Abstract
Originally described as a nuclear protein that bends DNA, the high mobility group box 1 protein (HMGB1) has recently emerged as a necessary and sufficient late mediator of severe sepsis. HMGB1 is therefore a molecular target that provides a wide window for clinical intervention in sepsis. Vasoactive intestinal peptide (VIP) and urocortin are two well known anti-inflammatory neuropeptides that protect against several immune disorders by regulating a wide panel of inflammatory mediators. In this study, we demonstrate the therapeutic effect of VIP and urocortin in various models of established sepsis: both agents reduced lethality induced by cecal ligation and puncture or by injection of live Escherichia coli. The therapeutic effect of VIP and urocortin was accompanied by a decrease in systemic levels of HMGB1. In addition, administration of recombinant HMGB1 completely reversed the protective effect of VIP and urocortin in experimental sepsis. In vitro and ex vivo studies show that both VIP and urocortin down-regulate translocation of HMGB1 from the nucleus to the cytoplasm and its subsequent secretion by activated macrophages, suggesting that macrophages are major targets in the inhibitory activity of these neuropeptides. To our knowledge, VIP and urocortin are the first endogenous inhibitors of HMGB1 secretion shown to improve sepsis survival in a clinically relevant time frame.
High mobility group box 1 (HMGB1), a chromatin-binding protein, was recently described as a late inflammatory factor secreted by monocytes and macrophages.1 Numerous evidences indicate that HMGB1 is a necessary and sufficient late mediator of severe sepsis.2 Patients and animals with sepsis or endotoxemia present high levels of systemic HMGB1, and administration of HMGB1 to mice causes epithelial cell dysfunction and lethal multiple organ damage.1,3,4 In addition, blocking of HMGB1 improves survival and prevents organ failure in septic mice.4,5 Therefore, the late kinetic action of HMGB1 provides a wider time frame for the treatment of sepsis. Anti-inflammatory mediators are secreted by the host innate immune system during the ongoing process to restore homeostasis. However, the endogenous factors involved in the control of HMGB1 secretion are poorly known.
Vasoactive intestinal peptide (VIP) and urocortin (UCN) are two neuropeptides widely distributed that exert multiple functions in the body. VIP and UCN are produced by several immune cells, especially under inflammatory stimuli, and have potent anti-inflammatory effects.6 The capacity of these neuropeptides to regulate a wide range of inflammatory mediators makes them attractive therapeutic candidates for the treatment of inflammatory and autoimmune diseases, such as endotoxemia, rheumatoid arthritis, and inflammatory bowel disease.6 The aim of this work was to investigate the effect of VIP and UCN on the secretion of HMGB1 and their potential therapeutic effect in severe established sepsis.
Materials and Methods
Animal Models
Animal experimental protocols were reviewed and approved by the Ethical Committee of the Spanish Council of Scientific Research. To induce endotoxemia, BALB/c mice (6 to 8 weeks old; Jackson Laboratories, Campbell, CA) were injected i.p. with lipopolysaccharide (LPS) (100 μg/mouse, Sigma-Aldrich, St. Louis, MO), or with a bacterial suspension containing 108 live Escherichia coli (DH5α). To induce sepsis, cecum of anesthetized BALB/c mice was ligated 5.0 mm from the cecal tip and punctured once with a 22 gauge needle, and the stool was then extruded (1 mm). Vehicle (controls), VIP (1 nmol, American Peptides, Sunnyvale, CA), or UCN (1 nmol, American Peptides) were administered i.p. starting at 12 or 24 hours after the cecal ligation and puncture (CLP), 2 hours after E. coli injection or 30 minutes after LPS infusion. The effective concentrations of neuropeptides used in the study were chosen based on previous experiments performed in our laboratory. In some experiments, recombinant HMGB1 (100 μg/mouse, HMGBiotech, Milan, Italy) was administered i.p. in VIP- and UCN-treated animals 18 hours after CLP. Animals were monitored daily for survival and clinical signs (ruffled fur, lethargy, diarrhea, and piloerection). Sera were obtained at different time points by cardiac puncture.
Cell Culture
BALB/c peritoneal macrophages or RAW264.7 cells were cultured at 106 cells/ml in RPMI medium 1640 (with 10% heat-inactivated fetal bovine serum, 2 mmol/L glutamine, and antibiotic-antimycotic mixture) for 2 hours, washed with Opti-MEM medium (Invitrogen, Carlsbad, CA) 2 hours later, and stimulated for 24 hours with LPS in the presence or absence of VIP or UCN in Opti-MEM. Supernatants were precipitated with trichloroacetic acid for HMGB1 determination.
Cytokine Determination
Cytokine contents in sera were determined by Multiplex (Bio-Rad, Hercules, CA) and BD CBA Flex Set (Becton Dickinson) assays following the manufacturer’s recommendations.
HMGB1 Western Blot Analysis
Serum was filtered and concentrated through Centricon YM-100 and YM-10 (Millipore, Billerica, MA), respectively. Proteins in concentrated sera and cell culture supernatants were separated on 12% SDS-polyacrylamide gels and transferred to immunoblot membranes. Blots were blocked with 5% dry milk in PBS-Tween, incubated with a rabbit anti-HMGB1 antibody (BD PharMingen, 1:5000) and with a peroxidase-conjugated anti-rabbit antibody (DakoCytomation, Carpinteria, CA), and developed with ECL plus substrate (Amersham, Piscataway, NJ). HMGB1 expression was expressed as densitometric units relative to CLP or LPS control samples on the same blot.
Immunofluorescence
Macrophages adhered to coverslides were fixed in 4% paraformaldehyde for 20 minutes at room temperature and then incubated with glycine 30 mmol/L for 5 minutes. After washing three times with PBS, cells were permeabilized with 0.2% Triton X-100 for 15 minutes, and blocked with 2% bovine serum albumin for 1 hour. Cells were incubated with rabbit anti-HMGB1 antibody (dilution 1:2000 in PBS/2% bovine serum albumin) for 12 hours at 4°C. Slides were then washed and incubated with FITC-labeled goat anti-rabbit antibody (Invitrogen, Molecular Probes, 1:500). After extensive washing, samples were mounted in 4′,6-diamidino-2-phenylindole-containing Vectashield medium (Vector Laboratories, Burlingame, CA) and acquisition was performed with a microscope system (Cell R IX81; Olympus, Center Valley, PA), 63× and 100× objectives, illumination system (MT20; Olympus), and camera (Orca CCD; Hamamatsu).
Results
Late Administration of UCN and VIP Protect against Severe Sepsis
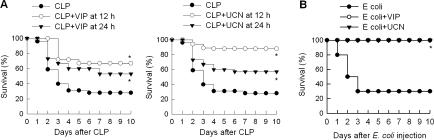
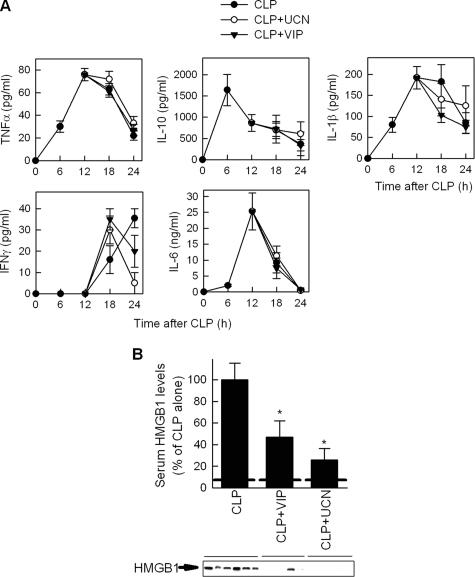
Because VIP and UCN inhibit the production of certain inflammatory mediators,6 we first investigated the potential therapeutic action of both neuropeptides in the murine CLP model. CLP is a clinically relevant model for human sepsis that causes a polymicrobial peritonitis, bacteremia, and sepsis, and is considered a critical preclinical test for any new treatment of severe sepsis.7,8,9 Delayed administration of VIP or UCN to mice with severe sepsis significantly reduced the mortality caused by cecal perforation (Figure 1A). We did not observe any late death (up to 3 weeks), indicating that VIP or UCN treatments confer lasting protection against experimental sepsis. Even when the initiation of the treatment was delayed to 24 hours after the induction of sepsis, VIP and UCN increased the survival from 20%, to 53% and 57%, respectively (Figure 1A). The delayed administration of the neuropeptides also attenuated the clinical signs of sepsis, including lethargy, diarrhea, huddling, and piloerection. Furthermore, administration of UCN or VIP to animals with sepsis induced with E. coli increased survival from 30% to 100% (Figure 1B). Previous studies showed that VIP and UCN prevent lethal endotoxemia by down-regulating a wide spectrum of early inflammatory mediators, including tumor necrosis factor-alpha (TNFα), interleukin (IL)-6, IL-1β, nitric oxide, and several chemokines.6 However, the therapeutic effect shown in the present study by VIP and UCN on established sepsis was observed when neuropeptides were administered after the peak of early inflammatory mediators.10,11 In fact, delayed administration of VIP or UCN did not significantly affect the systemic levels of pro-inflammatory (TNFα, IL-1β, IL-10 and IL-6) and anti-inflammatory (IL-10) cytokines in animals with severe sepsis (Figure 2A). Thus, these data suggest that the therapeutic effect of UCN and VIP in sepsis is mediated by the control of a late mediator other than the early inflammatory cytokines.
Figure 1.
Delayed administration of UCN and VIP improve survival in established sepsis. A: Sepsis was induced by CLP, and mice were treated with vehicle or with UCN or VIP (1 nmol every 8 hours for 3 days) starting at 12 hours or 24 hours after sepsis induction. Survival was monitored for 10 days. *P < 0.05 versus CLP alone (two-tailed Fisher’s exact test). n = 14 to 32 mice/group. B: UCN and VIP protect against lethality induced by i.p. injection of 108 live E. coli (DH5α). UCN or VIP (1 nmol) were injected 2, 5, and 8 hours after bacterial injection. *P < 0.05 vs. E. coli alone (two-tailed Fisher’s exact test). n = 10 mice/group.
Figure 2.
VIP and UCN inhibit HMGB1 release in sepsis. Mice were subjected to CLP and treated with vehicle or with VIP or UCN (1 nmol) at 12 and 18 hours after sepsis induction. A: Serum was collected at different times after CLP, and the levels of different cytokines determined as described in Materials and Methods. Data are mean ± SEM No significant differences were found between untreated or neuropeptide-treated mice. n = 5 to 10 mice per point. B: Serum was collected 24 hours after sepsis induction and the HMGB1 levels were determined by Western blot (HMGB1-specific band is indicated by an arrow) and expressed as band densities relative to control samples (CLP alone) on the same blot. Dashed line represents basal serum HMGB1 levels in normal mice. Data are mean ± SEM *P < 0.05 versus CLP alone (Mann-Whitney test). n = 7 to 10 mice per group.
UCN and VIP Down-regulate HMGB1 Levels in Septic Mice
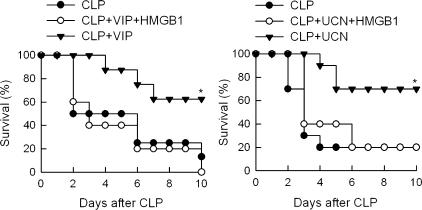
Although the pathophysiology of sepsis is unlikely attributable to a single molecule, several studies suggest that HMGB1 is a necessary and sufficient late mediator of the lethal multiple organ failure associated with severe sepsis.1,4,5 Therefore, we next investigated whether VIP and UCN could attenuate circulating HMGB1 levels during sepsis. Consistent with previous observations,4 sepsis induction resulted in increased systemic levels of HMGB1 (Figure 2B). Delayed treatment of septic mice with VIP or UCN significantly reduced the circulating levels of HMGB1 (Figure 2B). Interestingly, the relative efficiency in their protective effects on sepsis lethality (Figure 1A) correlated with the decrease on HMGB1 levels caused by both neuropeptides (Figure 2B). Moreover, exogenous administration of recombinant HMGB1 totally reversed the therapeutic effect of VIP and UCN in CLP-induced sepsis (Figure 3). Taken together these results indicate that VIP and UCN rescue mice from septic death by down-regulating the release of HMGB1.
Figure 3.
HMGB1 reversed the therapeutic effect of VIP and UCN in sepsis. Sepsis was induced by CLP and mice were treated with vehicle (CLP) or with UCN or VIP (1 nmol every 8 hours for 3 days) starting at 12 hours after sepsis induction. Recombinant HMGB1 (100 μg/mouse) was added to VIP- or UCN-treated animals at 18 hours after CLP. Survival was monitored for 10 days. *P < 0.05 versus CLP alone and versus HMGB1-treated animals (two-tailed Fisher’s exact test). n = 5 to 10 mice per group.
UCN and VIP Inhibit the Secretion of HMGB1 by Activated Macrophages
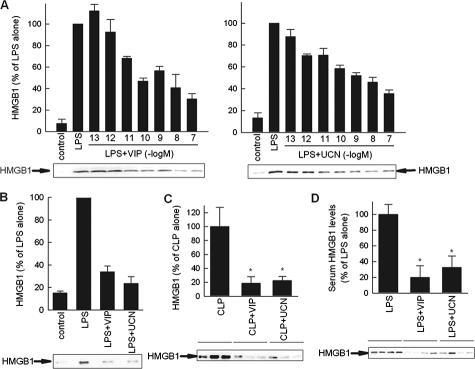
The late appearance of HMGB1 in the serum of septic mice parallels the kinetic of HMGB1 release by activated macrophages.1,4 In addition, macrophages have been previously described as the major targets in the anti-inflammatory effect of VIP and UCN.6 Therefore, we next evaluated whether macrophages are direct cell targets of the inhibitory effect of VIP and UCN on the secretion of HMGB1. We treated macrophages with LPS in the presence or absence of a range of UCN or VIP concentrations for 24 hours and determined the presence of HMGB1 in the culture supernatants by Western blot. Whereas resting macrophages scarcely secreted HMGB1, stimulation with the bacterial endotoxin LPS induced the release of high levels of HMGB1 (Figure 4A and B). VIP and UCN dose-dependently inhibited LPS-induced HMGB1 release, showing a maximal effect at 10−7 M (Figure 4A and B), a concentration that is within the physiological range.6 Although several administrations of VIP and UCN were needed to achieve a significant therapeutic effect in vivo, probably due to the short half-life of these peptides in circulation, a single administration of both neuropeptides was enough to signal macrophages and exert a long-term inhibition of HMGB1 in vitro. Thus, similar to their effect in other cytokines,6 VIP and UCN still down-regulated HMGB1 secretion when they were added 4 hours after LPS stimulation, and when macrophages were exposed to VIP or UCN for 1 hour, and then extensively washed and stimulated with LPS (data not shown).
Figure 4.
VIP and UCN reduce HMGB1 secretion by targeting macrophages. A and B: VIP and UCN inhibit the secretion of HMGB1 in endotoxin-activated RAW 264.7 (A) or peritoneal (B) macrophages were cultured with medium (control) or stimulated with LPS (1 μg/ml) and different concentrations of VIP or UCN (10−7 M for peritoneal macrophages) for 24 hours. HMGB1 content in culture supernatants was assayed by Western blotting and expressed as densitometric units relative to the LPS-treated condition on the same blot (HMGB1-specific band is indicated by an arrow). Data are mean ± SEM (n = 3 to 5). C: VIP and UCN deactivate peritoneal macrophages during sepsis. Mice were subjected to CLP and treated i.p. with VIP or UCN (1 nmol/mouse) 12 hours later. Peritoneal lavage was obtained 18 hours after sepsis induction. Peritoneal macrophages were isolated and cultured with medium at 106 cells/ml for 36 hours. The concentration of HMGB1 in the culture supernatants was determined by Western blotting and expressed as band densities relative to control samples (CLP alone) on the same blot. Data are mean ± SEM n = 8 per group *P < 0.001 versus CLP alone (Mann-Whitney test). D: VIP and UCN down-regulate LPS-induced HMGB1 secretion in vivo. Endotoxemia was induced by i.p. injection of LPS (100 μg/mouse). Mice were treated 30 minutes later with medium (controls) or with VIP (1 nmol) or UCN (1 nmol). Serum was collected 24 hours after endotoxin administration and circulating HMGB1 levels were determined by Western blot (HMGB1-specific band is indicated by an arrow), and expressed as band densities relative to control samples (LPS alone) on the same blot. Data are mean ± SEM *P < 0.05 versus LPS alone (Mann-Whitney test). n = 7 to 10 mice per group.
To determine whether our in vitro findings were relevant to the secretion of HMGB1 by macrophages during sepsis, we evaluated the impact of the treatment with VIP or UCN on the activation of peritoneal macrophages of septic mice ex vivo. Although macrophages isolated from septic animals spontaneously produced high amounts of HMGB1, macrophages isolated from animals treated with VIP or UCN secreted much lower levels of HMGB1 (Figure 4C). Because endotoxemia induced by LPS injection triggers a systemic inflammatory response characterized by the secretion of HMGB1 in the systemic circulation,1 we then asked whether UCN and VIP could also decrease the release of HMGB1 induced by LPS in vivo. We administered UCN or VIP simultaneously with a LD40 injection of LPS and determined the serum HMGB1 levels 24 hours after the induction of endotoxemia. Consistent with previous observations,1,12,13 LPS alone induced the release of HMGB1 into the serum, and both UCN and VIP reduced the levels of circulating HMGB1 (Figure 4D).
Taken together, these results suggest that the deactivation of resident and infiltrating macrophages could be the major mechanism involved in the therapeutic action of VIP and UCN on sepsis.
UCN and VIP Inhibit the Translocation of HMGB1 from the Nucleus to the Cytoplasm
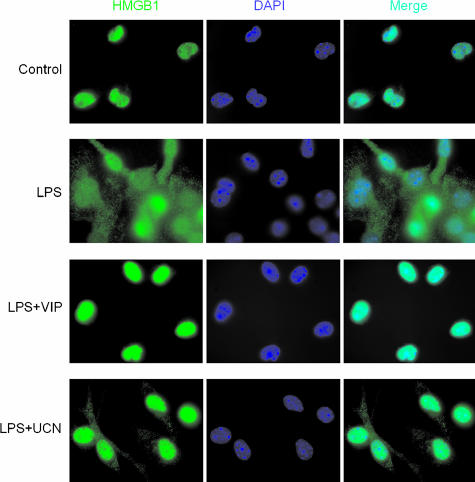
HMGB1 lacks a secretory signal peptide and is secreted via a nonclassic secretory pathway.14 Activation of monocytes and macrophages results in the accumulation of HMGB1 into cytoplasmic vesicles that display the features of secretory lysosomes.14 Because UCN and VIP inhibit HMGB1 release by activated macrophages, we sought to determine whether both neuropeptides affect the relocalization of HMGB1 in LPS-stimulated peritoneal macrophages. Cells were cultured for 12 hours with LPS in the absence or presence of UCN or VIP, double stained with anti-HMGB1 antibodies and with the nuclear dye 4′,6-diamidino-2-phenylindole, and analyzed by fluorescence microscopy. Nonstimulated cells displayed a strong staining of HMGB1 mostly restricted to the nucleus, as indicated by the colocalization with 4′,6-diamidino-2-phenylindole. LPS stimulation resulted in an increased translocation of HMGB1 into the cytoplasm, where it displayed a punctuate staining (Figure 5). Both neuropeptides prevented HMGB1 redistribution to the cytoplasm and retained the protein in the nucleus (Figure 5).
Figure 5.
VIP and UCN impair cytoplasmic translocation of HMGB1 in activated macrophages. Peritoneal macrophages were cultured with medium (control), or stimulated with LPS (100 ng/ml) in the absence or presence of VIP (10−7 M) or UCN (10−7 M). After 12 hours, macrophages were fixed, permeabilized, stained with 4′,6-diamidino-2-phenylindole (blue channel) and anti-HMGB1 antibody (green channel), and the nuclear-cytoplasmic translocation of HMGB1 was determined by fluorescent microscopy (percentages of cells with HMGB1 translocated to the cytoplasm were: 11% unstimulated cells, 74% LPS-stimulated cells, 36% UCN-treated cells, and 13% VIP-treated cells). Data are representative of four independent experiments.
Discussion
Sepsis is a major cause of morbidity and mortality in neonatal and medical intensive care units with an annual incidence of 750,000 patients per year in the United States.15 Sepsis results from excessive stimulation of the host immune system by pathogen components to produce various pro-inflammatory cytokines, and their overproduction causes a systemic inflammation that can lead to lethal multiple organ damage. Despite continuing progress in the development of antibiotics and other supportive care therapies, there is a lack of effective therapy for sepsis.16 Indeed, therapies directed to neutralize pro-inflammatory cytokines can prevent the development of septic shock in animal models, but clinical trials of these therapies have failed to improve the outcome of patients with sepsis.17 The failure of these clinical trials may reside in the kinetics of cytokines such as TNFα and IL-1β, which are released early in the development of a systemic inflammatory response and normalize before the specific treatment is implemented. In recent years, various evidence indicates that HMGB1 is a necessary and sufficient late mediator of severe sepsis, and therefore, its targeting provides a wider window for clinical intervention.1,2,3,4,5 In the present work, we show that VIP and UCN are physiological inhibitors of HMGB1 release. Both neuropeptides protect from the lethal effect of E. coli and CLP-induced sepsis and this protection is paralleled by a decrease in the systemic levels of HMGB1.
The inhibitory effects of VIP and UCN on HMGB1 release by peritoneal macrophages in vitro resembled closely the HMGB1 profile in endotoxemic and septic mice treated with the neuropeptides. This suggests that the deactivation of resident and infiltrating macrophages could be the major mechanism involved in the therapeutic action of VIP and UCN on established sepsis. However, we cannot exclude the involvement of other cells such as dendritic cells, natural killer cells, pituicytes, enterocytes, and endothelial cells, which secrete HMGB1 on stimulation.2 Our results indicate that VIP and UCN inhibit HMGB1 release by interfering with its translocation from the nucleus to the cytoplasm, an essential step for HMGB1 secretion.14
We previously showed that VIP and UCN prevent endotoxin-induced production of TNF in vivo and in vitro.6 However, the therapeutic use of these neuropeptides to improve survival in established sepsis was unknown. Sepsis and septic shock are two different syndromes and are likely to be mediated by different agents, such as TNF and HMGB1, respectively.10,11 For example, therapies based in blockade of TNFα were efficient in endotoxemia,18,19 but failed in septic humans and mice.10,11 Furthermore, most septic patients do not have significant increased levels of TNFα, but they present high amounts of serum HMGB1.16,17 Here, we observe that VIP and UCN are still protective against septic lethality even if administered 24 hours after sepsis induction. This wide therapeutic window can be mainly explained by the inhibition of HMGB1 secretion. Indeed, the exogenous administration of HMGB1 to septic animals abrogated the therapeutic effect of both neuropeptides. Moreover, the therapeutic window of UCN and VIP in severe sepsis has been uniquely achieved by specific blocking of HMGB1, such as neutralizing antibodies against HMGB1, ethyl pyruvate and nicotine.4,5,12,13 However, antibody-blocking strategies increase the formation of antibody-antigen complexes and clinical studies designed to block cytokines were disappointing. Therefore, inhibition of HMGB1 secretion by VIP and UCN might represent a therapeutic advantage compared to anti-HMGB1 antibodies.
Besides sepsis, HMGB1 is also involved in the progression of other inflammatory and autoimmune diseases such as arthritis, inflammatory bowel disease, and ischemia/reperfusion.2 UCN and VIP have been shown to exert protective actions in these disorders.6 We have found that UCN reduces HMGB1 levels in serum of collagen-induced arthritic mice (A.C. and M.D., unpublished results). This finding supports the concept that VIP and UCN are endogenous inhibitors of HMGB1 release and suggests that inhibition of HMGB1 could be a general mechanism of action of both neuropeptides.
Of physiological relevance is the observation that the secretion of VIP and UCN dramatically increase in certain pathological inflammatory conditions, such as sepsis, endotoxemia and rheumatoid arthritis.6,20,21,22,23,24,25,26 Interestingly, both VIP and HMGB1 peaks coincide in time in sepsis. In addition, deficient mice for VIP or VIP-receptor are significantly more susceptible to endotoxic shock.27,28 Therefore, it is tempting to speculate that the body responds to an exacerbated inflammatory response by increasing the peripheral production of endogenous anti-inflammatory factors, including these neuropeptides, in an attempt to restore homeostasis. Thus, VIP and UCN emerge as natural anti-inflammatory peptides that regulate critical late events related to the overwhelmed systemic inflammatory response to infection that causes sepsis. The fact that the control of the systemic HMGB1 levels has lately emerged as one of the most promising therapeutic strategies for sepsis, point outs to neuropeptides such as VIP and UCN as feasible therapeutic agents for the treatment of this disorder.
Footnotes
Address reprint requests to Mario Delgado, Ph.D., Instituto de Parasitologia y Biomedicina, CSIC, Avd. Conocimiento, PT Ciencias de la Salud, Granada 18100, Spain. E-mail: mdelgado@ipb.csic.es.
This work was supported by grants from Fondo de Investigaciones Sanitarias (FIS) and Junta de Andalucia (Grupos de Excelencia), and a fellowship from FIS (to A.C.).
References
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, Yang H. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52–63. doi: 10.1038/nri1984. [DOI] [PubMed] [Google Scholar]
- Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Deitch E, Moldawer LL, Opal S, Redl H, van der Poll T. Preclinical models of shock and sepsis: what can they tell us? Shock. 2005;24(Suppl 1):1–6. doi: 10.1097/01.shk.0000191383.34066.4b. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Czura CJ, Yang H, Amella CA, Tracey KJ. HMGB1 in the immunology of sepsis (not septic shock) and arthritis. Adv Immunol. 2004;84:181–200. doi: 10.1016/S0065-2776(04)84005-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, Hariri RJ, Fahey TJ, 3rd, Zentella A, Albert JD. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Chatzaki E, Charalampopoulos I, Leontidis C, Mouzas IA, Tzardi M, Tsatsanis C, Margioris AN, Gravanis A. Urocortin in human gastric mucosa: relationship to inflammatory activity. J Clin Endocrinol Metab. 2003;88:478–483. doi: 10.1210/jc.2002-020853. [DOI] [PubMed] [Google Scholar]
- Kohno M, Kawahito Y, Tsubouchi Y, Hashiramoto A, Yamada R, Inoue KI, Kusaka Y, Kubo T, Elenkov IJ, Chrousos GP, Kondo M, Sano H. Urocortin expression in synovium of patients with rheumatoid arthritis and osteoarthritis: relation to inflammatory activity. J Clin Endocrinol Metab. 2001;86:4344–4352. doi: 10.1210/jcem.86.9.7827. [DOI] [PubMed] [Google Scholar]
- Uzuki M, Sasano H, Muramatsu Y, Totsune K, Takahashi K, Oki Y, Iino K, Sawai T. Urocortin in the synovial tissue of patients with rheumatoid arthritis. Clin Sci. 2001;100:577–589. [PubMed] [Google Scholar]
- Gravanis A, Margioris AN. The corticotropin-releasing factor (CRF) family of neuropeptides in inflammation: potential therapeutic applications. Curr Med Chem. 2005;12:1503–1512. doi: 10.2174/0929867054039008. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Oktedalen O, Kierulf P, Opstad PK. Elevated VIP and endotoxin plasma levels in human gram-negative septic shock. Regul Pept. 1989;24:37–44. doi: 10.1016/0167-0115(89)90209-7. [DOI] [PubMed] [Google Scholar]
- Delgado M, Abad C, Martinez C, Juarranz MG, Arranz A, Gomariz RP, Leceta J. Vasoactive intestinal peptide in the immune system: potential therapeutic role in inflammatory and autoimmune diseases. J Mol Med. 2002;80:16–24. doi: 10.1007/s00109-001-0291-5. [DOI] [PubMed] [Google Scholar]
- Delgado M, Martinez C, Pozo D, Calvo JR, Leceta J, Ganea D, Gomariz RP. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activation polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-alpha and IL-6. J Immunol. 1999;162:1200–1205. [PubMed] [Google Scholar]
- Szema AM, Hamidi SA, Lyubsky S, Dickman KG, Mathew S, Abdel-Razek T, Chen JJ, Waschek JA, Said SI. Mice lacking the VIP gene show airway hyperresponsiveness and airway inflammation, partially reversible by VIP. Am J Physiol Lung Cell Mol Physiol. 2006;291:L880–L886. doi: 10.1152/ajplung.00499.2005. [DOI] [PubMed] [Google Scholar]
- Martinez C, Abad C, Delgado M, Arranz A, Juarranz MG, Rodriguez-Henche N, Brabet P, Leceta J, Gomariz RP. Anti-inflammatory role in septic shock of pituitary adenylate cyclase-activating polypeptide receptor. Proc Natl Acad Sci USA. 2002;99:1053–1058. doi: 10.1073/pnas.012367999. [DOI] [PMC free article] [PubMed] [Google Scholar]