Abstract
Prion diseases are untreatable neurodegenerative disorders characterized by accumulation of PrPSc, an aggregated isoform of the normal prion protein PrPC. Here, we delivered the soluble prion antagonist PrP-Fc2 to the brains of mice by lentiviral gene transfer. Although naïve mice developed scrapie at 175 ± 5 days postintracerebral prion inoculation (dpi), gene transfer before inoculation delayed disease onset by 72 ± 4 days. At 170 days postintracerebral prion inoculation, PrPSc accumulation and prion infectivity in PrPFc-treated brains were reduced by 3.6 and 4.2 logs, respectively. When PrP-Fc2 was delivered 30 days after prion inoculation, survival of the treated animals was extended by 25 days. We then used tissue-specific recombination to express PrP-Fc2 in the entire central nervous system, in only astrocytes, or in only oligodendrocytes. Oligodendrocyte-restricted PrP-Fc2 expression impaired PrPSc deposition and delayed disease even though oligodendrocytes are completely resistant to prion infection, suggesting that PrP-Fc2 affords protection via noncell autonomous mechanisms. These results suggest that somatic gene transfer of prion antagonists may be effective for postexposure prophylaxis of prion diseases.
To date, no efficacious treatments for prion diseases are available in clinical settings.1,2 Anti-PrP antibodies can delay scrapie after peripheral challenge of prions.3,4 However, this strategy does not target PrPSc replication in the brain, and intracerebral delivery of anti-PrPC antibodies may be neurotoxic.5 Active immunization strategies are meeting with little success most likely because endogenous PrPC induces robust immune tolerance.6,7 Transgenic expression of a soluble dimeric PrP, termed PrP-Fc2, affects prion propagation and scrapie pathogenesis by interfering with the buildup of PrPSc.8 The high solubility and stability of PrP-Fc2 prompted us to explore its therapeutic potential. We therefore used lentiviral vectors9 to investigate in a scrapie mouse model whether sustained intracerebral expression of PrP-Fc2 inhibits prion replication.
Materials and Methods
Mice
Mouse lines tg550Stop, carrying a loxP-flanked transcription-termination cassette upstream of the open reading frame of PrPFc, and tg588CMV, in which the transcription-termination cassette was eliminated, were described previously.8 Mice carrying the Cre recombinase under the transcriptional control of the Nestin, glial fibrillary acidic protein (GFAP), or proteolipid protein (PLP) regulatory elements have been described elsewhere.10,11,12 tg550Stop × PLP/Cre-ERT2, tg550Stop (but Cre-negative), and PLP/Cre-ERT2 (but tg550Stop-negative) littermates were treated with tamoxifen dissolved in a sunflower oil/ethanol (10:1) mixture at 10 mg/ml. Twice 1 mg of tamoxifen per day for 5 consecutive days was injected intraperitoneally, as described.12
Vector Production and Injection
PrPFc cDNA was cloned into the lentiviral vector pRRL.sin.cPPT.hCMV.GFP.Wpre9 in place of GFP. Vectors stocks were generated as described9 by co-transfection of 293T with pCMV-ΔR8.91,13 pMD.G,13 and pRRL.sin.cPPT.hCMV.PrPFc vectors. 293T-conditioned media were filtered and ultracentrifuged to concentrate the vector.9 Particle content was measured by HIV-1 p24 antigen immunocapture.9 HIV-1 p24 concentration was between 500 to 600 μg/ml (corresponding to 100,000 infectious units per ng of p24). C57BL/6 mice were slowly injected with 5 μl of the lentiviral preparations or phosphate-buffered saline (PBS) stereotaxically into the hippocampus or 30 μl intracerebrally into the right telencephalic hemisphere. Integration of the transgene was detected by polymerase chain reaction (PCR) with standard conditions using primers amplifying the PrP and Fc regions (Table 1).
Table 1.
Sequences of PCR Primers and PrPFc Open Reading Frame
| Forward primer recognizing PrP-moiety of PrPFc |
| 5′-AACGACTGGGAGGACCGCTA-3′ |
| Reverse primer recognizing Fc-moiety of PrPFc |
| 5′-CCTTGCCATTCAGCCAGTCC-3′ |
| Open reading frame of PrPFc |
| 5′-ATGGCGAACCTTGGCTACTGGCTGCTGGCCCTCTTTGTGACTATGTGGACTGATGTCGGCCTCTGCAAAAAGCGGCCAAAGCCTGGAGGGTGGAA-CACCGGTGGAAGCCGGTATCCCGGGCAGGGAAGCCCTGGAGGCAACCGTTACCCACCTCAGGGTGGCACCTGGGGGCAGCCCCACGGTGGTGGC-TGGGGACAACCCCATGGGGGCAGCTGGGGACAACCTCATGGTGGTAGTTGGGGTCAGCCCCATGGCGGTGGATGGGGCCAAGGAGGGGGTACC-CATAATCAGTGGAACAAGCCCAGCAAACCAAAAACCAACCTCAAGCATGTGGCAGGGGCTGCGGCAGCTGGGGCAGTAGTGGGGGGCCTTGGTGG-CTACATGCTGGGGAGCGCCATGAGCAGGCCCATGATCCATTTTGGCAACGACTGGGAGGACCGCTACTACCGTGAAAACATGTACCGCTACCCTA-ACCAAGTGTACTACAGGCCAGTGGATCAGTACAGCAACCAGAACAACTTCGTGCACGACTGCGTCAATATCACCATCAAGCAGCACACGGTCACC-ACCACCACCAAGGGGGAGAACTTCACCGAGACCGATGTGAAGATGATGGAGCGCGTGGTGGAGCAGATGTGCGTCACCCAGTACCAGAAGGAGT-CCCAGGCCTATTACGACGGGAGAAGATCCGGGGGCCTCGACAAAACTCACACATGCCCACCGTGCCCAGCACCTGAAGCCGAGGGGGCACCG-TCAGTCTTCCTCTTCCCCCCAAAACCCAAGGACACCCTCATGATCTCCCGGACCCCTGAGGTCACATGCGTGGTGGTGGACGTGAGCCACGAA-GACCCTGAGGTCAAGTTCAACTGGTACGTGGACGGCGTGGAGGTGCATAATGCCAAGACAAAGCCGCGGGAGGAGCAGTACAACAGCACGTACCG-TGTGGTCAGCGTCCTCACCGTCCTGCACCAGGACTGGCTGAATGGCAAGGAGTACAAGTGCAAGGTCTCCAACAAAGCCCTCCCAGCCTCCA-TCGAGAAAACCATCTCCAAAGCCAAAGGGCAGCCCCGAGAACCACAGGTGTACACCCTGCCCCCATCCCGGGATGAGCTGACCAAGAACCAGGT-CAGCCTGACCTGCCTGGTCAAAGGCTTCTATCCCAGCGACATCGCCGTGGAGTGGGAGAGCAATGGGCAGCCGGAGAACAACTACAAGACCAC-GCCTCCCGTGTTGGACTCCGACGGCTCCTTCTTCCTCTACAGCAAGCTCACCGTGGACAAGAGCAGGTGGCAGCAGGGGAACGTCTTCTCA-TGCTCCGTGATGCATGAGGCTCTGCACAACCACTACACGCAGAAGAGCCTCTCCCTGTCTCCGGGTAAA-3’ |
Target sequences of the primers are underlined.
Western Blot Analysis and NaPTA Precipitation
Sodium phosphotungstate precipitation and quantitative Western blot were performed as described.8 Brain and spleen samples were digested for 30 minutes at 37°C with 50 μg/ml and 20 μg/ml of proteinase K, respectively. Antibody incubations were done in 1% Top-Block (Sigma-Aldrich Chemia GmbH, Buchs, Switzerland) in Tris-buffered saline Tween 20 with ICSM183 or POM114 for PrP and PrP-Fc2 for 1 hour at room temperature or overnight at 4°C. The samples were analyzed with a VersaDoc digital imager (model 5000; Bio-Rad, Hercules, CA).
Bone Marrow (BM) Chimeric Mice
BM cells (5 × 106) were isolated from tibiae and femurs, and injected into tail veins of 8- to 10-week-old recipients conditioned by whole-body irradiation (1100 rad). Several paradigms were generated: wt BM was injected into wt or tg588-irradiated mice, and tg588 BM was injected into wt or tg588-irradiated mice. The tg588CMV line (for brevity tg588) was used in the BM reconstitution experiments because of the sixfold higher expression of PrP-Fc2 in spleen in tg588 compared to tg550.8 Eight weeks after grafting, reconstitution was assessed by enzyme-lined immunosorbent assay (ELISA) analysis of peripheral blood taken from the retro-orbital plexus of ether-anesthetized mice.
ELISA Analysis
ELISA to detect PrP-Fc2 was performed as described.3,4 Briefly, plates (Maxisorp; Nunc, Roskilde, Denmark) were coated with monoclonal anti-PrP antibody ICSM 18, washed with PBS containing 0.1% (v/v) Tween 20 (PBST), and blocked with 5% bovine serum albumin (BSA). After washing, plates were incubated with 30 μl of twofold serially diluted serum (1:20 prediluted) in PBST containing 1% BSA and then probed with horseradish peroxidase-conjugated goat anti-human IgG-Fc horseradish peroxidase (1:1000 dilution; Rockland Immunochemicals, Gilbertsville, PA). Plates were developed with 2,2′-azino-diethyl-benzothiazolinsulfonate, and optical density was measured at 405 nm. Titer was defined as the highest dilution showing an OD more than two times the technical background, which was calculated as the average of uncoated wells and wells incubated omitting serum. For detection of anti-human Fc and anti-GFP antibodies in serum of lentivirus-treated mice, the plates were coated with purified human Fc and GFP-His,15 respectively. The antibodies from the serum were detected in the plates by using rabbit anti-mouse IgG + A + M (H + L)-horseradish peroxidase conjugate (Zymed/Invitrogen AG, Basel, Switzerland).
Histopathology
Brains were fixed in 4% paraformaldehyde, paraffin-embedded, and cut into 2-μm sections. Sections were stained with hematoxylin and eosin (H&E) and with anti-GFAP.16 Histoblots were performed according to published protocols.17 For immunohistochemistry, cryosections were fixed 5 minutes in paraformaldehyde, 2 minutes in 50% acetone, 2 minutes in 100% acetone, 2 minutes in 50% acetone, PBS-washed, and blocked for 30 minutes in 5% donkey serum. Rabbit anti-human Fcγ (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted 1:1000 in 0.5% BSA was used as primary antibody and biotinylated donkey anti-rabbit (Jackson ImmunoResearch Laboratories) was used as a secondary antibody diluted 1:100 in 0.5% BSA. In our experience, histoimmunochemical detection of PrP-Fc2 in tissue sections proved difficult because PrP-Fc2 is a molecule secreted from cells.
Prion Inoculations
Mice were inoculated intracerebrally or intraperitoneally with 3 × 102 or 103 LD50 infectious units of Rocky Mountain Laboratory strain (RML, passage 5) scrapie prions prepared as described.8 Mice were sacrificed on the day of onset of terminal clinical signs of scrapie. Significance P values were derived by comparing mean survival (Student’s t-test; two-tailed distribution, two-sample unequal variance). Survival data were plot by using GraphPad Prism 4.
Infectivity Bioassays with tga20 Mice
Assays were performed on 1% homogenates of brain or of spleen tissues as described.18 Tissues were homogenized in 0.32 mol/L sucrose with a homogenizer and diluted 1:10 in 5% BSA in PBS. Supernatants (30 μl) were inoculated intracerebrally into groups of three to four tga20 mice.19 Infectivity titers were calculated as described.20
Scrapie Cell Assay in Endpoint Format (SCEPA)
For SCEPA, highly RML prion-susceptible neuroblastoma cells (subclone N2aPK121) were exposed to prion samples for 3 days in 96-well plates, and split three times 1:3 every 2 days, and three times 1:10 every 3 days. After reaching confluence, 25,000 cells from each well were filtered onto the membrane of white Immobilon P plate (Millipore, Billerica, MA) treated with PK, denatured, and individual infected (PrPSc-positive) cells were detected by ELISA using antibody POM-1 to PrP. After reaching confluence, 25,000 cells from each well were processed as above. Wells were counted positive if the spot number was clearly exceeding background. From the proportion of negative to total wells the number of infectious tissue culture (TCI) units per aliquot was calculated by the Poisson equation as described previously.21 The potency of the SCEPA is based on the finding that the proportion of infected cells, and with it the signal-to-background ratio, increases on average ∼25% per day during culturing. The sensitivity of the assay can be further enhanced by increasing the number of replicate samples and the number of 1:10 splits.
Preparation of Cerebellar Granule Cells, Oligodendrocytes, and Astrocytes
Cerebellar granule neurons were prepared from 7- to 8-day-old mice as previously described.22 Contamination with glial cells was <5%. Mixed glial cell cultures containing oligodendrocytes and astrocytes were produced from 1-day-old neonatal mice as described.23,24 Cultures were prepared with high-glucose Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum and replenished on day 4 and every 3 to 4 days thereafter for 10 days, with Dulbecco’s modified Eagle medium plus 10% heat-inactivated horse serum. Oligodendrocytes were purified from mixed glial cultures by differential detachment and negative selection of microglia by adherence to hydrophobic plastic. Purified oligodendrocytes were then plated onto glass or plastic culture chambers coated, respectively, with 100 μg/ml or 10 μg/ml poly-l-lysine, whereas astrocytes were kept in the same dish. Oligodendrocyte precursors were expanded with platelet-derived growth factor- and fibroblast growth factor-supplemented SATO medium for 2 days and subsequently differentiated with 1% horse serum-supplemented SATO medium for 3 days.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde in PBS for 15 minutes at room temperature. The cultures were permeabilized and blocked in PBS supplemented with 0.1% Triton X-100 and 10% fetal calf serum. Cells were then incubated with antibodies against neuronal-specific nuclear protein (NeuN, 1:50), glial fibrillary acidic protein (GFAP, 1:100; DAKO, Glostrup, Denmark), and myelin-associated glycoprotein (MAG; 1:100; Chemicon, Temecula, CA) diluted in 1% BSA in PBS at 4°C overnight. After washing, cells were incubated with goat anti-mouse secondary antibodies conjugated with Alexa 546 (1: 200; Molecular Probes, Eugene, OR) or with donkey anti-rabbit secondary antibodies conjugated with fluorescein isothiocyanate (1:50; Jackson Laboratory, Bar Harbor, ME). Nuclear staining was performed with DAPI.
Results
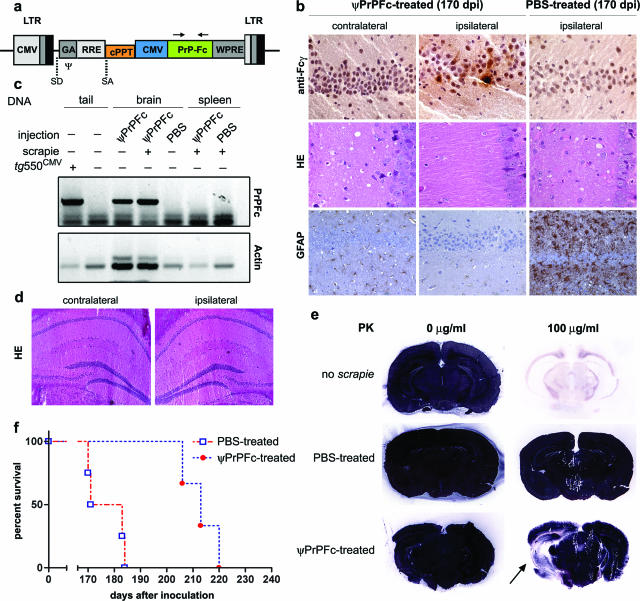
We generated a recombinant lentiviral vector transducing PrP-Fc2 (henceforth termed ψPrPFc; Figure 1a). In a first series of experiments we investigated whether ψPrPFc, when precisely targeted to a defined group of cells, would afford local protection. We therefore injected a relatively small amount of ψPrPFc (3 × 108 infectious units in 5 μl) into a group of five mice intrahippocampally under stereotaxic guidance. Controls were injected with PBS (n = 5). One mouse from each group was sacrificed 6 months after virus injection to provide a first indication about disease progression. PrP-Fc2 was detected by immunohistochemistry in the injected hippocampus, but not in the contralateral hemisphere (Figure 1b, top row). Accordingly, lentiviral integration in the brain was demonstrated by PCR analysis (Figure 1c). No adverse reactions were detected by conventional histology (Figure 1d), nor by immunohistochemistry with various astroglial, microglial, and inflammatory markers (GFAP, CD11b, Iba-1; data not shown).
Figure 1.
Intrahippocampal lentiviral transfer of PrP-Fc2. a: Schematic representation of the transfer vector used in this study. b: Hippocampal PrP-Fc2 expression in the injected hippocampus (top row) coincided with suppressed prion pathogenesis in ψPrPFc-treated mice sacrificed at 170 dpi (middle and bottom rows), as assessed by immunohistochemistry using an anti-human Fcγ antibody. c: PCR using primers indicated in a of various tissues and genotypes showing brain-specific lentiviral integration. Genomic actin amplification served for normalization of the PrPFc signal (concentration of genomic DNA in crude extract from the tissue samples was not controlled in this experiment). d: No pathology in the brain was associated with the lentiviral injection as determined by H&E staining and by various markers (GFAP, CD11b, Iba-1; data not shown) in nonprion inoculated mice. e: Histoblots showed a broad area of decreased PrPSc deposition extending well beyond the site of ψPrPFc injection (arrow). In contrast, PrPSc signals were saturated in the contralateral hemisphere and in a PBS-treated brain. f: Survival plots displaying the incubation time until development of terminal scrapie in PBS- and ψPrPFc-treated mice after intracerebral challenge. Median disease onset was delayed by 36 days in intrahippocampally ψPrPFc-treated mice. LTR, long terminal repeats; CMV, cytomegalovirus; SD, splice donor; SA, splice acceptor; Ψ, packaging sequence; GA, 5′ portion of gag gene with truncated reading frame including extended packaging signal; RRE, rev response element (RRE); cPPT, central polypurine tract; WPRE, posttranscriptional regulatory element from the genome of the woodchuck hepatitis virus.
Twenty days after virus injection, four ψPrPFc-treated mice and four PBS controls were inoculated intracerebrally with 3 × 102 LD50 RML prions. All PBS-injected control animals succumbed to disease [177 ± 8 days postintracerebral prion inoculation (dpi)], whereas none of the ψPrPFc-treated animals showed clinical signs at 177 dpi (Figure 1f). One clinically unaffected ψPrPFc-treated mouse was sacrificed at 170 dpi and analyzed for the presence of PrPSc by histoblotting. Whereas PBS-treated animals showed massive PrPSc accumulation, the ψPrPFc-injected mouse showed markedly reduced accumulation of PrPSc in the injected hippocampus. Importantly, ipsilateral reduction in PrPSc signal intensity extended well beyond the track of the stereotaxic needle, suggesting that ψPrPFc exerts long-range antiprion effects. In the contralateral hemisphere, however, PrPSc deposition was indistinguishable from that of PBS-treated controls (Figure 1e). Histological analysis failed to reveal any vacuolation and astrogliosis in the ipsilateral hippocampus (Figure 1b, middle and bottom rows). The contralateral side presented with rather strong vacuolation and astrogliosis, although the corresponding region of a terminally sick PBS-treated animal may have been more strongly affected (Figure 1b, middle and bottom rows). ψPrPFc-injected mice succumbed to disease at 213 ± 6 dpi (Figure 1f), which is remarkable, considering substantial vacuolation and PrPSc deposits detected in the contralateral side of the ψPrPFc-injected mice sacrificed for analysis at 170 dpi (see above; Figure 1b, middle row; and Figure 1e, bottom row). Therefore, a single unilateral hippocampal injection sufficed to delay onset of disease by 36 days (P < 0.01) as compared to PBS-injected controls.
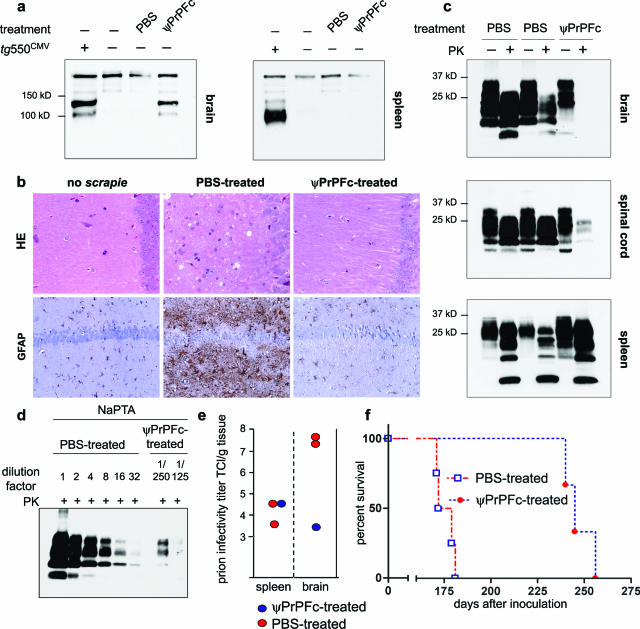
The above results, along with the observation that the antiprion action of PrP-Fc2 is dose-dependent in transgenic mice,8,25 encouraged us to explore whether nonstereotaxic delivery of a larger number of ψPrPFc particles would enhance protection. We injected 30 μl of ψPrPFc (1.5 × 109 infectious units) intracerebrally into the right telencephalic hemisphere of mice (n = 4), and inoculated them with 3 × 102 LD50 RML prions intracerebrally 20 days later. Six months after virus injection, immunoblot analysis revealed sustained PrP-Fc2 expression in brains of ψPrPFc-treated mice (Figure 2a).
Figure 2.
Intracerebral lentiviral vector administration. a: Western blot analysis of frontal brain of transgenic PrP-Fc2 mice,8 and PBS-treated and ψPrPFc-treated animals. Using anti-PrP antibody,3 sustained PrP-Fc2 expression was detected 6 months after virus injection in the brain of ψPrPFc-treated animals, but not in the spleen. b: Severe spongiosis and astrogliosis in terminally sick PBS-treated animals, but no sign of prion-associated pathology in a ψPrPFc-treated animal sacrificed 170 dpi, similarly to age-match uninoculated mice. Sections were stained with H&E and immunostained with GFAP for detection of astrocytosis. c. Whereas PrPSc signals were visible in wt brains and spinal cords at terminal stage, no or weak signals were present in the brain and spinal cord of a ψPrPFc-treated animal sacrificed at the same time point. However, spleens of treated and controls animals exhibited similar quantities of PrPSc. d: NaPTA-enhanced quantitative Western analysis showed that PrPSc accumulation in the tested ψPrPFc-treated brain was 0.03% of the tested PBS-treated brain. To reach a comparable intensity level for Western blot quantification, control samples were diluted linearly up to 32 times, whereas for ψPrPFc-treated brains, PrPSc was concentrated by NaPTA precipitation from 125- or 250-fold more starting material than was used directly for Western blot in the case of PBS-treated mouse, lane 1. e: Prion infectivity, determined in the brain of one ψPrPFc-treated animal by the scrapie cell assay in endpoint format (SCEPA), was reduced by 4.2 log TCl/g tissue compared to PBS-treated animals. f: Survival of scrapie-infected mice treated with PBS or ψPrPFc. Whereas PBS-treated mice (n = 4) succumbed to disease at 175 ± 5 dpi, ψPrPFc-treated animals (n = 3) had an extended survival of 72 days (247 ± 8 dpi).
We sacrificed one presymptomatic ψPrPFc-treated mouse and two terminally sick PBS-treated mice at 171 dpi to estimate prion pathogenesis at this experimental paradigm. Profound histopathological changes were identified in PBS-treated brains (Figure 2b). In contrast, the ψPrPFc-treated brain displayed no spongiform changes ipsi- or contralaterally. Astrogliosis, estimated by GFAP immunoreactivity, was similar to age-matched uninfected mice (Figure 2b).
Brain, spinal cord, and spleen homogenates were subjected to proteinase K (PK) digestion and analyzed for accumulation of PrPSc by Western blot analysis. Surprisingly, whereas wild-type (wt) animals showed extensive buildup of PrPSc, no PrPSc was detectable in the brain of the ψPrPFc-treated animal and only traces thereof in the spinal cord (Figure 2c). Conversely, spleens from all animals showed similar accumulation of PrPSc, suggesting that protection was restricted to the central nervous system (CNS) (Figure 2c). To increase the sensitivity of PrPSc detection, we concentrated PrPSc by differential precipitation with sodium phosphotungstic acid (NaPTA). Quantitative Western blot analysis4 showed that PrPSc accumulation in ψPrPFc-treated brain was 0.03% of PBS-treated brain. To reach a comparable intensity level for Western blot quantification, control samples were diluted linearly up to 32 times, whereas for ψPrPFc-treated brains, PrPSc was concentrated by NaPTA precipitation from 125- or 250-fold more starting material than is was used directly for Western blot in the case of PBS-treated mouse, lane 1 (Figure 2d). Prion infectivity titers determined by end-point scrapie cell assay21 were similar in spleens of PBS- and ψPrPFc-treated mice [4.1 and 4.6 log infectious tissue culture units (TCI U)/g tissue, respectively, Figure 2e]. In contrast, prion titers in control brains (n = 2) reached 7.6 log TCI U/g tissue, but only 3.4 log TCI U/g tissue in the treated animal (Figure 2e). Typical progressive signs of scrapie, including ataxia and kyphosis, led to terminal disease in all PBS-treated mice by 175 ± 5 dpi (n = 4). In contrast, ψPrPFc-treated animals succumbed to prion disease at 247 ± 8 dpi (n = 3), suggesting remarkable resistance to the disease (72 days) and extending their lifespan by more than 40% (Figure 2f). Therefore, single-dose gene transfer of PrPFc provides very potent protection against prion disease, and suppresses prion replication by >10,000-fold.
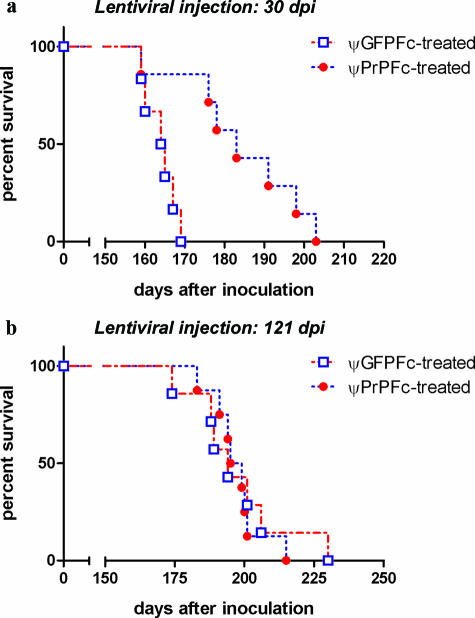
Next, we tested whether PrP-Fc2 might afford postexposure prophylaxis. Mice were inoculated with 3 × 102 LD50 RML prions intracerebrally and, 30 days later, intracerebrally treated with ψPrPFc (1.5 × 109 IU; n = 7). Control animals injected with ψGFPFc (0.8 × 109 IU; n = 6) succumbed to scrapie at 163 ± 4 dpi, whereas ψPrPFc-treated animals survived 188 ± 11 days (P < 0.01) (Figure 3a). Terminally sick mice from both groups showed similar PrPSc accumulation in their brains (data not shown). We then administered ψPrPFc at 121 dpi, when scrapie-induced brain damage is already evident by histology (A.A., unpublished data). ψGFPFc (n = 7)- and ψPrPFc (n = 8)-treated animals succumbed with similar incubation times (197 ± 17 dpi and 197 ± 9 dpi, respectively), suggesting that treatment at this stage is ineffective (Figure 3b). Importantly, we detected by ELISA anti-GFP antibodies in serum of the ψGFPFc-treated mice and anti-human Fc antibodies in serum of the ψGFPFc-treated mice and ψPrPFc-treated mice (data not shown). This indicates that secreted GFP-Fc2 and PrP-Fc2, both fused to human Fc, crossed the blood-brain-barrier, and were processed by the murine immune system as immunogens. Induction of the transgene-specific antibodies may affect prion propagation. It has been reported that chronic activation of the immune system in the periphery facilitates prion pathogenesis.26,27,28 This may also explain faster onset of disease seen for the groups treated with the same lentivirus at 30 dpi compared to 121 dpi. (Figure 3, a and b).
Figure 3.
Intracerebral lentiviral vector transfer during prion disease progression. a: Survival plots displaying the incubation time until development of terminal scrapie in ψGFPFc and ψPrPFc (n = 7) mice treated 30 days after RML prion inoculation. Median disease onset was delayed by 25 days in ψPrPFc-treated mice. b: When prion-inoculated mice were injected with ψPrPFc 121 dpi, they succumbed at the same time as ψGFPFc-treated mice (respectively, 197 ± 17 dpi and 197 ± 9 dpi).
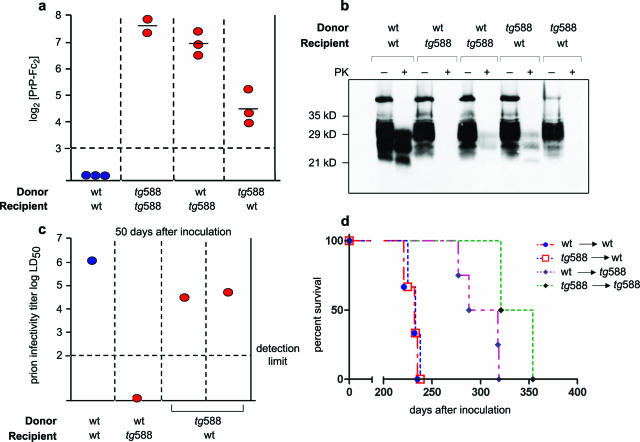
We then sought to determine whether peripherally expressed PrP-Fc2 contributes to the antiprion effect. We used transplantation of genetically modified BM progenitors, because this technique may represent a gene therapy vehicle. BM of PrP-Fc2-expressing mice (tg588CMV)8 or of wt mice was transferred to wt or to tg588CMV mice. Eight weeks after BM grafting, the concentration of PrP-Fc2 was assessed in peripheral blood by ELISA (Figure 4a). Blood PrP-Fc2 concentrations of tg588CMV mice that had received wt BM approached those of tg588CMV→tg588CMV mice and were four to six times higher than those of wt mice reconstituted with tg588CMV BM, indicating that circulating PrP-Fc2 is mainly contributed by nonhematopoietic radioresistant cells (Figure 4a).
Figure 4.
Effects of hematopoietically expressed PrP-Fc2. a: PrP-Fc2 titers (relative units) in blood of chimeric mice. tg588→wt reconstituted mice showed weaker PrP-Fc2 expression than tg588 mice. b: Fifty days after RML prion inoculation, spleens from tg588→wt and wt→ tg588 showed decreased accumulation of PrPSc compared to wt→wt spleens. c: Infectivity titer of wt→ tg588 (n = 1) was below detectability and those of tg588→wt (n = 2) were 1.2 log lower than wt→wt (n = 2, average is represented) controls. d: wt→wt and tg588→wt reconstituted mice succumbed to scrapie at the same stage, whereas wt→ tg588 and tg588→ tg588 mice were retarded.
Twelve weeks after reconstitution, chimeric mice were challenged intraperitoneally with 103 LD50 RML prions. At 50 dpi, immunoblot analysis showed that PrPSc accumulation was reduced in spleens of tg588CMV→wt (n = 2) and wt→tg588CMV (n = 2) compared to wt→wt mice (Figure 4b). Accordingly, splenic prion titers in tg588CMV→wt (n = 2) were 1.2 log lower than wt→wt (n = 2) (Figure 4c) and wt→tg588 spleen (n = 1) showed no detectable infectivity (Figure 4c). Wt→wt mice (n = 3) and tg588CMV→wt mice (n = 3) developed scrapie with identical latency (232 ± 7 dpi and 232 ± 6 dpi, respectively), whereas wt→tg588CMV (n = 4) and tg588CMV→tg588CMV (n = 2) were affected later (300 ± 21 dpi and 337 ± 25 dpi, respectively) (Figure 4d). These results indicate that PrP-Fc2-expressing hematopoietic cells antagonized PrPSc accumulation and prion replication, yet failed to modify the clinical course of prion disease.
Replication-defective lentiviral vectors are not expected to spread throughout the brain after a single unilocular injection.29 PrP-Fc2 may therefore owe its potent antiprion efficacy to secretion and extracellular spread. This scenario assumes that PrP-Fc2 would be capable of antagonizing PrPSc deposition and prion replication in a noncell autonomous manner. We tested this hypothesis in tg550Stop mice, which bear a PrP-Fc2 transgene preceded by a loxP-flanked transcriptional stop cassette.8 tg550Stop mice8 were bred to nestin/Cre10 (tg550Nestin), GFAP/Cre11 (tg550GFAP), or PLP/CreERT212 (tg550PLP) recombinator mice, activating expression of PrP-Fc2 in the entire CNS, in astrocytes, or in oligodendrocytes, respectively. Before prion inoculation, tg550Stop × PLP/Cre-ERT2, tg550Stop (but Cre-negative), and PLP/Cre-ERT2 (but tg550Stop-negative) littermates were treated daily with 2 mg of tamoxifen intraperitoneally for 5 days to activate Cre recombination.12
Restriction of transgene expression to the specified cell populations was tested on primary cell cultures of cerebellar granule neurons, astrocytes, and oligodendrocytes from tg550Nestin, tg550GFAP, and tg550PLP mice. Purity in these primary cultures was typically >95%, as determined by specific cellular marker stains (see Supplemental Figure S1 at http://ajp.amjpathol.org). Cells were lysed and subjected to Western blot analysis for PrP-Fc2 (see Supplemental Figure S2 at http://ajp.amjpathol.org). Expression of PrP-Fc2 was relatively weak in tg550PLP oligodendrocytes (maybe because the Prnp promoter is weakly active in oligodendrocytes30,31), but undetectable in neurons and astrocytes (see Supplemental Figure S2 at http://ajp.amjpathol.org). Tg550Nestin showed sustained generalized PrP-Fc2 expression in the CNS, whereas PrP-Fc2 was mainly secreted by tg550GFAP astrocytes (see Supplemental Figure S2 at http://ajp.amjpathol.org).
Tg550Nestin, tg550GFAP, and littermate control mice were inoculated with 3 × 102 LD50 RML prions intracerebrally or 103 LD50 RML prions intraperitoneally. Typical signs of scrapie, including ataxia and kyphosis, occurred in all inoculated mice. However, tg550Nestin and tg550GFAP mice showed delayed disease after intracerebral inoculation (151 and 65 days, respectively; P < 0.01) (Figure 5, a and c) and after intraperitoneal inoculation (90 and 65 days, respectively; P < 0.01) (Figure 5, b and d). Astrocytes have been shown to be competent for prion replication,32 although the toxicity of astrocytic PrPSc to neurons is somewhat controversial.33,34
Figure 5.
Protection against scrapie can be mediated both by neuronally and glially restricted expression of PrP-Fc2. a–f: Survival plots of scrapie-infected transgenic mice expressing PrP-Fc2 in the entire CNS (a, b), in astrocytes (c, d), or in oligodendrocytes (e, f) after intracerebral (a, c, e) or intraperitoneal RML prion inoculation (b, d, f). Expression of PrP-Fc2 in oligodendrocytes, which are inherently resistant to scrapie, delayed prion disease, suggesting a noncell autonomous antiprion effect. g: Immunoblot analysis revealed reduced levels of PrPSc in brains of transgenic mice expressing PrP-Fc2 cells specifically. h: GFAP staining indicated diminished pathological changes, in agreement with delayed disease progression.
To determine whether PrP-Fc2 exerts its antiprion effect when produced by cells resistant to prion infection, we inoculated tg550PLP, which expressed PrP-Fc2 selectively in oligodendrocytes (see Supplemental Figure S2 at http://ajp.amjpathol.org) and Schwann cells.12 Transgenic mice expressing PrP under the control of the myelin basic protein-promoter are resistant to prion infection, suggesting that oligodendrocytes and Schwann cells do not support cell-autonomous prion replication and neural spread of prions.31
We found that tg550PLP mice (n = 8), which expressed low amounts of PrP-Fc2, showed delayed disease over tamoxifen-treated tg550Stop and PLP/Cre-ERT2 mice. The delay was 35 days (P < 0.01) after intracerebral inoculation (Figure 5e) and 50 days (P < 0.01) after intraperitoneal inoculation (Figure 5f). Terminal illness was unambiguously accompanied by clinical scrapie signs. Spleens from tg550Nestin, tg550GFAP, tg550PLP, and controls did not show any PrP-Fc2 expression (see Supplemental Figure S3a at http://ajp.amjpathol.org) nor any difference in their accumulation of PrPSc (see Supplemental Figure S3b at http://ajp.amjpathol.org).
Determination of prion titers19 50 days after intracerebral inoculation demonstrated that all spleens from tg550Nestin, tg550GFAP, and tg550PLP and their littermate controls contained approximately the same infectivity, confirming the similar prion replication rate in spleens of each genotype (see Supplemental Table S1 at http://ajp.amjpathol.org). Although brains of wt mice contained prion titers between 2.7 to 4.5 log LD50/g tissue after 50 dpi intracerebrally, tg550Nestin, tg550GFAP, and tg550PLP brain titers were below detectability. At 100 dpi, tg550Nestin brain titers were still reduced compared to wt controls (see Supplemental Table S1 at http://ajp.amjpathol.org).
Immunoblot analysis of PrPSc in tg550Nestin, tg550GFAP, tg550PLP, and wt littermate brains revealed similar PrPSc deposition at terminal disease (Figure 5g). In contrast, tg550Nestin, tg550GFAP, and tg550PLP sacrificed at the time at which wt controls mice had developed terminal scrapie, showed markedly reduced PrPSc accumulation (Figure 5g). With the caveat that Western blot analyses are only semiquantitative, this suggests that PrPSc deposition is counteracted even when PrP-Fc2 is expressed by CNS cells unable to replicate prions.
Next, we analyzed neuropathological changes during the course of the disease. At terminal stage (181/183/224 dpi), all control animals showed pathological evidence of neuroinvasive CNS scrapie infection to a similar extent (Figure 5h and data not shown). At these time points, tg550Nestin mice showed only weak astrogliosis, vacuolation, and PrPSc deposition (Figure 5h and data not shown), whereas in tg550GFAP and tg550PLP mice, neuropathological changes were more advanced, as reflected by astrocytosis, PrPSc deposition, and spongiform degeneration (Figure 5h and data not shown), but were still reduced in comparison to wt sick mice. Therefore, PrP-Fc2 not only delays PrPSc accumulation in the brain, but also retards histopathological changes. The entirety of the above results strongly suggests that PrP-Fc2 delays PrPSc accumulation and prion disease in a noncell autonomous manner.
Discussion
We have explored in vivo gene therapy in a mouse model of prion disease. Our approach resulted in sustained expression of a soluble prion antagonist in the brain, impaired replication of disease-associated PrPSc, effective neuropathological protection, and delayed disease progression. Even though lentiviral transduction is limited to the site of injection,29 PrPSc accumulation was decreased in most of the brain. The most plausible interpretation is that secreted PrP-Fc2 was released into the cerebrospinal fluid, where it antagonized PrPSc replication noncell autonomously, as suggested in a previous report.8
Why was ψPrPFc less efficient to delay prion pathogenesis when delivered during disease progression? Transgene expression may take 4 to 6 weeks before reaching its maximum.35 Therefore, after injection at 30 dpi, high expression of PrP-Fc2 was achieved in the brain ∼60 to 75 dpi, when prion infectivity and PrPSc have already colonized the brain. Indeed, we detected PrPSc in the brain by Western blot as early as 58 dpi (data not shown). Because there is a competitive interaction between PrP-Fc2 and PrPC for PrPSc,8 the presence of substoichiometric PrP-Fc2 may not be sufficient to efficiently prevent PrPSc formation and may only weakly slow down its accumulation.
Direct gene delivery of a soluble prion antagonist into the CNS by lentiviral vectors may be an effective strategy against prion diseases. In contrast, almost all previous therapeutic interventions only showed benefits after extracerebral injection, probably reflecting the difficulty of most of the drugs to cross the blood-brain barrier.3,36,37,38 Because variant Creutzfeld-Jakob disease can be diagnosed before death on lymphoid tissues39 and patients may display extraneural PrPSc long before neuroinvasion,40 gene delivery of prion antagonists directly in the brain may be sufficient to block PrPSc before it has already colonized the CNS. In this context, our findings may provide a promising new approach to prion therapeutics.
Acknowledgments
We thank D. Robay and G. Bosshard for technical help, Harald Seeger for determination of prion titers by scrapie-cell assay, S. Marino for GFAP/Cre mice, M. Kawe and A. Plückthun for purified GFP-His, A. Audigé for p24 ELISA, M. Glatzel and F. Heppner for mouse inoculation, C. Weissmann for critical reading of the manuscript, and the Lentiviral Vector Production Unit of the Swiss Institute of Technology Lausanne (EPFL, supported by the Association Française contre les Myopathies) for providing lentivirus production.
Footnotes
Address reprint requests to Adriano Aguzzi, Institute of Neuropathology, Schmelzbergstr. 12, CH-8091 Zürich, Switzerland. E-mail: adriano.aguzzi@usz.ch.
See Related Commentary on page 1171
Supported by the Bundesamt für Bildung und Wissenschaft (to A.A.), the Swiss National Foundation (to A.A. and U.S.), the National Center for Competence in Research on “Neural Plasticity and Repair” (to A.A. and U.S.), the Koetser Research Foundation (to N.G.), and the Roche Research Foundations (to N.G.).
Supplementary material for this article can be found on http://ajp.amjpathol.org.
Current address of N.G.: Gene Expression Laboratory, The Salk Institute, San Diego CA; current address of M.P.: Department of Neuropathology, University of Freiburg, Freiburg, Germany; and current address of D.O.: Institute of Chemical Sciences and Engineering, Lausanne, Switzerland.
References
- Aguzzi A, Sigurdson CJ. Antiprion immunotherapy: to suppress or to stimulate? Nat Rev Immunol. 2004;4:725–736. doi: 10.1038/nri1437. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Glatzel M, Montrasio F, Prinz M, Heppner FL. Interventional strategies against prion diseases. Nat Rev Neurosci. 2001;2:745–749. doi: 10.1038/35094590. [DOI] [PubMed] [Google Scholar]
- White AR, Enever P, Tayebi M, Mushens R, Linehan J, Brandner S, Anstee D, Collinge J, Hawke S. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature. 2003;422:80–83. doi: 10.1038/nature01457. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Musahl C, Arrighi I, Klein MA, Rulicke T, Oesch B, Zinkernagel RM, Kalinke U, Aguzzi A. Prevention of scrapie pathogenesis by transgenic expression of anti-prion protein antibodies. Science. 2001;294:178–182. doi: 10.1126/science.1063093. [DOI] [PubMed] [Google Scholar]
- Solforosi L, Criado JR, McGavern DB, Wirz S, Sanchez-Alavez M, Sugama S, DeGiorgio LA, Volpe BT, Wiseman E, Abalos G, Masliah E, Gilden D, Oldstone MB, Conti B, Williamson RA. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science. 2004;303:1514–1516. doi: 10.1126/science.1094273. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Kratke O, Burwinkel M, Riemer C, Schultz J, Henklein P, Bamme T, Baier M. Immunisation with a synthetic prion protein-derived peptide prolongs survival times of mice orally exposed to the scrapie agent. Neurosci Lett. 2003;350:187–189. doi: 10.1016/s0304-3940(03)00907-8. [DOI] [PubMed] [Google Scholar]
- Polymenidou M, Heppner FL, Pellicioli EC, Urich E, Miele G, Braun N, Wopfner F, Schaetzl H, Becher B, Aguzzi A. Humoral immune response to native eukaryotic prion protein correlates with anti-prion protection. Proc Natl Acad Sci USA. 2004;101:14670–14676. doi: 10.1073/pnas.0404772101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P, Genoud N, Prinz M, Maissen M, Rulicke T, Zurbriggen A, Raeber AJ, Aguzzi A. Soluble dimeric prion protein binds PrP(Sc) in vivo and antagonizes prion disease. Cell. 2003;113:49–60. doi: 10.1016/s0092-8674(03)00201-0. [DOI] [PubMed] [Google Scholar]
- Follenzi A, Naldini L. Generation of HIV-1 derived lentiviral vector. Methods Enzymol. 2002;346:454–465. doi: 10.1016/s0076-6879(02)46071-5. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- Leone DP, Genoud S, Atanasoski S, Grausenburger R, Berger P, Metzger D, Macklin WB, Chambon P, Suter U. Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Mol Cell Neurosci. 2003;22:430–440. doi: 10.1016/s1044-7431(03)00029-0. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- Polymenidou M, Stoeck K, Glatzel M, Vey M, Bellon A, Aguzzi A. Coexistence of multiple PrPSc types in individuals with Creutzfeldt-Jakob disease. Lancet Neurol. 2005;4:805–814. doi: 10.1016/S1474-4422(05)70225-8. [DOI] [PubMed] [Google Scholar]
- Kawe M, Plückthun A. GroEL walks the fine line: the subtle balance of substrate and co-chaperonin binding by GroEL. A combinatorial investigation by design, selection and screening. J Mol Biol. 2006;357:411–426. doi: 10.1016/j.jmb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Genoud N, Behrens A, Miele G, Robay D, Heppner FL, Freigang S, Aguzzi A. Disruption of Doppel prevents neurodegeneration in mice with extensive Prnp deletions. Proc Natl Acad Sci USA. 2004;101:4198–4203. doi: 10.1073/pnas.0400131101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandner S, Raeber A, Sailer A, Blattler T, Fischer M, Weissmann C, Aguzzi A. Normal host prion protein (PrPC) is required for scrapie spread within the central nervous system. Proc Natl Acad Sci USA. 1996;93:13148–13151. doi: 10.1073/pnas.93.23.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büeler H, Raeber A, Sailer A, Fischer M, Aguzzi A, Weissmann C. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol Med. 1994;1:19–30. [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Rülicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Klein MA, Schwarz P, Aguzzi A. Efficient lymphoreticular prion propagation requires prp(c) in stromal and hematopoietic cells. J Virol. 2001;75:7097–7106. doi: 10.1128/JVI.75.15.7097-7106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöhn PC, Stoltze L, Flechsig E, Enari M, Weissmann C. A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc Natl Acad Sci USA. 2003;100:11666–11671. doi: 10.1073/pnas.1834432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JB, Weller M, Klockgether T. Potassium deprivation-induced apoptosis of cerebellar granule neurons: a sequential requirement for new mRNA and protein synthesis. ICE-like protease activity, and reactive oxygen species. J Neurosci. 1996;16:4696–4706. doi: 10.1523/JNEUROSCI.16-15-04696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J, Schachner M. Cells positive for the O4 surface antigen isolated by cell sorting are able to differentiate into astrocytes or oligodendrocytes. Brain Res Dev Brain Res. 1989;46:115–122. doi: 10.1016/0165-3806(89)90148-x. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masel J, Genoud N, Aguzzi A. Efficient inhibition of prion replication by PrP-Fc(2) suggests that the prion is a PrP(Sc) oligomer. J Mol Biol. 2005;345:1243–1251. doi: 10.1016/j.jmb.2004.10.088. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Heikenwalder M. Pathogenesis of prion diseases: current status and future outlook. Nat Rev Microbiol. 2006;4:765–775. doi: 10.1038/nrmicro1492. [DOI] [PubMed] [Google Scholar]
- Klein MA, Kaeser PS, Schwarz P, Weyd H, Xenarios I, Zinkernagel RM, Carroll MC, Verbeek JS, Botto M, Walport MJ, Molina H, Kalinke U, Acha-Orbea H, Aguzzi A. Complement facilitates early prion pathogenesis. Nat Med. 2001;7:488–492. doi: 10.1038/86567. [DOI] [PubMed] [Google Scholar]
- Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112:7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Consiglio A, Quattrini A, Martino S, Bensadoun JC, Dolcetta D, Trojani A, Benaglia G, Marchesini S, Cestari V, Oliverio A, Bordignon C, Naldini L. In vivo gene therapy of metachromatic leukodystrophy by lentiviral vectors: correction of neuropathology and protection against learning impairments in affected mice. Nat Med. 2001;7:310–316. doi: 10.1038/85454. [DOI] [PubMed] [Google Scholar]
- Moser M, Colello RJ, Pott U, Oesch B. Developmental expression of the prion protein gene in glial cells. Neuron. 1995;14:509–517. doi: 10.1016/0896-6273(95)90307-0. [DOI] [PubMed] [Google Scholar]
- Prinz M, Montrasio F, Furukawa H, van der Haar ME, Schwarz P, Rülicke T, Giger O, Häusler KG, Glatzel M, Aguzzi A. Intrinsic resistance of oligodendrocytes to prion infection. J Neurosci. 2004;24:5974–5981. doi: 10.1523/JNEUROSCI.0122-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeber AJ, Race RE, Brandner S, Priola SA, Sailer A, Bessen RA, Mucke L, Manson J, Aguzzi A, Oldstone MB, Weissmann C, Chesebro B. Astrocyte-specific expression of hamster prion protein (PrP) renders PrP knockout mice susceptible to hamster scrapie. EMBO J. 1997;16:6057–6065. doi: 10.1093/emboj/16.20.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. doi: 10.1126/science.1090187. [DOI] [PubMed] [Google Scholar]
- Jeffrey M, Goodsir CM, Race RE, Chesebro B. Scrapie-specific neuronal lesions are independent of neuronal PrP expression. Ann Neurol. 2004;55:781–792. doi: 10.1002/ana.20093. [DOI] [PubMed] [Google Scholar]
- Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- Cashman NR, Caughey B. Prion diseases—close to effective therapy? Nat Rev Drug Discov. 2004;3:874–884. doi: 10.1038/nrd1525. [DOI] [PubMed] [Google Scholar]
- Montrasio F, Frigg R, Glatzel M, Klein MA, Mackay F, Aguzzi A, Weissmann C. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science. 2000;288:1257–1259. doi: 10.1126/science.288.5469.1257. [DOI] [PubMed] [Google Scholar]
- Mabbott NA, McGovern G, Jeffrey M, Bruce ME. Temporary blockade of the tumor necrosis factor receptor signaling pathway impedes the spread of scrapie to the brain. J Virol. 2002;76:5131–5139. doi: 10.1128/JVI.76.10.5131-5139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AF, Zeidler M, Ironside J, Collinge J. Diagnosis of new variant Creutzfeldt-Jakob disease by tonsil biopsy. Lancet. 1997;349:99–100. doi: 10.1016/S0140-6736(97)24002-X. [DOI] [PubMed] [Google Scholar]
- Hilton DA, Fathers E, Edwards P, Ironside JW, Zajicek J. Prion immunoreactivity in appendix before clinical onset of variant Creutzfeldt-Jakob disease. Lancet. 1998;352:703–704. doi: 10.1016/S0140-6736(98)24035-9. [DOI] [PubMed] [Google Scholar]