Abstract
Cigarette smoke (CS) induces recruitment of inflammatory cells in the lungs leading to the generation of reactive oxygen species (ROS), which are involved in lung inflammation and injury. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a multimeric system that is responsible for ROS production in mammalian cells. We hypothesized that NADPH oxidase-derived ROS play an important role in lung inflammation and injury and that targeted ablation of components of NADPH oxidase (p47phox and gp91phox) would protect lungs against the detrimental effects of CS. To test this hypothesis, we exposed p47phox−/− and gp91phox−/− mice to CS and examined inflammatory response and injury in the lung. Surprisingly, although CS-induced ROS production was decreased in the lungs of p47phox−/− and gp91phox−/− mice compared with wild-type mice, the inflammatory response was significantly increased and was accompanied by development of distal airspace enlargement and alveolar destruction. This pathological abnormality was associated with enhanced activation of the TLR4-nuclear factor-κB pathway in response to CS exposure in p47phox−/− and gp91phox−/− mice. This phenomenon was confirmed by in vitro studies in which treatment of peritoneal macrophages with a nuclear factor-κB inhibitor reversed the CS-induced release of proinflammatory mediators. Thus, these data suggest that genetic ablation of components of NADPH oxidase enhances susceptibility to the proinflammatory effects of CS leading to airspace enlargement and alveolar damage.
Emphysema is characterized by permanent destruction of peripheral airspace distal to the terminal bronchioles, and the accumulation of inflammatory cells, such as macrophages and neutrophils, in bronchioles and alveolar structure. Pathologically distinct structural alterations of the small airways (airway remodeling),1 as well as systemic and local inflammation are seen in lungs of patients with chronic obstructive pulmonary disease (COPD).2 However, the molecular and cellular mechanisms that are responsible for the development of COPD are not well understood. Cigarette smoke (CS) contains an estimated 1015 to 1017 oxidants/free radicals and ∼4700 different chemical compounds, including reactive aldehydes, quinones, and semiquinones, per puff.3,4 It has been shown that CS is the major risk factor for the development of COPD, and that chronic exposure to CS leads to lung inflammation with an increase of inflammatory cells, such as macrophages, neutrophils, dendritic cells, and CD8+ T lymphocytes.5 These cells are capable of releasing reactive oxygen species (ROS), inflammatory mediators, and proteinases, which are believed to play a critical role in the progressive destruction of the lung, leading to emphysema.6,7
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a highly regulated membrane-bound enzyme present in a variety of phagocytic and nonphagocytic cells. In phagocytes, it is composed of six subunits: p40phox (phox for phagocytic oxidase), p47phox, p67phox, Rac, p22phox, and gp91phox. The latter two are membrane associated, and together constitute the flavocytochrome b558, whereas the other components are located in the cytoplasm of resting cells. On activation, the cytoplasmic components translocate to the cell membrane where they bind to flavocytochrome b558, thus forming the active NADPH oxidase.8,9 Once active NADPH oxidase forms, it catalyzes the transfer of electrons from NADPH to molecular oxygen producing the superoxide anion (O2·−), hydroxyl radical, and hydrogen peroxide.9,10
NADPH oxidase is the main cellular source of ROS in mononuclear and granulocytic leukocytes and is involved in host defense.9 It has been shown that CS induces endothelial O2·− production via NADPH oxidase activation in cell culture systems,11,12 which was associated with CS-induced lung inflammation and injury. Recent studies have shown that NADPH oxidase subunits are under tight regulatory control in physiological conditions.13,14 For example, a specific disruption of Rac1 in mouse embryonic fibroblasts leads to an increase in ROS level that is attributable to a compensatory increase in Rac3, another component of NADPH oxidase.13 Furthermore, Rac1 was up-regulated in hematopoietic cells deficient in Rac2.14 Nevertheless, the role of NADPH oxidase in vivo in CS-mediated lung inflammation and injury is not known. We, and others, have shown that leukocytes isolated from patients with COPD release high levels of O2·,15,16 which was associated with increased systemic levels of inflammatory mediators.17,18 Hence, inhibition of CS-induced generation of ROS would confer protection against oxidant-mediated lung inflammation.
In light of the above, we hypothesized that inhibition of cellular ROS release by targeted ablation of components of NADPH oxidase (p47phox and gp91phox) would protect lungs against detrimental effects of CS. To test this hypothesis, we exposed p47phox−/− and gp91phox−/− mice to both acute (3 days) and subchronic (8 weeks) CS exposure, and determined the role of ROS in the protection against CS-induced inflammatory and injurious responses in the lung.
Materials and Methods
CS Exposure
Wild-type C57BL/6J mice and p47phox−/− and gp91phox−/− (C57BL/6J background) mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and housed and bred in the vivarium facility at the University of Rochester. Eight to ten weeks of age wild-type (WT) and p47phox−/− and gp91phox−/− mice (8 to 10 mice per group) were used for acute (3 days) and subchronic (8 weeks) CS exposure. In brief, mice were placed in an individual compartment of a wire cage, which was placed inside a closed plastic box connected to the smoke source. Research grade cigarettes [2R4F (total particulate matter per cubic meter of air (TPM) concentration 11.7 mg/cigarette, tar 9.7 mg/cigarette, nicotine 0.85 mg/cigarette), University of Kentucky, Lexington, KY] were used to generate smoke and mice were exposed according to the Federal Trade Commission protocol (1 puff/minute of 2-second duration and 35-ml volume) using a Baumgartner-Jaeger CSM2072i automatic cigarette smoking machine (CH Technologies, Westwood, NJ). Mainstream CS was diluted with filtered air and directed into the exposure chamber. The smoke exposure (TPM) was monitored in real time with a MicroDust pro-aerosol monitor (Casella CEL, Bedford, UK) and verified daily by gravimetric sampling.19,20,21,22 The smoke concentration was set at a nominal value of ∼300 mg/m3 TPM by adjusting the flow rate of the diluted medical air.20,21,22,23 Mice received two 1-hour exposures, 1 hour apart, for 3 consecutive days or 8 consecutive weeks (5 days/week), and were sacrificed 24 hours after last exposure. Control mice were exposed to filtered air in an identical chamber according to the same protocol described for CS exposure. The concentration of carbon monoxide in the chamber was 297 ppm. All animal procedures were approved by the Animal Research Committee of the University of Rochester.
Bronchoalveolar Lavage (BAL)
Mice were intraperitoneally injected with 100 mg/kg (body weight) of pentobarbiturate (Abbott Laboratories, Abbott Park, IL) and euthanized by exsanguination. The heart and lungs were removed en bloc, and the lungs were lavaged three times with 0.5 ml of 0.9% sodium chloride. The lavage fluid was centrifuged, and the cell-free supernatants were frozen at −80°C for later analysis. The BAL cell pellet was resuspended in 1 ml of 0.9% sodium chloride, and the total cell number was determined by counting on a hemocytometer. Differential cell counts (minimum of 500 cells per slide) were performed on cytospin-prepared slides (Thermo Shandon, Pittsburgh, PA) stained with Diff-Quik (Dade Behring, Newark, DE).
Hematoxylin and Eosin (H&E) Staining and Mean Linear Intercept Analyses
Mouse lungs (which had not been lavaged) were inflated by 1% low-melting agarose at a pressure of 25 cm H2O, and then fixed with 4% neutral buffered formalin. Tissues were embedded in paraffin, sectioned (4 μm), and stained with H&E. Alveolar size was estimated from the mean linear intercept (Lm) of the airspace as described.24,25,26,27 In brief, Lm was calculated for each sample based on 10 random fields observed at a magnification of ×200 using a cross-line.
Immunohistochemistry for Macrophages and 8-Hydroxy-2′-Deoxyguanosine (8-OHdG)
The formalin-fixed, paraffin-embedded lung sections were deparaffinized and rehydrated by passing through a series of xylene and graded alcohol. Endogenous peroxidase activity was quenched by exposure to 3% H2O2 in methanol for 30 minutes. Nonspecific binding of antibodies to the tissues was blocked by incubating the tissue with 5% normal goat serum in 0.5% bovine serum albumin in phosphate-buffered saline (PBS) for 30 minutes. For the detection of macrophages, rat anti-mouse Mac-3 monoclonal antibody (BD Pharmingen, Franklin Lakes, NJ) at a titer of 1:50 was used. A biotinylated goat anti-mouse/rabbit Ig (DAKO Corp., Santa Barbara, CA) was used at a titer of 1:100. Tissues were incubated with primary antibodies overnight at 4°C. After being washed, tissues were incubated with secondary antibody for 30 minutes. 3,3′-Diaminobenzidine (DAKO) was used as peroxidase substrate. In each instance, sections from different time points were processed together, with equal time for color development. Tissues were counterstained with hematoxylin. The number of Mac-3-positive cells in the lung sections (five random microscopic fields per lung section in three different sections) were counted manually under microscope with ×200 magnification and averaged.27,28,29 Similarly, 8-OHdG was detected by immunohistochemistry in paraffin-embedded sections pretreated with 100 μg·ml−1 ribonuclease A in PBS to inhibit nonspecific binding to RNA using a mouse monoclonal antibody (Oxis International Inc., Foster City, CA). The number of 8-OHdG-positive cells in the lung sections (three random microscopic fields per lung section in three different sections) was also counted manually in a blinded manner.
Periodic Acid-Schiff (PAS) Staining
Airway mucus was identified by the PAS (Sigma-Aldrich, St. Louis, MO) staining. PAS staining was assessed by a semiquantitative method. In brief, airways were examined under light microscopy and assigned a score between 0 and 3 based on the following criteria30: 0, no staining; 1, PAS staining <25% of airway perimeter; 2, PAS staining 25 to <50% of airway perimeter; and 3, PAS staining >50% of airway perimeter.
Lipid Peroxidation Products Assay in Lung Homogenate
Lung tissues were homogenized with ice-cold 20 mmol/L Tris-HCl (pH 7.4) and diluted to ∼10% (w/v). The homogenates were centrifuged at 3000 rpm for 10 minutes at 4°C, and the supernatants were collected. Butylated hydroxytoluene (5 mmol/L, Sigma-Aldrich) was added to the supernatant to prevent further peroxidation and the samples were immediately frozen in liquid nitrogen. Measurement of lipid peroxidation products [4-hydroxy-2-nonenal (4-HNE) and malondialdehyde (MDA)] was performed using a lipid peroxidation kit (Calbiochem, Foster City, CA). To measure MDA and 4-HNE, 200 μl of supernatants was added to 650 μl of 10.3 mmol/L N-methyl-2-phenylindole in acetonitrile and vortexed immediately, and 150 μl of 15.4 mmol/L methanesulfonic acid was added and incubated at 45°C for 40 minutes. The samples were cooled on ice and measured at 586 nm using a spectrophotometer.
ROS Determination by 2′,7′-Dichlorofluorescin-Diacetate (DCF-DA) in BAL Cells
ROS release was determined using DCF-DA, which is an oxidant-sensitive fluorescent probe. Inside the cell, the probe is deacetylated by esterases and formed H2DCF, which is oxidized by ROS to DCF, a highly fluorescent compound.31 ROS level in BAL cells was assessed using the probe DCF-DA. Briefly, the cytospin slides were incubated with 10 μmol/L DCF-DA (Molecular Probes, Eugene, OR) for 15 minutes at room temperature. After being rinsed with PBS, the slides were observed under the fluorescent microscope and assessed with a score between 1 and 3 based on the following criteria: 1, weak staining; 2, moderate staining; 3, intense staining.
Myeloperoxidase (MPO) Assay
The lung tissues were homogenized in 10 vol of 100 mmol/L phosphate buffer (pH 7.4) containing protease inhibitors. To determine the MPO activity in enzymatic extract, we used a spectrophotometric method with tetramethylbenzidine (Sigma-Aldrich). Briefly, the assay mixture consisted of 400 μl of phosphate buffer (100 mmol/L, pH 5.4) with 0.5% hexadecyltrimethylammonium bromide (Sigma-Aldrich), 40 μl of tetramethylbenzidine (20 mmol/L in dimethyl sulfoxide, prepared fresh and protected from light), and H2O (23 μl). To this assay mixture, 20 μl of enzyme extract was added and the mixture was incubated at 37°C for 3 minutes, and 17 μl of H2O2 (0.03%) was added. The mixture was further incubated for 3 minutes. The reaction was stopped by adding 2 ml of acetate buffer (0.2 mol/L, pH 3.2). The tubes were kept on ice and the absorbance was read at 655 nm. The appropriate reagent blank was prepared by using a buffer instead of the tissue extract. Activity was expressed in the samples as U/mg protein.
Protein Extraction from Lung Tissue
One hundred mg of lung tissue was mechanically homogenized in 0.5 ml of buffer A [10 mmol/L HEPES (pH 7.8), 10 mmol/L KCl, 2 mmol/L MgCl2, 1 mmol/L dithiothreitol, 0.1 mol/L ethylenediaminetetraacetic acid, 0.2 mmol/L NaF, 0.2 mmol/L Na orthovandate, 1% (v/v) Nonidet P-40, 0.4 mmol/L phenylmethyl sulfonyl fluoride, and 1 μg/ml leupeptin] on ice. The homogenate was centrifuged at 2000 rpm in a benchtop centrifuge for 30 seconds at 4°C to remove cellular debris. The supernatant was then transferred to a 1.7-ml ice-cold Eppendorf tube and further centrifuged for 30 seconds at 13,000 rpm at 4°C. The supernatant was collected as a cytoplasmic extract. The pellet was resuspended in 200 μl of buffer C [50 mmol/L HEPES (pH 7.8), 50 mmol/L KCl, 300 mmol/L NaCl, 0.1 mol/L ethylenediaminetetraacetic acid, 1 mmol/L dithiothreitol, 10% (v/v) glycerol, 0.2 mmol/L NaF, 0.2 mmol/L Na orthovanadate, and 0.6 mmol/L phenylmethyl sulfonyl fluoride] and placed on the rotator in the cold room for 30 minutes. After centrifugation at 13,000 rpm in an Eppendorf tube for 5 minutes, the supernatant was collected as the nuclear extract and kept frozen at −80°C. Whole cell lysate was extracted from lung tissue after homogenization in RIPA buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 0.25% deoxycholate, 1 mmol/L Na3VO4, 1 mmol/L NaF, 1 μg of leupeptin/ml, 1 μg of aprotinin/ml, and 1 mmol/L phenylmethyl sulfonyl fluoride).
Proinflammatory Mediators: KC, Interleukin (IL)-6, MCP-1, Tumor Necrosis Factor (TNF)-α, and GM-CSF Assays
The levels of KC, IL-6, MCP-1, TNF-α, and GM-CSF in lung homogenates (10%) were measured by the Luminex100 using the Beadlyte mouse multicytokine beadmaster kit (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer’s instructions. The assays permit simultaneous cytometric quantitation of multiple chemokines/cytokines with minimal sample volume. The assays use microspheres as the solid support for immunoassays.
In peritoneal macrophages, the culture medium was collected after treatment and centrifuged at 2500 rpm for 5 minutes to pellet the cells. The levels of MCP-1 and KC in the supernatant were determined by enzyme-linked immunosorbent assay from respective duo-antibody kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instruction.
Protein Assay
Protein level was measured with a BCA kit (Pierce, Rockford, IL). Protein standards were obtained by diluting a stock solution of bovine serum albumin. Linear regression was used to determine the actual protein concentration of the samples.
Western Blot Analysis
Lung tissue homogenate/lysate samples, including cytoplasmic and nuclear proteins were separated on a 7.5 to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Equivalent loading of the gel was determined by quantitation of protein as well as by reprobing membranes for actin. Separated proteins were electroblotted onto nitrocellulose membranes (Amersham, Arlington Heights, IL), and blocked for 1 hour at room temperature with 5% nonfat dry milk. The membranes were then probed with a 1:400 to 1:1000 diluted antibodies of a polyclonal phospho-nuclear factor κB (NF-κB) p65 (ser276), monoclonal phospho-NF-κB p65 (ser536) (Cell Signaling Technology, Beverly, MA), or polyclonal RelA/p65 (Santa Cruz Biotechnology, Santa Cruz, CA) for nuclear protein, polyclonal IκBα (Santa Cruz Biotechnology) for cytoplasmic protein and polyclonal TLR4, gp91phox, Nox3, Nox4, monoclonal p47phox for lung homogenate/lysate (Santa Cruz Biotechnology) at room temperature for 1 hour. After three washing steps (15 minutes each), the levels of protein were detected using anti-rabbit, anti-mouse, or anti-goat antibody (1:5000 dilution in 2.5% nonfat dry milk in TBS for 1 hour) linked to horseradish peroxidase (DAKO), and bound complexes were detected using the enhanced chemiluminescence method (Perkin Elmer, Waltham, MA).
RNA Extraction and mRNA Analysis
For reverse transcriptase-polymerase chain reaction (RT-PCR), lungs were submerged immediately after excision in the RNAlater stabilization reagent (Qiagen, Valencia, CA) at 37°C for 24 hours, and then total RNA was extracted from lungs using the RNeasy mini kit (Qiagen). Two μg of RNA was reverse-transcribed using oligo(dT) (Promega, Madison, WI) and Maloney murine leukemia virus reverse transcriptase (M-MLV RT) (Promega) according to the supplier’s recommendation. The following primers were used in RT-PCR: GAPDH, 5′-ACGACCCCTTCATTGAC-3′ (sense) and 5′-CCACGACATACTCAGCAC-3′ (antisense)32; p47phox, 5′-CTGCTGTTGAAGAGGACGAGATG-3′ (sense) and 5′-AGCCGGTGATATCCCCTTTCC-3′ (antisense)33; gp91phox, 5′-CACCCATTCACACTGACCTCTG-3′ (sense) and 5′-CTTATCACAGCCACAAGCATTGAA-3′ (antisense)33; Nox3, 5′-CTCGTTGCCTACGGGATAGC-3′ (sense) and 5′-CCTTCAGCATCCTTGGCCT-3′ (antisense)34; Nox4, 5′-ACAACCAAGGGCCAGAATACTACTAC-3′ (sense) and 5′-GGATGAGGCTGCAGTTGAGG-3′ (antisense).34 RT-PCR was performed in total volume of 25 μl in 10× PCR buffer in the presence of 0.2 mmol/L dNTP (Promega), 4 μmol/L of each primer, 1.5 mmol/L MgCl2 (Invitrogen, Carlsbad, CA), and 7 U of Taq polymerase (Invitrogen). PCR conditions for each primer couple were as follows: GAPDH, 94°C for 15 seconds, 55°C for 30 seconds, and 72°C for 30 seconds during 32 cycles; p47phox and gp91phox, 95°C for 30 seconds, 54°C for 3 minutes, and 72°C for 5 minutes during 40 cycles; Nox3 and Nox4, 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds during 40 cycles with a final extension at 72°C for 10 minutes in a PTC-200 DNA thermal cycler (MJ Research, Waltham, MA). Samples were analyzed by gel electrophoresis on 1.2% agarose gels and stained with SYBR safe dye. Real-time PCR was performed with iQ SYBR Green Supermix in an iCycler iQ System (Bio-Rad, Hercules, NY) using the SYBR Green detection protocol. The specific sense and antisense primer were used for murine TLR4: 5′-GCAATGTCTCTGGCAGGTGTA-3′ (sense) and 5′-CAAGGGATAAGAACGCTGAGA-3′ (antisense).35 Total reaction volume was 20 μl, and PCR condition was as follows: 95°C for 45 seconds, 61°C for 45 seconds, and 72°C for 45 seconds during 33 cycles with a final extension at 72° for 5 minutes. Mouse GAPDH gene was amplified as an internal control.
Preparation of CS Extract
Research grade cigarettes (1R3F) were obtained from the Kentucky Tobacco Research and Development Center at the University of Kentucky (Lexington, KY). The composition of 1R3F/cigarettes was: TPM, 17.1 mg; tar, 15 mg; and nicotine, 1.16 mg. Cigarette smoke extract (CSE, 10%) was prepared by bubbling smoke from one cigarette into 10 ml of RPMI 1640 media at a rate of one cigarette/2 minutes as described previously,19,36 using a modification of the method described earlier by Carp and Janoff.37 The pH of the CSE was adjusted to 7.4, and was sterile-filtered through a 0.45-μm filter (25 mm Acrodisc; Pall Corporation, Ann Arbor, MI). CSE preparation was standardized by measuring the absorbance (OD 0.74 ± 0.05) at a wavelength of 320 nm. The pattern of absorbance (spectrogram) observed at λ320 showed very little variation between different preparations of CSE. CSE was freshly prepared for each experiment and diluted with culture media supplemented with 1% fetal bovine serum immediately before use. Control medium was prepared by bubbling air through 20 ml of serum-free RPMI, adjusting pH to 7.4, and sterile filtering as described above.
Elicitation and Culture of Peritoneal Macrophages
The mice were subjected to peritoneal lavage with PBS 4 days after injection with 2 ml of 4% autoclaved, 2 weeks aged thioglycollate broth (Sigma-Aldrich). The peritoneal macrophages were washed with PBS and grown in a six-well plate with RPMI 1640 medium (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT), 2 mmol/L l-glutamine, 100 μg/ml penicillin, 100 U/ml streptomycin, 1% nonessential amino acids, 1% sodium pyruvate, 1 μg/ml human holo-transferrin, and 1 mmol/L oxaloacetic acid at 37°C in a humidified atmosphere containing 5% CO2. After 2 hours of incubation, wells were gently pipetted to remove nonadherent cells, adherent cells were continuously cultured overnight before treatment.
Treatment of Peritoneal Macrophages
After starvation for 6 hours with RPMI 1640 medium containing 1% fetal bovine serum before treatment, the cells were treated with CSE (0.25%, 0.5%, 1%) with or without NF-κB inhibitor BAY117082 (10 μmol/L; Biomol, Plymouth Meeting, PA) for 24 hours at 37°C with 5% CO2. All treatments were performed in duplicate. Culture media from these peritoneal macrophages were collected and stored at −80°C for KC and MCP-1 assay. The cells were washed with cold sterile Ca2+/Mg2+-free PBS and lysed with RIPA buffer before being used to assay TLR4 protein levels and interaction of TLR4 with p47phox and gp91phox.
TLR4 Immunoprecipitation and Its Interaction with p47phox and gp91phox
Peritoneal macrophages were treated with CSE (0.25%, 0.5%, and 1%) for 24 hours at 37°C as described above, and the whole cell lysate was used to immunoprecipitate TLR4 using the antibody for TLR4 (1:40 dilution, Santa Cruz Biotechnology), which was added to 80 μg of protein in a final volume of 400 μl and incubated for 1 hour. Protein-A/G agarose beads (20 μl) (Santa Cruz Biotechnology) were added to each sample and left overnight at 4°C on a rocker. The samples were then centrifuged at 2000 rpm at 4°C for 5 minutes. The supernatant was discarded, and the beads were washed three times and then resuspended in 40 μl of lysis buffer. For Western blots, 80 μg of the immunoprecipitated TLR4 agarose bead suspension were added to 10 μl of 5× sample buffer, boiled, and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Negative alone (beads only) was used as negative control. To demonstrate the interaction of TLR4 protein with p47phox or gp91phox, the membranes of immunoprecipitated TLR4 were blotted against p47phox or gp91phox (Santa Cruz Biotechnology), respectively.
Statistical Analysis
The results are shown as the mean ± SEM. Statistical analysis of significance was calculated using one-way analysis of variance followed by Tukey’s posthoc test for multigroup comparisons using Statview (SAS Institute, Cary, NC).
Results
Genetic Ablation of p47phox and gp91phox Enhanced Inflammatory Cell Influx into the Lungs after CS Exposure
To determine the role of p47phox and gp91phox in proinflammatory effect of CS, p47phox−/− and gp91phox−/− and WT mice were exposed to CS for 3 days (acute) and 8 weeks (subchronic). Inflammatory cell influx into BAL fluid and lung tissue was assessed using Diff-Quick and histological staining, respectively. Exposure to CS resulted in significant neutrophil influx in BAL fluid of WT mice, which was augmented in p47phox−/− and p91phox−/− mice (Figure 1, A and B). CS exposure did not result in any significant change in total BAL macrophage numbers in p47phox−/− mice whereas the total number of macrophages was significantly decreased in p91phox−/− mice (Figure 1, C and D). Genetic ablation of gp91phox further decreased the total number of macrophages in BAL fluid in response to both acute and subchronic CS exposures (Figure 1, C and D). Lung H&E staining showed a significantly increased interstitial inflammation with prominent neutrophils and macrophages in both WT and knockout (KO) mice in response to acute (3 days) and subchronic (8 weeks) CS exposures (Figure 2, A and B). In addition, the neutrophil and macrophage influx into the lung of CS-exposed p47phox−/− and gp91phox−/− mice were further augmented compared to that of the CS-exposed WT mice (Figure 2, A and B). Similarly, macrophage staining by immunohistochemistry using anti-Mac-3 antibody was significantly increased in p47phox−/− and gp91phox−/− mice compared to WT mice in response to both acute and subchronic CS exposure (Figure 3, A and B). Acute and subchronic CS exposure did not change the index of alveolar destruction, as assessed by mean linear intercept (Lm), in WT mice, which was consistent with previous studies showing that emphysema starts to develop after 4 months of CS exposure in C57BL/6J mice.7,38 However, increased Lm was noticed in both p47phox−/− and gp91phox−/− mice as compared to C57BL/6J WT mice in response to 8 weeks, but not 3 days of CS exposure (Figure 2, A and B). It was also interesting to find the increased inflammation and alveolar destruction in air-exposed p47phox−/− and gp91phox−/− mice compared to air-exposed WT mice at 8 weeks of air exposure (Figure 2B and Figure 3B). In addition, increased spontaneous emphysema (alveolar destruction and airspace enlargement) in p47phox−/− and gp91phox−/− mice was noticed at the age of 3 months with the index of emphysema score persistently increased at 6 and 9 months after the birth in an unchallenged state (data not shown). These data suggested that NADPH oxidase plays an important role in maintaining lung integrity. Consistent with the histological results, genetic ablation of p47phox and gp91phox had increased MPO activity, which is used to assess neutrophil infiltration in lungs in response to CS exposure (Figure 4, A and B). These results suggested that there was an increased lung inflammation in p47phox−/− and gp91phox−/− mice as compared to WT mice in response to CS exposure.
Figure 1.
Increased neutrophil and decreased macrophage counts in the BAL fluid of p47phox−/− and gp91phox−/− mice in response to CS exposure. The number of neutrophils in BAL fluid from 3-day (A) and 8-week (B) air- or CS-exposed mice. The number of neutrophils in BAL fluid was significantly increased in p47phox−/− and gp91phox−/− mice compared to WT mice in response to CS exposure. Lavaged macrophages from 3-day (C) and 8-week (D) air- or CS-exposed mice. The number of macrophages in BAL fluid was significantly decreased in gp91phox−/− mice compared to WT mice in response to CS exposure. Data are shown as mean ± SEM (n = 3 to 4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective air-exposed groups; +P < 0.05, ++P < 0.01, +++P < 0.001, significant compared with CS-exposed WT mice; #P < 0.05, significant compared with air-exposed WT mice.
Figure 2.
Increased inflammatory cell influx into the lungs of p47phox−/− and gp91phox−/− mice exposed to CS. Figure shown is H&E-stained lung sections from 3-day (A) and 8-week (B) air- or CS-exposed mice (n = 3 per group). Lung sections from p47phox−/− and gp91phox−/− mice show increased inflammatory cell infiltration (A and B) and alveolar destruction and airspace enlargement (B) compared to WT mice. Black arrows indicate inflammatory cells, white arrows indicate alveolar destruction. *P < 0.05, **P < 0.01, significant compared with respective air-exposed groups; +P < 0.05, ++P < 0.01, significant compared with CS-exposed WT mice; #P < 0.05, ##P < 0.05, significant compared with air-exposed WT mice. Original magnifications, ×200.
Figure 3.
Increased macrophage influx into lungs of p47phox−/− and gp91phox−/− mice exposed to CS. Immunohistochemical detection of macrophages (arrows) using rat anti-mouse Mac-3 antibody in lung from 3-day (A) and 8-week (B) air- or CS-exposed mice. The lung sections from CS-exposed p47phox−/− and gp91phox−/− mice showed more macrophages than those from CS-exposed WT counterparts. In addition, there were more alveolar macrophages in air-exposed p47phox−/− and/or gp91phox−/− mice than those from air-exposed WT mice. The pictures represent at least three separate experiments. **P < 0.01, ***P < 0.001, significant compared with respective air-exposed groups; +P < 0.05, ++P < 0.01, significant compared with CS-exposed WT mice; #P < 0.05, ##P < 0.01, significant compared with air-exposed WT mice. Original magnifications, ×200.
Figure 4.
Genetic ablation of p47phox and gp91phox increased lung activity of MPO in response to CS exposure. The activity of MPO in lungs was significantly increased in p47phox−/− and gp91phox−/− mice as compared to WT mice in response to 3-day (A) and 8-week (B) CS exposure. Data are shown as mean ± SEM (n = 3 to 4 per group). *P < 0.05, ***P < 0.001, significant compared with respective air-exposed group; +P < 0.05, +++P < 0.001, significant compared with CS-exposed WT mice; #P < 0.05, significant compared with air-exposed WT mice.
Targeted Disruption of p47phox and gp91phox Increased Proinflammatory Mediator Release in Lungs in Response to CS Exposure
Patients with COPD have increased levels of proinflammatory mediators, such as IL-6, TNF-α, KC, MCP-1, and GM-CSF in the lungs.1,15,18 It is known that the ROS generated by NADPH oxidase can activate NF-κB, leading to NF-κB-dependent release of proinflammatory cytokines. Therefore, we investigated whether p47phox−/− and gp91phox−/− mice exhibited the altered levels of cytokines/chemokines, which were measured by Luminex multiplex assay in lung homogenates in response to both acute and subchronic CS exposures. Levels of KC, MCP-1, IL-6, and TNF-α were significantly increased in response to CS exposure at 3 days in the lungs of WT mice (Figure 5A). Furthermore, targeted disruption of p47phox and gp91phox further increased these mediators in lung tissue in response to CS exposure (Figure 5A). Similarly, the levels of MCP-1 and GM-CSF in lung tissue were augmented in p47phox−/− and/or gp91phox−/− mice compared to WT mice exposed to CS for 8 weeks (Figure 5B). Although the levels of KC were not affected in the lungs of WT mice exposed to CS for 8 weeks, the level was still significantly increased in p47phox−/− and gp91phox−/− mice (Figure 5B). It was interesting to note that the basal lung levels of GM-CSF, IL-6, KC, and MCP-1 were significantly increased in p47phox−/− and/or gp91phox−/− mice compared to that of WT mice exposed to air at 8 weeks, however, the levels of these cytokines were not as high as CS exposure to NADPH oxidase-deficient mice (Figure 5B). These data further confirmed that genetic ablation of p47phox and gp91phox augmented lung inflammatory response in the lung in response to both acute and subchronic CS exposure.
Figure 5.
Increased lung proinflammatory mediators release in p47phox−/− and gp91phox−/− mice exposed to CS. A: Acute CS exposure increased the levels of KC, MCP-1, IL-6, and TNF-α in the lungs of WT mice that were significantly increased in p47phox−/− and gp91phox−/− mice. B: Lung levels of GM-CSF, MCP-1, and KC were significantly increased in p47phox−/− and gp91phox−/− mice compared to WT mice exposed to CS for 8 weeks. A and B: Genetic ablation of p47phox and gp91phox also led to increased basal levels of GM-CSF, MCP-1, KC, and IL-6 in the lungs. The levels of proinflammatory mediators were measured by the Luminex100 using the Beadlyte mouse multicytokine beadmaster kit. Data are shown as mean ± SEM (n = 4 to 5 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective air-exposed group; ++P < 0.01, +++P < 0.001, significant compared with CS-exposed WT mice; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with air-exposed WT mice.
Increased Mucus Production in the Airways of p47phox−/− and gp91phox−/− Mice after CS Exposure
Neutrophils and macrophages are thought to contribute to mucus secretion through the release of neutrophil elastase and proinflammatory mediators.39,40,41,42,43 We found that increased lung neutrophil and macrophage influx occurred in NADPH oxidase-deficient mice. Therefore, we determined the amount of mucus production in the airways of NADPH oxidase-deficient and WT mice in response to subchronic CS exposure by PAS staining. The mucus production in the airways was significantly increased in WT mice in response to CS exposure for 8 weeks (Figure 6), but not 3 days (data not shown). Targeted disruption of p47phox and gp91phox enhanced the basal (but not the same extent as CS exposure) and CS-induced mucus production as compared to corresponding WT animals (Figure 6). These findings corroborate the above observations showing hyperinflammatory response in lungs of p47phox−/− and gp91phox−/− mice exposed to CS.
Figure 6.
Increased mucus production shown by PAS staining in the airways of p47phox−/− and gp91phox−/− mice compared to WT mice in response to 8 weeks of CS exposure. Targeted disruption of p47phox and gp91phox increased mucus production in response to 8 weeks of CS exposure. In addition, increased mucus production was seen in the airways of air-exposed p47phox−/− and gp91phox−/− mice compared to air-exposed WT mice. The PAS staining pictures shown are from a representative experiment with three to four mice per group. Arrows indicate the mucus (purple) in the airway. *P < 0.05, significant compared with respective air-exposed group; +P < 0.05, significant compared with CS-exposed WT mice; #P < 0.05, compared with air-exposed WT mice. Original magnifications, ×200.
Genetic ablation of p47phox and gp91phox Decreased ROS Production in BAL Cells, Levels of Lipid Peroxidation Products, and 8-OHdG expression in Lung Tissue in Response to CS Exposure
We hypothesized that genetic ablation of NADPH oxidase leads to abrogation of ROS by CS. Therefore, we assessed ROS levels by DCF-DA staining in BAL cells obtained from WT, and from both p47phox−/− and gp91phox−/− mice in response to CS exposure after 3 days (acute) and 8 weeks (subchronic). CS exposure led to a significantly increased level of intracellular ROS, reflected by enhanced DCF fluorescent intensity, in BAL cells from WT compared to air-exposed WT mice at both time points (Figure 7, A and B). However, the ROS level was decreased in BAL cells from both p47phox−/− and gp91phox−/− mice when they were exposed to CS as compared to CS-exposed WT mice. CS exposure, however, resulted in a slight increase in ROS release from BAL cells obtained from gp91phox−/− mice, but not from p47phox−/− mice, compared to respective air-exposed NADPH oxidase subunit KO mice (Figure 7, A and B). Consistent with reduced levels of ROS in BAL cells, the levels of lipid peroxidation products, 4-HNE and MDA, were decreased in lungs of both p47phox−/− and gp91phox−/− mice compared with WT mice exposed to CS at both the time points (Figure 8, A and B). Furthermore, a marker for oxidative DNA damage, 8-OHdG, was also decreased in lungs in p47phox−/− and gp91phox−/− mice compared to WT mice exposed to CS for 8 weeks (Figure 9). Thus, CS induces ROS release, causes lipid peroxidation and DNA oxidation, which were, in part, mediated by NADPH oxidase activation in the lung. However, it is interesting to note that ROS level, oxidative damage of DNA, and lipid were not altered in lungs of these null mice compared to WT mice exposed to air suggesting that the stimuli are required to exacerbate oxidative stress in these mice.
Figure 7.
ROS production is reduced in BAL cells of p47phox−/− and gp91phox−/− mice in response to CS exposure. Genetic ablation of p47phox and gp91phox decreased ROS production in BAL cells in response to 3-day (A) and 8-week (B) CS exposure. ROS production (arrows) in BAL cells was detected with DCF-DA fluorescence. The pictures shown are from a representative experiment with three mice per group. *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective air-exposed group; +P < 0.05, ++P < 0.01, +++P < 0.001, significant compared with CS-exposed WT mice. Original magnifications, ×400.
Figure 8.
Levels of lipid peroxidation products (4-HNE and MDA) are decreased in lungs of p47phox−/− and gp91phox−/− mice exposed to CS. The levels of 4-HNE and MDA were significantly reduced in p47phox−/− and gp91phox−/− mice compared to WT mice exposed to 3 days (A) and 8 weeks (B) of CS. Data are shown as mean ± SEM (n = 4 to 5 per group). **P < 0.01, ***P < 0.001, significant compared with respective air-exposed mice; +P < 0.05, +++P < 0.001, significant compared with CS-exposed WT mice.
Figure 9.
8-OHdG expression is decreased in lungs of p47phox−/− and gp91phox−/− mice exposed to CS. Immunohistochemical detection 8-OHdG (arrows) using anti-mouse monoclonal antibody in lungs of mice exposed to air or CS for 8 weeks. The lung sections from CS-exposed p47phox−/− and gp91phox−/− mice showed decreased 8-OHdG-positive cells than those from CS-exposed WT counterparts. The pictures are representative of at least three separate experiments. **P < 0.01, ***P < 0.001, significant compared with respective air-exposed groups; ++P < 0.01, significant compared with CS-exposed WT mice. Original magnifications, ×200.
Targeted Disruption of p47phox and gp91phox Increased the Levels of RelA/p65 and Its Phosphorylation on Ser276 and Ser536 Residues after CS Exposure
NF-κB activation plays an important role in CS-mediated lung inflammation.19,44 In light of our data showing heightened CS-induced lung inflammatory response in NADPH oxidase subunit-deficient mice, we determined the nuclear levels of RelA/p65 and its phosphorylation on ser276 and ser536 residues in lungs of mice exposed to CS. Immunoblot analysis showed CS exposure increased the levels of RelA/p65 and its phosphorylation on ser276 and ser536 residues in nuclear protein of WT mice (Figure 10). Targeted disruption of p47phox and gp91phox further increased the levels of RelA/p65 and its phosphorylation on ser276 and ser536 residues in response to CS exposure (Figure 10). Furthermore, the basal levels of RelA/p65 and its phosphorylation on ser276 and ser536 residues were also increased in p47phox−/− and gp91phox−/− mice compared to air-exposed WT mice (Figure 10). These results suggested phosphorylation of RelA/p65 may be a contributing factor for CS-mediated NF-κB-dependent proinflammatory cytokine release in NADPH oxidase-deficient mice.
Figure 10.
Increased levels of RelA/p65 and its phosphorylation on ser276 and ser536 in lung nuclear protein of p47phox−/− and gp91phox−/− mice in response to 3 days and 8 weeks of CS exposure. Acute and subchronic CS exposure increased the levels of RelA/p65 and its phosphorylation on ser276 and ser536 in lung nuclear protein of WT mice that were further increased in p47phox−/− and gp91phox−/− mice. In addition, the basal levels of RelA/p65 and its phosphorylation at ser276 and ser536 in the lungs were significantly increased in p47phox−/− and gp91phox−/− mice as compared to air-exposed WT mice. Gel pictures shown are representative of at least three separate experiments. The protein levels of RelA/p65 and its phosphorylation on ser276 and ser536 were assayed by Western blotting.
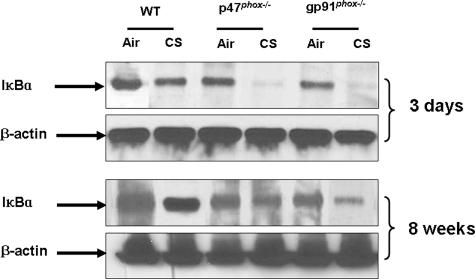
Degradation of IκBα Was Increased in Lung Cytoplasmic Fraction of p47phox−/− and gp91phox−/− Mice in Response to CS Exposure
In resting status, NF-κB activity is mainly controlled by its tight association with the IκB proteins, which prevents NF-κB from entering into the nucleus.45,46 To further investigate NF-κB activity, IκBα levels were determined by Western blot in lung cytoplasmic fractions of WT, p47phox−/−, and gp91phox−/− mice exposed to CS. IκBα was degraded in lung of WT, and p47phox−/− and gp91phox−/− mice exposed to CS (Figure 11). However, the degree of degradation in p47phox−/− and gp91phox−/− mice was more pronounced than those in WT mice exposed to CS (Figure 11). Moreover, the basal level of IκBα was also decreased in p47phox−/− and gp91phox−/− mice compared to air-exposed WT mice (Figure 11). These results demonstrated that genetic ablation of p47phox and gp91phox led to increased phosphorylation and nuclear translocation of RelA/p65 associated with IκBα degradation in response to CS exposure.
Figure 11.
Degradation of IκBα in lung cytoplasmic fraction is increased in p47phox−/− and gp91phox−/− mice as compared to WT mice in response to 3 days and 8 weeks of CS exposure. Acute and subchronic CS exposure led to degradation of IκBα in lung cytoplasmic fraction that was increased in p47phox−/− and gp91phox−/− mice. Moreover, the basal level of IκBα in the lungs was significantly decreased in p47phox−/− and gp91phox−/− mice as compared to air-exposed WT mice. Gel pictures shown are representative of at least three separate experiments. Protein levels of IκBα were determined by Western blotting.
Increased Release of Proinflammatory Mediators in Peritoneal Macrophages from p47phox−/− and gp91phox−/− Mice via Activation of NF-κB in Response to CSE Treatment
To further investigate whether enhanced NF-κB activation is one of the mechanisms underlying hyperinflammatory response seen in lungs of these KO mice in response to CS exposure, we isolated peritoneal macrophages from WT and NADPH oxidase-deficient mice, and treated with CSE. Consistent with the in vivo results, CSE (0.25%, 0.5%, and 1%) significantly increased the release of MCP-1 and KC from peritoneal macrophages in WT mice which was augmented in these cells derived from p47phox−/− and gp91phox−/− mice (Figure 12). To investigate whether CSE-mediated MCP-1 and KC release from peritoneal macrophages is dependent on NF-κB activation, we pretreated peritoneal macrophages with a specific inhibitor of NF-κB (BAY-117082)44 and assessed the effect on CSE-mediated MCP-1 and KC release. We found that BAY-117082 (10 μmol/L) significantly inhibited CSE-induced release of MCP-1 and KC in peritoneal macrophages from all WT, p47phox−/−, and gp91phox−/− mice (Figure 12). This suggests that CSE-mediated MCP-1 and KC release from peritoneal macrophages is dependent on NF-κB activation.
Figure 12.
Increased levels of MCP-1 and KC in peritoneal macrophages from p47phox−/− and gp91phox−/− in response to CSE treatment that was partially inhibited by NF-κB inhibitor. Peritoneal macrophages were isolated from WT, p47phox−/−, and gp91phox−/− mice, and treated with CSE (0.25%, 0.5%, and 1%) in the presence or absence of NF-κB inhibitor (BAY-117082). CSE (0.25%, 0.5%, and 1%) induced the release of MCP-1 and KC from peritoneal macrophages of WT that was further increased in peritoneal macrophages derived from p47phox−/− and gp91phox−/− mice. Treatment of peritoneal macrophages with the NF-κB inhibitor significantly decreased CSE-mediated release of MCP-1 and KC. Data are shown as mean ± SEM (n = 4 to 5 per group). **P < 0.01, ***P < 0.001, significant compared with respective control group; +P < 0.05, ++P < 0.01, +++P < 0.001, significant compared with respective medium or CSE-treated peritoneal macrophages from WT mice; #P < 0.05, ##P < 0.01, compared with respective CSE (0.5%)-treated group.
Increased Levels of TLR4 mRNA and Protein in Lungs of p47phox−/− and gp91phox−/− Mice after CS Exposure
TLR4 plays a critical role in CS-induced lung inflammation by activating the NF-κB pathway.47,48 We therefore determined whether genetic ablation of p47phox and gp91phox alters the levels of TLR4 mRNA and protein in the lung in response to CS exposure. We used a sensitive real-time RT-PCR approach to quantify the changes in TLR4 mRNA levels in the lung. The cycle threshold values were generated for each sample using a standard curve for TLR4 and GADPH. After normalization to GAPDH levels, acute and subchronic CS exposure significantly increased the level of TLR4 mRNA in lungs of WT mice that was further increased in p47phox−/− and gp91phox−/− mice (Figure 13A). Similarly, targeted disruption of p47phox and gp91phox increased TLR4 protein levels in lungs in response to both acute and subchronic CS exposures (Figure 13B). These results implicate that genetic ablation of NADPH oxidase leads to activation of TLR4, and this increase may be the mechanism of hyperinflammation seen in lungs of NADPH oxidase-deficient mice in response to CS exposure via NF-κB activation.
Figure 13.
Increased TLR4 mRNA and protein expression in lung of p47phox−/− and gp91phox−/− mice in response to CS exposure. Genetic knockout of gp91phox and p47phox increased lung levels of TLR4 mRNA (A) and protein (B) as compared to WT mice in response to CS exposure. The levels of TLR4 mRNA (A) and protein (B) were also increased in air-exposed p47phox−/− and/or gp91phox−/− mice compared to air-exposed WT mice. Results shown are from a representative with three to four mice per group. The TLR4 mRNA and protein was assayed by real-time RT-PCR and Western blotting, respectively. *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective air-exposed group; +P < 0.05, ++P < 0.01, +++P < 0.001, significant compared with CS-exposed WT mice; #P < 0.05, ##P < 0.01, compared with air-exposed WT mice.
Disassociation of TLR4 from NADPH Oxidase Subunits, and Increased Levels of TLR4 Protein in Peritoneal Macrophages from p47phox−/− and gp91phox−/− Mice in Response to CSE Treatment
It has been shown that lipopolysaccharide induces the interaction of TLR4 with gp91phox in RAW 264.7 cells that can be inhibited by NADPH oxidase inhibitor (diphenylene iodonium, DPI).49 In the present study, we found that CSE (0.5%) also caused interaction of TLR4 with p47phox and gp91phox in peritoneal macrophages from WT mice that was completely abrogated in peritoneal macrophages from p47phox−/− and gp91phox−/− mice, respectively (Figure 14, A and B). Consistent with the in vivo results, CSE (0.25%, 0.5%, and 1%) increased the level of TLR4 protein in peritoneal macrophages from WT mice that was further augmented in p47phox−/− and gp91phox−/− mice (Figure 15) suggesting that elevated and available TLR4 acts as a sensor for CS components to trigger downstream signaling such as NF-κB pathway for inflammatory response seen in lungs of p47phox−/− and gp91phox−/− mice in response to CS exposure.
Figure 14.
CSE causes the interaction of TLR4 with p47phox and gp91phox in peritoneal macrophages obtained from WT mice but this interaction is abrogated in p47phox−/− and gp91phox−/− mice. Peritoneal macrophages were isolated from WT, p47phox−/−, and gp91phox−/− mice, and treated with CSE (0.5%). CSE caused the interaction of TLR4 with p47phox (A) and gp91phox (B) in peritoneal macrophages from WT mice but this interaction was abrogated in p47phox−/− and gp91phox−/− mice, respectively. Gel pictures shown represent at least three separate experiments. The interaction of TLR4 with p47phox and gp91phox in peritoneal macrophages was analyzed using immunoprecipitation assay.
Figure 15.
Increased levels of TLR4 protein in peritoneal macrophages from p47phox−/− and gp91phox−/− mice in response to CSE treatment. The levels of TLR4 protein were significantly increased in peritoneal macrophages obtained from p47phox−/− and gp91phox−/− compared to that from WT mice in response to CSE (0.25%, 0.5%, and 1%) treatments. Moreover, genetic ablation of p47phox and gp91phox increased the basal level of TLR4 protein. Results shown are from a representative of three separate experiments. The level of TLR4 protein was assayed with Western blotting. *P < 0.05, ***P < 0.001, significant compared with respective control group; ++P < 0.01, +++P < 0.001, significant compared with respective CSE-treated peritoneal macrophages from WT mice; #P < 0.05, compared with control group in WT mice.
Up-Regulation of Nox4 in Lungs of p47phox−/− and gp91phox−/− Mice after CS Exposure
It has been shown that NADPH oxidase subunits are under tight regulatory control in physiological condition.13,14 It is possible that one of the subunits is compensated because of the loss of other subunits. Therefore, we determined the mRNA and protein levels of NADPH oxidase major components such as p47phox, gp91phox, Nox3, and Nox4 in lungs of WT, p47phox−/−, and gp91phox−/− mice in response to air and CS exposure. As expected, no bands of p47phox and gp91phox mRNA and protein were seen in lungs of p47phox- and gp91phox-deficient mice, respectively. However, CS exposure led to an increase in mRNA and protein of p47phox, gp91phox, Nox3, and Nox4 in lungs of WT mice. Furthermore, the mRNA and protein levels of p47phox, gp91phox, and Nox3 in lungs were not altered in the null mice compared to WT mice exposed to CS. Interestingly, CS exposure resulted in augmentation of both mRNA expression and protein levels of Nox4 in lungs of p47phox- and gp91phox-deficient mice compared to WT exposed to CS suggesting a compensatory role of Nox4 in these NADPH oxidase subunit-deficient mice in response to CS (Figure 16, A and B).
Figure 16.
Compensatory regulation of NADPH oxidase subunits in lung of p47phox−/− and gp91phox−/− mice in response to CS exposure. Genetic knockout of gp91phox and p47phox increased the lung levels of Nox4 mRNA (A) and protein (B) at 3 days and 8 weeks by CS exposure as compared to WT mice. However, the mRNA (A) and protein (B) levels of p47phox, gp91phox, and/or Nox3 were not altered in p47phox−/− and/or gp91phox−/− mice exposed to CS as compared to WT mice. CS exposure led to an increase in mRNA and protein of p47phox, gp91phox, Nox3, and Nox4 in lungs of WT mice. Results shown are from a representative with three to four mice per group. The TLR4 mRNA and protein was assayed by RT-PCR and Western blotting, respectively.
Discussion
CS is a strong inflammatory stimulus that induces proinflammatory mediators and recruits macrophages and neutrophils to the lung tissue.19,20,21,22,50,51 We, and others, have shown that O2·− release is increased from leukocytes isolated from smokers and in patients with COPD,15,16 as well as from endothelial cells in response to CS that is mediated via NADPH oxidase activation.11,12 However, the role of NADPH oxidase in CS-induced lung inflammation is not known. We hypothesized that inhibition of cellular ROS release by targeted ablation of components of NADPH oxidase (p47phox and gp91phox) would protect lungs against detrimental effects of CS. To determine the role of NADPH oxidase-derived O2·−, we exploited the use of p47phox−/− and gp91phox−/− mice in which the phagocytic cells are incapable of respiratory burst and release of ROS. We observed that both acute and subchronic CS exposures resulted in an increase of inflammatory cell infiltration, MPO activity, and mucus secretion in lungs of WT mice, and these responses were augmented in both p47phox−/− and gp91phox−/− mice. In addition, the levels of KC, MCP-1, IL-6, TNF-α, and/or GM-CSF were significantly increased in lungs of p47phox−/− and/or gp91phox−/− mice compared to WT mice in response to CS exposure, particularly after acute CS exposure. These findings were consistent with previous studies showing that enhanced inflammatory response occurs in the lungs of p47phox−/− and gp91phox−/− mice challenged to LPS when compared to WT mice.52,53 Thus, genetic ablation of p47phox and gp91phox enhanced the inflammatory response in lungs in response to CS exposure. However, a little discrepancy in inflammatory response was observed between the two strains of knockout mice in response to CS exposure. The mechanism of this disparity is not clear at this juncture, but it may be related to different cellular distributions and functions of p47phox and gp91phox besides their role in ROS production as reviewed by Bedard and Krause.54 Although the basis of increased inflammatory cell influx into the lungs of NADPH oxidase-deficient mice is not yet clear, a possible explanation of this finding is that there is an accumulation of ingested particles that cannot be phagositized, and/or decreased apoptosis in inflammatory cells, both of which are attributable to the defect in NADPH oxidase functioning.55,56 Furthermore, the increased inflammatory cell migration into the lung tissue of NADPH oxidase-deficient mice could be a source of the higher cytokine and chemokine release, which in turn could attract more peripheral monocytes and neutrophils into the lungs in response to CS exposure.5,21 It is interesting to note that the neutrophil infiltration and the levels of proinflammatory mediators in the lungs were decreased in subchronic CS-exposed mice as compared to acute CS-exposed mice, which corroborates our recent observation showing a similar biphasic proinflammatory mediator release in rat lungs after CS exposure.57 Nevertheless, these results suggested that CS induces the dynamics of inflammatory response in the lungs in vivo. Although the levels of chemokines were increased in both BAL fluid and lung tissue in response to CS exposure, the levels of chemokines in BAL fluid were much lower than that of lung tissue (data not shown). Thus, chemokine gradient between BAL fluid and lung tissue attracted more macrophages into the lungs (interstitium), which could contribute to the decrease in numbers of macrophages in BAL fluid whereas the number of macrophages was increased in lung interstitium in response to CS exposure. The other possibility would be that macrophages are trapped in the alveolar compartments because acute and subchronic CS exposure induced up-regulation of adhesion molecules and chemokines, and hence led to a reduction in number of macrophages recovered from the airspace.
The expression of proinflammatory mediators is regulated by the transcription factor NF-κB.58 It has been shown that NF-κB activity is mainly controlled by its association with the inhibitory IκB proteins, which prevents NF-κB from entering into the nucleus.45,46,58,59 Our results demonstrated that genetic ablation of p47phox and gp91phox increased cytoplasmic IκBα degradation induced by CS exposure, thus explaining the increased translocation of RelA/p65 into the nucleus in the KO mice. Additionally, posttranslational modification of NF-κB members is believed to represent a second level of regulating NF-κB activity. It has been shown that different phosphorylation sites have been mapped for the activating NF-κB RelA/p65 subunit.45,59 We observed an increase in phosphorylation of RelA/p65 on ser276 and ser536 in the nuclear extract of WT mice in response to CS exposure, and the phosphorylation on these sites on RelA/p65 was increased in p47phox−/− and gp91phox−/− mice. Previous studies demonstrated that phosphorylation of RelA/p65 on ser276 is required for association with CBP and NF-κB transcriptional activity.60,61 Therefore, increased RelA/p65 expression is possibly because of increased phosphorylation of RelA/p65 on ser276 as our results show. Consistent with in vitro studies,62 CS exposure also induced the phosphorylation of RelA/p65 on ser536 in the lungs. Furthermore, the basal level of phosphorylation of RelA/p65 on ser536 was also increased in p47phox−/− and gp91phox−/− mice compared to the air-exposed control WT mice. Recently, it has been shown that phosphorylation of ser536 is a prerequisite for acetylation of RelA/p65 on lysine310.19,63 We have also shown that acetylation of RelA/p65 plays an important role in sustained inflammatory gene transcription.19 Thus, the phosphorylation of RelA/p65 at ser276 and ser536 seen in p47phox−/− and gp91phox−/− mice would hypothetically lead to sustained and prolonged inflammatory response. Overall, NF-κB activation was enhanced in lungs of NADPH oxidase-deficient mice compared to WT mice in response to CS exposure. This was also confirmed by in vitro studies showing that the release of NF-κB-dependent proinflammatory mediators (MCP-1 and KC) in peritoneal macrophages from p47phox−/− and gp91phox−/− mice was increased as compared to that from WT mice in response to CSE treatment and this response was partially inhibited by NF-κB inhibitor (BAY117082).
TLRs have been implicated in a number of lung-associated immune responses and pathologies.64 It has been recently shown that neutralization of TLR4 with anti-human TLR4 antibody significantly blocked CSE-mediated proinflammatory mediator release by human macrophages.48 Furthermore, NF-κB activation induced by CSE was also attenuated in human macrophages pretreated with anti-human TLR4 antibody.48 Moreover, subacute (5 weeks) and chronic (26 weeks) CS exposure resulted in increased pulmonary expression of TLR4 in WT mice, and targeted disruption of TLR4 protected against acute and chronic CS-induced lung inflammatory response.47,65 Therefore, the TLR4-NF-κB signal pathway plays an important role in CS-mediated lung inflammatory response. Our findings corroborate with the above studies that acute and subchronic CS exposure led to increased levels of TLR4 mRNA and protein in the lungs of C57BL/6J mice. However, targeted disruption of p47phox and gp91phox augmented both mRNA and protein levels of TLR4 in lungs and peritoneal macrophages in response to CS, which is possibly because of TLR4 protein stabilization (posttranslational control) and/or enhanced TLR4 transcription. In addition, CSE treatment induced the interaction of TLR4 with p47phox and gp91phox in peritoneal macrophages obtained from WT mice that was completely abrogated leading to increased levels of TLR4 in peritoneal macrophages from p47phox−/− and gp91phox−/− mice. These results suggested that elevated TLR4 acts as a sensor for CS components (oxidants and aldehydes) to trigger downstream NF-κB inflammatory signaling. This would be the potential mechanism for proximal signaling and the hyperinflammatory response seen in the null mice in response to CS exposure.
Previously, it has been shown that lipopolysaccharide (LPS)-induced TLR4 mRNA expression and its stabilization was decreased by DPI and apocynin in human aortic smooth muscle cells66 suggesting that the inhibition of NADPH oxidase leads to attenuation of TLR4 mRNA expression. The reason of discrepancy between this study and our findings in the present study is not known. It is possible that this may be related to different models used in our study (CS exposure in vivo using genetic-deficient mice) whereas in other studies (LPS treatment using DPI and apocynin in vitro). However, another study demonstrated that DPI inhibited the interaction of TLR4 with gp91phox in RAW 264.7 cells in response to LPS,49 which is consistent with our finding of dissociation of TLR4 in gp91phox cells. In this study, the protein levels of TLR4 in membrane fraction were not altered in peritoneal macrophages obtained from gp91phox−/− mice compared to that of WT mice in response to LPS treatment.49 However, we showed that the level of TLR4 was increased in whole cell lysate of lung and peritoneal macrophages from p47phox−/− and gp91phox−/− mice in response to CS. These data suggested that TLR4 can be not only localized in membrane but also in cytosol,67 and genetic ablation of p47phox and gp91phox leads to dissociation of active TLR4 from a TLR4-NADPH oxidase complex to activate downstream proinflammatory signaling in response to CS.
In contrast to above studies, Zhang and colleagues34 have recently shown that genetic ablation of TLR4 led to enlargement of distal airspace at 3 months of age, which is distinct from our finding showing that elevated TLR4 acts as a sensor for CS components to trigger downstream NF-κB inflammatory signaling. The reason for this differential finding is not known. One possible explanation would be that our studies used the mice deficient in NADPH oxidase subunits as opposed to Zhang and colleagues34 who used TLR4-deficient mice in which ROS generation was different. Furthermore, our findings suggested the involvement of inflammatory mechanisms (non-ROS-dependent) is crucial for CS-induced airspace enlargement in these mice.
We further observed increased inflammatory cell infiltration and proinflammatory mediator release in the lungs, as well as destruction of alveolar architecture in air-exposed p47phox−/− and gp91phox−/− mice than those of air-exposed WT mice exposed subchronically to CS. These data are in agreement with the previous observation that gp91phox mutant mice develop spontaneous, progressive emphysema when their age is more than 6 months.68 The mechanism underlying these findings is not clear but it might be related to a defect in apoptotic cell removal by macrophages in these null mice in response to CS exposure because there is increasing evidence that apoptotic clearance mechanisms are less effective in lungs of COPD patients and that macrophages from such lungs show a defect in recognizing and ingesting apoptotic cells.69,70 These results suggest that ROS participate in tissue homeostasis, because the progression of lung injury in the p47phox−/− and gp91phox−/− mice in the absence of an excess exogenous stimulus implies that ROS derived from NADPH oxidase is engaged in the normal turnover of alveolar extracellular matrix. However, the basal levels of NADPH oxidase subunits (p47phox, gp91phox, and/or Nox3), ROS, and oxidative damage of DNA and lipid were not altered in these null mice compared to WT mice suggesting that stimuli are required to exacerbate the oxidative stress. Indeed, CS-mediated ROS release, lipid peroxidation, and oxidative DNA damage were decreased in lungs of p47phox−/− and gp91phox−/− mice although a compensatory increase in mRNA and protein of Nox4, but not in p47phox, gp91phox, or Nox3, was observed in these null mice exposed to CS.
In summary, enhanced inflammatory response was observed in lungs of p47phox−/− and gp91phox−/− mice compared to WT mice exposed to CS. Furthermore, genetic ablation of p47phox and gp91phox led to development of distal airway enlargement accompanied by alveolar destruction as early as 8 week of CS exposure. In addition, targeted disruption of p47phox and gp91phox led to increased TLR4 and activation of RelA/p65 by CS exposure that may be the underlying mechanism of increased proinflammatory effects of CS in lungs of these mice. These data suggest that genetic ablation of p47phox and gp91phox, which decreases ROS production but enhances susceptibility to proinflammatory effects of CS leading to airspace enlargement in these mice. Thus, the long-term use of NADPH oxidase inhibitor as proposed previously for redressing the oxidant/antioxidant imbalance in COPD may be deleterious.
Acknowledgments
We thank Chengyu Wu, Department of Pathology, University of Rochester Medical Center, for his assistance in the real-time RT-PCR assay in mouse lung tissue.
Footnotes
Address reprint requests to Dr. Irfan Rahman, Department of Environmental Medicine, Lung Biology and Disease Program, University of Rochester Medical Center, Box 850, 601 Elmwood Ave., Rochester, NY 14642. E-mail: irfan_rahman@urmc.rochester.edu.
Supported by the National Institutes of Health (grant R01-HL085613), Institute for Science and Health, National Institute of Environmental Health Sciences grant ES01247, and National Institute of Environmental Health Sciences toxicology training grant ES07026.
References
- Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:176–183. doi: 10.1513/pats.200402-009MS. [DOI] [PubMed] [Google Scholar]
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasday JD, Bascom R, Costa JJ, Fitzgerald T, Dubin W. Bacterial endotoxin is an active component of cigarette smoke. Chest. 1999;115:829–835. doi: 10.1378/chest.115.3.829. [DOI] [PubMed] [Google Scholar]
- Bracke KR, D’Hulst AI, Maes T, Moerloose KB, Demedts IK, Lebecque S, Joos GF, Brusselle GG. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol. 2006;177:4350–4359. doi: 10.4049/jimmunol.177.7.4350. [DOI] [PubMed] [Google Scholar]
- Demedts IK, Brusselle GG, Bracke KR, Vermaelen KY, Pauwels RA. Matrix metalloproteinases in asthma and COPD. Curr Opin Pharmacol. 2005;5:257–263. doi: 10.1016/j.coph.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003;163:2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Dodd OJ, Pearse DB. Effect of the NADPH oxidase inhibitor apocynin on ischemia-reperfusion lung injury. Am J Physiol. 2000;279:H303–H312. doi: 10.1152/ajpheart.2000.279.1.H303. [DOI] [PubMed] [Google Scholar]
- Orosz Z, Csiszar A, Labinskyy N, Smith K, Kaminski PM, Ferdinandy P, Wolin MS, Rivera A, Ungvari Z. Cigarette smoke-induced proinflammatory alterations in the endothelial phenotype: role of NAD(P)H oxidase activation. Am J Physiol. 2007;292:H130–H139. doi: 10.1152/ajpheart.00599.2006. [DOI] [PubMed] [Google Scholar]
- Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol. 2004;24:1031–1036. doi: 10.1161/01.ATV.0000127083.88549.58. [DOI] [PubMed] [Google Scholar]
- Debidda M, Williams DA, Zheng Y. Rac1 GTPase regulates cell genomic stability and senescence. J Biol Chem. 2006;281:38519–38528. doi: 10.1074/jbc.M604607200. [DOI] [PubMed] [Google Scholar]
- Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med. 1999;159:473–479. doi: 10.1164/ajrccm.159.2.9804080. [DOI] [PubMed] [Google Scholar]
- Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154:1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanisms. Cell Biochem Biophys. 2005;43:167–188. doi: 10.1385/CBB:43:1:167. [DOI] [PubMed] [Google Scholar]
- Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol. 2007;170:855–864. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher TH, McHugh NA, Egan RW, Chapman RW, Hey JA, Turner CK, Redonnet MR, Seweryniak KE, Sime PJ, Phipps RP. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol. 2005;289:L322–L328. doi: 10.1152/ajplung.00039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, Macnee W, Rahman I. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol. 2004;31:633–642. doi: 10.1165/rcmb.2004-0006OC. [DOI] [PubMed] [Google Scholar]
- Foronjy RF, Mirochnitchenko O, Propokenko O, Lemaitre V, Jia Y, Inouye M, Okada Y, D’Armiento JM. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am J Respir Crit Care Med. 2006;173:623–631. doi: 10.1164/rccm.200506-850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwick JA, Stevenson CS, Giddings J, MacNee W, Butler K, Rahman I, Kirkham PA. Cigarette smoke disrupts VEGF165-VEGFR-2 receptor signaling complex in rat lungs and patients with COPD: morphological impact of VEGFR-2 inhibition. Am J Physiol. 2006;290:L897–L908. doi: 10.1152/ajplung.00116.2005. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Paul JL, Thurlbeck WM. The effect of age on lung structure in male BALB/cNNia inbred mice. Am J Anat. 1984;170:1–21. doi: 10.1002/aja.1001700102. [DOI] [PubMed] [Google Scholar]
- Imai K, Mercer BA, Schulman LL, Sonett JR, D’Armiento JM. Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J. 2005;25:250–258. doi: 10.1183/09031936.05.00023704. [DOI] [PubMed] [Google Scholar]
- Foronjy RF, Mercer BA, Maxfield MW, Powell CA, D’Armiento J, Okada Y. Structural emphysema does not correlate with lung compliance: lessons from the mouse smoking model. Exp Lung Res. 2005;31:547–562. doi: 10.1080/019021490951522. [DOI] [PubMed] [Google Scholar]
- Castro P, Legora-Machado A, Cardilo-Reis L, Valenca S, Porto LC, Walker C, Zuany-Amorim C, Koatz VL. Inhibition of interleukin-1beta reduces mouse lung inflammation induced by exposure to cigarette smoke. Eur J Pharmacol. 2004;498:279–286. doi: 10.1016/j.ejphar.2004.07.047. [DOI] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins CS, Pouladi MA, Fattouh R, Dawe DE, Vujicic N, Richards CD, Jordana M, Inman MD, Stampfli MR. Mainstream cigarette smoke exposure attenuates airway immune inflammatory responses to surrogate and common environmental allergens in mice, despite evidence of increased systemic sensitization. J Immunol. 2005;175:2834–2842. doi: 10.4049/jimmunol.175.5.2834. [DOI] [PubMed] [Google Scholar]
- LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Shimizu C, Fuda H, Yanai H, Strott CA. Conservation of the hydroxysteroid sulfotransferase SULT2B1 gene structure in the mouse: pre- and postnatal expression, kinetic analysis of isoforms, and comparison with prototypical SULT2A1. Endocrinology. 2003;144:1186–1193. doi: 10.1210/en.2002-221011. [DOI] [PubMed] [Google Scholar]
- Lund AK, Peterson SL, Timmins GS, Walker MK. Endothelin-1-mediated increase in reactive oxygen species and NADPH oxidase activity in hearts of aryl hydrocarbon receptor (AhR) null mice. Toxicol Sci. 2005;88:265–273. doi: 10.1093/toxsci/kfi284. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116:3050–3059. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van ’t Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol. 2002;168:1286–1293. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J. 2004;18:1897–1899. doi: 10.1096/fj.04-1506fje. [DOI] [PubMed] [Google Scholar]
- Carp H, Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978;118:617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- van der Strate BW, Postma DS, Brandsma CA, Melgert BN, Luinge MA, Geerlings M, Hylkema MN, van den Berg A, Timens W, Kerstjens HA. Cigarette smoke-induced emphysema: a role for the B cell? Am J Respir Crit Care Med. 2006;173:751–758. doi: 10.1164/rccm.200504-594OC. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000;164:1546–1552. doi: 10.4049/jimmunol.164.3.1546. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Jung B, Shim JJ, Burgel PR, Dao-Pick T, Ueki IF, Protin U, Kroschel P, Nadel JA. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am J Physiol. 2001;280:L165–L172. doi: 10.1152/ajplung.2001.280.1.L165. [DOI] [PubMed] [Google Scholar]
- Leikauf GD, Borchers MT, Prows DR, Simpson LG. Mucin apoprotein expression in COPD. Chest. 2002;121:166S–182S. doi: 10.1378/chest.121.5_suppl.166s. [DOI] [PubMed] [Google Scholar]
- Sommerhoff CP, Nadel JA, Basbaum CB, Caughey GH. Neutrophil elastase and cathepsin G stimulate secretion from cultured bovine airway gland serous cells. J Clin Invest. 1990;85:682–689. doi: 10.1172/JCI114492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer R, Lucey EC, Stone PJ, Christensen TG, Snider GL. Proteolytic activity of human neutrophil elastase and porcine pancreatic trypsin causes bronchial secretory cell metaplasia in hamsters. Exp Lung Res. 1985;9:167–175. doi: 10.3109/01902148509061535. [DOI] [PubMed] [Google Scholar]
- Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol. 2006;291:L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- Schmitz ML, Bacher S, Kracht M. I kappa B-independent control of NF-kappa B activity by modulatory phosphorylations. Trends Biochem Sci. 2001;26:186–190. doi: 10.1016/s0968-0004(00)01753-9. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Role of transcription factors in inflammatory lung diseases. Thorax. 1998;53:601–612. doi: 10.1136/thx.53.7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes T, Bracke KR, Vermaelen KY, Demedts IK, Joos GF, Pauwels RA, Brusselle GG. Murine TLR4 is implicated in cigarette smoke-induced pulmonary inflammation. Int Arch Allergy Immunol. 2006;141:354–368. doi: 10.1159/000095462. [DOI] [PubMed] [Google Scholar]
- Karimi K, Sarir H, Mortaz E, Smit JJ, Hosseini H, De Kimpe SJ, Nijkamp FP, Folkerts G. Toll-like receptor-4 mediates cigarette smoke-induced cytokine production by human macrophages. Respir Res. 2006;7:66–76. doi: 10.1186/1465-9921-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Wang X, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med. 2004;170:492–498. doi: 10.1164/rccm.200404-511OC. [DOI] [PubMed] [Google Scholar]
- Churg A, Dai J, Tai H, Xie C, Wright JL. Tumor necrosis factor-alpha is central to acute cigarette smoke-induced inflammation and connective tissue breakdown. Am J Respir Crit Care Med. 2002;166:849–854. doi: 10.1164/rccm.200202-097OC. [DOI] [PubMed] [Google Scholar]
- Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH, Malik AB. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J Immunol. 2002;168:3974–3982. doi: 10.4049/jimmunol.168.8.3974. [DOI] [PubMed] [Google Scholar]
- Koay MA, Christman JW, Segal BH, Venkatakrishnan A, Blackwell TR, Holland SM, Blackwell TS. Impaired pulmonary NF-kappaB activation in response to lipopolysaccharide in NADPH oxidase-deficient mice. Infect Immun. 2001;69:5991–5996. doi: 10.1128/IAI.69.10.5991-5996.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bylund J, Goldblatt D, Speert DP. Chronic granulomatous disease: from genetic defect to clinical presentation. Adv Exp Med Biol. 2005;568:67–87. doi: 10.1007/0-387-25342-4_5. [DOI] [PubMed] [Google Scholar]
- Kobayashi SD, Voyich JM, Braughton KR, Whitney AR, Nauseef WM, Malech HL, DeLeo FR. Gene expression profiling provides insight into the pathophysiology of chronic granulomatous disease. J Immunol. 2004;172:636–643. doi: 10.4049/jimmunol.172.1.636. [DOI] [PubMed] [Google Scholar]
- Stevenson CS, Docx C, Webster R, Battram C, Hynx D, Giddings J, Cooper PR, Chakravarty P, Rahman I, Marwick JA, Kirkham PA, Charman C, Richardson DL, Nirmala NR, Whittaker P, Butler K. Comprehensive gene expression profiling of rat lung reveals distinct acute and chronic responses to cigarette smoke inhalation. Am J Physiol. 2007;293:L1183–L1193. doi: 10.1152/ajplung.00105.2007. [DOI] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Anrather J, Racchumi G, Iadecola C. Cis-acting, element-specific transcriptional activity of differentially phosphorylated nuclear factor-kappa B. J Biol Chem. 2005;280:244–252. doi: 10.1074/jbc.M409344200. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Sakon S, Sasazuki T, Sakurai H, Doi T, Yagita H, Okumura K, Nakano H. Phosphorylation of serine 276 is essential for p65 NF-kappaB subunit-dependent cellular responses. Biochem Biophys Res Commun. 2003;300:807–812. doi: 10.1016/s0006-291x(02)02932-7. [DOI] [PubMed] [Google Scholar]
- Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- Syed DN, Afaq F, Kweon MH, Hadi N, Bhatia N, Spiegelman VS, Mukhtar H. Green tea polyphenol EGCG suppresses cigarette smoke condensate-induced NF-kappaB activation in normal human bronchial epithelial cells. Oncogene. 2007;26:673–682. doi: 10.1038/sj.onc.1209829. [DOI] [PubMed] [Google Scholar]
- Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol. 2004;286:L887–L892. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- Doz E, Noulin N, Boichot E, Guenon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, Couillin I. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180:1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- Lin FY, Chen YH, Tasi JS, Chen JW, Yang TL, Wang HJ, Li CY, Chen YL, Lin SJ. Endotoxin induces toll-like receptor 4 expression in vascular smooth muscle cells via NADPH oxidase activation and mitogen-activated protein kinase signaling pathways. Arterioscler Thromb Vasc Biol. 2006;26:2630–2637. doi: 10.1161/01.ATV.0000247259.01257.b3. [DOI] [PubMed] [Google Scholar]
- Powers KA, Szaszi K, Khadaroo RG, Tawadros PS, Marshall JC, Kapus A, Rotstein OD. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J Exp Med. 2006;203:1951–1961. doi: 10.1084/jem.20060943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassim SY, Fu X, Liles WC, Shapiro SD, Parks WC, Heinecke JW. NADPH oxidase restrains the matrix metalloproteinase activity of macrophages. J Biol Chem. 2005;280:30201–30205. doi: 10.1074/jbc.M503292200. [DOI] [PubMed] [Google Scholar]
- Henson PM, Vandivier RW, Douglas IS. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc. 2006;3:713–717. doi: 10.1513/pats.200605-104SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson PM, Cosgrove GP, Vandivier RW. State of the art. Apoptosis and cell homeostasis in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:512–516. doi: 10.1513/pats.200603-072MS. [DOI] [PMC free article] [PubMed] [Google Scholar]