Abstract
Findings from previous magnetic resonance imaging studies of sex differences in gray matter have been inconsistent, with some showing proportionally increased gray matter in women and some showing no differences between the sexes. Regional sex differences in gray matter thickness have not yet been mapped over the entire cortical surface in a large sample of subjects spanning the age range from early childhood to old age. We applied algorithms for cortical pattern matching and techniques for measuring cortical thickness to the structural magnetic resonance images of 176 healthy individuals between the ages of 7 and 87 years. We also mapped localized differences in brain size. Maps of sex differences in cortical thickness revealed thicker cortices in women in right inferior parietal and posterior temporal regions even without correcting for total brain volume. In these regions, the cortical mantle is up to 0.45 mm thicker, on average, in women than in men. Analysis of a subset of 18 female and 18 male subjects matched for age and brain volume confirmed the significance of thicker gray matter in temporal and parietal cortices in females, independent of brain size differences. Further analyses were conducted in the adult subjects where gender differences were evaluated using height as a covariate, and similar sex differences were observed even when body size differences between the sexes were controlled. Together, these results suggest that greater cortical thickness in posterior temporal inferior parietal regions in females relative to males are independent of differences in brain or body size. Age-by-sex interactions were not significant in the temporoparietal region, suggesting that sex differences in these regions are present from at least late childhood and then are maintained throughout life. Male brains were larger than female brains in all locations, though male enlargement was most prominent in the frontal and occipital poles, bilaterally. Given the large sample and the large range of ages studied, these results help to address controversies in the study of central nervous system sexual dimorphisms.
Keywords: brain size, gender, gray matter, MRI, parietal lobes, temporal lobes
Introduction
On average, men have larger brains than women, a sex difference that cannot be explained entirely by differences in body size (Peters and others 1998). Although brain size is highly variable across subjects, and many women have larger brains than do many men, substantial interest in sex differences in neural structures has been generated, in part, by observations of sex differences in cognitive functions (Kimura 2000). A male advantage for spatial abilities has been observed widely in humans and other animals (Jones and others 2003). This advantage may be specific to cognitive functions geared toward simultaneous or holistic processing (Halpern and Tan 2001), and it is exemplified in tasks such as mental rotation (Roberts and Bell 2000). A female advantage has been noted for verbal abilities such as verbal fluency and verbal memory (Sommer and others 2004), perhaps because these tasks require sequential processing, which is thought to afford performance advantages to women (Halpern and Tan 2001). Given the apparent specificity of differences in male and female cognitive advantages, and regional specificity of brain–behavior relationships, global differences in brain size between the sexes that have been readily observed with relatively gross methods might not be the most relevant structural dimorphism when investigating neural substrates of sex differences in cognition.
Quantitative magnetic resonance imaging (MRI) studies of intracranial gray matter volumes have yielded inconsistent findings in adults. Some show that females actually have increased total gray matter relative to males after controlling for the overall increase in male brain size (Gur and others 1999; Goldstein and others 2001; Lemaitre and others 2005); whereas others report no sex difference in total gray matter volume in adults after controlling for brain size (Filipek and others 1994; Blatter and others 1995; Courchesne and others 2000; Good and others 2001b; Ge and others 2002); yet others show decreased gray matter in women relative to men after brain size correction (Resnick and others 2000; Sullivan and others 2004). Regional differences in cortical volumes across the sexes have shown that women have increased gray matter (controlling for overall brain size) compared with men in frontal (Goldstein and others 2001; Gur and others 2002), parietal (Nopoulos and others 2000; Allen and others 2003), and occipital (Allen and others 2003) cortices. Studies measuring cortical thickness and gray matter density have also shown proportional local increases in gray matter in women, primarily in the parietal lobes (Good and others 2001a; Luders and others 2006; Narr and others 2005; Im and others 2006) and temporal lobes (Luders and others 2006; Im and others 2006). Thus, whereas a consensus of studies of total gray matter volume have tended to show no sex differences, the regional volumetric and gray matter distribution patterns tend to show an enlargement in females when controlling for brain size.
In children, postmortem studies have shown larger brain sizes in males emerging during the first 4 or 5 years of life (Dekaban 1978), findings that have been confirmed in numerous developmental imaging studies (Caviness and others 1996; Giedd and others 1997, 1999; Courchesne and others 2000; De Bellis and others 2001; Sowell, Trauner, and others 2002). Without correcting for overall brain size, some studies have reported that volumes of cortical gray matter are increased in boys relative to girls (Caviness and others 1996; Giedd and others 1999; Courchesne and others 2000), and some have shown no differences across sexes (De Bellis and others 2001; Sowell, Trauner, and others 2002). Similar to findings in the adult literature, when controlling for the enlargement of the male brain, findings of sex differences in gray matter volume in children have been inconsistent, with some reporting no sex difference (De Bellis and others 2001), and some showing enlargement in females, most prominently in the medial and lateral temporal cortices (Sowell, Trauner, and others 2002).
Interactions between age and sex in measures of overall and regional brain volumes are also of interest in studies of development and aging, particularly given the robust differences between the sexes in levels of steroid hormones and in cognitive functions that emerge across the life span. Few studies have explicitly examined sex differences in the correlations of gray matter volumes with age in children. One prominent study from Giedd and others suggested that nonlinear age-related changes in absolute volumes of gray matter may peak earlier in girls than boys across most of the brain regions evaluated (Giedd and others 1999), although the nonlinear (quadratic) interaction of sex with age in gray matter volumes was not statistically significant, indicating that gray matter changes with age actually progress at similar rates in boys and girls. Similar results of nonsignificant statistical interactions of age with sex in children and young adults were reported by Pfefferbaum and others (1994). Others have reported that maturation of gray matter proceeds more rapidly in boys than in girls (De Bellis and others 2001), as evidenced by significant age-by-sex interactions.
The interactions of sex with age in studies of brain volume have been more widely examined in adults and the elderly. As with the studies in children, findings have been inconsistent. Many have reported more prominent age effects on gray matter structures in men (Coffey and others 1998; Xu and others 2000; Raz and others 2004; Sullivan and others 2004; DeCarli and others 2005). Raz and others have shown that sex differences were more prominent in younger than in older adults (Raz and others 2004), but their findings did not support the hypothesis that the effect of aging is accelerated in men (Raz and others 1997). In a longitudinal study from the same group, no age-by-sex interactions for cortical volumes were observed (Raz and others 2005). Other investigators have also failed to find age-by-sex interactions in adult and elderly populations (Salat and others 2004; Lemaitre and others 2005). One group reported accelerated volume loss in men in the frontal and temporal lobes but accelerated loss in women in the parietal lobes (Murphy and others 1996).
Differences in findings between studies may stem from differences in the age range of the subjects evaluated. Although sex differences in volumes of cortical gray matter have been studied in children and adults, no study to our knowledge has thus far investigated sex differences in cortical thickness across the human life span from young childhood to old age. This is the focus of the current report, in which we apply methods for cortical pattern matching and measure cortical thickness in a group of 176 normal individuals aged 7–87 years. Because our methods ensure that anatomically homologous points are matched across the entire surface of each brain hemisphere, we were able to evaluate cortical thickness in millimeters without scaling brain data sets into a standard template space. To determine whether differences in cortical thickness between sexes were independent of the larger volumes of the male brains, we also assessed differences in cortical thickness in a subset of 18 female subjects who were closely matched, subject by subject, to 18 male subjects for age and total brain volume. Given our large sample size across such a wide age range, we also assessed age-by-sex interactions in cortical thickness across the cerebrum. Generally, we expected thicker cortices in females, given our previous findings from independent samples using similar methods for matching cortical patterns (Luders and others 2006; Narr and others 2005). Additionally, we investigated localized sex differences in brain size to assess regional specificity in the male pattern of brain enlargement.
Methods
Subjects
Brain imaging data were collected from 176 normal control subjects 7–87 years of age. Eighty-six women were studied (mean age 33.9 years, standard deviation [SD] = 22.3; 83 were right handed) and 90 men (mean age 31.0 years, SD = 21.3; 79 were right handed). The same subjects were studied in our previous assessment of the age correlates of gray matter density across the lifespan (Sowell, Peterson, and others 2003), although sex effects were not evaluated in that prior report. All subjects were recruited from community households randomly selected from a tele-marketing database. Subjects were excluded from participation if they had a history of concussion, substance abuse, or seizure disorder. Further, subjects were thoroughly screened for neurological impairments, psychiatric illness, history of learning disability, or developmental delay using a structured diagnostic interview either administered or reviewed by a board-certified child and adult psychiatrist (B.S.P.). Informed consent was obtained from all subjects and for the children from their parents as well.
MRI Scan Acquisition
All subjects were scanned (by B.S.P.) with a single 1.5-T superconducting magnet (Signa; General Electric, Milwaukee, WI) located at Yale University. The MRI protocol collected was a whole-brain spoiled gradient recalled acquisition in the steady state T1-weighted series collected in the sagittal plane with repetition time = 24 ms, echo time = 5 ms, number of excitations = 2, flip angle = 45 degrees, field of view of 30 cm, 124 slices with section thickness of 1.2 mm, and no gaps.
Image Processing
Details of the image analysis procedures have been described previously (Sowell, Thompson, and others 2001, 2003; Sowell, Thompson, Mattson, and others 2002; Sowell and others 2004; Thompson and others 2004). Briefly, the magnetic resonance images from each individual were analyzed with a series of manual and automated procedures that included 1) transforming brain volumes into a standardized 3-dimensional (3D) coordinate space (Mazziotta and others 1995) using a 12-parameter, linear, automated image registration algorithm (Woods and others 1998); 2) Semiautomated tissue segmentation was conducted for each volume data set to classify voxels based on signal intensity as most representative of gray matter, white matter, or cerebrospinal fluid (CSF) (Sowell and others 1999); 3) removing nonbrain tissue (i.e., scalp, orbits) and cerebellum and excluding the left hemisphere from the right; 4) automatically extracting the cortical surface of each hemisphere, which was represented as a high-resolution mesh of 131 072 triangulated elements spanning 65 536 surface points in each hemisphere (MacDonald and others 1994); 5) tracing 35 sulcal and gyral landmarks on the lateral and interhemispheric surfaces of each hemisphere; 6) transforming image volumes back into their own native image acquisition space by mathematically inverting the transformation that took them into standard space (step (1) above); 7) spatially registering with a rigid-body 6-parameter transformation all segmented images and brain surfaces for each individual by defining 80 standardized, manually defined anatomical landmarks (40 in each hemisphere, the first and last points on each of 20 of the 35 sulcal lines drawn in each hemisphere) (Sowell, Thompson, Rex, and others 2002; Sowell, Peterson, and others 2003); 8) measuring cortical thickness in millimeters averaged within a 15-mm sphere attached to each point on the cortical surface (see below); and 9) estimating relative local brain growth measured at each cortical surface point in each hemisphere (see below) (Sowell and others 2004).
Image analysts (J.Y. and E.K.) who were blind to subject sex and age traced each of 17 sulci (Sylvian fissure and central, precentral, postcentral, superior temporal sulcus (STS) main bodies, STS ascending branch, STS posterior branch, primary intermediate sulcus, secondary intermediate sulcus, inferior temporal, superior frontal, inferior frontal, intraparietal, transverse occipital, olfactory, occipitotemporal, and collateral sulci) on the lateral brain surface in each hemisphere of each subject’s brain. An additional set of 12 sulci were outlined on each interhemispheric surface (callosal sulcus, inferior callosal outline, superior rostral sulcus, inferior rostral sulcus, paracentral sulcus, anterior, and posterior segments of the cingulate sulcus, outer segment double parallel cingulate sulcus when present, parieto-occipital sulcus, anterior and posterior segments of the calcarine sulcus, and the subparietal sulcus). In addition to contouring the major sulci, a set of 6 midline landmark curves bordering the longitudinal fissure were outlined in each hemisphere to establish hemispheric gyral limits. Spatially registered grayscale image volumes in coronal, axial, and sagittal planes were available simultaneously to help clarify brain anatomy. We have developed detailed criteria for delineating the cortical lines and for starting and stopping points for each sulcus using brain surface atlases as references (Ono and others 1990; Duvernoy and others 1991). These criteria have been described previously along with reliability measures (Sowell, Thompson, Rex, and others 2002). Complete details of the written anatomical protocol can be obtained from the authors.
Gray matter thickness was calculated using the Eikonal fire equation (Sapiro 2001; Thompson and others 2004). Although the brain image volumes acquired for this study had voxel dimensions of approximately 1 × 1 × 1.2 mm, we supersampled the image data to create voxel dimensions of 0.33 mm3. The 3D Eikonal equation was applied only to voxels that segmented as gray matter, and a smoothing kernel was used to average gray matter thickness within a 15-mm sphere at each point on the cortical surface. This allowed us to calculate cortical thickness for each subject with an effective resolution much finer than the original voxel size, given that the error associated with localizing anatomy on the inner and outer cortical surfaces was averaged with the unbiased error of all other voxels within the smoothing kernel. Points on the cortical surfaces surrounding and between the sulcal contours drawn on each individual’s brain surface were calculated using the averaged sulcal contours as anchors to drive 3D cortical surface mesh models from each subject into correspondence using fluid warping parameters described in more detail in another report (Thompson and others 2004). This allows the creation of average surface models and the creation of maps of sex differences on gray matter thickness or local brain size. To map gray matter thickness onto the surface rendering of each subject, the coordinate for each point on the brain surface for each individual (anatomically matched across individuals) was mapped to the same anatomical location in their ‘thickness’ volume, and the average gray matter thickness value within the 15-mm sphere was calculated. The average gray matter thickness value within the sphere was then doubled to estimate the maximum thickness at each point on the cortical surface. In a previous report, we helped to establish the validity of these methods by showing close regional correspondence between the cortical thickness maps created for normally developing children in vivo (Sowell and others 2004) and for the 1929 postmortem data of Von Economo (Von Economo 1929). In our earlier report (Sowell and others 2004), we also assessed test–retest reliability of cortical thickness measures among individuals with 2 image volumes acquired at short intervals and showed maximal error estimates of 0.15 mm.
Local sex differences in brain size were also assessed using the ‘distance from the center of the hemisphere’ (DFC-H) measure (Sowell and others 2004). DFC-H is a measure of radial expansion calculated in millimeters for the distance from the group average center of mass within each hemisphere (in the nonscaled image data) to each of the 65 536 matched hemisphere surface points in each individual.
Statistical Analyses
After the basic preprocessing steps were conducted for each individual, total brain volume, total gray matter volume, total white matter volume, and total CSF volume were calculated for each individual from the segmented volumes. Statistical significance of sex differences in the total volumes was assessed with t-tests.
Statistical maps of differences between sexes were created for gray matter thickness and DFC-H for the entire sample. In these analyses, the correlation (Pearson’s r) between group membership (i.e., male or female) and gray matter thickness or DFC-H at each point on the brain surface was calculated for the comparison of males with females. A significance threshold of P = 0.05 was used to illustrate local changes in gray matter thickness or DFC-H at each point on the cortical surface.
Statistical maps were also generated to evaluate age-by-sex interactions and the effects of sex when body height was statistically controlled. To evaluate the age-by-sex interaction, analysis of variance was used to compare a full model, which included sex-by-age and sex-by-age2 interactions, with a reduced model that did not include these interactions. The main effects of sex, age, and age2 were included in both of these linear models. F-ratios were computed at each point on the cortical surface and were converted to uncorrected P values. An uncorrected threshold of P = 0.05 was used to illustrate the regions where interactions between sex and one or both of the age terms (age or age2) should be considered. Similar analyses were conducted to hold body height constant while evaluating sex differences.
The statistical maps (uncorrected) are crucial for allowing us to visualize the spatial patterns of sex difference and age-by-sex interactions in gray matter thickness and brain size. Nevertheless, permutation methods (Bullmore and others 1999) were used to assess the significance of the statistical maps of the main effects of sex on cortical thickness and brain size and to correct for multiple comparisons (i.e., for the statistical tests at each of 65 536 surface points in each hemisphere) as follows. Nine coarse regions of interest (ROIs) for each hemisphere were created for each individual from a probabilistic atlas (Evans and others 1996) for the frontal lobe (ventral and dorsal regions separated by an axial plane passing through the intersection of the posterior extent of the inferior frontal sulcus and the precentral sulcus in each hemisphere), parietal lobe, temporal lobe, and occipital lobe by transforming the probabilistic ROIs from standardized space back into the resliced space of each individual using an automated 12-parameter affine transformation (Woods and others 1998). The new ROIs for all individuals were then averaged to create regional masks, and the ventral and dorsal frontal, parietal, and occipital ROIs were separated into medial and lateral regions. In the permutation analyses, subjects were randomly assigned to sex groups for 10 000 new correlation analyses at each surface point in each ROI, and the number of significant results (i.e., gray matter thickness or DFC-H at any surface point that significantly differed between sexes at the threshold of P = 0.05) that occurred in the real test for group differences was compared with the null distribution of significant results that occurred by chance. In other words, the threshold for assessing the significance of statistical maps based on the permutation tests (within each ROI) was determined objectively by calculating the surface area (number of surface points) of significant effects in the real test of group differences. That surface area within any tested ROI was used as the threshold for comparison with the random tests for that ROI, and if fewer than 5% (i.e., P < 0.05) of the results from random tests reached or exceeded the surface area of the real test, the statistical map (within ROIs) was considered significant.
It is not possible to perform an exact permutation test that separates the effects of sex-by-age interactions from the main effects of sex and age (given that by definition, the main effects of sex, age, and age2 must be held constant). The same is true for the evaluation of sex differences when body height is also included in the equation. However, approximate permutation tests, based on permutation of the residuals of these statistical models, have been described and validated (Freedman and Lane 1983; Anderson and Legendre 1999; Anderson and Ter Braak 2003). While holding constant the portions of variance in cortical thickness that are attributed by the reduced model to sex, age, and age2, the associated residual variances in thickness not attributable to these factors were permuted randomly across all subjects irrespective of sex or age. The new set of observations generated by combining the permuted contribution to cortical thickness with the nonpermuted contribution associated with the main effects of sex and age was then analyzed using the same statistical model as was used to analyze the original data. By combining a large number of such permutations (N = 10 000), a distribution for the number of brain locations where the uncorrected P value was less than 0.05 was created. Based on this distribution and the number of such points observed in the original data, a P value was assigned for each of the same 9 ROIs (per hemisphere) described above for permutation of the main effects of sex. Despite the fact that only residuals were permuted, this analysis was analogous to the exact permutation testing performed for the main effects of sex under the assumption of no significant age-by-sex interactions. Similar permutation analyses were conducted to evaluate the effects of sex when height was residualized out.
Results
Volume Analyses
As shown in Table 1, males have larger total brain, gray matter, and white matter volumes than do females (P < 0.001 for all). Although the groups did not differ significantly on mean age, we conducted analyses using both age and sex to predict total volumes so as to ensure that volume differences were not attributable to age differences between the groups. The unique predictive value of sex on the total volumes was still significant while controlling for the variance associated with age.
Table 1.
Volumetric results for sex differences in total brain volume, total gray matter, total white matter, and total CSF
| Volume differences
| ||||
|---|---|---|---|---|
| Total volume (cm3) | Gray matter volume (cm3) | White matter volume (cm3) | CSF volume (cm3) | |
| Female | 1355.9 (SD 97.9) | 744.2 (SD 85.3) | 444.3 (SD 62.9) | 164.7 (SD 48.8) |
| Male | 1544.2 (SD 118.8) | 844.9 (SD 84.5) | 518.4 (SD 76.6) | 177.6 (SD 53.3) |
| Sex difference | t = 11.487, P < 0.001 | t = 7.860, P < 0.001 | t = 7.039, P < 0.001 | t = 1.673, P = 0.096 |
Gray Matter Thickness
Maps of differences in cortical thickness between males and females can be seen in Figure 1A. The greatest difference between the sexes occurred in right inferior parietal and posterior temporal regions where the female cortex on average was approximately 0.45 mm thicker than the male cortex in the unscaled brain image data, even without controlling for brain size. A similar, though less robust, pattern of thicker cortices in females was observed in the left posterior temporal and left ventral frontal regions. Statistical maps in Figure 1B show that the difference in cortical thickness between males and females was statistically significant in these regions on a point-by-point basis (uncorrected for multiple comparisons). The cortex of the males was significantly thicker than that of the females only in small regions of the anterior temporal and orbitofrontal regions in the right hemisphere (shown in white in Fig. 1B).
Figure 1.
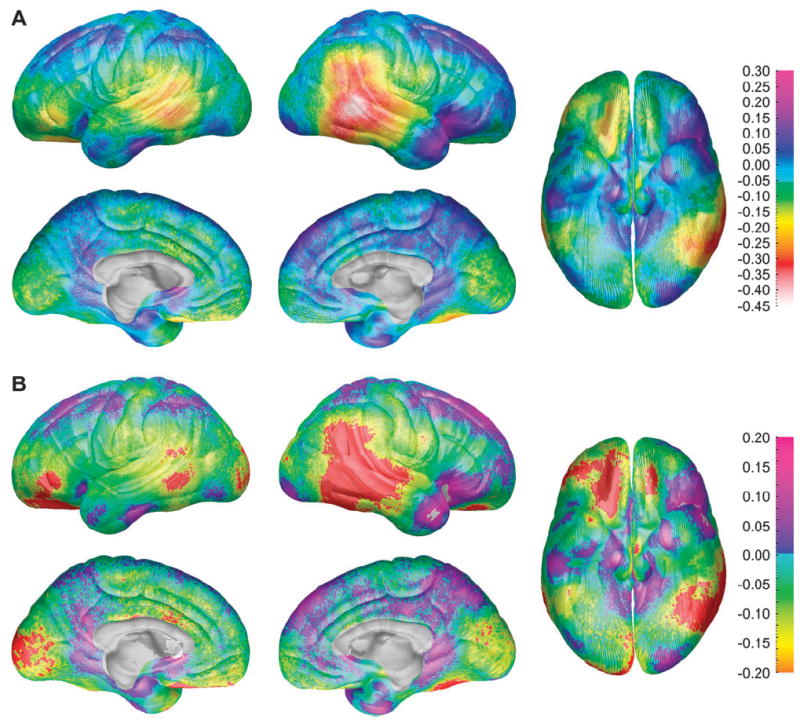
(A) Maps of differences between the sexes in thickness of gray matter (males coded 1, females coded 0 for all maps displayed) for the entire group of 176 subjects showing differences in gray matter (in millimeters) between the male and female subjects according to the color bar on the right. Warmer colors (<0 on the color bar) are regions where gray matter thickness is greater in the female than in the male subjects, and cooler colors (>0) are regions where the males have greater gray matter thickness than the female subjects. Note the approximately 0.45 mm increase in cortical thickness in females in the right posterior temporal lobe. These maps are constructed without any brain scaling, so represent absolute thickness increases in women. (B) Maps of statistically significant differences in gray matter thickness between the sexes for the entire group of 176 subjects according to the color bar on the right (Pearson’s correlation coefficients ranging from −0.2 to 0.2 ranging from orange on the negative end to pink on the positive end). Regions in red correspond to correlation coefficients that show significant increase in gray matter thickness in the female subjects at a threshold of P = 0.05 and those in white correspond to significant increase in the male subjects at a threshold of P = 0.05.
Results of ROI permutation analyses (shown in Table 2) confirm the significance of sex differences in cortical thickness in right lateral parietal (P = 0.048), right lateral temporal (P = 0.024), and left medial occipital (P = 0.017) regions. Female cortices were thicker at trend levels of significance in the left lateral ventral frontal and left lateral occipital regions. Male cortices were not significantly thicker than females in any region in these permutation analyses.
Table 2.
Permutation results for gray matter thickness in the entire group of 176 subjects
| Gray matter sex difference for 176 subjects
| ||
|---|---|---|
| Region | Females thicker
|
|
| L | R | |
| Medial dorsal frontal | 0.171 | 1.00 |
| Medial ventral frontal | 0.157 | 0.349 |
| Medial occipital | 0.017 | 0.066 |
| Medial parietal | 0.162 | 0.382 |
| Lateral dorsal frontal | 0.429 | 0.351 |
| Lateral ventral frontal | 0.068 | 0.251 |
| Lateral occipital | 0.083 | 0.188 |
| Lateral parietal | 0.216 | 0.048 |
| Lateral temporal | 0.208 | 0.024 |
Only the results for female thicker than male are shown separately for each ROI. Male thicker than female results were not significant in any of the ROIs. The numbers presented are P values representing a ratio of the number of random tests that matched or exceeded the number of significant surface points (at P = 0.05) in the real test to the total number of randomizations run (i.e., 10 000).
Because measures of cortical thickness were assessed in the native, unscaled image space without correcting for total brain or total gray matter volume and therefore because subtle differences between groups in overall brain size could have confounded comparisons of cortical thickness, maps of differences between the sexes in cortical thickness were created for the subset of 36 subjects (18 males and 18 females) who had been individually matched for total brain volume and age. Sex differences for total gray and total white matter volumes for the matched subjects were no longer significant in this subsample (P = 0.98 for white matter and P = 0.69 for gray matter). Maps of differences in thickness of gray matter between these matched subjects are shown in Figure 2A, and they demonstrate that even after brain size was controlled, and when total volume of gray matter did not differ between the sexes, females had thicker cortices (up to 0.6 mm) than did males in right lateral frontal, parietal, and temporal cortices. Statistical maps in Figure 2B show the significance of these effects, and permutation results are shown in Table 3. The pattern of results in the subset of matched subjects is similar to that observed in the whole group, and the effects are considerably more robust, despite the reduced sample size.
Figure 2.
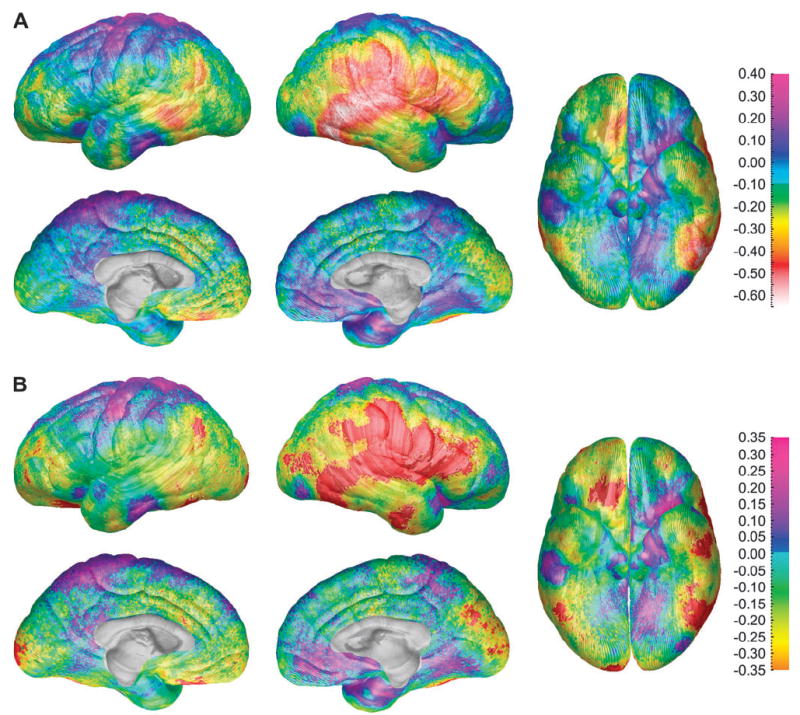
(A) Gray matter thickness sex difference maps for the subgroup of 36 age- and brain volume–matched subjects showing differences in gray matter (in millimeters) between the male and female subjects according to the color bar on the right. Warmer colors (<0 on the color bar) are regions where females have thicker gray matter than the males, and cooler colors (<0) are regions where the males have thicker gray matter than the females. Note the approximately 0.6-mm thicker cortices in females in the right posterior temporal lobe. (B) Statistical differences between the sexes in gray matter thickness for the subgroup of 36 age- and brain volume–matched subjects showing the significance of gray matter thickness differences between the male and female subjects according to the color bar on the right (Pearson’s correlation coefficients ranging from −0.2 to 0.2). Regions overlaid in red correspond to correlation coefficients that show significant increase in gray matter thickness in the female subjects at a threshold of P = 0.05. There were no regions where the male subjects had thicker cortex than the females at a threshold of P = 0.05.
Table 3.
Permutation results for gray matter thickness in the subgroup of 36 subjects matched for age and total brain volume
| Gray matter sex difference for 36 age- and brain volume–matched subjects
| ||
|---|---|---|
| Region | Females thicker
|
|
| L | R | |
| Medial dorsal frontal | 0.251 | 0.385 |
| Medial ventral frontal | 0.278 | 0.349 |
| Medial occipital | 0.129 | 0.065 |
| Medial parietal | 1.00 | 0.382 |
| Lateral dorsal frontal | 1.00 | 0.066 |
| Lateral ventral frontal | 0.111 | 0.006 |
| Lateral occipital | 0.138 | 0.043 |
| Lateral parietal | 0.222 | 0.008 |
| Lateral temporal | 0.309 | 0.010 |
Only the results for female thicker than male are shown separately for each ROI. Male thicker than female results were not significant in any of the ROIs. The numbers presented were calculated in the same way as those presented in Table 2.
Because the effects of differences in body size on cortical thickness between the sexes may be different than the effects of total brain size, we also conducted analyses where we used sex, body height, and sex-by-body height interactions to predict cortical thickness. Unlike brain volume and age, height and age were highly correlated between 7 and 20 years (r = 0.89), and height effects could not be differentiated during this part of the age range. Thus, these analyses were conducted only with adult subjects greater than 20 years of age (n = 119) where the correlation between age and body height was nonsignificant. As shown in Figure 3A, even when body height is controlled, adult females have thicker cortex in left and right inferior parietal (permutation results, P = 0.019 and 0.026, respectively), left and right lateral temporal (permutation results P = 0.0098 and 0.0172, respectively), and left inferior frontal cortices (permutation results P = 0.023).
Figure 3.
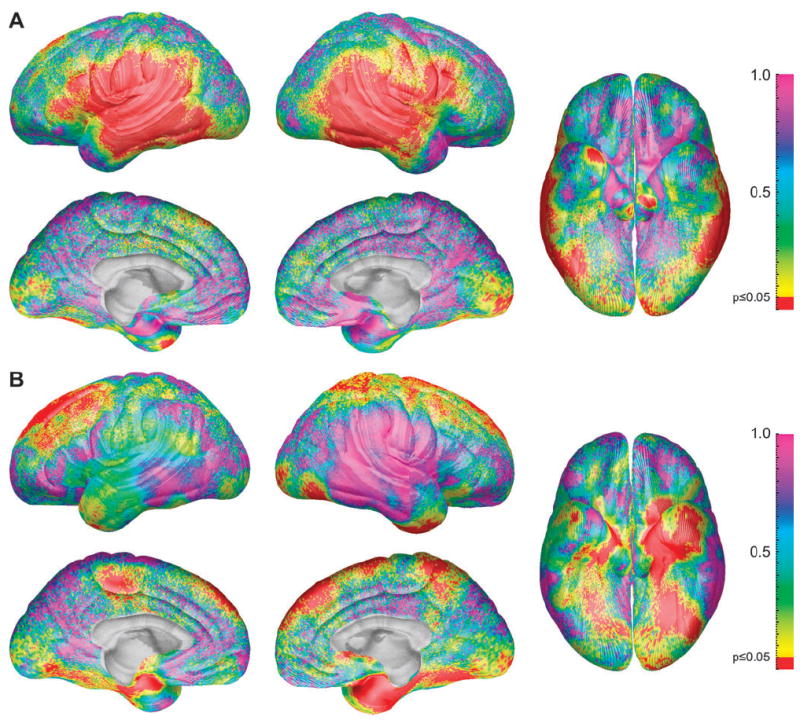
(A) Statistical P maps of the sex difference in cortical thickness in adults (aged >20 years) with height differences partialled out. The map is color coded according to the bar, and regions in red are statistically significant at a P value of 0.05 or less. Regions in pink and blue (i.e., on the dorsal and medial surfaces of the brain) do not approach significance with P values between 0.5 and 1.0. (B) Statistical P maps of the combined interaction terms age by sex and age2 by sex. The map is color coded according to the bar, and regions in red are statistically significant at a P value of 0.05 or less. Regions in pink (i.e., on the lateral surface of the right temporal and parietal lobes) do not approach significance with P values near 1.0.
Because the age range of our subjects was so large, we assessed for the interactions of age or age2 with sex in predicting cortical thickness within the entire sample. As shown in Figure 3B, statistical maps for the interaction were not significant in the temporoparietal cortices where the main effects of sex were largest (shown in Fig. 1A, 2A). This suggests that the thicker female cortices in the temporoparietal region are present from at least late childhood (the youngest age sampled in the present report) and consistent through old age. Statistical maps show significant interactions of sex with age2 in bilateral dorsal frontal and right inferior temporal regions (Fig. 3B). Permutation analyses were significant in both the left (P = 0.01) and right (P = 0.03) dorsal frontal regions, the right temporal region (P = 0.007), and the medial dorsal frontal region in the right hemisphere (P = 0.03). The interactions were somewhat complex to deconstruct, but visual examination of the quadratic curves in the dorsal frontal regions shown in Figure 4 suggests that older women tend to show more linear effects of aging than do men in these regions. In other words, the initial downward slopes are steeper in the males than the females, perhaps supporting the notion that age effects are more prominent in men as suggested in numerous other reports (Coffey and others 1998; Xu and others 2000; Raz and others 2004; Sullivan and others 2004; DeCarli and others 2005). At the very least, we can conclude that sex differences in these regions are not stable throughout the life span.
Figure 4.
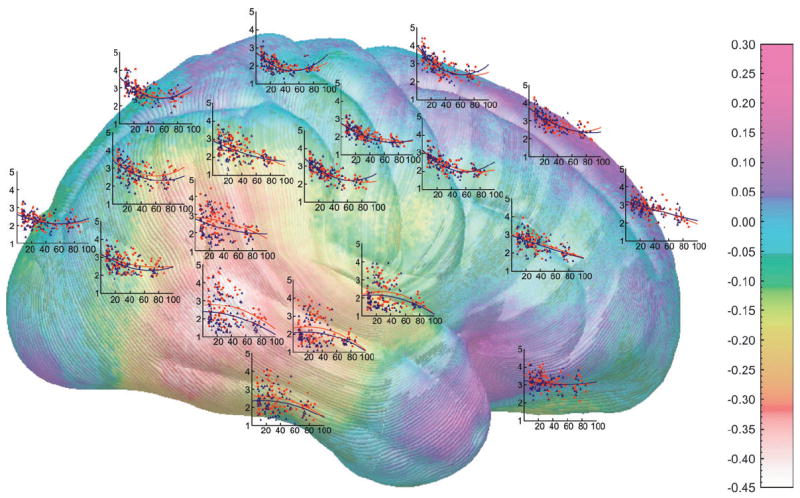
Shown here is the same map as in Figure 1A (right hemisphere) with scatterplots that show how gray matter thickness depends on age, in men and women separately, at various points over the brain surface situated approximately where the measurements were taken. On each of the y axes, cortical thickness is displayed in millimeters and age (7–87 years) is represented on the x axes. The axes are identical for all graphs. Male subjects are represented by blue points and the blue regression lines, and females are represented by the red points and the red regression lines.
Brain Size
Maps of local brain size differences (DFC-H) between males and females can be seen in Figure 5. The greatest difference between the sexes occurs bilaterally in the frontal and occipital poles, where male brains extend on average up to 6 mm beyond the female brains. Statistical maps (not shown) reveal that the difference in DFC-H between males and females is significant in most locations. DFC-H is not significantly greater in females than in males at any location. ROI permutation results were highly significant in all ROIs (P values from 0.0001 to 0.0006) and thus are not shown in tabular format.
Figure 5.
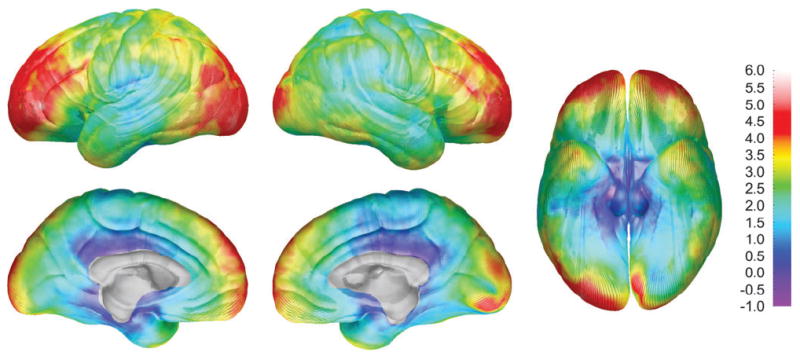
DFC-H sex difference maps showing mean differences in DFC-H (in millimeters) between the male and female subjects according to the color bar on the right. Note the brain size increases up to 6 mm in the male subjects at the frontal and occipital poles bilaterally.
Discussion
This study confirms the presence of regionally specific sex differences in gray matter thickness over the human lifespan. It shows that the cortical ribbon is actually thicker in some brain regions in females, despite the fact that females tend to have smaller bodies, and smaller brains, including smaller overall gray and white matter volumes than do men. The regions in which cortices are most prominently thicker in females than in males are in the right hemisphere association cortices, particularly the temporal and parietal lobes. In these regions, the cortex is up to 0.45 mm thicker in women; permutation analyses confirmed that these effects were not attributable to chance. The nonsignificant age-by-sex interactions in these regions suggest that the sex differences in temporoparietal cortices are stable across the life span. Rather than scaling the imaging data or statistically controlling for overall brain size as other research groups have done, we paired female subjects individually with male subjects matched on age and brain volume in a subset of 36 of the original 176 subjects studied. Notably, sex effects in this subgroup in the temporal and parietal cortices were even more significant than they were in the larger group, confirming that differences in cortical thickness are not mediated by differences in overall brain size. The findings of increased significance in the matched sample, despite the considerably reduced statistical power, may be consistent with findings in other samples showing increases in regional gray matter volumes that emerge only after controlling for overall differences in brain size between the sexes (Gur and others 1999; Goldstein and others 2001).
The pattern of results from analyses where body height was statistically controlled in adult subjects was similar to the pattern of results from simple correlations between sex and cortical thickness in the entire sample and in the brain size–matched sample. That is, independent of body size differences between males and females, females still have thicker cortex than males in numerous lateral cortical regions. Whereas in no case did group differences extend beyond the lateral surfaces of the temporal, parietal, and frontal lobes, variation in the pattern of results occurred when brain volume and height were used to control brain and body size differences between the sexes. Specifically, when the whole group and brain volume–matched group were evaluated, right hemisphere effects were more prominent than left, but when height was controlled in adult subjects, the pattern of results was bilateral. Thus, while height and brain volume are correlated in this sample (r = 0.32), and in other samples (r = 0.55 [Baare and others 2001]), there are apparent differences in the pattern of results whether brain size correction or body size correction is used. Age differences in the different subsets of subjects used for the simple correlation maps, the brain size–corrected maps, and the height-corrected maps could result in the different pattern of results. However, age-by-sex interactions were not significant in the lateral cortices in either hemisphere where gender effects were observed in the various analyses (as shown in Figs 3B and 4). This means that regardless of the age evaluated, gender differences should be comparable, at least in the lateral cortices of the temporal, frontal, and parietal lobes. Inferring actual differences based on qualitative visual comparison of the different maps may be inappropriate anyway given that we did not statistically test the difference between the simple correlation maps and the maps where height or brain volume were controlled.
The regional pattern of increased thickness of cortical gray matter in females is similar to the findings reported in independent young adult samples using similar surface-based methods for studying cortical thickness (Luders and others 2006; Im and others 2006). In both of these previous reports, women were observed to have thicker parietal cortices than men. Our results are consistent with prior volumetric findings of increased parietal lobe gray matter in women (Nopoulos and others 2000; Allen and others 2003) and with findings of greater gray matter density in women detected with voxel-based analyses (Good and others 2001a).Frederikse and others (1999), in contrast, reported increased volumes in inferior parietal lobes in men, although they did not measure gray matter separately, rendering their results difficult to compare with ours. In addition to the parietal lobe effects, we observed large regions of increased cortical thickness in women in the posterior temporal regions, consistent with prior studies that measured cortical thickness directly (Luders and others 2006; Im and others 2006) but not consistent with a prior voxel-based morphometry study (Good and others 2001a). Age differences in the samples assessed could have led to discrepancies in findings. Our findings of nonsignificant age-by-sex interactions in the posterior temporal and inferior parietal regions where the main effects of sex were most robust for cortical thickness suggest that the sex differences in this region are independent of age in our subjects. Methodological differences between studies using conventional volumetric measures, voxel-based morphometry, and measures of cortical thickness using surface-based methods could also have produced inconsistencies in findings. Specifically, surface-based methods are likely to improve anatomical correspondence between subjects and, thus, may provide increased sensitivity to detect group differences in regions where anatomical variability can yield poorly matched anatomy from the signal-based averaging of images that is employed in voxel-based morphometry studies. Further, conventional volumetric studies are limited to evaluating sex differences in regions that can be visually identified and anatomically defined, which may not represent the actual boundaries of regions, such as those identified here that have the largest sex differences.
The cellular bases for the thicker cortices in women compared with men cannot be determined using current in vivo imaging technologies. Nevertheless, the pattern of regional differences in thickness across the sexes may be consistent with postmortem findings of increased neuronal density and increased cortical volumes in the posterior temporal cortex of women (Witelson and others 1995; Harasty and others 1997). Regional cortical thickening in women may also be consistent with the profile of cognitive differences long observed between the sexes, particularly the female advantage on language tasks that may be attributable to their thicker cortices in posterior perisylvian language regions. Gur and others (1999) have suggested that more cortical gray matter in women may provide a computational advantage (compared with possible differences in white matter, which would affect speed of information transfer). This hypothesis may also be consistent with our findings, although we should note that thicker cortices were most prominent in the right hemisphere, which is usually nondominant for language.
A thicker cortex, however, may not necessarily be better than a thinner one. Studies of normal development, for example, have consistently shown cortical thinning to occur with age as part of normal brain maturation (Jernigan and others 1991; Giedd and others 1999; Sowell and others 2004). Thinning of frontal and parietal cortices in normally developing children, moreover, is associated with improvements in performance on language tasks (Sowell, Delis, and others 2001; Sowell and others 2004). Cortical thinning during childhood and adolescence is thought to derive both from progressive changes in myelination (Yakovlev and Lecours 1967; Benes and others 1994) and from regressive changes, such as synaptic pruning (Huttenlocher and de Courten 1987). Both of these cellular changes are thought to improve computational speed and efficiency as redundant synapses are eliminated and oft-used cortical circuits are insulated with myelin. The parietal cortex subserves visuospatial functions, and thus, thinner cortices in men within temporoparietal regions, if it indeed arises from greater pruning and myelination during development and more efficient computational processing, may contribute to the superior visuospatial skills of men. This hypothesis is testable through longitudinal studies in which detailed cognitive assessments would accompany detailed morphological studies. Differences in the rates of cortical maturation between males and females, and relationships between cortical maturation and changes in cognitive function, could be assessed across the cerebrum.
Most of the analyses in this report were focused on sex effects on the entire sample of 176 subjects collapsed across the age range from 7 to 87 years. The nonsignificant interactions of age with sex in regions where sex differences were large (i.e., posterior temporal and inferior parietal regions) suggest that sex effects were stable throughout the lifespan. Interactions between the quadratic age term and sex were significant in bilateral dorsal frontal and temporal regions, indicating that sex differences in cortical thickness vary depending on the age of the participant and that sex must be considered when evaluating age effects in these regions. Furthermore, these age-by-sex interactions could account for discrepancies in reported sex differences in these regions depending on the age composition of the groups in the study. Our findings suggest that the effects of aging may be more prominent in males in dorsal frontal and temporal cortices. However, the combined linear and quadratic interactions were complex and confirm only that sex differences in these regions are not stable over the wide range of ages studied. Our results may be consistent with those of others who have failed to detect age-by-sex interactions in adult populations (Raz and others 1997; Lemaitre and others 2005), although to our knowledge no other reports have evaluated age-by-sex interactions from childhood through old age.
As can be seen in Figure 4, the quadratic regression line curves slightly upward in the oldest subjects. Gray matter is unlikely to actually increase after the eighth decade, rather, the oldest subjects studied were likely not representative of all individuals of that age. In other words, only individuals with above-average cognitive and physical functions were likely capable of participating in the study, and more frail and less cognitively adept subjects did not volunteer. This is probably the case for most studies of normal aging that require on-site participation. Unfortunately, we do not have the data to test this explanation in the current sample. Future studies should include detailed measures of cognitive functioning and health status to help determine whether the most elderly subjects actually differ from their younger counterparts.
We also mapped for the first time differences in brain size between males and females. Permutation analyses showed that male brains are significantly larger than female brains in every region assessed, and our maps showed that the differences are more pronounced at the frontal and occipital poles (up to approximately 6 mm increased DFC-H in males) and less pronounced on the lateral surfaces of the brain at the extremes of the temporal and parietal lobes (up to approximately 2 mm increased DFC-H in males). The possible cognitive correlates of these local size differences may be less transparent than those described for cortical thickness, given that cognitive differences observed between the sexes are not functions typically associated with anterior frontal and occipital lobes.
The regions where the differences in size were smallest are the same locations where sex differences in cortical thickness were most pronounced. Occasionally in imaging studies, artifacts are observed where MRI signal intensity suffers falloff in brain regions that are furthest from the center of the field of view. This signal falloff can affect segmentation of gray and white matters and decrease volumes of measured gray matter in the cortex; therefore, it could perhaps also artificially decrease cortical thickness in these regions in men, whose lateral temporal lobes extend further from the center of the field of view than do the corresponding cortices in women. The regions where male brains are largest, however, are not the regions where sex differences in cortical thickness were statistically significant. More importantly, we conducted sex analyses for cortical thickness in a subgroup of subjects who were carefully matched, male-for-female, on total brain volume and age. The pattern of sex effects on cortical thickness in these analyses agreed with the pattern found in the larger group of subjects, where differences in brain size between sexes were a potential confound. Thus, we are confident that the observed differences in localized cortical thickness are independent of localized differences in brain size.
Acknowledgments
Funding support for this work was provided by National Institute of Mental Health (NIMH) K01 MH01733 and National Institute on Drug Abuse R21 DA015878 and R01 DA017830 awarded to ERS; National Institutes of Health (NIH)/National Center for Research Resources grant P41 RR013642, NIH Roadmap for Medical Research Grant U54 RR021813, and National Institute on Neurological Disorders and Stroke grant NS3753 awarded to AWT; NIH AG016570, LM05639, EB01651, and RR019771 supported PMT; and NIMH MHK02-74677, MH59139, and MH068318 awarded to BSP.
Footnotes
Conflict of Interest: None declared.
References
- Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage. 2003;18:880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Legendre P. An Empirical comparison of permutation methods for tests of partial regression coefficients in a linear model. J Stat Comput Simul. 1999;62:271–303. [Google Scholar]
- Anderson MJ, Ter Braak CJ. Permutation tests for multi-factorial analysis of variance. J Stat Comput Simul. 2003;73:85–113. [Google Scholar]
- Baare WF, Hulshoff Pol HE, Boomsma DI, Posthuma D, de Geus EJ, Schnack HG, van Haren NE, van Oel CJ, Kahn RS. Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex. 2001;11:816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Parker N, Kurth S, Horn SD. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. Am J Neuroradiol. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, Bryan RN. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol. 1998;55:169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Cabanis EA, Vannson JL. The human brain: surface, three-dimensional sectional anatomy and MRI. Vienna, Austria: Springer-Verlag; 1991. [Google Scholar]
- Evans AC, Collins DL, Holmes CJ. Automatic 3D regional MRI segmentation and statistical probabilistic anatomical maps. New York: Academic Press; 1996. [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Frederikse ME, Lu A, Aylward E, Barta P, Pearlson G. Sex differences in the inferior parietal lobule. Cereb Cortex. 1999;9:896–901. doi: 10.1093/cercor/9.8.896. [DOI] [PubMed] [Google Scholar]
- Freedman D, Lane D. A Nonstochastic interpretation of reported significance levels. J Bus Econ Stat. 1983;1:292–298. [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part II: quantitative magnetization transfer ratio histogram analysis. Am J Neuroradiol. 2002;23:1334–1341. [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001a;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001b;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cereb Cortex. 2002;12:998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern DF, Tan U. Stereotypes and steroids: using a psychobiosocial model to understand cognitive sex differences. Brain Cogn. 2001;45:392–414. doi: 10.1006/brcg.2001.1287. [DOI] [PubMed] [Google Scholar]
- Harasty J, Double KL, Halliday GM, Kril JJ, McRitchie DA. Language-associated cortical regions are proportionally larger in the female brain. Arch Neurol. 1997;54:171–176. doi: 10.1001/archneur.1997.00550140045011. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6:1–9. [PubMed] [Google Scholar]
- Im K, Lee JM, Lee J, Shin YW, Kim IY, Kwon JS, Kim SI. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Jones CM, Braithwaite VA, Healy SD. The evolution of sex differences in spatial ability. Behav Neurosci. 2003;117:403–411. doi: 10.1037/0735-7044.117.3.403. [DOI] [PubMed] [Google Scholar]
- Kimura D. Sex and cognition. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- Lemaitre H, Crivello F, Grassiot B, Alperovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage. 2005;26:900–911. doi: 10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Woods RP, Deluca H, Jancke L, Toga AW. Gender effects on cortical thickness and the influence of scaling. Hum Brain Mapp. 2006;27:314–324. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D, Avis D, Evans A. Multiple surface identification and matching in magnetic resonance images. Proc Vis Biomed Comput. 1994;2359:160–169. [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, Schapiro MB, Grady CL, Horwitz B, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53:585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O’Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98:1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD. Atlas of the cerebral sulci. Stuttgart: G. Thieme Verlag; 1990. [Google Scholar]
- Peters M, Jancke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. Unsolved problems in comparing brain sizes in Homo sapiens. Brain Cogn. 1998;37:254–285. doi: 10.1006/brcg.1998.0983. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Bell MA. Sex differences on a mental rotation task: variations in electroencephalogram hemispheric activation between children and college students. Dev Neuropsychol. 2000;17:199–223. doi: 10.1207/S15326942DN1702_04. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sapiro G. Geometric partial differential equations and image analysis. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- Sommer IE, Aleman A, Bouma A, Kahn RS. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127:1845–1852. doi: 10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002;12:856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Rex D, Kornsand D, Tessner KD, Jernigan TL, Toga AW. Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: maturation in perisylvian cortices. Cereb Cortex. 2002;12:17–26. doi: 10.1093/cercor/12.1.17. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging. 2004;25:185–192. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, et al. Mapping cortical change in Alzheimer’s disease, brain development, and schizophrenia. Neuroimage. 2004;23(Suppl 1):S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- Von Economo CV. The cytoarchitectonics of the human cerebral cortex. London: Oxford Medical Publications; 1929. [Google Scholar]
- Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. J Neurosci. 1995;15:3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Xu J, Kobayashi S, Yamaguchi S, Iijima K, Okada K, Yamashita K. Gender effects on age-related changes in brain structure. Am J Neuroradiol. 2000;21:112–118. [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]


