as myocardial protein and mRNA levels of angiogenic mediators were assessed.
Results
Endothelial dysfunction was observed in all hypercholesterolemic animals as impaired microvessel relaxation in response to adenosine diphosphate and VEGF. VEGF administration improved baseline-adjusted collateral-dependent perfusion at rest(−0.03±0.05 vs. −0.12±0.04, VEGF vs. placebo, p=0.09), but FGF-2 delivery caused a significantly greater improvement in perfusion compared to either group (+0.15±0.03, p<0.05 vs. HC-placebo and HC-VEGF) at rest. Molecular analysis revealed increased eNOS expression (135% ± 8%, p=0.03 vs. placebo) in all growth factor treated animals and increased expression of FGF-2 receptor, FGFR1, (65 ± 26%, p = 0.04 vs. placebo) in FGF-2 treated animals. No significant changes were demonstrated in other angiogenic mediators including Akt, Syndecan-4.
Conclusions
In the setting of hypercholesterolemic endothelial dysfunction, FGF-2 is more effective than VEGF at enhancing collateral-dependent perfusion and thus, may be a better candidate than VEGF for angiogenic therapy in patients with end-stage CAD.
Keywords: Endothelial Dysfunction, Vascular Endothelial Growth Factor, Fibroblast Growth Factor, Myocardial Ischemia, Angiogenesis, Molecular Biology
Introduction
Therapeutic angiogenesis, utilizing growth factors or cell populations, remains an attractive modality for the treatment of end-stage coronary artery disease (CAD). Pre-clinical studies and clinical trials, conducted over the past decade, have highlighted the challenges of translating the significant angiogenic response to these therapies observed in animal models to clinically meaningful and measurable benefits for patients[1]. Two important issues, among others, have been identified over these years of investigation. The first is the realization that angiogenesis is a complex process, which involves interaction between a number of pro-angiogenic growth factors, anti-angiogenic mediators, and the extracellular matrix[2]. As such, it is likely that combinations of therapies utililizing multiple growth factor and cell combinations will be needed to maximize the therapeutic potential of these agents. Howvever, a number of critical issues including dosing, timing, interactions, and combined toxicities of multiple pro-angiogenic agents need to be further studied in in-vitro or small animal models prior to pre-clinical evaluation of combination growth factor therapy. Although a variety of growth factors have been studied in-vitro and in animal models, only members of the vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) families have been used in clinical trials[3]. Furthermore, a comparative evaluation of these growth factors has not been performed in the clinical setting.
Another important realization has been that findings from pre-clinical animal models have not been reproducible in clinical trials. This lack of reproducibility may be due, in significant part, to the presence of endothelial dysfunction, which is a common finding in patients with CAD[4], but is not present in the young, healthy animals used for pre-clinical studies. We and others have previously demonstrated, in a swine model, that the presence of endothelial dysfunction states, such as hypercholesterolemia and diabetes, impairs the angiogenic response to chronic ischemia[5–7] and to exogenous growth factor therapy[8], which can be rescued, in part, by pro-endothelial agents, such as l-arginine[9] and insulin[10].
The aim of this study, therefore, was to perform a comparison of the angiogenic potential of VEGF and FGF-2 in an established and clinically relevant swine model of diet-induced hypercholesterolemia and endothelial dysfunction. The key outcomes for this study included coronary microvascular function, collateral-dependent myocardial perfusion, as well as an exploration of the molecular pathways involved.
Materials and Methods
Animal Model and Study Design
Yucatan miniswine (Sinclair Research, Columbia, MO, USA) were fed a hypercholesterolemic diet (n = 19), composed of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow, continued throughout the experimental period (total 20 weeks). A separate group of swine were fed regular chow (NORM, n = 7) and served as controls. After 13 weeks of dietary modification, all animals underwent ameroid constrictor placement on the proximal left circumflex coronary artery (LCx). For all surgical procedures, anesthesia was induced with ketamine (10 mg/kg IM), thiopental (5–10 mg/kg IV), and thiopental 2.5%, and maintained with a gas mixture of oxygen at 1.5–2 L/min and isoflurane at 0.75 to 3.0 %. The animals were intubated and mechanically ventilated at 12–20 breaths/minute. During the first procedure, the pericardium was opened through a left minithoracotomy, and gold-labelled mircrospheres (Biophysics Assay laboratory, Worcester, MA, USA) were injected in the left atrium during temporary occlusion of the LCx to determine, by shadow-labeling, the exact myocardial territory at risk. Thus, the myocardial territory with the lowest count of gold-labeled microspheres represent the area at highest risk of ischemia with circumflex artery occlusion. Next, a titanium ameroid constrictor (1.75 mm in internal diameter) was placed around the proximal left circumflex artery at the site of the temporary occlusion.
Three weeks after ameroid placement, the animals were anesthetized and coronary angiography was performed through an 8F sheath (Cordis corporation, Miami, FL, USA) introduced into the femoral artery using a catheter with the appropriate distal angulation. High atomic weight contrast (Mallinckrodt Inc., St. Louis, MO, USA) was injected after selective cannulation of the right and left coronary ostia to confirm occlusion of the circumflex artery by the ameroid constrictor. A repeat small left thoracotomy was performed and microspheres were injected in the left atrium to determine myocardial perfusion at rest and with atrial pacing (150 bpm). This served as the baseline perfusion measurement following ameroid occlusion. Following microsphere injection, the animals were divided into three groups. In group 1 (HC-VEGF, n = 6), an osmotic pump (Alzet Inc.–model 2ML4, Cupertino, CA, USA) containing human recombinant vascular endothelial growth factor (VEGF165) was used to provide sustained intramyocardial delivery of the growth factor using a microcatheter implanted in the ischemic territory. VEGF (2μg) was mixed with 50U of heparin was delivered as a 2 mL solution over 4 weeks at a rate of 3μL/hour in the ischemic territory, as previously described[11]. In group 2, (HC-FGF, n = 6), 10 sterile heparin-alginate sustained-release beads each containing 10 μg of bovine FGF-2 (Chiron, Emeryville, CA, USA) were implanted in the subepicardium and myocardium surrounding the proximal and mid-circumflex coronary artery as previously described[12]. In group 3 (HC-placebo, n = 7), heparin alginate beads containing no growth factors were implanted.
Seven weeks after ameroid placement, the animals were again anesthetized and the heart exposed through a sternotomy. Microspheres were then injected at rest and with pacing and euthanasia was performed with injections of saturated potassium chloride solution. The heart was harvested and two 1-cm-thick transversal slices were cut at the midventricular level, and then sectioned into 8 segments identified clockwise starting from the anterior junction of the right and left ventricles. Samples from the anterior and left lateral walls were divided and rapidly frozen in liquid nitrogen (molecular studies), kept in 4°C Krebs solution (microvessel reactivity studies). Separate samples were weighed and dried in a 60 °C oven (microsphere perfusion analyses). Plasma cholesterol levels were obtained from serum samples of all animals using an enzymatic method (AccelLAB, Montreal, PQ, Canada).
In Vitro Assessment of Coronary Microvessel Reactivity
After cardiac harvest, epicardial coronary arterioles (80 to 180 μm in diameter and 1 to 2 mm in length) originating from branches of the left anterior descending (LAD) were dissected from the surrounding tissue with a 40x microscope and examined in isolated microvessel chambers as described previously[13]. The microvascular responses to sodium nitroprusside (SNP) (1 nmol/L to 100 μmol/L, endothelium-independent cGMP-mediated vasodilator) and adenosine 5’ diphosphate (ADP, endothelium-dependent vasodilator, 1 nmol/L to 10 μmol/L) and VEGF (1 fM to 1 nM) were evaluated. Briefly, microvessels were cannulated with dual glass micropipettes and pressurized to 40 mmHg using 2 burettes containing Krebs solution. Vessels were bathed in Krebs solution and preconstricted by 20–50% of the baseline diameter with the thromboxane A2 analog, U46619 (0.1–1 μM). All drugs were applied extraluminally. Relaxation responses were defined as the percent relaxation of the preconstricted diameter. Six vessels were examined in each group with uniform levels of preconstriction.
Myocardial Perfusion Analysis
Myocardial perfusion was determined during each procedure with isotope-labeled microspheres (ILMs) (BioPAL, Worcester, MA), 15 μm in diameter, using previously reported methods[14]. Briefly, 1.5 x 107 gold-labeled microspheres were injected during temporary LCx occlusion at the time of ameroid placement to identify myocardial samples that originated from the LCx distribution (those with the lowest count of gold-labeled microspheres). 1.5 x 107 Samarium and 1.5 x 107 Europium-labeled ILMs were used during the second procedure to determine baseline blood flow in the LCx territory 3 weeks after ameroid placement at rest and with pacing. 1.5 x 107 Lutetium and 1.5 x 107 Lanthanum-labeled ILMs were injected at the final procedure, 7 weeks after ameroid placement. Following euthanasia, 10 transmural left ventricular sections were collected for ILM assays in each animal, weighed, and dried. Each sample was exposed to neutron beams and microsphere densities measured in a gamma counter. Adjusted myocardial blood flow (at rest and with pacing), reflecting changes in lateral myocardial perfusion, was determined from the 2 myocardial samples which showed the lowest count of gold-labeled microspheres by using the following equations:
Molecular Studies
Western blotting was performed as previously described[6]. Briefly, whole-cell lysates were isolated from the homogenized myocardial samples with a RIPA buffer (Boston Bioproducts, Worcester, MA, USA) and centrifuged at 12,000g for 10 min at 4 °C to separate soluble from insoluble fractions. Protein concentration was measured spectrophotometrically at a 595-nm wavelength with a DC protein assay kit (Bio-Rad, Hercules, CA, USA). Forty micrograms of total protein were fractionated by 4–20%gradient, SDS polyacrylamide gel electrophoresis (Invitrogen, San Diego, CA, USA) and transferred to PVDF membranes (Millipore, Bedford, MA). Each membrane was incubated with specific antibodies as follows: anti-VEGF antibody (Calbiochem, San Diego, CA, USA), anti-FGF-2 antibody (US Biological, Swampscott, MA, USA), anti-FGFR1 antibody and anti-Syndecan-4 antibody (Zymed, San Francisco, CA, USA), anti-Akt antibody (Cell Signaling, Beverly, MA, USA), and anti-eNOS antibody (BD Biosciences, San Jose, CA). Then the membranes were incubated for 1 hour in diluted appropriate secondary antibody (Jackson Immunolab, West Grove, PA). Immune complexes were visualized with the enhanced chemiluminescence detection system (Amersham, Piscataway, NJ, USA). Bands were quantified by densitometry of radioautograph films.
Northern blot analysis was performed as previously described[15]. Briefly, myocardial samples from the anterior and lateral myocardial territories were homogenized for 60 seconds on ice; total RNA was then isolated with a Tri-Reagent solution (Sigma, St. Louis, MO, USA). A 10μg RNA pellet was dissolved in RNase free water, fractionated on a 1.3% formaldehyde-agarose gel, and transferred to a GeneScreen Plus filter (Perkin Elmer, Boston, MA, USA). Complementary DNA probes of VEGF receptor-1 (VEGFR1) and VEGF receptor-2 (VEGFR2) were labeled with α32P-dCTP (Amersham, Arlington Heights, IL, USA) with the use of a random-priming labeling kit (Boehringer, Indianapolis, IN, USA) and purified from unincorporated nucleotides with G-50 Quick Spin Columns (Boehringer). The specific activity of the probes used was 1 to 2 x 109 cpm/μg. The blots were hybridized at 68°C for 16 hours in UltraHyb solutions (Ambion, Austin, TX, USA). After hybridization, the blots were washed twice in 2X SSC, placed in 0.1% SDS for 15 minutes at room temperature, and then rewashed twice in 0.1X SSC, 0.1% SDS for 15 minutes at 60°C. Autoradiography was carried out at −80°C for 16 to 20 hours.
Data Analysis
All results are expressed as mean ± SEM. Microvessel responses are expressed as percentage relaxation of the preconstricted diameter and were analyzed using two way, repeated measures analysis of variance. Western blots were analyzed after digitalization of x-ray films using a flat-bed scanner (ScanJet 4c; Hewlett Packard, Palo Alto, CA, USA) and NIH ImageJ 1.33 software (National Institute of Health, Bethesda, MD, USA). Comparisons between samples were analyzed by unpaired, two-tailed t-tests using GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA, USA). P values less than 0.05 were considered significant.
Animal Care
Animals were cared for in compliance with the Harvard Medical Area Institutional Animal Care and Use Committee and in accordance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” (NIH publication No.5377-3 1996).
Results
Animal Model
Three animals (11%) did not complete the entire experimental protocol. One animal in the NORM group died one day following ameroid placement. Two animals died due to intractable ventricular arrhythmias at the time of the second procedure, one at the time of contrast injection in the right coronary artery (HC-FGF group), and another following rapid atrial pacing (HC-VEGF).
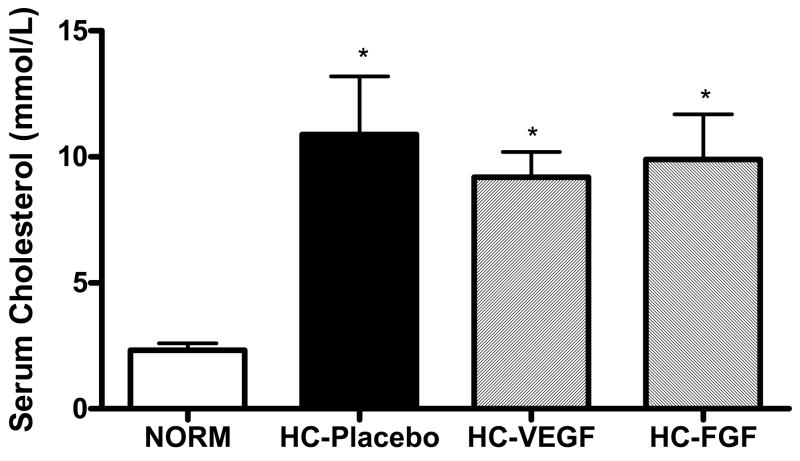
Serum cholesterol levels were significantly elevated in all animals fed a hypercholesterolemic diet (HC-Placebo: 10.9 ± 2.3, HC-VEGF: 9.2 ± 1.0, HC-FGF: 9.9 ± 1.8 mmol/L) compared to normocholesterolemic controls (NORM: 2.3 ± 0.3, p < 0.001), with no significant differences between the three hyperholesterolemic groups. Figure 1 depicts the total cholesterol levels within the different study groups.
Figure 1.
Serum cholesterol levels were significantly elevated in animals fed a hypercholesterolemic diet (HC) compared to a normal diet (NORM) with no significant differences between the hypercholesterolemic groups. * - p < 0.001
Coronary Microvessel Reactivity
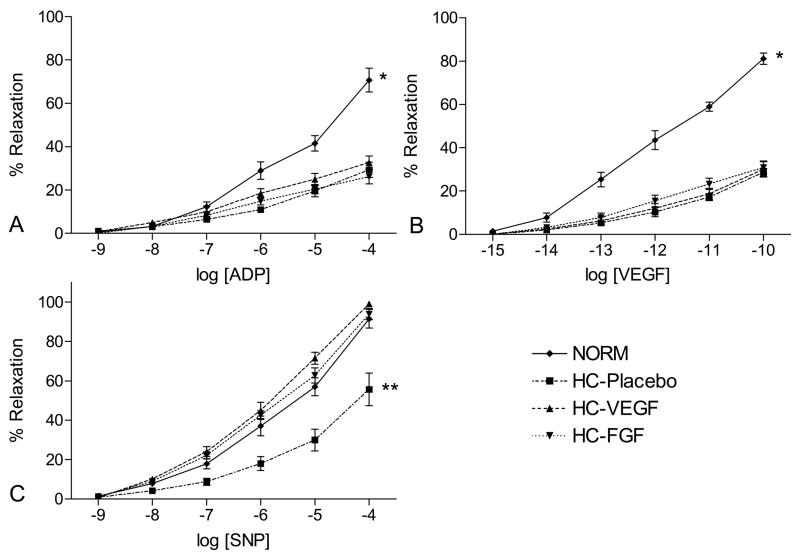
Coronary microvessel relaxation responses are depicted in Figure 2. Baseline diameters of coronary arterioles were similar in all groups (NORM: 125 ± 12, HC-Placebo: 151 ± 19, HC-VEGF: 141 ± 11, HC-FGF: 134 ± 9 μm, ANOVA p = 0.45), as was the amount of pre-constriction (NORM: 41 ± 6, HC-Placebo: 34 ± 2, HC-VEGF: 38 ± 3, HC-FGF: 39 ± 2% of baseline, ANOVA p = 0.52). Hypercholesterolemic animals (HC) demonstrated a marked impairment in the relaxation responses to endothelium dependent vasodilators, ADP and VEGF (both p < 0.001) compared to the NORM group. This observation was consistent and similar in magnitude (58% and 63% reduction in response to ADP[10−4] and VEGF[10−10], respectively) across all the hypercholesterolemic groups with no significant differences between groups. Interestingly, the response to SNP, an endothelium-independent vasodilator, was impaired in the HC-placebo animals (p < 0.01) but recovered in the growth-factor treated animals (HC-VEGF, HC-FGF).
Figure 2.
Coronary microvessel relaxation responses (depicted as % relaxation of pre-constricted diameter) to (A, B) endothelium dependent (ADP, VEGF) and (C) endothelium independent (SNP) vasodilators in NORM, HC-Placebo, HC-VEGF, and HC-FGF animals. * - p < 0.001, ** - p < 0.01.
Myocardial Perfusion
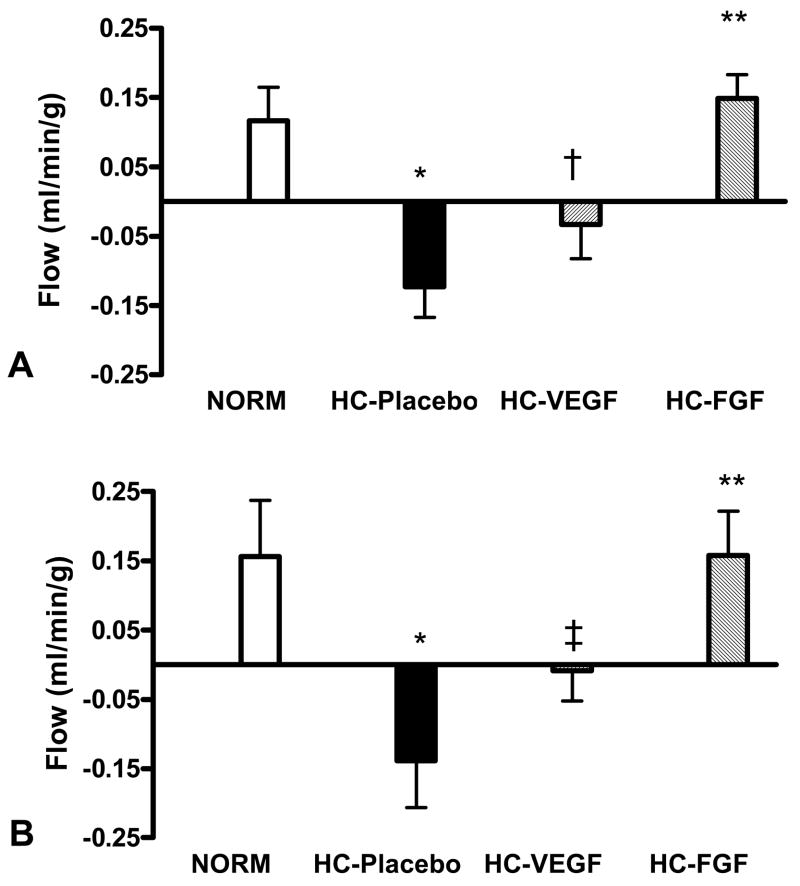
Baseline myocardial perfusion of the circumflex territory, determined 3 weeks following ameroid placement, was marginally higher in the NORM group (0.65 ± 0.07 ml/min/g) compared to the HC groups (HC-Placebo: 0.48 ± 0.05, HC-VEGF: 0.52 ± 0.05, HC-FGF: 0.42 ± 0.18 ml/min/g) but this difference was not statistically significant (ANOVA p = 0.08). More importantly, baseline circumflex perfusion was similar between the three HC groups (ANOVA p = 0.52). As seen in Figure 3A, after four weeks of treatment, the NORM group demonstrated an increase in baseline-adjusted circumflex territory perfusion, whereas hypercholesterolemic animals (HC-Placebo) demonstrated reduced perfusion (0.12 ± 0.05 vs. −0.12 ± 0.04 ml/min/g, NORM vs. HC-Placebo, p < 0.001). VEGF treatment led to an increase in circumflex territory perfusion compared to placebo (−0.03 ± 0.05 ml/min/g, p = 0.09), but FGF-2 treatment led to a significantly greater increase in circumflex territory perfusion (0.15 ± 0.03, p < 0.05 vs. both HC-Placebo and HC-VEGF). Similar values of baseline-adjusted circumflex territory perfusion were seen under stress conditions with rapid atrial pacing (NORM: 0.16 ± 0.08; HC-Placebo: −0.14 ± 0.07; HC-VEGF: −0.01 ± 0.04, and HC-FGF: 0.16 ± 0.06; Figure 3B).
Figure 3.
Baseline-adjusted circumflex territory perfusion was significantly reduced in HC-Placebo group compared to NORM, both at rest (A) and with pacing (B, * - p < 0.001). VEGF treatment was associated with a small increase in flow compared to placebo († - p = 0.09, ‡ - p = 0.10), but FGF treatment resulted in a significant increase in ischemic territory perfusion (** - p < 0.05 vs. HC-Placebo and HC-VEGF)
Molecular Studies
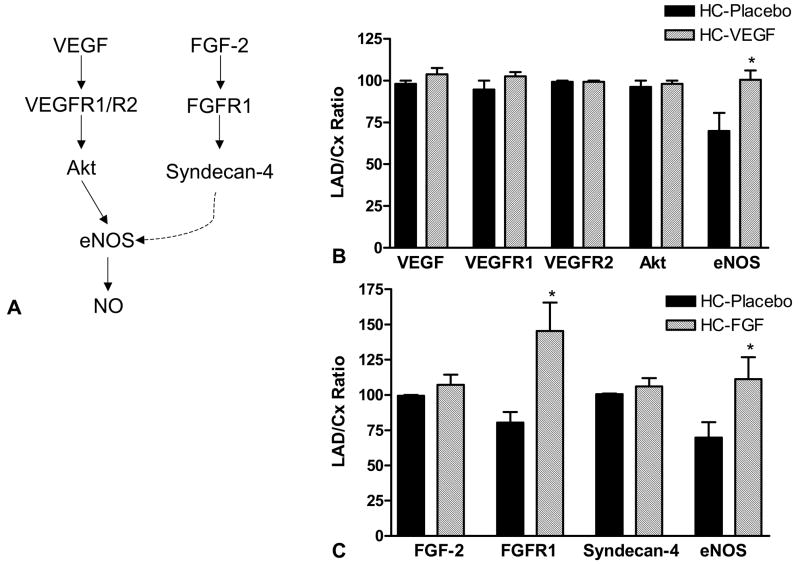
In order to further explore potential mechanisms for the functional differences, the myocardial expression of growth factors, their receptors and downstream mediators in the placebo, VEGF, and FGF-2 treated hypercholesterolemic animals were examined. A schematic diagram of VEGF and FGF-2 signaling pathways is depicted in Figure 4A. Myocardial expression of VEGF was similar in the HC-Placebo and HC-VEGF treated animals (p = 0.65) and the expression of FGF-2 was similar between HC-Placebo and HC-FGF animals (p = 0.63). While levels of VEGF receptors, VEGFR1 and VEGFR2 were similar between the placebo and HC-VEGF groups, FGF-2 treatment was associated with a significant increase in the expression of its receptor, FGFR1 (65 ± 26%, p = 0.04). There were no differences in Akt and Syndecan-4 expression. Growth factor treatment, with both VEGF and FGF-2, was associated with greater myocardial expression of endothelial nitric oxide synthase compared to the HC-Placebo animals (eNOS, both p < 0.05).
Figure 4.
(A) VEGF and FGF-2 signaling pathways. (B) Expression of VEGF, its receptors, VEGFR1 and VEGFR2, and downstream mediators Akt and eNOS in placebo and VEGF treated animals. (C) Expression of FGF-2, its receptor, FGFR1, and downstream mediators, syndecan-4 and eNOS, in placebo and FGF-2 treated animals. (* - p < 0.05; VEGF–vascular endothelial growth factor, PI3K–Phosphatidylinositol-3 kinase, eNOS–endothelial nitric oxide synthase, FGF–fibroblast growth factor)
Discussion
Using a clinically relevant swine model of hypercholesterolemia-induced endothelial dysfunction and chronic myocardial ischemia, this study evaluated the comparative therapeutic efficacy of VEGF and FGF-2 in inducing myocardial angiogenesis. The major findings of this study were, firstly, that diet induced hypercholesterolemia, over a period of 20 weeks, led to a profound reduction in NO bioavailability in the coronary vasculature, as indicated by significant impairments in the relaxation response to ADP and VEGF. Secondly, hypercholesterolemic animals in the placebo group demonstrated a significant reduction in collateral-dependent perfusion compared to normocholesterolemic controls. Treatment with local, sustained-release VEGF led to an improvement in ischemic territory perfusion, but this did not reach statistical significance. FGF-2, on the other hand, led to a much greater increase in perfusion of the ischemic circumflex territory. Molecular studies demonstrated that there was a sustained increase in FGF receptor expression in FGF-2 treated animals, and a relative increase in eNOS expression in the ischemic territory of growth factor treated animals compared to placebo treated hypercholesterolemic animals.
A number of agents have been proposed and evaluated for the induction of myocardial angiogenesis. These have included growth factors, delivered either as proteins or genes, as well as a variety of cell populations. Isoforms of VEGF and FGF are the only growth factors that have been evaluated in clinical trials to date[3]. However, their therapeutic efficacies have not been compared in the clinical setting. Although VEGF and FGF families of growth factors share characteristics in common, important differences in downstream signaling and mechanisms of action exist (Figure 4A). VEGFs bind to their tyrosine kinase receptor, VEGFR2, which activates PI3 kinase leading to the phosphorylation of Akt (protein kinase B) and subsequent phosphorylation and activation of eNOS and the production of NO[16]. FGF signaling is initiated by its binding to its receptor FGFR1 leading to the activation of protein kinase C (alpha and epsilon isoforms) and also involves syndecan-4 as a downstream mediator[17,18]. While FGF signaling also involves NO, its angiogenic effects are less clearly tied to NO compared to VEGF. Hughes et al evaluated VEGF and FGF-2 in a swine model of myocardial ischemia and found significant increases in myocardial blood flow in response to both growth factors, when delivered intramuscularly[19]. However, this comparison was performed in young, healthy swine which do not reflect the pathophysiologic state of patients with CAD.
We and others have previously demonstrated that diet-induced hypercholesterolemia results in endothelial dysfunction, which appears to be associated with increased production of reactive oxygen species (ROS)[5]. These ROS can combine with and consume NO, thereby reducing its bioavailability. Endothelial dysfunction, due to reduced NO bioavailability, and oxidative stress are also key features of CAD in patients[20]. Furthermore, these animals also have increased myocardial expression of anti-angiogenic protein, endostatin, which has a selective inhibitory effect on VEGF receptors[21]. Thus, the increased dependence of VEGF on NO availability combined with increased endostatin expression in these animals may explain the limited angiogenic benefit of VEGF in this model. FGF, which is less NO dependent and acts via additional pathways, may be a better angiogenic growth factor for use in patients with CAD.
The molecular studies in our model revealed that growth factor expression was similar in placebo and treated animals. This is consistent with the known short half-life of growth factors and the reason why sustained release systems were used for delivery rather than single injections. Another important finding of the study was that FGF-2 treatment was associated with a persistent elevation in FGFR1 levels, despite similar levels of FGF-2 protein in the placebo and FGF-2 treated animals. This prolonged effect of FGF-2 on receptor expression may be another reason for its enhanced angiogenic effect. Treatment with both growth factors resulted in upregulation of eNOS in the myocardium, which is consistent with previous reports in in-vitro models[22]. Lastly, while we did not find significant differences in the protein or mRNA expression of other angiogenic mediators, it is important to realize that many of these proteins may be regulated at the post-translation level through phosphorylation and these phosphorylation states were not specifically assessed in this study.
Strengths and limitations of the model
The swine model of chronic myocardial ischemia provides physiologically relevant measures of myocardial blood flow and coronary vascular function. Furthermore, diet-induced hypercholesterolemia captures many of the pathophysiologic abnormalities of patients with CAD. However, this study is limited by the fact that the intramyocardial delivery systems for the growth factors used were different for VEGF and FGF which may affect their efficacy. Unfortunately, the desired sustained release method (heparin alginate beads) were not available for VEGF and thus, an osmotic pump was utilized instead. Additionally, only one dose was utilized for each group. While the chosen doses were similar to those utilized in clinical studies, additional studies comparing higher doses may reveal important differences in growth factor efficacy. Lastly, molecular studies were only performed at a single timepoint, four weeks following the initiation of therapy, and may not capture changes in the expression of angiogenic mediators in the early period following initiation of growth factor treatment.
Conclusions
In a clinically relevant model of hypercholesterolemic endothelial dysfunction, intramyocardial FGF-2 treatment was significantly more efficacious that VEGF in improving collateral-dependent myocardial blood flow. These differences may be due to greater NO dependency of VEGF and suggest that FGF-2 may be a better angiogenic agent for use in patients with CAD.
Acknowledgments
This study was funded by a grant from the National Institutes of Health (R01 HL69024).
Footnotes
Presented at the 21st EACTS Meeting in Geneva, Switzerland (15th -18th September, 2007)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, Laham RJ, Li W, Pike M, Sellke FW, Stegmann TJ, Udelson JE, Rosengart TK. Clinical trials in coronary angiogenesis: issues, problems, consensus: An expert panel summary. Circulation. 2000;102:E73–86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 3.Boodhwani M, Sodha NR, Laham RJ, Sellke FW. The future of therapeutic myocardial angiogenesis. Shock. 2006;26:332–341. doi: 10.1097/01.shk.0000225318.08681.a7. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Zhao SP, Li XP, Gao M, Zhou QC. Endothelium-dependent and -independent functions are impaired in patients with coronary heart disease. Atherosclerosis. 2000;149:19–24. doi: 10.1016/s0021-9150(99)00288-9. [DOI] [PubMed] [Google Scholar]
- 5.Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, Feng J, Michael K, Li J, Sellke FW. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81:634–641. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 6.Boodhwani M, Sodha NR, Mieno S, Xu SH, Feng J, Ramlawi B, Clements RT, Sellke F. Functional, Cellular, and Molecular Characterization of the Angiogenic Response to Chronic Myocardial Ischemia in Diabetes. Circulation. 2007 doi: 10.1161/CIRCULATIONAHA.106.680157. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang JJ, Ho HK, Kwan HH, Fajardo LF, Cooke JP. Angiogenesis is impaired by hypercholesterolemia: role of asymmetric dimethylarginine. Circulation. 2000;102:1414–1419. doi: 10.1161/01.cir.102.12.1414. [DOI] [PubMed] [Google Scholar]
- 8.Ruel M, Wu GF, Khan TA, Voisine P, Bianchi C, Li J, Laham RJ, Sellke FW. Inhibition of the cardiac angiogenic response to surgical FGF-2 therapy in a Swine endothelial dysfunction model. Circulation. 2003;108(Suppl 1):II335–340. doi: 10.1161/01.cir.0000087903.75204.ad. [DOI] [PubMed] [Google Scholar]
- 9.Voisine P, Li J, Bianchi C, Khan TA, Ruel M, Xu SH, Feng J, Rosinberg A, Malik T, Nakai Y, Sellke FW. Effects of L-arginine on fibroblast growth factor 2-induced angiogenesis in a model of endothelial dysfunction. Circulation. 2005;112:I202–207. doi: 10.1161/CIRCULATIONAHA.104.526350. [DOI] [PubMed] [Google Scholar]
- 10.Boodhwani M, Sodha NR, Mieno S, Ramlawi B, Xu SH, Feng J, Clements RT, Ruel M, Sellke FW. Insulin Treatment Enhances the Myocardial Angiogenic Response in Diabetes. J Thorac Cardiovasc Surg. 2007 doi: 10.1016/j.jtcvs.2007.08.025. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boodhwani M, Mieno S, Voisine P, Feng J, Sodha N, Li J, Sellke FW. High-dose atorvastatin is associated with impaired myocardial angiogenesis in response to vascular endothelial growth factor in hypercholesterolemic swine. J Thorac Cardiovasc Surg. 2006;132:1299–1306. doi: 10.1016/j.jtcvs.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 12.Sellke FW, Laham RJ, Edelman ER, Pearlman JD, Simons M. Therapeutic angiogenesis with basic fibroblast growth factor: technique and early results. Ann Thorac Surg. 1998;65:1540–1544. doi: 10.1016/s0003-4975(98)00340-3. [DOI] [PubMed] [Google Scholar]
- 13.Tofukuji M, Metais C, Li J, Franklin A, Simons M, Sellke FW. Myocardial VEGF expression after cardiopulmonary bypass and cardioplegia. Circulation. 1998;98:II242–246. II247–248. [PubMed] [Google Scholar]
- 14.Boodhwani M, Nakai Y, Voisine P, Feng J, Li J, Mieno S, Ramlawi B, Bianchi C, Laham R, Sellke FW. High-dose atorvastatin improves hypercholesterolemic coronary endothelial dysfunction without improving the angiogenic response. Circulation. 2006;114:I402–408. doi: 10.1161/CIRCULATIONAHA.105.000356. [DOI] [PubMed] [Google Scholar]
- 15.Voisine P, Bianchi C, Ruel M, Malik T, Rosinberg A, Feng J, Khan TA, Xu SH, Sandmeyer J, Laham RJ, Sellke FW. Inhibition of the cardiac angiogenic response to exogenous vascular endothelial growth factor. Surgery. 2004;136:407–415. doi: 10.1016/j.surg.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Toyota E, Matsunaga T, Chilian WM. Myocardial angiogenesis. Mol Cell Biochem. 2004;264:35–44. doi: 10.1023/b:mcbi.0000044372.65864.18. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Li J, Partovian C, Sellke FW, Simons M. Syndecan-4 modulates basic fibroblast growth factor 2 signaling in vivo. Am J Physiol Heart Circ Physiol. 2003;284:H2078–2082. doi: 10.1152/ajpheart.00942.2001. [DOI] [PubMed] [Google Scholar]
- 18.Detillieux KA, Sheikh F, Kardami E, Cattini PA. Biological activities of fibroblast growth factor-2 in the adult myocardium. Cardiovasc Res. 2003;57:8–19. doi: 10.1016/s0008-6363(02)00708-3. [DOI] [PubMed] [Google Scholar]
- 19.Hughes GC, Biswas SS, Yin B, Coleman RE, DeGrado TR, Landolfo CK, Lowe JE, Annex BH, Landolfo KP. Therapeutic angiogenesis in chronically ischemic porcine myocardium: comparative effects of bFGF and VEGF. Ann Thorac Surg. 2004;77:812–818. doi: 10.1016/j.athoracsur.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 20.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 21.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 22.Cudmore M, Ahmad S, Al-Ani B, Hewett P, Ahmed S, Ahmed A. VEGF-E activates endothelial nitric oxide synthase to induce angiogenesis via cGMP and PKG-independent pathways. Biochem Biophys Res Commun. 2006;345:1275–1282. doi: 10.1016/j.bbrc.2006.04.031. [DOI] [PubMed] [Google Scholar]