Abstract
Repeated stimulation of the laryngeal mucosa occurs during speech. Single stimuli, however, can elicit laryngeal adductor responses (LAR). Our hypothesis was that the LAR to repeated rapid air pressure stimuli are centrally suppressed in humans. Hooked wire electrodes were inserted into the thyroarytenoid and cricothyroid muscles bilaterally and into the posterior cricoarytenoid muscle on one side. Pairs of air puff stimuli were presented to the mucosa over the arytenoids at pressure levels three times threshold with inter-stimulus intervals from 250 to 5000 ms. Bilateral thyroarytenoid responses occurred at around 150 ms to over 70% of initial stimuli. With repeated presentation at intervals of 2 seconds or less, the percent occurrence decreased to less than 40% and response amplitudes were reduced by 50%. Central suppression of adductor responses to repeated air puff stimuli may allow speakers to produce voice without eliciting reflexive spasms which could disrupt speech.
Keywords: air pressure, conditioning, laryngeal adductor reflex, larynx, sensation, spasmodic dysphonia, thyroarytenoid muscles
Introduction
Laryngeal Adductor Responses (LAR) have been demonstrated when afferents in the laryngeal mucosa supplied by the superior laryngeal nerve are stimulated[1, 2]. Electromyographic (EMG) thyroarytenoid (TA) recordings during electrical stimulation of the internal branch of the superior laryngeal nerve (iSLN) yield a dual response, with an early ipsilateral R1 at approximately 18 milliseconds and a later bilateral R2 at approximately 68 milliseconds.[3] Among the afferents in the larynx are mechanoreceptors in the mucosa which are responsive to airflow and air pressure changes[4].
Aviv, et. al. developed a system for delivering airpuff pressure stimuli to the laryngeal mucosa through a channeled endoscope for the purpose of studying laryngeal sensory thresholds[5]. Using a similar system to deliver air puff stimuli to the laryngeal mucosa, Bhabu et. al.[6] found the EMG characteristics in response to air puff to be very different from those in response to electrical stimuli. The response latencies were much later, beginning around 150–175 milliseconds. The investigators predicted that the responses they were observing were all R2 responses involving the same pathways as the R2 responses seen in response to electrical stimulation of the iSLN. These differences would make the responses similar to those found in the corneal reflex[7].
Repeated stimulation of the laryngeal mucosa occurs during speech, yet people do not normally have reflexive spasms associated with the stimulation that occurs during normal vocal communication. This suggests that central mechanisms, invoked by the onset of afferent stimulation, suppress muscle responses from continuing to occur with sustained afferent stimulation. Such central conditioning effects were first shown to modify long latency R2 responses of the blink reflex in response to electrical stimulation. [8] Similar conditioning effects were shown to alter the R2 component of the LAR when repeated electrical stimuli were presented the superior laryngeal nerve[9].
The purpose of this study was to examine the EMG responses to the delivery of repeated rapid air pressure to the laryngeal mucosa, in order to detect any conditioning that may take place. Our hypothesis was that LAR responses to repeated rapid air pressure stimuli are centrally suppressed in humans.
Methods
Subjects
The study was reviewed and approved by the Internal Review Board of the National Institute of Neurological Disorders and Stroke. The data was acquired during the same experimental session as a previous study published by this laboratory[6]. Normal subjects who met the requirements for inclusion gave informed consent to participate in the study. Ten nonsmoking volunteers without history or evidence of neurological or laryngological disorders met the criteria for participation. None were on neuroleptic medications or on ones that would affect the sensory function of the larynx. All were determined by flexible nasolaryngoscopy to have a normal larynx without evidence of structural, voice, or swallowing problems and none had signs or symptoms of an upper respiratory infection or laryngopharyngeal reflux. There were nine males and one female ranging from 27 to 68 years old with a mean age of 42.4 years.
Laryngeal Electromyography
Throughout the study, all subjects underwent continuous EMG monitoring of multiple laryngeal muscles. After injecting a small amount of subcutaneous 2% lidocaine with 1:100,000 of epinephrine, a bipolar concentric needle was placed percutaneously to locate each of the muscles with the aid of verification gestures. Hooked wire electrodes were then placed in the manner of Hirano[10] and the verification gestures repeated.
The gestures that verified thyroarytenoid (TA) muscle placement included sustained activation during prolonged and effortful closure (prolonged and repeated/i/)[10]. Recordings with a muscle burst of activation prior to, and following phonation, but not during phonation and with phasic activation during exhalation, were identified as the lateral cricoarytenoid (LCA) muscles. The cricothryoids (CT) were identified as those muscles with increases during high pitch phonation but not during low pitch phonation[10]. The posterior cricoarytenoids (PCA) were located using the lateral approach introduced by Blitzer [11] and were verified by activation on a sniff gesture and suppression during swallowing. If there were large increases during head raising the muscle was identified as a “strap” muscle. The patient was transferred to a sitting position for the remainder of the study.
Air pulse apparatus and calibration
The air puff pressures used during testing were generated and calculated in the same manner used in previous studies published by this laboratory with an apparatus designed specifically for this purpose [6]. A tank of compressed breathable air with cascaded high and low pressure regulators provided the driving pressure, which was adjustable. A tube connected this to a half liter air reservoir near the subject. A digital manometer measured and displayed the reservoir pressure to allow trial by trial variations. A digitally controlled pnuematic valve, connecting the reservoir to the endoscope, generated rectangular air pulses by gating air flow. This set up allowed rapid and precisely-timed air pulse onset and offset. Air pressure waveforms were measured online at the inlet to the endoscope using a Validyne DP45-30 pressure gauge (60 mmHg full scale.)
The pressures created by the airpuffs at the level of the mucosa were also calculated in the same manner previously used in this laboratory [6]. Pressures were measured 2 mm from the tip of the endoscope with a solid state pressure transducer (ALL Sensors model 10 inch D-4V) for calibration purposes. The pressure readings were converted to millimeters of mercury pressure at 2 mm from the tip of the nasoendoscope on the basis of the air pressure calibration procedures.
Conditioning Testing
Prior to conditioning testing, the sensory threshold pressure levels were determined for each patient using the method described in Bhabu, et. al.[6]. A Pentax FNL-10RAP channeled flexible laryngoscope was inserted after nasal administration of a decongestant without topical anesthesia. A 100 ms air pulse was varied in pressure and administered via the working channel of the endoscope. The air puff was delivered when the tip of the endoscope was approximately 2 mm from the mucosa. This distance was judged to be when the mucosal capillaries were visible and only those trials in which mucosal indentation was noted, were accepted. The bracketing approach was used to determine the thresholds[12]. Pressures descended in 2–5 mmHg increments until the subject could no longer feel the air pulse. Pressures were then increased in 2–5 mmHg increments until they were felt again. Intermittent sham trials were inserted, in which a click was heard, but no air puff was delivered. The lowest pressure at which the subject could reliably feel the pulse was determined to be the sensory threshold.
After the determination of the sensory threshold to airpuff stimuli, the Validyne pressure reading was set to a level of three times threshold to ensure the most reliable obtainable response for that subject. Applying the same technique and criteria used in acquisition of the threshold data, pairs of air puff stimuli were presented to the laryngeal mucosa. Only trials where the position of the endoscope remained the same for both stimuli were accepted. The order of presentation of the inter-stimulus intervals was 500 ms, 5 s, 250 ms, 2 s, 750 ms, and 1 s to assure sampling from different intervals at regular intervals. At least two trials of each inter-stimulus interval were recorded for each subject. The conditioning paradigm used was similar to that which this laboratory has used in the past[9].
Electromyographic Analysis
The electromyographic analysis was performed in the manner previously described by Bhabu[6]. Digital data were recovered from the tape and processed for presentation by custom software written in Matlab. The software automatically extracted the sections of data in which air puffs were presented and segregated those data into separate data sets for analysis. The investigators were able to view the data in a variety of ways to mark the EMG baseline and response onsets and offsets.
Before identifying responses to the air puff, we filtered the signals to remove DC offset. The EMG recordings were visually inspected, and the beginning and end of muscle responses were labeled. The period just prior to the air puff stimulus was chosen for measuring baseline activity. The labeling of responses was inspected by a second author, and only those responses agreed upon by both authors were included for the study. All responses were marked and reviewed by the same two authors. Individual trials were excluded from the study if the investigators had recorded that the trial was not acceptable because of placement inconsistencies or if the air pressure curve for that trial registered a pressure that was incorrect for that trial.
The signals were full-wave-rectified before computation of the latency of a response and the integral of the EMG signal within the interval between the response onset and the response offset. The mean baseline activity was integrated for the same time interval as the response and then subtracted from the response integral to compute the residual integral of the response.
Statistical Anaysis
The frequency of responses to the first stimulus of each pair was compared across muscle types (thyroarytenoid, cricothyroid and posterior cricoarytneoid) using Chi Square computations for the ipsilateral and contralateral response to one side of stimulation. Single factor ANOVAs were computed to compare the mean response latencies between the three muscle types on the ipsilateral and contralateral sides to stimulation. Repeated ANOVAs compared the percent occurrence of muscle responses to the first and second air puff stimuli (repeated dependent variable) with the effects of the independent factors of muscle type (thyroarytenoid versus cricothyroid), side (ipsilateral versus contralateral), and inter-stimulus interval (250, 500 750, 1000, 2000, 5000 ms). In addition the interaction between the repeated dependent variable and the inter-stimulus interval was considered.
Similarly, to compare conditioning effects on the amplitude of muscle responses, repeated ANOVAs were computed comparing the integral of responses to the first and second air puff stimuli (repeated dependent variable) with the effects of the independent factors of muscle type (thyroarytenoid versus cricothyroid), side (ipsilateral versus contralateral), and inter-stimulus interval (250, 500 750, 1000, 2000, 5000 ms). In addition the interaction between the repeated dependent variable and the inter-stimulus interval was considered.
Results
After verification gestures were examined, accurate electromyographic recordings were obtained in the thyroarytenoid muscle bilaterally in 9 of the 10 subjects, and unilateral in one. Accurate unilateral cricothyroid and posterior cricoarytenoid muscle recordings were obtained in 6 of the 10 subjects, and one of these subjects also had bilateral cricothyroid recordings.
Comparison of Frequency of Responses in Different Muscles
The thyroarytenoid muscles showed responses ipsilateral to the airpuff stimulus in all of the subjects. Three of the 10 subjects had cricoarytenoid responses, five had posterior cricoarytenoid responses and all had thyroarytenoid responses. On the contralateral side, three had cricoarytenoid responses, five had posterior cricoarytenoid responses and 9 of the 10 had thyroarytenoid responses. When the frequency of responses to the initial stimulus was compared across muscles for ipsilateral responses, they differed significantly between muscles both ipsilateral (Chi Square= 44.78, p < 0.0005) and contralateral (Chi Square= 51.34, p < 0.0005) to the side of stimulation (Figure 2). On both sides, responses were more frequent in the cricothyroid and the thyroarytenoid muscles than in the posterior cricoarytenoid.
Figure 2.
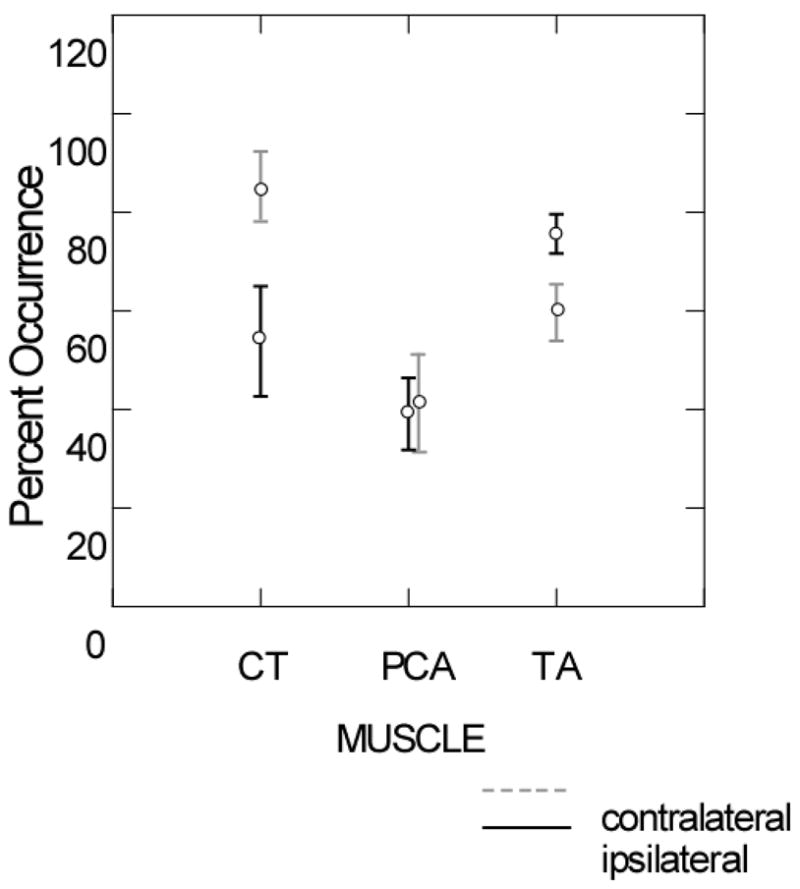
Mean and standard errors of the percent of first stimuli to the mucosa overlying the arytenoids cartilages on either the ipsilateral (black lines) or the contralateral sides (grey lines) eliciting responses in the cricothyroid (CT), posterior cricoarytenoid (PCA or the thyroarytenoid (TA) muscles.
Latency of Muscle Responses
When the latencies of the responses to the initial stimulus for each of the muscles were compared, there were significant differences between the three muscles on both the ipsilateral side (F= 17.153, p < 0.0005) and on the contralateral side (F= 21.043, p < 0.0005). These differences were mainly due to the latency of posterior cricoarytenoid responses being much later than those for both the cricothyroid and the thyroarytenoid (Figure 3).
Figure 3.
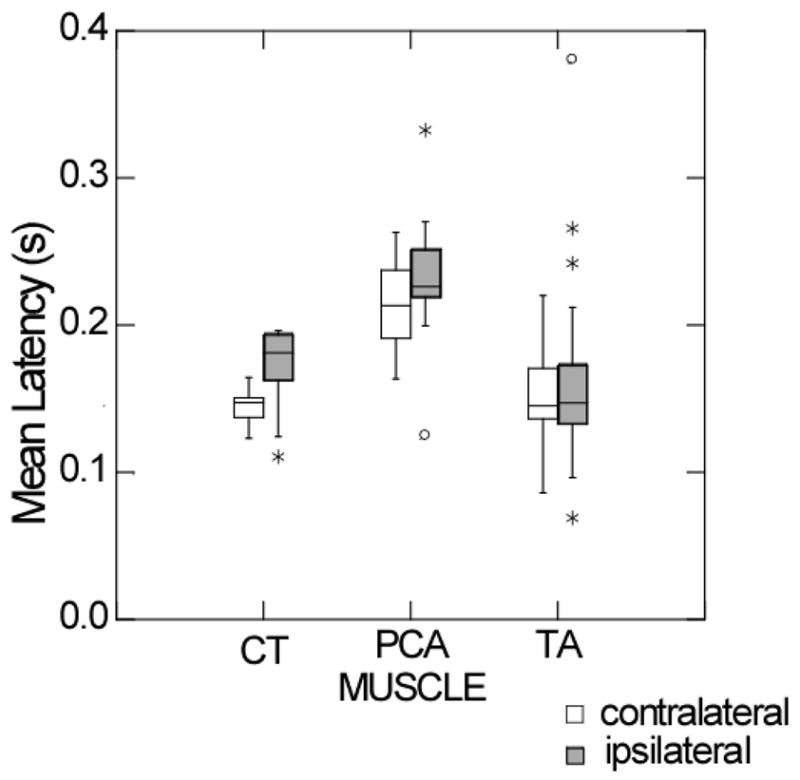
Box plots of the quartile distribution around the median of the latencies of muscle responses to first stimuli presented on either the ipsilateral (white boxes) or the contralateral sides (grey boxes) in the cricothyroid (CT), posterior cricoarytenoid (PCA or the thyroarytenoid (TA) muscles.
Conditioning Effects—Changes in Frequency of Response
We compared the frequency of responses to the initial air puff with the frequency of responses to the second air puff, as a measure of conditioning in the thyroarytenoid and cricothyroid muscles. Because only 50% of the first stimuli yielded posterior cricoarytenoid responses (Figure 2), this muscle was not included in the analysis. For the thryoarytenoid and cricothyroid muscles, the percentage of muscles responses averaged approximately 68% to the first stimulus and approximately 50 % for the second stimulus. This difference was consistent across different inter-stimulus intervals (Figure 4). A repeated ANOVA comparing the percent occurrence of muscle responses to the first and the second (conditioned) air puff stimulus was statistically significant (F = 17.28, p < 0.0005) and did not interact with muscle—thyroarytenoid versus cricothyroid (F = 0.003, p = .960) side—ipsilateral versus contralateral (F = 0.896, p = .346) or inter-stimulus interval (F = 1.267, p = .262). As shown in Figure 4, the decrease in numbers of responses following a conditioning stimulus tended to be greatest at intervals of 250 ms (F = 14.152, p = 0.002) 500 ms (F = 16.418, p = 0.001) and 2s (F = 5.331, p = 0.031) and were not significant at 750 ms, 1 s, and 5 s.
Figure 4.
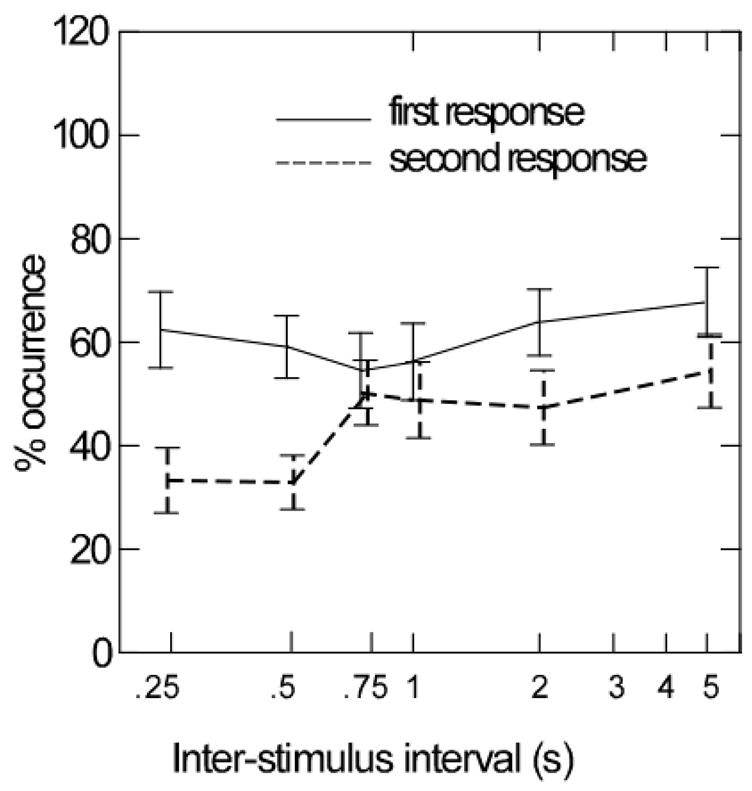
Mean and standard errors of the percent of responses to the first stimulus (solid lines) and the percent of responses to the second stimulus (hatched lines) in the cricothyroid or the thyroarytenoid muscles to air puff stimuli to the mucosa over-lying the arytenoids cartilages on either side.
Conditioning Effects—Changes in Response Amplitude
To determine if suppression of the response amplitude occurred following a conditioned stimulus, we compared the amplitude of the responses occurring following the conditioning stimulus with responses occurring to the initial unconditioned stimulus on a repeated ANOVA. The effects of conditioning on response amplitude (the integral) was non-significant (F = 1.388, p = .243), however, there was a significant conditioning by side (ipsilateral versus contralateral) interaction (F = 7.376, p = 0.009). None of the other effects such as muscle (F = 0.599, p = .442) were significant. As shown in Figure 5, the amplitude conditioning effects differed between the ipsilateral and contralateral sides, the percent of the unconditioned response amplitude occurring in the second response on the ipsilateral side was less than 50 percent at the inter-stimulus interval of 500 ms (F= 4.62, p= 0.055) and 80% at 2 s (F=3.43, p= 0.087).
Figure 5.
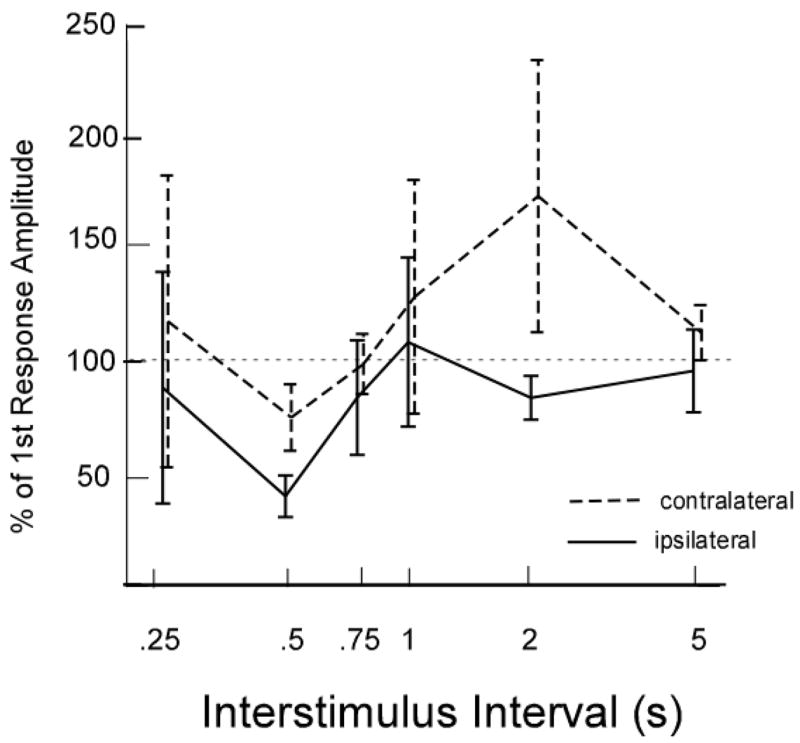
Mean and standard errors of the percent of the amplitude of the first response occurring in the second response to stimuli in muscles either on ipsilateral side to the stimulus (solid lines) or on the contralateral side to the stimulus (hatched lines) in the cricothyroid or the thyroarytenoid muscles to air puff stimuli to the mucosa over-lying the arytenoids cartilages.
Discussion
The finding of more frequent responses occurring in the cricothyroid and in the thyroarytenoid muscles than in the posterior cricoarytenoid is in agreement with the observation that this is an adductor response to air puff stimulation[6, 13]. Fewer responses occurred in the posterior cricoarytenoid and those that did occur had a much slower latency than in either the thyroarytenoid and cricothyroid muscles. These differences in latency were on the order of 50 to 60 ms and were most likely central because both the thyroarytenoid and the posterior cricoarytenoid muscles are innervated via the recurrent laryngeal nerve. Given that average latencies of the thyroarytenoid and cricothyroid responses on the side ipsilateral to the stimulus were 157 and 172 ms respectively and 153 and 145 on the contralateral side, the differences in nerve length would account for only a couple of ms and would not impact on the response latencies[14]. These latencies tended to be quite variable (Figure 3) and indicated a considerable amount of central processing time in the elicitation of these adductor responses, particularly given that electrical stimulation to the superior laryngeal nerve elicits a rapid response at around 17 ms on the ipsilateral side[3].
In a previous study we observed the differences in latency between responses to electrical stimulation to the superior laryngeal nerve and responses to air puffs. The latency of thyroarytenoid responses to the air puff stimuli was much later than either the R1 responses to electrical stimulation that occur around 18 ms [6] or the R2 responses which occur around 68 ms. We proposed that the air puff responses were similar to the R2 responses even though the average latency of these air puff responses are about 80 milliseconds later, around 150 and 175 ms[3]. To address this hypothesis, we needed to determine if there were conditioning effects on the frequency and amplitude of these muscle responses to an air puff, similar to the conditioning effects seen with repeated electrical stimulation in R2 responses[12]. In the current study, we found significant conditioning effects on these responses with the frequency of response to the second (conditioned) stimulus being reduced by an average of 20 percent. This was particularly evident at the inter-stimulus intervals of 250 and 500 ms. Similarly, the amplitudes of the second responses were reduced by about 50% at the inter-stimulus intervals of about 500 ms.
These findings demonstrate that on the side ipsilateral to afferent stimulation, central suppression, either within the medulla or from higher centers, reduces motor neuron firing for the thyroarytenoid and cricothyroid muscles following an initial response to afferent stimulation. The duration of this suppression was greatest up to 500 ms. This suppression may play a role in normal coughing; when coughing is repetitive, the average duration between two coughs is between 500 ms and 1 s [15].
In normal voice production, vibration generated within the larynx impacts upon the laryngeal mucosa throughout the glottis. High speed imaging has demonstrated that tissue vibration occurs beyond the medial edge of the vocal folds, deflecting mucosa throughout the larynx including regions overlying the arytenoids [16–18]. This is the region that we stimulated during this study, particularly because the highest density of mechanoreceptors are found in the mucosa in this area [19]. In a previous study, we found that laryngeal adductor responses only occur when this mucosa is intact and are abolished when the mucosa is removed even though the same displacement continued to be applied to the thyroarytenoid muscle and the cricoarytenoid joint [20]. Because mucosal deflection of these mechanoreceptors can elicit laryngeal adductor responses and such deflection occurs during voicing, it has been questioned why such responses are not elicited during speech [12, 21]. Our findings demonstrate active central suppression of laryngeal adductor responses with repeated stimulation of the laryngeal mucosa in normal volunteers. Previously, using electrical stimulation of the superior laryngeal nerve, we found that this suppression is reduced in both adductor and abductor spasmodic dysphonia when repeated electrical stimuli were applied to the superior laryngeal nerve [22, 23].
The demonstration of active suppression of muscle responses to repeated air puff stimuli in normal volunteers demonstrates that central mechanisms normally prevent the elicitation of laryngeal adductor responses with continued stimulation, such as occurs during voicing. Impaired suppression of such central mechanisms might account for the elicitation of spasmodic muscle bursts that disrupt voice production in spasmodic dysphonia.
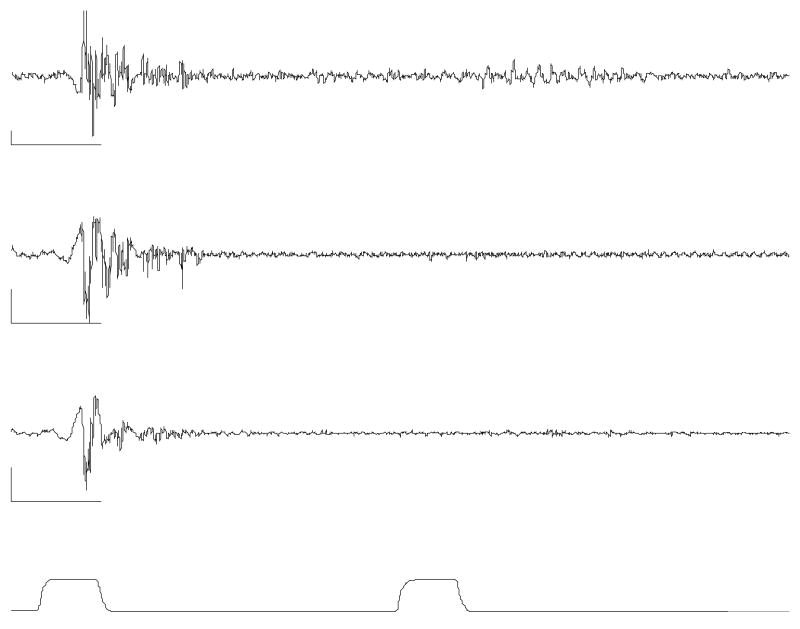
Figure 1.
This example illustrates the conditioning effect at an interpulse-interval of 500 ms. The top two traces are filtered recordings from the ipsilateral TAs and the third trace is from the contralateral TA. The bottom trace is the airpuff stimulus pressure. The calibration bars indicate 100 ms in time and 100 μV in EMG amplitude. The traces reveal that the response to the second stimulus is diminished or eliminated on both sides by the conditioning effect.
References
- 1.Murakami Y, Kirchner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81(1):59–71. doi: 10.1177/000348947208100106. [DOI] [PubMed] [Google Scholar]
- 2.Boushey HA, et al. The response of laryngeal afferent fibres to mechanical and chemical stimuli. J Physiol. 1974;240(1):153–75. doi: 10.1113/jphysiol.1974.sp010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludlow CL, Van Pelt F, Koda J. Characteristics of late responses to superior laryngeal nerve stimulation in humans. Ann Otol Rhinol Laryngol. 1992;101(2 Pt 1):127–34. doi: 10.1177/000348949210100204. [DOI] [PubMed] [Google Scholar]
- 4.Sant'Ambrogio G, Brambilla-Sant'Ambrogio F, Mathew OP. Effect of cold air on laryngeal mechanoreceptors in the dog. Respir Physiol. 1986;64(1):45–56. doi: 10.1016/0034-5687(86)90059-9. [DOI] [PubMed] [Google Scholar]
- 5.Aviv JE, et al. Air pulse quantification of supraglottic and pharyngeal sensation: a new technique. Ann Otol Rhinol Laryngol. 1993;102(10):777–80. doi: 10.1177/000348949310201007. [DOI] [PubMed] [Google Scholar]
- 6.Bhabu P, et al. Thyroarytenoid muscle responses to air pressure stimulation of the laryngeal mucosa in humans. Ann Otol Rhinol Laryngol. 2003;112(10):834–40. doi: 10.1177/000348940311201002. [DOI] [PubMed] [Google Scholar]
- 7.Esteban A. A neurophysiological approach to brainstem reflexes. Blink reflex. Neurophysiol Clin. 1999;29(1):7–38. doi: 10.1016/S0987-7053(99)80039-2. [DOI] [PubMed] [Google Scholar]
- 8.Sanes JN, Ison JR. Habituation and sensitization of components of the human eyeblink reflex. Behav Neurosci. 1983;97(5):833–6. doi: 10.1037//0735-7044.97.5.833. [DOI] [PubMed] [Google Scholar]
- 9.Ludlow CL, et al. Abnormalities in long latency responses to superior laryngeal nerve stimulation in adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1995;104(12):928–35. doi: 10.1177/000348949510401203. [DOI] [PubMed] [Google Scholar]
- 10.Hirano M, Ohala J. Use of hooked-wire electrodes for electromyography of the intrinsic laryngeal muscles. J Speech Hear Res. 1969;12(2):362–73. doi: 10.1044/jshr.1202.362. [DOI] [PubMed] [Google Scholar]
- 11.Blitzer A, et al. Abductor laryngeal dystonia: a series treated with botulinum toxin. Laryngoscope. 1992;102(2):163–7. doi: 10.1288/00005537-199202000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Ludlow CL, Swisher LP. The audiometric evaluation of adult aphasics. J Speech Hear Res. 1971;14(3):535–43. doi: 10.1044/jshr.1403.535. [DOI] [PubMed] [Google Scholar]
- 13.Aviv JE, et al. Laryngopharyngeal sensory discrimination testing and the laryngeal adductor reflex. Ann Otol Rhinol Laryngol. 1999;108(8):725–30. doi: 10.1177/000348949910800802. [DOI] [PubMed] [Google Scholar]
- 14.Sims S, et al. An evaluation of the use of magnetic stimulation to measure laryngeal muscle response latencies in normal subjects. Otolaryngology-Head and Neck Surgery. 1996;114:761–767. doi: 10.1016/S0194-59989670099-2. [DOI] [PubMed] [Google Scholar]
- 15.Ludlow CL, Lou G. Observations on human laryngeal muscle control. In: Fletcher N, Davis P, editors. Controlling complexity and chaos: 9th Vocal Fold Physiology Symposium. Singular Press; Sandiego, CA: 1996. pp. 201–218. [Google Scholar]
- 16.Yumoto E, Kadota Y. Pliability of the vocal fold mucosa in relation to the mucosal upheaval during phonation. Arch Otolaryngol Head Neck Surg. 1998;124(8):897–902. doi: 10.1001/archotol.124.8.897. [DOI] [PubMed] [Google Scholar]
- 17.Larsson H, et al. Vocal fold vibrations: high-speed imaging, kymography, and acoustic analysis: a preliminary report. Laryngoscope. 2000;110(12):2117–22. doi: 10.1097/00005537-200012000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Hertegard S, Larsson H, Wittenberg T. High-speed imaging: applications and development. Logoped Phoniatr Vocol. 2003;28(3):133–9. doi: 10.1080/14015430310015246. [DOI] [PubMed] [Google Scholar]
- 19.Davis PJ, Nail BS. Quantitative analysis of laryngeal mechanosensitivity in the cat and rabbit. J Physiol (Lond) 1987;388:467–485. doi: 10.1113/jphysiol.1987.sp016625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreatta RD, et al. Mucosal afferents mediate laryngeal adductor responses in the cat. J Appl Physiol. 2002;93(5):1622–9. doi: 10.1152/japplphysiol.00417.2002. [DOI] [PubMed] [Google Scholar]
- 21.Davis PJ, Bartlett D, Luschei ES. Coordination of the respiratory and laryngeal systems in breathing and vocalization. In: Titze IR, editor. Vocal Fold Physiology. Singular Publishing Group, Inc; San Diego: 1993. pp. 189–226. [Google Scholar]
- 22.Ludlow CL, et al. Abnormalities in long latency responses to superior laryngeal nerve stimulation in adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1995;104:928–935. doi: 10.1177/000348949510401203. [DOI] [PubMed] [Google Scholar]
- 23.Deleyiannis FW, et al. Laryngeal long latency response conditioning in abductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1999;108(6):612–9. doi: 10.1177/000348949910800615. [DOI] [PubMed] [Google Scholar]