Abstract
Objective
Calcific deposits develop in 20–40% of children with juvenile dermatomyositis (juvenile DM), contributing to disease morbidity and mortality. This study was undertaken to define the structure and composition of these deposits and to characterize their association with chronic inflammation.
Methods
We examined calcific deposits from 5 children with juvenile DM (2 boys and 3 girls). The crystal structure and mineral content of the deposits was analyzed by x-ray diffraction, Fourier transform infrared spectroscopy, and imaging. The protein content of the deposits, following solubilization, was assayed by Western blotting.
Results
All 5 children had both a young age at disease onset (mean ± SD 3.3 ± 1.9 years) and, despite therapy, persistent cutaneous inflammation (mean ± SD duration 81.3 ± 58.7 months). The bone proteins, osteopontin, osteonectin, and bone sialoprotein, were identified in the protein extracts; the only mineral detected was hydroxyapatite, but the tissue was distinct from bone, with an extremely high mineral content and an irregular distribution of mineral.
Conclusion
These results indicate that chronic cutaneous inflammation may contribute to the formation of hydroxyapatite-containing pathologic calcifications in children with juvenile DM.
Pathologic calcifications in soft tissue, similar to those present in calcified heart valves (1), are a complication of several connective tissue diseases, increasing morbidity and mortality (2,3). In contrast to adults with dermatomyositis (DM), in whom calcification is relatively uncommon, it is estimated that 20–40% of children with juvenile DM have calcific deposits (3). In juvenile DM, calcifications are more frequent at anatomic sites that are normally exposed to daily minor trauma but not usually mineralized, such as the elbows or behind the knees. Synchrotron diffraction studies of these deposits have indicated that they contain bone-like hydroxyapatite (4). Although other pathologic calcifications contain osteopontin, osteonectin, and bone sialoprotein, which each have direct effects on hydroxyapatite crystal formation and growth, there is little information pertaining to these proteins in calcifications from children with juvenile DM. This report describes the composition and structure of the calcifications in children with juvenile DM, as determined by x-ray diffraction, Fourier transform infrared spectroscopy and microscopic imaging, and chemical analysis. The impact of clinical and genetic factors on pathologic calcification in juvenile DM is also considered.
PATIENTS AND METHODS
Patient population
Five children (2 boys and 3 girls; all white race) with definite juvenile DM (5) underwent surgical removal of some of their more troublesome calcifications, which were stored at −80°C. Demographic and clinical data on the patients are presented in Table 1. The children provided age-appropriate approval, and their parents signed the institutional review board–approved consent for research.
Table 1.
Demographic and clinical characteristics of the children with juvenile dermatomyositis and pathologic calcifications*
| Patient | Sex | Age at juvenile DM onset, years | Duration of disease at calcification removal, months | Age at calcification removal, years | DAS, skin | DAS, muscle | TNFα –308 | DQA1*0501 |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 2.9 | 78 | 9.3 | 5 | 1.5 | GA | + |
| 2 | F | 1.1 | 155 | 13.8 | 5 | 7 | GA | + |
| 3 | F | 6.2 | 125 | 16.4 | 6 | 0 | GG | − |
| 4 | F | 2.5 | 29 | 4.9 | 4 | 2 | GG | − |
| 5 | M | 4.0 | 20 | 5.6 | 4 | 2 | GG | − |
| Mean ± SD | 3.3 ± 1.9 | 81.3 ± 58.7 | 10.0 ± 5.0 | 4.8 ± 0.8 | 2.5 ± 2.7 |
DAS = Disease Activity Score for children with juvenile dermatomyositis (juvenile DM) (scale of 0–9 for skin and 0–11 for muscle); TNFα = tumor necrosis factor α.
Clinical definitions
The total Disease Activity Score for children with juvenile DM (DAS) (6) was assessed by 1 physician (LMP) at the clinic visit closest to the time of surgery. This validated scoring system rates the active involvement of both skin and muscle (20 points). The separate scoring for skin (9 points) and muscle (11 points) has also been validated (Pachman LM, et al: unpublished observations); for all scores, a higher DAS represents higher disease activity. Disease onset was defined as the time the first definite symptom of juvenile DM (rash or weakness) was recognized. The duration of disease was defined as the interval of time between the first symptom (disease onset) and removal of the calcifications.
Diagnostic testing
Testing for myositis-specific antibodies and myositis-associated antibodies was performed at the laboratory of Dr. Ira Targoff’s (Oklahoma City, OK) and the Children’s Memorial Hospital Immunology Laboratory.
Genetic testing
Primers were synthesized at Invitrogen (Carlsbad, CA). Anticoagulated (EDTA) whole blood was aliquoted and stored at −80°C until DNA isolation was performed using a Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN).
Genetic typing for tumor necrosis factor α (TNFα) –308 and DQA1*0501
The TNFα –308 polymorphism consists of a single-basepair substitution of an A for the more common G. Polymerase chain reaction was used to amplify a 107-bp fragment containing an Nco I restriction site as previously described (7). Digestion with Nco I confirmed the genotype as GG, GA, or GG. DQA1*0501 was identified as previously described (8).
Characterization of pathologic calcifications obtained from children with juvenile dermatomyositis
Western blotting
Tissue samples (~5 gm) from 3 patients were washed with 15% NaCl for 1 hour at 4°C. Lipids were removed, and the extraction procedure was performed at 4°C, with protease inhibitors added to all solutions to minimize protein degradation as previously described (1). Extracted proteins (5 μg) separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis were transferred to a nitrocellulose membrane for Western blot analysis. Monoclonal antibodies (Developmental Studies Hybridoma Bank, Iowa City, IA) were used as primary antibodies to bind the specific antigens, followed by treatment with horseradish peroxidase–conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) combined with enhanced chemiluminescence substrate for detection. Antibodies to osteopontin (MPIIIB10), osteonectin (AON-1), and bone sialoprotein (WVID1) were used. The material extracted from the pathologic calcifications was compared with that from normal bone removed from children during the course of surgery, with informed consent.
X-ray diffraction
Aliquots of lyophilized fresh-frozen tissue from all 5 patients, ground in a liquid nitrogen–cooled freezer mill (Spex, Metuchen, NJ), were analyzed by wide-angle x-ray diffraction using a Bruker AX-8 diffractometer (Bruker, Madison, WI) with Cu Kα radiation. Scans were recorded from 20° to 40° 2θ, the line-width of the 002 peak measured, and c-axis crystallite size determined using manufacturer-supplied software (AXS Topas P, version 2; Bruker).
Infrared spectroscopy and spectroscopic imaging
The infrared spectrum of KBr pellets from deposits in all 5 children (~1 weight percent dry deposit) was obtained using a Thermo-Nicolet 4700 spectrometer (Thermo Electronics, Madison, WI) with a mercury tellurium selenide detector as described elsewhere (9). Aliquots of the fresh-frozen tissue were fixed in 90% ethanol and embedded in polymethyl methacrylate. Sections of the embedded tissue were cut at 2–4-μm thickness and examined by infrared imaging spectroscopy using a Perkin-Elmer (Shelton, CT) spotlight imaging system. Parameters determined were mineral:matrix ratio (a parameter linearly related to ash weight), mineral crystal size and perfection, and collagen maturity (9). Spectra of adult mouse cortical bone were used for comparison. Images were displayed using the same color scale for each parameter unless the values in the sample were >5 times that in the control. In addition to the parameters calculated for the infrared microspectroscopic data, the ratio of the area of the carbonate band (840–890 cm−1) to the phosphate band (~916–1180 cm−1), as an index of carbonate substitution in the mineral, was determined. Multivariate analyses of the images were performed with ISYS software (Spectral Dimensions, Olney, MD).
RESULTS
Clinical data
All 5 children had definite juvenile DM (5), with the characteristic rash, symmetric proximal weakness, and classic perifascicular atrophy with capillary occlusion seen on muscle biopsy, but no visceral involvement. At the time of removal of the calcified deposit, 2 of the patients had normal muscle enzyme levels, despite earlier elevations. All of the children were negative for both myositis-specific and myositis-associated antibodies. Two were positive for both DQA1*0501, and the TNFα –308A allele (Table 1). All patients had had active cutaneous inflammation for a prolonged period (mean ± SD 81.3 ± 58.7 months), with a mean ± SD DAS skin score of 4.8 ± 0.8 (maximum possible score 9).
Bone matrix proteins
Western blot analysis of the extracted calcification samples documented the presence of bone matrix proteins, including the 41-kd osteopontin, 62-kd bone sialoprotein, and 32-kd osteonectin (Figure 1) (molecular sizes similar to those of normal bone proteins). The calcifications contained relatively more osteonectin but less osteopontin than are found in bone.
Figure 1.
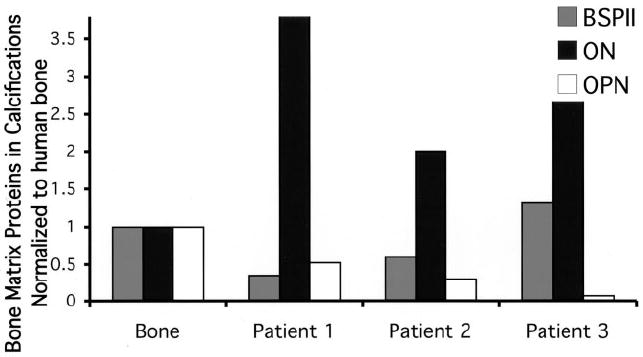
Quantification of the results of Western blot analysis performed on calcific deposits from 3 of the 5 patients with juvenile dermatomyositis, documenting the presence of bone sialoprotein (BSPII), osteonectin (ON), and osteopontin (OPN). Protein concentration is normalized to that in human bone (set at 1).
Crystalline apatite
X-ray diffraction and synchrotron x-ray microdiffraction studies confirmed that the mineral present in the deposits was a poorly crystalline hydroxyapatite. The mean crystal size was comparable with that of bone mineral and smaller than that of highly crystalline hydroxyapatite (Figure 2).
Figure 2.
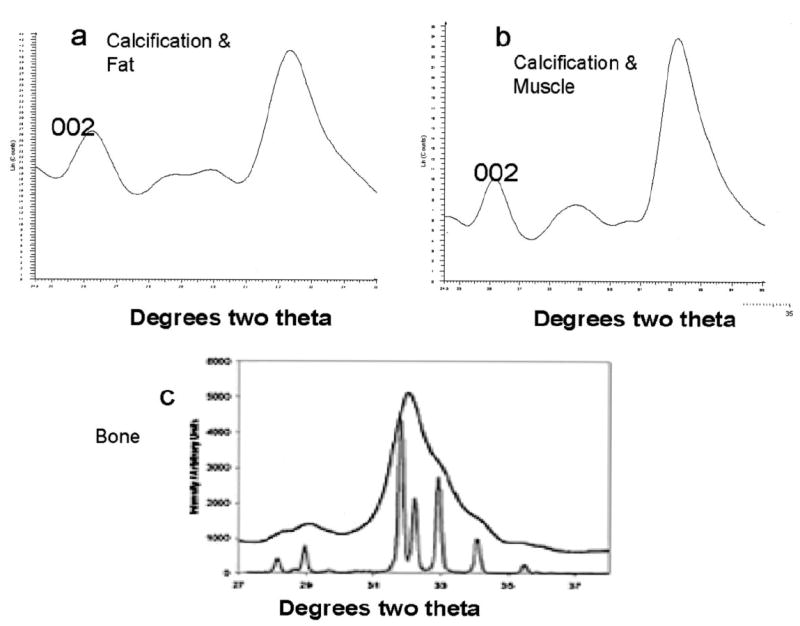
Fat (a) and muscle (b) x-ray diffraction patterns typical of bone hydroxyapatite, in calcific deposits removed from a patient with juvenile dermatomyositis. For comparison, the pattern obtained with highly crystalline hydroxyapatite (sharp lines) is also shown (c). The c-axis length, calculated from line-broadening of the 002 reflection in these patterns, was 18Å in fat and 14Å in the muscle deposit. Bone c-axis lengths range from 15Å to 20Å.
Differences in the qualities of calcified tissue from juvenile DM patients and those of bone
Infrared imaging was used to determine whether the deposits were similar to bone in terms of apatite mineral content as well as matrix composition. Mouse (4 months old) cortical bone, which has a crystal size comparable with that of human bone, was used to image an area of similar size. Figure 3a compares typical spectra from bone and from a calcific deposit. The protein (amide I) band in the deposit was significantly smaller than that in bone, while the mineral bands were comparable in shape as well as area. Figures 3c and d compare the mineral:matrix ratio in the deposit with that in cortical bone. Values for the deposit were shifted markedly to the right, indicating that it had a significantly higher mineral content than bone. The 1660:1690 collagen crosslink ratio, an indicator of collagen maturity (9), was slightly higher in the deposit than in bone, with the maximum values in the center of the deposit rather than on the periphery, as was seen in cortical bone (Figures 3e and f). Crystallinity was slightly lower in the deposit than in bone, and pixel histograms showed that the pixel distribution of crystallinity was much sharper in the deposit (Figures 3g and h). The carbonate:phosphate ratio in the deposit mineral was comparable with that in bone, while the pixel distribution in the deposit was sharper.
Figure 3.
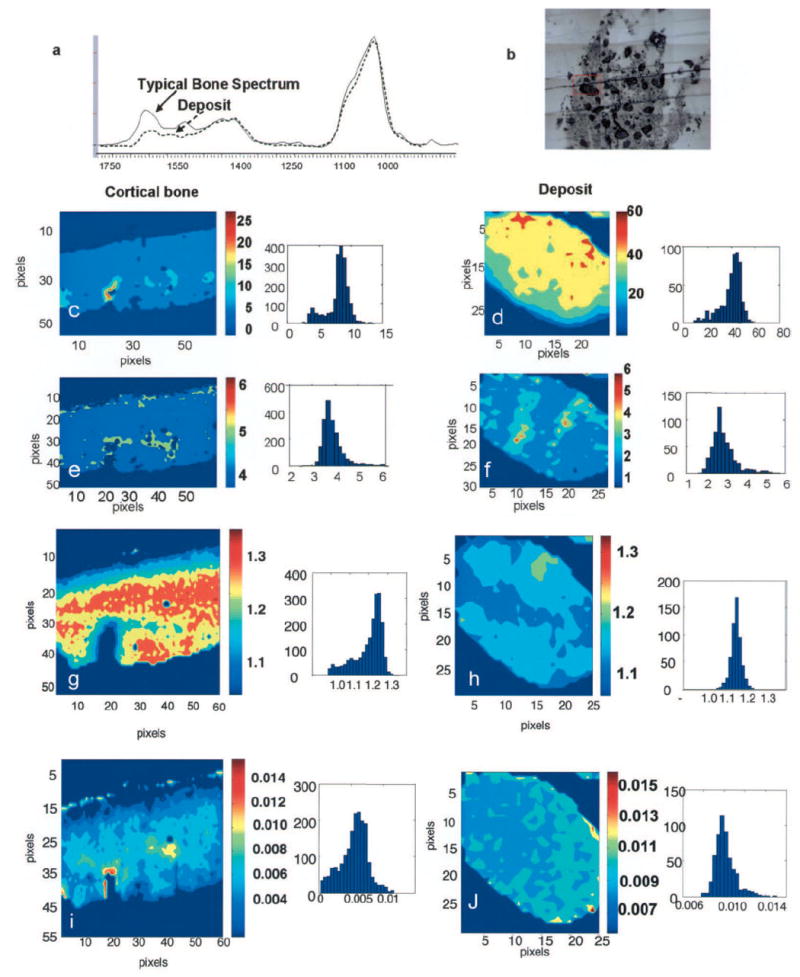
Infrared imaging analysis of a calcium deposit from a patient with juvenile dermatomyositis, compared with normal mouse cortical bone. a, Typical infrared spectra obtained with cortical bone and the deposit. Note the decreased protein (amide I) band in the spectrum from the deposit. Spectra were normalized so maximum intensity of the phosphate band is equivalent for the 2 samples. b, Photomicrograph of the deposit. Boxed area indicates the area imaged in d, f, h, and j. c–j, Images of mouse cortical bone (c, e, g, and i), and the calcific deposit (d, f, h, and j), and their respective pixel histograms. c and d, Mineral:matrix ratio. e and f, Collagen maturity. g and h, Crystallinity. i and j, Carbonate:phosphate ratio. Each image includes a color scale; for mineral:matrix ratio, the scales for the deposit and bone are not identical because the mineral:matrix ratio in the deposit is almost 10 times that in the bone. Sample sizes were 400 × 344 μm2 for cortical bone and 312 × 375 μm2 for the calcific deposit.
DISCUSSION
Juvenile DM is a rare disease, and delay in diagnosis is associated with development of pathologic calcification (7), indicating that chronic inflammation and/or tissue injury plays a role (10). In this study, as in others, persistent skin involvement in juvenile DM was associated with calcifications, but only 2 of the 5 patients were positive for TNFα –308A, which is often associated with increased TNFα production (7); this implies that the chronicity of the inflammation is a critical element. DQA1*0501, which is found in ~70% of patients with juvenile DM (Pachman LM: unpublished observations), was present in 2 of 5 patients in the present study, and may be more relevant to disease onset than to calcification.
The deposits contained hydroxyapatite mineral, which was comparable in crystal size with that found in bone, but distinct from bone. They were composed of relatively more mineral than matrix and had a sharper distribution of constituents. The deposits, like bone, contained the proteins osteopontin, osteonectin, and bone sialoprotein, seen in other soft tissue calcifications (1).
Calcific deposits occurring at sites where they are unwanted, as opposed to physiologic calcifications, are usually designated as “pathologic” or, when associated with cell death, as “dystrophic” in nature. Formation of physiologic and pathologic calcifications may share similar mechanisms, which include elevated local calcium and/or phosphate concentrations, injury, infection, inflammation, and decreased presence of calcification inhibitors. In this study, the mean age at onset of the first symptoms of juvenile DM was 3.3 years, compared with a mean age of 6.9 years in US patients overall (11). The association between calcifications and infection such as tuberculosis is well known, and infection appears to be a component of calcified atherosclerotic lesions (12). In >60% of 286 patients with new-onset juvenile DM, families reported a history of infection (respiratory and gastrointestinal) before the onset of disease, although a specific agent was not identified (11).
The calcifications from these patients with juvenile DM contained more osteonectin than is found in human bone (Figure 1), perhaps related to increased vascular components. While the deposit mineral was a poorly crystalline carbonated hydroxyapatite similar to that found in bone, the relative amount of mineral in the deposit far exceeded that in bone tissue. The association of bone matrix proteins with the deposit implies a process similar to bone mineralization, which may be involved either in the initial formation of the deposit or in the regulation of its growth.
Prolonged cutaneous inflammation in young children with juvenile DM is associated with pathologic calcifications. Identification of osteonectin, osteopontin, and bone sialoprotein, as well as hydroxyapatite, in these deposits suggests that crystal formation is a regulatory protein–mediated process involving tissue damage and an attempted healing mechanism. We speculate that the mineral deposition–modulating mechanism in inflammation-associated calcifications differs from normal bone formation.
Acknowledgments
Dr. Pachman’s work was supported in part by grants from the NIH (R01-AR-48289), the National Arthritis Foundation, the Greater Illinois Chapter of the Arthritis Foundation, and the Cure Juvenile Myositis Association. Dr. Veis’s work was supported in part by National Institute of Dental and Craniofacial Research grant DE-001374. Dr. Boskey’s work was supported in part by NIH grant DE-04141 and Core Center grant AR-046121.
Footnotes
Presented in part at the 5th Annual Meeting of the Federation of Clinical Immunology Societies, Boston, MA, May 2005, and the Annual Meeting of the American Association of Immunologists, Boston, MA, May 2006.
References
- 1.Gura T, Wright K, Veis A, Webb C. Identification of specific calcium-binding noncollagenous proteins associated with glutaraldehyde preserved bovine pericardium in the rat subdermal model. J Biomed Mater Res. 1997;35:483–95. doi: 10.1002/(sici)1097-4636(19970615)35:4<483::aid-jbm8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 2.Boulman N, Slobodin G, Rozenbaum M, Rosner I. Calcinosis in rheumatic diseases. Semin Arthritis Rheum. 2005;34:805–12. doi: 10.1016/j.semarthrit.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Rider LG. Calcinosis in juvenile dermatomyositis: pathogenesis and current therapies. Pediatric Rheumatology Online Journal [serial online] 2003;1(2) URL: www.pedrheumonlinejournal.org.
- 4.Stock SR, Ignatiev K, Lee PL, Abbott K, Pachman LM. Pathological calcification in juvenile dermatomyositis (JDM): microCT and synchrotron x-ray diffraction reveal hydroxyapatite with varied microstructures. Calcif Tissue Res. 2004;45:248–56. doi: 10.1080/03008200490903066. [DOI] [PubMed] [Google Scholar]
- 5.Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 6.Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM. Disease Activity Score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum. 2003;49:7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- 7.Pachman LM, Liotta-Davis MR, Hong DK, Kinsella TR, Mendez EP, Kinder JM, et al. TNFα-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor α, disease duration, and pathologic calcifications. Arthritis Rheum. 2000;43:2368–77. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. 1992;41:119–34. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 9.Boskey AL, Moore DJ, Amling M, Canalis E, Delany AM. Infrared analysis of the mineral and matrix in bones of osteonectin-null mince and their wildtype controls. J Bone Miner Res. 2005;18:1005–11. doi: 10.1359/jbmr.2003.18.6.1005. [DOI] [PubMed] [Google Scholar]
- 10.Pachman LM, Abbott K, Sinacore JM, Amoruso L, Dyer A, Lipton R, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatr. 2006;148:247–253. doi: 10.1016/j.jpeds.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Pachman LM, Lipton R, Ramsey-Goldman R, Shamiyeh E, Abbott K, Mendez EP, et al. History of infection before the onset of juvenile dermatomyositis: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Research Registry. Arthritis Rheum. 2005;53:166–72. doi: 10.1002/art.21068. [DOI] [PubMed] [Google Scholar]
- 12.Muller BT, Huber R, Henrich B, Adams O, Berns G, Siebler MJ, et al. Chlamydia pneumoniae, herpes simplex virus and cytomegalovirus in symptomatic and asymptomatic high-grade internal carotid artery stenosis: does infection influence plaque stability? Vasa. 2005;34:163–9. doi: 10.1024/0301-1526.34.3.163. [DOI] [PubMed] [Google Scholar]


