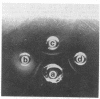
Abstract
Mutant strains of Rhizobium japonicum that were unable to allow the Corsoy cultivar of soybean to reduce acetylene or fix N2 were isolated. These strains grow as well as the wild type in a variety of media. Mutant strains SM1 and SM2 did not form nodules on the host plant; however, they reduced acetylene in the nonsymbiotic assay. Strains SM3 and SM4 produced nodules that did not have the characteristic pink pigment caused by leghemoglobin. The nodules formed by these strains also were small. One mutant strain, SM5, produced large pink nodules. The lesion in this strain seems to be in the gene that specifies nitrogenase component II.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen F. J., Turner G. L., Appleby C. A. Studies of the physiological role of leghaemoglobin in soybean root nodules. Biochim Biophys Acta. 1973 Jan 18;292(1):271–282. doi: 10.1016/0005-2728(73)90271-5. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Evans H. J., Daniel R. M., Hampton R. O. Immunological evidence for the capability of free-living Rhizobium japonicum to synthesize a portion of a nitrogenase component. Biochim Biophys Acta. 1975 Feb 13;381(2):248–256. doi: 10.1016/0304-4165(75)90231-7. [DOI] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Hubbell D. H. Antigenic differences between infective and noninfective strains of Rhizobium trifolii. Appl Microbiol. 1975 Aug;30(2):172–177. doi: 10.1128/am.30.2.172-177.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. J. Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim Biophys Acta. 1966 Oct 31;127(2):285–294. doi: 10.1016/0304-4165(66)90383-7. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Koch B., Klucas R. Preparation of nitrogenase from nodules and separation into components. Methods Enzymol. 1972;24:470–476. doi: 10.1016/0076-6879(72)24092-7. [DOI] [PubMed] [Google Scholar]
- Gordon J. K., Brill W. J. Derepression of nitrogenase synthesis in the presence of excess NH4+. Biochem Biophys Res Commun. 1974 Aug 5;59(3):967–971. doi: 10.1016/s0006-291x(74)80074-4. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsten R. D., Burns R. C., Hardy R. W., Hebert R. R. Establishment of symbiosis between Rhizobium and plant cells in vitro. Nature. 1971 Jul 16;232(5307):173–176. doi: 10.1038/232173a0. [DOI] [PubMed] [Google Scholar]
- Johnston A. W., Beringer J. E. Identification of the rhizobium strains in pea root nodules using genetic markers. J Gen Microbiol. 1975 Apr;87(2):343–350. doi: 10.1099/00221287-87-2-343. [DOI] [PubMed] [Google Scholar]
- Keister D. L. Acetylene reduction by pure cultures of Rhizobia. J Bacteriol. 1975 Sep;123(3):1265–1268. doi: 10.1128/jb.123.3.1265-1268.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondorosi A., Barabás I., Sváb Z., Orosz L., Sik T., Hotchkiss R. D. Evidence for common genetic determinants of nitrogenase and nitrate reductase in Rhizobium meliloti. Nat New Biol. 1973 Dec 5;246(153):153–154. doi: 10.1038/newbio246153a0. [DOI] [PubMed] [Google Scholar]
- Murphy P. M., Koch B. L. Compatibility of the components of nitrogenase from soybean bacteroids and free-living nitrogen-fixing bacteria. Biochim Biophys Acta. 1971 Nov 2;253(1):295–297. doi: 10.1016/0005-2728(71)90257-x. [DOI] [PubMed] [Google Scholar]
- Nagatani H. H., Shah V. K., Brill W. J. Activation of inactive nitrogenase by acid-treated component I. J Bacteriol. 1974 Nov;120(2):697–701. doi: 10.1128/jb.120.2.697-701.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Nitrogenase. IV. Simple method of purification to homogeneity of nitrogenase components from Azotobacter vinelandii. Biochim Biophys Acta. 1973 May 30;305(2):445–454. doi: 10.1016/0005-2728(73)90190-4. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis I. C., Gordon J. K., Orme-Johnson W. H., Brill W. J. Nitrogenase. 3. Nitrogenaseless mutants of Azotobacter vinelandii: activities, cross-reactions and EPR spectra. Biochim Biophys Acta. 1973 Jan 18;292(1):246–255. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Brill W. J. Mutant of Clostridium pasteurianum that does not fix nitrogen. J Bacteriol. 1971 Jan;105(1):65–69. doi: 10.1128/jb.105.1.65-69.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John R. T., Johnston H. M., Seidman C., Garfinkel D., Gordon J. K., Shah V. K., Brill W. J. Biochemistry and genetics of Klebsiella pneumoniae mutant strains unable to fix N2. J Bacteriol. 1975 Mar;121(3):759–765. doi: 10.1128/jb.121.3.759-765.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjepkema J., Evans H. J. Nitrogen fixation by free-living Rhizobium in a defined liquid medium. Biochem Biophys Res Commun. 1975 Jul 22;65(2):625–628. doi: 10.1016/s0006-291x(75)80192-6. [DOI] [PubMed] [Google Scholar]