Abstract
We tested the hypothesis that children with Tourette syndrome (TS) would exhibit aberrant brain lateralization compared to a healthy control (HC) group in an attention-modulation version of a verbal dichotic listening task using consonant-vowel syllables. The modulation of attention to focus on the right ear stimulus in the dichotic listening situation is thought to involve the same prefrontal attentional and executive functions that are involved in the suppression of tics, whereas, performance when focusing attention on the left ear stimulus additionally involves a callosal transfer of information. In light of presumed disturbances in transfer of information across the corpus callosum, we hypothesized that children with TS would, however, have difficulty modulating the functional lateralization that ensues through a shift of attention to the left side. This hypothesis was tested by exploring the correlations between CC size and left ear score in the forced-left condition.
Twenty boys with TS were compared with 20 age- and handedness-matched healthy boys. Results indicated similar performance in the TS and HC groups for lateralization of hemispheric function. TS subjects were also able to shift attention normally when instructed to focus on the right ear stimulus. When instructed to focus attention on the left ear stimulus, however, performance deteriorated in the TS group. Correlations with CC area further supported the hypothesized presence of deviant callosal functioning in the TS group.
Keywords: Tourette syndrome, Dichotic listening, Corpus callosum, Executive functions
1. Introduction
Tourette syndrome (TS) is characterized by motor and phonic tics that fluctuate in severity (American Psychiatric Association, 1994). Cortico-Striato-Thalamo-Cortical (CSTC) circuits are thought to contribute both to the generation and the suppression of tics (Leckman, 2002). Early reports of altered basal ganglia asymmetries in subjects with TS (Peterson et al., 1993; Singer et al., 1993) suggested that anatomical and functional hemispheric asymmetries may be disrupted in persons with TS. In addition, the size the corpus callosum has been shown repeatedly to be altered in TS compared with control subjects (Baumgardner et al., 1996; Moriarty et al., 1997; Mostofsky, Wendlandt, Cutting, Denckla, & Singer, 1999; Peterson et al., 1994). A recent study from our laboratory (Plessen et al., 2004) found that children with TS have smaller areas of the midsagittal CC compared with control children and that a smaller CC area is associated with less severe tics. In addition, inverse correlations between prefrontal cortex and callosal area were significantly more prominent in the TS group. This study stimulated further interest in studies of hemispheric laterality in children TS, as the callosum is thought to be the brain structure that supports functional brain lateralization (Banich, 2003). Dichotic listening (DL) is an experimental paradigm that permits the non-invasive study of how lateralized information is processed in the two hemispheres of the brain (Bryden, 1988; Hugdahl, 2003; Kimura, 1961). Dichotic listening has to our knowledge not been studied previously in children with TS.
Numerous studies have shown a consistent right ear advantage (REA) during performance of the DL task in healthy individuals (Hugdahl, 2003). The classic structural model (Kimura, 1967) posits that the phenomenon of REA arises as follows: first, auditory input is more strongly represented in the contralateral hemisphere than in the ipsilateral one. Second, the left hemisphere is specialized for language in most individuals. Third, auditory information sent along the ipsilateral pathways seems to be suppressed or blocked by information from contralateral pathways. Finally, the right ear advantage results from the fact that information reaches the right cerebral hemisphere via transfer across the corpus callosum to the contralateral (left) cerebral hemisphere language area for processing.
REA can be modified by instructing the individual to attend to the stimulus in either the right or left ear (Bryden, Munhall, & Allard, 1983; Hugdahl & Andersson, 1986), thus adding a “top-down” component to an originally “bottom-up” processing of lateralized auditory stimuli. Therefore, when focusing on the right ear stimulus (“forced-right” condition), REA actually increases, whereas, it decreases or even disappears during attentional focus on the left ear (“forced-left” condition), thus creating a left ear advantage (LEA). Thus, DL is regarded as a measure of auditory processing in the temporal lobe (Spreen & Strauss, 1991), and as a measure for frontal lobe functioning when combined with instructions of attentional shift (Hugdahl et al., 2003).
This study thus aimed to assess functional brain asymmetry in children with TS using a variant of the DL paradigm that also allows study of the effects of attention and executive functions to modulate lateralization (Hugdahl et al., 2003). The attention-modulated DL paradigm instructs the subject explicitly to focus attention on a stimulus in the right or left ear and to report the perceived syllable (Hugdahl & Andersson, 1986). The task assesses experimentally the “top-down” modulation of a stimulus-driven, or “bottom-up”, laterality effect.
Functional MRI studies have shown that prefrontal cortices activate strongly during the voluntary suppression of tics (Peterson et al., 1998), and the frequent need to suppress tics is thought to induce a compensatory hypertrophy of frontal cortices in which the degree of hypertrophy corresponds with the degree of control over symptoms in persons with TS (Peterson et al., 2001). The forced attention condition in the DL paradigm is also considered a test of the attentional aspects of executive functioning mediated by the prefrontal cortex, in that the degree to which an individual is able to direct attention voluntarily to one ear or the other depends on the ability to overrule a bottom-up, stimulus-driven laterality effect, an ability that has been shown to be compromised in clinical populations with an impaired attentional focus (Hugdahl et al., 2003).
We therefore propose that shifting attention to the right ear stimulus could be regarded in children with TS as tapping the same regulatory circuits that subserve the top-down modulation or suppression of tic behaviors. Shifting attention to the left ear stimulus processed in the contralateral hemisphere, on the other hand, could be regarded as a test of callosal transfer of information, given that left ear performance in the forced-left attention condition depends on transfer of regulatory control across the CC (Milner, Taylor, & Sperry, 1968; Pollmann, Maertens, von Cramon, Lepsien, & Hugdahl, 2002). We predicted that callosal transfer would be impaired in children with TS as a consequence of their previously documented reduction in callosal size, similar to altered transfer in other conditions with abnormal morphologies of the CC (Reinvang, Bakke, Hugdahl, Karlsen, & Sundet, 1994).
We thus tested three specific hypotheses for the TS group compared with healthy control subjects. We predicted that the TS group would evidence reduced measures of a normal functional brain asymmetry, as well as an intact ability to shift attention actively towards the right ear stimulus, and finally an impaired callosal transfer during the forced-left attention condition. The first hypothesis was tested in the non-forced condition, the second in the forced-right, and the third in the forced-left condition, as well as by correlating the left ear scores with CC size.
2. Methods
2.1. Subjects
TS subjects were recruited from the Department of Child and Adolescent Psychiatry at the Haukeland University Hospital, University of Bergen, Norway, and from outpatient clinics in the greater Bergen area. All children met DSM-IV criteria for a diagnosis of TS (American Psychiatric Association, 1994). HC children were recruited by contacting local schools in the same geographic area. Controls were matched for age and gender with the children in the patient group. Written informed consent was obtained from all participants, and the study was clarified by the Regional Committee for Medical Research Ethics, West-Norway.
Exclusion criteria for the control group were a lifetime history of Tic Disorder, Obsessive Compulsive Disorder (OCD), Attention Deficit Hyperactivity Disorder (ADHD), or a current DSM-IV Axis I disorder. Additional exclusion criteria for both groups were epilepsy, head trauma with loss of consciousness, former or present substance abuse, or an IQ below 70, as measured with the WISC-III (Wechsler, 1996).
Parents and children were interviewed by a child and adolescent psychiatrist using the “Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version” (Kaufman et al., 1997). The psychiatric diagnoses were established through review of all available study materials in a best estimate consensus procedure (Leckman, Sholomskas, Thompson, Belanger, & Weissman, 1982). OCD symptoms were quantified using the Yale Brown Obsessive Compulsive Scale (Goodman et al., 1989; Scahill et al., 1997), and the severity of tics was rated with Yale Global Tic Severity Scale (YGTSS) (Leckman et al., 1989). Socioeconomic status (SES) was estimated from the level of parental education (JAACAP, 2005).
We enrolled 20 consecutively recruited subjects into the study who met diagnostic criteria for TS, without any criteria for exclusion. (Two girls with TS were recruited but had to be excluded prior to data analysis because of motion artifacts on their MR scans.) The final sample thus consisted of two male groups: 20 TS and 20 HC boys, 9–17 years of age. The groups were of comparable age (TS=13.6 years, ±1.9; HC=13.4 years, ±2.4; t=−0.3; p=.77) and SES. The groups, however, differed in full scale IQ (TS=94.5 ± 10.2; HC=105.7 ± 9.2; t=3.6; p<.001), verbal IQ (TS=94.4 ± 11.4; HC=104.4 ± 10.5; t=2.9; p<.006) and performance IQ (TS=95.6 ± 10.8; HC=106.1 ± 12.1; t=2.9; p<.006).
Five of the subjects in the TS group had comorbid combined-type ADHD, and four others had comorbid OCD. In each group were two left-handed individuals (left-handed individuals differed between groups in their age by 1 and 12 months, respectively), all others being right-handed with a laterality index of 80% or above as measured by the Edinburgh handedness inventory (Oldfield, 1971). Nine subjects in the TS group were taking medication, either neuroleptics (n=4), alpha agonists (n=2), selective serotonin uptake inhibitors (n=1), or stimulants (n=2). HC subjects were not taking any psychotropic medication. Tic severity at the time of investigation in the TS group was 11.4 ± 2.9 for motor and 9.2 ± 3.4 for phonic tics, with lifetime-worst ever scores 15.5 ± 5.1 for motor and 13.7 ± 5.5 for phonic tics (possible range 0–25 in each category).
2.2. Dichotic listening and the forced-attention paradigm
The auditory stimuli of the DL paradigm consisted of the six stop-consonants, together with vowel /a/, to form six consonant-vowel syllables: /ba/; /da/; /ga/; /pa/; /ta/; /ka/. The recorded consonant-vowels (CV) were without any semantic content, spoken by a male voice and were simultaneously presented to both ears via computer (Hugdahl & Andersson, 1986). CV syllables were paired with one another in all combinations, thereby yielding 36 bi-auricular combinations (six trials were homonymic conditions, where the same consonant-vowel was presented to both ears). These DL stimuli were administered under three attentional conditions: the non-forced (NF) condition, in which the subject was asked to report the passively heard syllable or the forced-right (FR) or forced-left (FL) conditions in which the subject was to report syllables heard by attending selectively to the right (FR) or left ear (FL). Subjects were instructed to report the syllable heard best, if they heard both syllables. The NF condition was always administered first, whereas, the order of the two forced conditions was counterbalanced between subjects.
Before beginning the DL task, five trials were administered to ensure that the probands understood the task. During the task, subjects recorded the syllable heard on a sheet of paper placed in front of them that listed all possible syllables. Results were given as the correct scores for each ear within each of the attention conditions separately. In addition, a score was calculated for each ear to estimate the effects of attentional shifting; the baseline score of the right ear in the NF condition was subtracted from the right ear score in the FR condition (RE(FR)–RE(NF)), and similarly for the left ear (LE(FL)–LE(NF)). This score, termed an “effort score”, can be interpreted as the ability of an individual to modulate the stimulus-driven asymmetry through effortful shift of attention.
2.3. MRI scanning and image analysis
MR images were acquired on a Siemens Symphony, 1.5 Tesla scanner. Head positioning was standardized using canthomeatal landmarks. T1-weighted, sagittal 3D volume MPRage anatomical images were acquired for all subjects, with repetition time (TR)=1910 ms, echo time (TE)=3.93 ms, flip angle (FA)=4°, image matrix=256 × 192, field of view (FOV)=256 mm, slice thickness=1 mm, with 176 contiguous slices acquired.
MR image analysis for overall CC area, using the T1 weighted MPRage images, was performed using Analyze 6.0 software (Rochester, MN, USA) operating on a Linux workstation. Raters were blind to subject characteristics and group assignments. Each MR dataset was realigned to the midsagittal slice, which was identified using standard midline landmarks (callosal sulcus, cerebral aqueduct, pineal gland, peaked roof of the fourth ventricle, and minimal gray matter in the interhemispheric fissure), to minimize variability in CC size between subjects that may have been caused by individual differences in head positioning during scanning (Rauch & Jinkins, 1996). The midsagittal slice was magnified, and the CC contour was segmented automatically, using an isointensity contour function, with subsequent manually editing.
Whole brain volume (WBV) was determined using an automated segmentation procedures and manual editing with Analyze 7.5 software (Rochester, MN) on Sun Ultra 10 workstations. WBV included gray and white matter, ventricular cerebrospinal fluid (CSF), and cisterns, fissures, and cortical sulci. CSF was included using a connected components analysis (Analyze subroutine “delete holes”). WBV was used as a covariate in the statistical analyses to control for scaling effects within the brain (Arndt, Cohen, Alliger, Swayze, & Andreasen, 1991).
2.4. Statistical analyses
All statistical analyses were performed using SPSS (SPSS, 1999), Statistica (StatSoft, 2003) or R (Team, 2003). A three-way ANOVA was performed according to the design Group (TS group, HC group) × Attentional condition (NF, FR, FL) × Ear (right, left). Significant main effects and interactions, with multiple comparisons between means, were followed-up with a Fischer’s LSD test (because of directed hypotheses). Group differences in the forced-left conditions were addressed using a 2 × 2 ANOVA. Scores for attentional shift (effort scores) were compared between groups using a Student’s t-test for two independent groups. All tests for significance were of the two-tailed type and thresholded at p<.05.
2.5. Correlations of DL measures with IQ, symptom severity, and CC size
Correlations of DL measures with IQ scores, current tic severity (combined current motor and phonic tic severity), and overall CC size were computed for the TS and HC groups separately. Semipartial correlations were used in analyses that included CC size, by controlling CC size for WBV. Correlations with symptom scores were computed only for the TS group, given that the controls had no tic symptoms. A permutation test was conducted for testing the difference of semipartial correlations between CC area size (controlled for WBV) and left ear performance (FL condition) in the TS group versus the HC group.
2.6. Controlling for comorbidity
The effects of comorbid OCD or ADHD on our findings were assessed by excluding from the analyses individuals with either or both of these illnesses, recognizing that this procedure rendered the analyses susceptible to statistical Type II errors.
3. Results
3.1. Overall ANOVA
The ANOVA showed a significant main effect of Ear (F1,38=19.16, p<.001). This effect was caused by a higher number of correct reports for the right ear stimulus across groups and conditions, confirming in these subjects the statistical basis for the REA effect. A significant main effect of the Attention condition (NF, FR, FL) was also observed (F2,37=14.1; p<.001). This effect was caused by divergent correct reports in the right compared with the left ear depending on the attentional instructions. The significant two-way interaction of Ear × Attention condition (F2,37=15.1; p<.001) was also significant. Follow-up tests showed that this interaction reflected a significant REA in the NF and the FR conditions, but no ear advantage in the FL condition. The presence of this effect in both the TS and HC groups produced a nonsignificant interaction of Ear × Attention condition × Group. Finally, the main effect of Group showed a clear trend toward significance (F1,38=3.64; p=.06), with fewer overall correct reports in the TS group. Main effects and interactions are shown in Table 1. Means for Groups, Ear, and Attention conditions are depicted in Fig. 1, and scatter-plots with individual correlations for the right and left ear score are shown in Fig. 2. Table 2 documents the presence of more subjects with an REA (defined as at least one more correct item from the right ear) in the TS group compared to the HC group.
Table 1.
Main and interaction effects from the three-way ANOVA
| SS | DF | MS | F | p-Value | |
|---|---|---|---|---|---|
| Group | 29 | 1 | 29 | 3.64 | .064 |
| Condition | 85 | 2 | 42 | 14.14 | .000 |
| Condition × Group | 0.2 | 2 | 0.8 | 0.03 | .974 |
| Ear | 1038 | 1 | 1038 | 19.16 | .000 |
| Ear × Group | 12 | 1 | 12 | 0.22 | .645 |
| Condition × Ear | 444 | 2 | 222 | 15.09 | .000 |
| Condition × Ear × Group | 1.2 | 2 | 0.6 | 0.04 | .961 |
Fig. 1.
Mean correct reports for the right and the left ear split for attentional condition and for the Tourette syndrome (TS) and the healthy control (HC) groups.
Fig. 2.
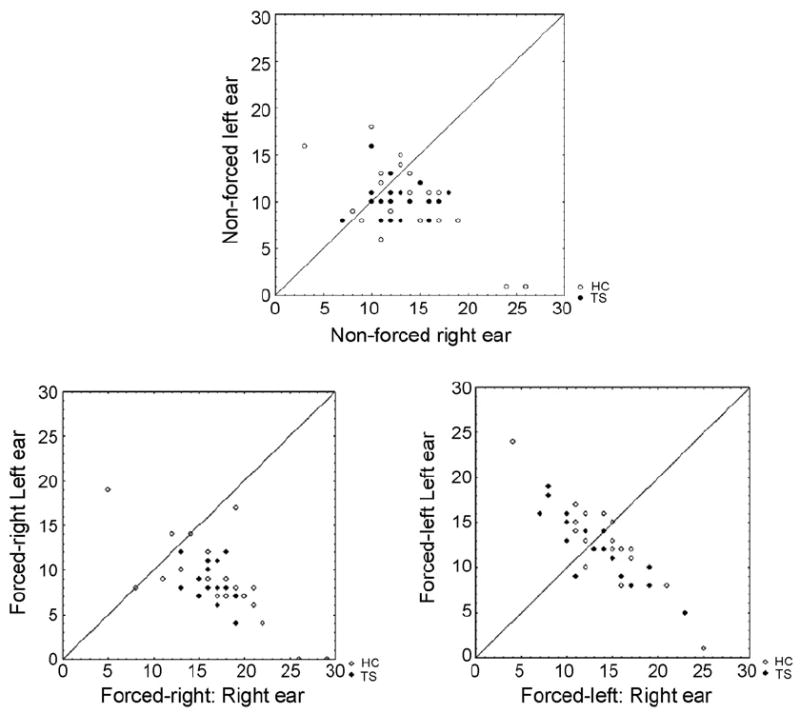
Scatter plots of individual performance for the three attention conditions. The diagonal line is a symmetry line=45°. All individuals falling below the line have a right ear advantage (REA) and all individuals falling above the line have a left ear advantage (LEA).
Table 2.
Absolute ear advantage (right, left, or none) for the two groups (TS and HC group) in the three conditions of testing
| Right | Left | None | ||
|---|---|---|---|---|
| Non-forced | HC group | 12 | 8 | 0 |
| TS group | 14 | 4 | 2 | |
| Forced-right | HC group | 16 | 2 | 2 |
| TS group | 20 | 0 | 0 | |
| Forced-left | HC group | 11 | 8 | 1 |
| TS group | 12 | 7 | 1 |
Measures for shift of attention (effort measures) did not differ between groups (effort measures Right Ear: HC 3.5 (S.D. 3.9); TS 3.5 (S.D. 2.4); t38≈0.0; p≈1.0 and Left Ear: HC 2.3 (S.D. 4.5); TS 2.0 (S.D. 4.5); t38=.21; p=.83).
3.2. Correlations with brain measures
Correlations of left ear performance in the FL attention with CC size (HC group 664.4 mm2 (S.D. 112.1) versus TS group 633.9 mm2 (S.D. 85.6) were in opposing directions in the two groups: correlations were positive in the HC (r=.30; p=.19) but inverse in the TS group (r=−.30; p=.20) when controlling CC size for whole brain volume (HC group 1549.2 cm3 (S.D. 115) versus TS group 1450.6 cm3 (S.D. 134)). Current symptom severity did not correlate significantly with CC size in the TS group (semipartial correlation r=−.18; p=.45).
3.3. Correlations of DL measures with symptoms
Effort measures from the FR condition correlated inversely with the severity of TS symptoms (r=−.06, p=.19); measures for the FL condition correlated positively, though not significantly, with severity (r=.30, p=.19).
3.4. Correlation of DL measures with IQ
No significant correlations between total IQ and right ear DL scores were observed for either group (NF r=−.18; FR r=.05) for the HC group and (NF r=−.01; FR r=.20) for the TS group. Neither were significant correlations of total IQ score with left ear performance found for the HC group (NF r=.09; FL r=−.40; p=.09). For the TS group, however, a significant inverse correlation was observed for total IQ with left ear performance in the FL condition (r=−.57; p<.009), but not for the NF condition (r=.01).
3.5. Excluding subjects with comorbid diagnoses
The primary findings from the 3 × 2 ANOVA were stable in the TS-only sample (n=11). Results for the FL attention condition, however, demonstrated impaired left ear performance in the TS group when excluding individuals with comorbid conditions (see Fig. 4). This was followed up in a 2 × 2 ANOVA for the FL condition for the TS-only subjects compared with the entire HC group (n=20). Results from this ANOVA showed a significant effect of Group (F1,33=5.28; p<.05), with a post hoc LSD test showing a significant lower left ear performance in TS-only subjects (p<.005).
Fig. 4.
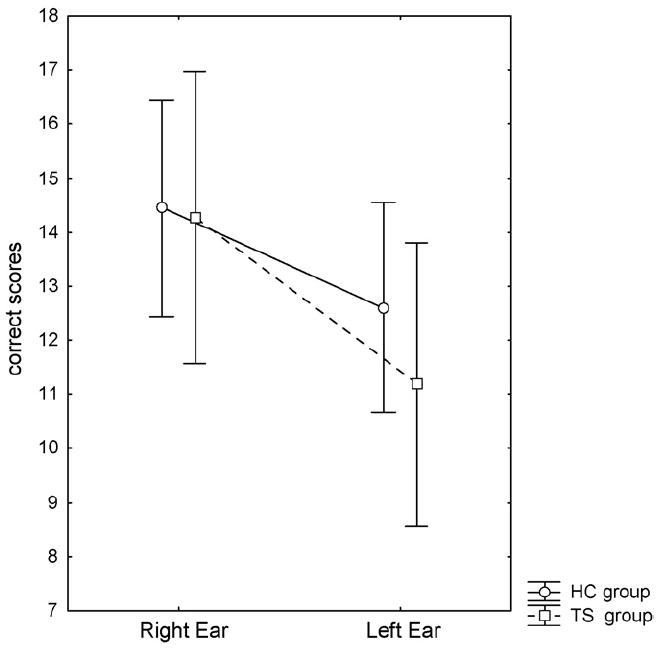
Mean correct reports for the right and the left ear in the forced-left condition for the Tourette syndrome (TS) and the healthy control (HC) groups.
Inspection of the results in each subgroup revealed that TS individuals with comorbid ADHD and not TS individuals with a comorbid OCD were responsible for the finding of better left ear performance in the FL condition for the entire TS group. Further inspection of the ANOVA results in the TS + ADHD group showed that this group had no discernible attentional modulation, as expected, but a LEA in both the forced-right and forced-left condition.
The effort measures for shift of attention did not differ between the groups when excluding individuals with ADHD, OCD, or both.
However, semipartial correlations between CC size (controlled for WBV) and left ear performance in the FL attention condition reached statistical significance when excluding individuals with ADHD (r=−.57; p=.04) (Fig. 3), and were indeed more prominent when excluding individuals with OCD and looking at the TS-only group (r=−.81; p<.003). The semi-partial correlations were significantly different in the TS only and HC groups (p<.05).
Fig. 3.
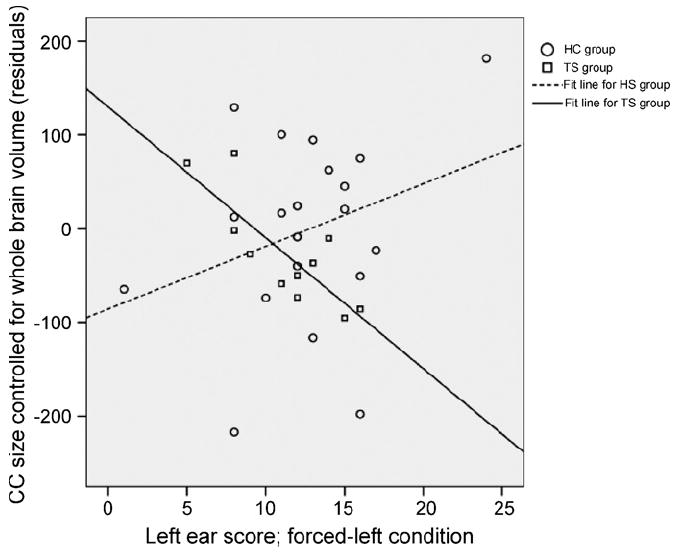
Residuals for left ear performance and CC size (controlled for whole brain volume), showing separate fit lines for the TS only and the HC group.
4. Discussion
The TS group did not differ from the healthy controls either in their degree of stimulus-driven laterality, or in their ability to modulate REA through attentional shifting to either the left or right ear stimulus. Thus, we were unable to confirm the first hypothesis, that the TS group would evidence reduced functional brain lateralization, which was tested in the NF condition. Our second hypothesis was confirmed, however, in that TS children modulated REA to the same degree as the HC children in the FR condition. Our third hypothesis also was confirmed, in that we found a reduced left ear-performance in the TS subjects who did not have comorbid ADHD or OCD.
4.1. Functional brain asymmetry
The absence of impairment of functional asymmetry is supported by recent brain imaging studies that have shown that the basal ganglia and cortices are not abnormally lateralized in TS children or adults (Peterson et al., 2001; Peterson et al., 2003), in contrast to findings from earlier studies which suggested abnormal asymmetries in subjects with TS (Peterson et al., 1993; Singer et al., 1993). These earlier studies, however, were based on small sample sizes that could have been vulnerable to individual differences that generate spurious findings. An earlier study reported reduced functional lateralization in TS adults compared with healthy controls (Yazgan, Peterson, Wexler, & Leckman, 1995). These subjects showed reduced laterality in a dual task with verbal and manual interference, a line bisection test, and a measure of turning bias; nevertheless, no abnormalities were detected in a DL task using fused rhymed words. A recent study reported impaired performance on the bimanual Purdue Pegboard test and on a verbal-manual dual task in adults with TS (Margolis, Donkervoort, Kinsbourne, & Peterson, 2006).
The subjects in the current study overall exhibited a relatively week degree of lateralization compared to adult populations (Hugdahl, Carlsson, & Eichele, 2001). In addition, children generally are less efficient in modulating the stimulus-driven ear advantage upon instruction (Hugdahl et al., 2001), which also was true in the present sample. The distribution of right and left ear advantage presented here is congruent with prior findings for the same DL task in a larger reference sample, comparing children and adults (Hugdahl et al., 2001).
4.2. Attentional modulation
The TS children were able to modify ear advantage in response to attentional instructions to about the same degree as the HC children. The ability to shift attention suggests the presence of a normal processing capacity in TS children for this particular task, which is generally acknowledged to be a test of executive functioning (Hugdahl et al., 2003). Impaired executive functioning in samples of TS subjects have been attributed primarily to the inclusion of TS patients who have comorbid ADHD, rather than to the pathophysiology of TS per se (Verte, Geurts, Roeyers, Oosterlaan, & Sergeant, 2005). Moreover, considering the attention-shift condition as a measure of executive and prefrontal functioning, the ability to shift attention in a stimulus-conflict situation may relate to the ability to suppress tics in persons with TS. Children with TS have larger volumes of dorsal prefrontal cortices (Peterson et al., 2001), and these regions have been found to activate strongly during the suppression of tics (Peterson et al., 1998; Stern et al., 2000). Thus, successful execution of strategies for tic suppression and self-regulatory control seems to be related to a functionally intact prefrontal cortex, which has been tested functionally in our study using an attentional shift to the right ear. Hence, children with TS were able to shift their attention to the right ear upon instruction. However, effort scores (NF-FR) were not correlated significantly with the current severity of tics.
4.3. Callosal transfer
The hypothesized impaired transfer of information across the callosum in the TS group was confirmed by demonstrating reduced left ear-performance in the TS subjects who did not have comorbid ADHD or OCD. In the present study, an inverse correlation between CC size and left ear performance was found in the FL attention condition in the TS group, most prominently in individuals with TS who did not have comorbid ADHD or OCD. Left ear performance in the forced-left condition can be regarded as deriving both from the ability to focus attention on the left ear (an ability that requires top-down executive processing), as well as deriving from the intact callosal transfer of auditory information from the right to the left hemisphere for processing (Pollmann et al., 2002). CC size and left ear performance is usually positively correlated (see Reinvang et al., 1994), which we confirmed in our sample of HC children. The inverse correlation, however, of CC size with left ear performance indicated that individuals in the TS group who had a smaller CC had better left ear performance. This latter finding may be understood as reflecting the influences of executive control on left ear performance in this condition, especially given that the ability to shift attention to the left ear was better in those individuals with TS who have a smaller CC.
In contrast to prior findings from a much larger sample of children with TS (Plessen et al., 2004), overall CC size in the present study was not smaller in the TS group. Nevertheless, reduced left ear performance in the TS group suggests the presence of impaired interhemispheric transfer of information in TS children that is independent of a generally normal size of the CC in this group.
A plastic reorganization of the CC in individuals with TS has been suggested to be a consequence of activity-dependent plastic modulation of the morphology of the CC that enhances the functions of frontal cortices that attenuate the severity of tics (Plessen et al., 2004; Spessot, Plessen, & Peterson, 2004). CC fibers themselves are primarily glutamatergic, yet via connection to inhibitory GABAergic interneurons (Carr & Sesack, 1998), reduced activity within axons of the CC may reduce cortical inhibition, in line with the increasingly recognized inhibitory characteristics of callosal functioning (Duque et al., 2005). Reduction in the number of interhemispheric axons in the TS group would therefore produce an overall net increase in activity of executive control centers within the frontal cortices, thus providing greater cortical reserve for the attenuation of tic symptoms (and the here tested attentional modulation).
5. Conclusion
We did not find evidence for altered brain lateralization in boys with TS. In addition, individuals in the TS group were able to modulate their ear advantage through instruction-driven attentional shifting to the right side, and thus they did not evidence problems with executive functioning. Nevertheless, left ear performance was impaired in the TS group when excluding subjects who had comorbid illnesses. The normal, positive correlation of CC size with left ear performance in the FL attention condition, which has been documented previously in healthy populations and which we demonstrated in our healthy controls, was reversed in children with TS. These correlations likely reflect altered callosal interhemispheric processing in children with TS, possibly as a consequence of the previously postulated, plastic reorganization of the CC that may facilitate modulation of tic severity within prefrontal cortices. Clearly this possibility must be borne out in future studies of the CC and cortex in larger numbers of TS children with and without comorbid ADHD.
Acknowledgments
We thank Tore Wentzel-Larsen, Centre for Clinical Research, Haukeland University Hospital, Bergen, Norway for statistical advice and Anne Øfsthus, Liv Heldal, Department of Biological and Medical Psychology, UiB, as well as Martin Ystad, Department of Biomedicine, UiB for technical assistance. This work was financially supported through grants from the Center for Child and Adolescent Mental Health, University of Bergen, Norway, the Dedichsen fond and the Nyquist legat to Kerstin Plessen, NIMH grants MH59139, MH068318, K02-74677 to Bradley Peterson, and grants from the Research Council of Norway, Oslo, and from the Health Authority of Western Norway, Bergen, to Kenneth Hugdahl.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; 1994. [Google Scholar]
- Arndt S, Cohen G, Alliger RJ, Swayze VW, II, Andreasen NC. Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Research. 1991;40(1):79–89. doi: 10.1016/0925-4927(91)90031-k. [DOI] [PubMed] [Google Scholar]
- Banich M. Interaction between the hemispheres and its implications for the processing capacity of the brain. In: Hugdahl K, Davidson RJ, editors. The asymmetrical brain. Cambridge, MA: MIT Press; 2003. pp. 261–302. [Google Scholar]
- Baumgardner TL, Singer HS, Denckla MB, Rubin MA, Abrams MT, Colli MJ, et al. Corpus callosum morphology in children with Tourette syndrome and attention deficit hyperactivity disorder. Neurology. 1996;47(2):477–482. doi: 10.1212/wnl.47.2.477. [DOI] [PubMed] [Google Scholar]
- Bryden MP. Correlates of the dichotic right-ear effect. Cortex. 1988;24(2):313–319. doi: 10.1016/s0010-9452(88)80039-x. [DOI] [PubMed] [Google Scholar]
- Bryden MP, Munhall K, Allard F. Attentional biases and the right-ear effect in dichotic listening. Brain and Language. 1983;18(2):236–248. doi: 10.1016/0093-934x(83)90018-4. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Callosal terminals in the rat prefrontal cortex: Synaptic targets and association with GABA-immunoreactive structures. Synapse. 1998;29(3):193–205. doi: 10.1002/(SICI)1098-2396(199807)29:3<193::AID-SYN1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cerebral Cortex. 2005;15(5):588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Goodman W, Price L, Rasmussen S, Mazure C, Fleischmann R, Hill C, et al. The Yale-brown obsessive compulsive scale. I. Development, use, and reliability. Archives of General Psychiatry. 1989;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Hugdahl K. Dichotic listening in the study of auditory laterality. In: Hugdahl K, Davidson RJ, editors. The asymmetrical brain. Cambridge: MIT Press; 2003. pp. 441–475. [Google Scholar]
- Hugdahl K, Andersson L. The “forced-attention paradigm” in dichotic listening to CV-syllables: A comparison between adults and children. Cortex. 1986;22(3):417–432. doi: 10.1016/s0010-9452(86)80005-3. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Carlsson G, Eichele T. Age effects in dichotic listening to consonant-vowel syllables: Interactions with attention. Developmental Neuropsychology. 2001;20(1):445–457. doi: 10.1207/S15326942DN2001_8. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Rund BR, Lund A, Asbjornsen A, Egeland J, Landro NI, et al. Attentional and executive dysfunctions in schizophrenia and depression: Evidence from dichotic listening performance. Biological Psychiatry. 2003;53(7):609–616. doi: 10.1016/s0006-3223(02)01598-6. [DOI] [PubMed] [Google Scholar]
- Journal of the American Academy of Child and Adolescent Psychiatry. 2005 Instructions for the authors from http://www.jaacap.com.
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children—present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of American Academy for Child Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kimura D. Some effects of temporal-lobe damage on auditory perception. Canadian Journal of Psychology. 1961;15:156–165. doi: 10.1037/h0083218. [DOI] [PubMed] [Google Scholar]
- Kimura D. Functional asymmetry of the brain in dichotic listening. Cortex. 1967;3:163–168. [Google Scholar]
- Leckman JF. Tourette’s syndrome. Lancet. 2002;360(9345):1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle M, Hardin M, Ort S, Swartz K, Stevenson J, et al. The Yale global tic severity scale: Initial testing of a clinician-rated scale of tic severity. Journal of American Academy for Child Adolescent Psychiatry. 1989;28(4):566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson W, Belanger A, Weissman M. Best estimate of lifetime psychiatric diagnosis: A methodological study. Archives of General Psychiatry. 1982;39(8):879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Margolis A, Donkervoort M, Kinsbourne M, Peterson BS. Interhemispheric connectivity and executive functioning in adults with Tourette syndrome. Neuropsychology. 2006;20(1):66–76. doi: 10.1037/0894-4105.20.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Taylor L, Sperry RW. Lateralized suppression of dichotically presented digits after commissural section in man. Science. 1968;161(837):184–186. doi: 10.1126/science.161.3837.184. [DOI] [PubMed] [Google Scholar]
- Moriarty J, Varma A, Stevens J, Fish M, Trimble M, Robertson M. A volumetric MRI study of Gilles de la Tourette’s syndrome. Neurology. 1997;49(2):410–415. doi: 10.1212/wnl.49.2.410. [DOI] [PubMed] [Google Scholar]
- Mostofsky S, Wendlandt J, Cutting L, Denckla M, Singer H. Corpus callosum measurements in girls with Tourette syndrome. Neurology. 1999;53(6):1345–1347. doi: 10.1212/wnl.53.6.1345. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Leckman J, Duncan J, Wetzles R, Riddle M, Hardin M, et al. Corpus callosum morphology from magnetic resonance images in Tourette’s syndrome. Psychiatry Research. 1994;55(2):85–99. doi: 10.1016/0925-4927(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Riddle MA, Cohen DJ, Katz LD, Smith JC, Hardin MT, et al. Reduced basal ganglia volumes in Tourette’s syndrome using three-dimensional reconstruction techniques from magnetic resonance images. Neurology. 1993;43(5):941–949. doi: 10.1212/wnl.43.5.941. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Archives of General Psychiatry. 1998;55(4):326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman J, et al. Regional brain and ventricular volumes in Tourette syndrome. Archives of General Psychiatry. 2001;58(5):427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, et al. Basal Ganglia volumes in patients with gilles de la Tourette syndrome. Archives of General Psychiatry. 2003;60(4):415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- Plessen KJ, Wentzel-Larsen T, Hugdahl K, Feineigle P, Klein J, Staib LH, et al. Altered interhemispheric connectivity in individuals with Tourette’s disorder. American Journal of Psychiatry. 2004;161(11):2028–2037. doi: 10.1176/appi.ajp.161.11.2028. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Maertens M, von Cramon DY, Lepsien J, Hugdahl K. Dichotic listening in patients with splenial and nonsplenial callosal lesions. Neuropsychology. 2002;16(1):56–64. doi: 10.1037//0894-4105.16.1.56. [DOI] [PubMed] [Google Scholar]
- Rauch R, Jinkins J. Variability of corpus callosal area measurements from midsagittal MR images: Effect of subject placement within the scanner. American Journal of Neuroradiology. 1996;17(1):27–28. [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. The R foundation for statistical computing; Vienna, Austria: 2003. [Google Scholar]
- Reinvang I, Bakke S, Hugdahl K, Karlsen NR, Sundet K. Dichotic listening performance in relation to callosal area on the MRI scan. Neuropsychology. 1994;8:445–450. [Google Scholar]
- Scahill L, Riddle M, McSwiggin-Hardin M, Ort S, King R, Goodman W, et al. Children’s Yale-brown obsessive compulsive scale: Reliability and validity. Journal of American Academy for Child Adolescent Psychiatry. 1997;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Singer H, Reiss A, Brown J, Aylward E, Shih B, Chee E, et al. Volumetric MRI changes in basal ganglia of children with Tourette’s syndrome. Neurology. 1993;43(5):950–956. doi: 10.1212/wnl.43.5.950. [DOI] [PubMed] [Google Scholar]
- Spessot AL, Plessen KJ, Peterson BS. Neuroimaging of developmental psychopathologies: the importance of self-regulatory and neuroplastic processes in adolescence. Annals of the New York Academy of Sciences. 2004;1021:86–104. doi: 10.1196/annals.1308.010. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests. New York: Oxford University press; 1991. [Google Scholar]
- SPSS. SPSS Base 10. 0 for Windows User’s Guide. Chicago, IL: SPSS Inc; 1999. [Google Scholar]
- StatSoft. Statistica (data analysis software system), version 6. StatSoft Inc.; 2003. http://www.statsoft.com. [Google Scholar]
- Stern E, Silbersweig DA, Chee KY, Holmes A, Robertson MM, Trimble M, et al. A functional neuroanatomy of tics in Tourette syndrome. Archives of General Psychiatry. 2000;57(8):741–748. doi: 10.1001/archpsyc.57.8.741. [DOI] [PubMed] [Google Scholar]
- Verte S, Geurts HM, Roeyers H, Oosterlaan J, Sergeant JA. Executive functioning in children with autism and Tourette syndrome. Development and Psychopathology. 2005;17:415–445. doi: 10.1017/s0954579405050200. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WISC-III Manual. Toronto: Psychological Corporation; 1996. [Google Scholar]
- Yazgan M, Peterson B, Wexler B, Leckman J. Behavioral laterality in individuals with Gilles de la Tourette’s syndrome and basal ganglia alterations: A preliminary report. Biological Psychiatry. 1995;38:386–390. doi: 10.1016/0006-3223(94)00302-J. [DOI] [PubMed] [Google Scholar]



