Abstract
The tumor necrosis superfamily (TNFSF) contains two soluble ligands that are involved in B lymphocyte development, BAFF (B cell activating factor, BlyS, TALL-1, CD257, TNFSF13B) and APRIL (a proliferation inducing ligand, CD256, TNFSF13). These two ligands signal through three receptors: the exclusive BAFF receptor (BAFF-R, CD268, TNFRSF17) and two receptors that recognize both BAFF and APRIL, TACI (transmembrane-activator-1 and calcium-modulator- and cyclophilin ligand-interactor CD267, TNFRSF13B) and BCMA (B cell maturation antigen, CD269, TNFRSF13C). All but BAFF-R are known to be synthesized in term placentas. In this study, expression of the ligands and receptors were distinguished in two embryologically discrete subpopulations of placental cells, villous cytotrophoblast (vCTB) cells and mesenchymal cells (MCs). Real-Time PCR showed that vCTB cells contain low levels of BAFF and APRIL transcripts whereas MCs contain high levels. Both Real-Time PCR and immunohistochemistry identified BAFF-R and BCMA mRNA and proteins in vCTB cells but essentially no TACI. By contrast, MCs contained readily detectable levels of all three receptors. These results illustrating potential autocrine and paracrine pathways for BAFF and APRIL signaling in human placentas suggest that lineage-specific regulation of placental cell viability, differentiation and/or other activities may be novel functions of these proteins.
Human placentas contain multiple messages and proteins encoded by genes of the tumor necrosis factor superfamily (TNFSF).1,2,3 Of these, three non-apoptosis-inducing TNFSF ligands have been identified, B lympho-cyte-activating factor (BAFF; also known as BlyS, TALL-1, CD257, TNFSF13B), a proliferation-inducing ligand (APRIL; also known as CD256, TNFSF13), and CD30L.3 Two of the three ligands, BAFF and APRIL, support B-lymphocyte survival and differentiation, and influence T lymphocytes as well.
Characteristics of BAFF and APRIL and their receptors have been extensively reviewed.4,5,6,7,8,9,10,11,12,13,14,15,16,17,18 BAFF is found in many tissues, where it is primarily produced by stimulated myeloid cells such as monocytes, macrophages, dendritic cells and neutrophils. Synthesis and release are facilitated by CD40L, interleukin-10, and interferon-α and -γ. Some of the same cell types may produce APRIL, which is also stimulated by cytokines. As with other TNFSF ligands, BAFF and APRIL form trimeric soluble complexes that recognize and signal through membrane-bound receptors. BAFF may also exist as a membrane-bound ligand. BAFF binds to three different receptors: BAFF receptor (BAFF-R; BR3, CD268, TNFRSF17), transmembrane activator-1 and calcium modulator- and cyclophilin ligand-interactor (TACI; CD267, TNFRSF13B), and B cell maturation antigen (BCMA; CD269, TNFRSF13C). Of these, only the BAFF-R is exclusive for BAFF.19 APRIL is recognized by both TACI and BCMA. Preferences that TACI and BCMA may exhibit for BAFF and APRIL remain unresolved.12,13
Separate and distinct events occur in B lymphocytes signaled through each of the three receptors. The BAFF/BAFF-R signaling system is anti-apoptotic via induction of nuclear factor κB and Bcl-2, acting at the transition stage of B-lymphocyte development and inducing the B cell differentiation markers, CD21 and CD23.16 As summarized by Woodland et al,17 BAFF-dependent survival signaling in B cells activates either the Pim 2 or the Akt/mTOR pathway and requires Mcl-1 for full protection. Regarding TACI, binding to this receptor in mice results in negative regulation of B-lymphocyte proliferation stimulated by BAFF, but this seems not to be the case in humans, since patients with TACI mutations have normal numbers of B lymphocytes.14 Treml et al18 have commented that TACI has its major impact on short-lived, proliferating B cells. In contrast to BAFF-R and TACI, BCMA is involved in late stages of B cell maturation, being important to the survival of plasmablasts and long-lived plasma cells in the bone marrow.13,14,18,20
An early study from our laboratory identified BAFF and APRIL messages and proteins in human placentas.3 Until recently, when Chang et al21 reported human monocyte binding of BAFF, B lymphocytes were the only described targets of these two cytokines. B cells in placentas are entirely restricted to blood vessels and are not known at any time to be permanent residents in normal placental villus stroma. Thus, in this study, we postulated that other types of cells that comprise placental villi, ie, villous cytotrophoblast (vCTB) cells encasing the villi and/or B lymphocyte-free preparations of mesenchymal cells (MCs) from the villous core, might comprise target cells for BAFF and/or APRIL. In experiments designed to test this idea, the results suggested that driving placental development and/or other functions may well be new and entirely unsuspected actions of these two normally B cell-influencing cytokines.
Materials and Methods
Tissue Collection and Processing
Human placentas were acquired from cesarean sections performed in the third trimester of pregnancy to avoid the possibility of fetal distress. These acquisitions were done in accordance with a protocol approved by the Human Subjects Committee of the University of Kansas Medical Center. Underlying pathology was not evident on histological examination of the samples. For immunohistochemistry experiments, samples of placentas were manually dissected and (1) fixed in freshly prepared 4% paraformaldehyde in PBS at 4°C for 4 hours followed by transfer into 18% sucrose overnight at 4°C, or (2) fixed in paraformaldehyde overnight. Paraformaldehyde/sucrose-fixed tissues were embedded into optimum cutting temperature tissue freezing medium (OCT; Triangle Biomedical Sciences, Durham, NC) and stored at −20°C until used. Paraformaldehyde-fixed tissues were embedded in paraffin and blocks were stored at 20°C. Five- to eight-micrometer sections were cut, placed on glass slides, and tested by immunohistology as previously described.22 For quantitative polymerase chain reaction studies, tissues were taken from term cesarean deliveries and processed as described below to separate vCTB cells and MCs.
Separation of vCTB Cells and MCs
The protocol for collecting vCTB cells from term placentas has been reported.23 In brief, samples were taken randomly from beneath the basal plate and minced finely. Of particular importance to the present studies, extensively minced tissues were washed thoroughly and then filtered through fine steel mesh to remove fetal and maternal blood. Cells were released from the tissues by enzyme digestion and purified by density gradient centrifugation over Percoll to remove the remaining cellular debris and red blood cells. The cells were then further purified over an affinity column using magnetic bead technology with the anti-human leukocyte antigen (HLA) class I monoclonal antibody, W6/32,24 to trap HLA class I-positive cells. The flow-through cells (vCTB) were washed and concentrated as needed for experiments. MC originally resident in the stroma of placental villi, which are HLA class I positive, were released from the affinity column, counted, and used as needed for experiments.
Purity of vCTB and MC Preparations
Villous CTB cells were analyzed for purity as described.23 In brief, the cells were centrifuged onto glass slides and immunostained to identify vCTB cells using mouse anti-cytokeratin-7 (Dako Corporation, Carpinteria, CA). An isotype-matched nonspecific monoclonal antibody (mAb) was used as a negative control (IgG1; BD Biosciences PharMingen, San Jose, CA). The results were evaluated by two independent readers. MCs were analyzed by flow cytometry to determine percentages of macrophages using a directly labeled fluorescein isothiocyanate-labeled anti-CD14 mouse monoclonal antibody (Chemicon International, Temecula, CA). CD14+ blood monocytes served as positive controls. An isotype-matched, nonspecific mAb was used as a negative control (IgG1; BD Biosciences PharMingen). Analysis was performed using FlowJo software from TreeStar Inc. (Ashland, OR). Villous CTB cells and MCs were further tested for B-lymphocyte contamination by semiquantitative PCR as described below.
Reverse Transcriptase (RT)-PCR
RNA isolated from vCTB cells and MCs were subjected to RT-PCR using CD19-specific primers to test for the presence of B lymphocytes. RNA from two human B-cell lines, L721.221 and Raji (American Type Culture Collection, Manassas, VA), were used as positive controls. RNA extraction was performed using TRIzol RNA Isolation Reagent (Invitrogen, Carlsbad, CA) and treated with deoxyribonuclease I (DNase; Sigma Chemical Co., St. Louis, MO) to remove contaminating DNA. One microgram of the DNase-treated RNA was reverse-transcribed in a 40-μl reaction using 400 U of Moloney-murine leukemia virus reverse transcriptase. The reaction contained 10 mmol/L dithiothreitol, 50 mmol/L Tris-HCl, pH 8.3, 75 mmol/L KCl, 3 mmol/L MgCl2, 0.5 mmol/L each of dGTP, dATP, dTTP, and dCTP, 1 μg of oligo(dT)12–18 and 1 U of RNaseOUT (all from Invitrogen). Reverse transcription was performed using a GeneAmp PCR System 2400 Thermocycler (PerkinElmer Corp., Norwalk, CT) for 1 hour at 37°C, 15 minutes at 70°C, and then chilled on ice. Four microliters of cDNA were amplified in a PCR using the following CD19-specific primers: CD19F, 5′-ACCTCCTCGCCTCCTCTTC-3′ (forward) and CD19R, 5′-TCCCCTTCCTCTTCTTCTG-3′ (reverse). A control experiment was performed using human β-actin primers, 5′-CGGGAAATCGTGCGTGACAT-3′ (forward) and 5′-GAACTTTGGGGGATGCTGGC-3′ (reverse). The PCR contained 0.2 μmol/L each primer, 20 mmol/L Tris-HCl, pH 8.4, 50 mmol/L KCl, 1 mmol/L MgCl2, 0.2 mmol/L dNTP mix (dATP, dTTP, dGTP, and dCTP), and 2 U of Taq DNA polymerase (all reagents from Invitrogen). The PCR was incubated for 3 minutes at 94°C, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 2 minutes. A final extension was conducted for 10 minutes at 72°C, then the PCR products were cooled to 4°C. The PCR products were resolved by electrophoresis in a 2% agarose/NuSieve gel (3:1; Amresco, Solon, OH), containing 0.1 μg/ml ethidium bromide in 1× Tris-acetate-EDTA buffer for 2 hours at 100 volts and examined using a UV transilluminator. The amplified PCR products were excised from the gel, extracted using a QIAquick Gel Extraction kit (Qiagen Inc., Valencia, CA) and sequenced to verify the identity.
Cell Lines Used as Controls in Real-Time PCR Experiments
RNA isolated from the cervical adenocarcinoma cell line, HeLa, and the HLA class I-negative lymphoblastoid cell line, L721.221 (American Type Culture Collection), were used as calibrators in real-time PCR experiments. The cells were grown in minimum essential medium (Eagle) with 2 mmol/L l-glutamine and Earle’s basic salt solution adjusted to contain 1.5 g/L sodium bicarbonate, 0.1 mmol/L nonessential amino acids, and 1.0 mmol/L sodium pyruvate and 10% fetal bovine serum (all from Cellgro, Mediatech, Inc., Herndon, VA) at 37°C in a humidified atmosphere containing 5% carbon dioxide. At confluence, the culture medium was aspirated and RNA was extracted from the cells using TRIzol Reagent (Invitrogen) as described by the manufacturer.
Real-Time PCR
Complementary DNA was synthesized from 1.0 μg of total RNA isolated from HeLa, L721.221, MCs, and vCTB cells as previously described.22 The cDNA was subjected to real-time PCR to determine the levels of BAFF, APRIL, and its three receptors, BAFF-R, TACI, and BCMA, using predeveloped TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA). Preliminary experiments showed that levels of BAFF, APRIL, and BAFF-R mRNAs in HeLa cells and levels of TACI and BCMA mRNAs in L721.221 lymphoblastoid B cells were appropriate for using these lines as calibrators. The relative quantitation method was used to estimate the levels of messages encoded by these genes in each sample, with glyceraldehyde-6-phosphate dehydrogenase (GAPDH) mRNA serving as the endogenous normalization control. The suitability of using this method for quantitation was validated by confirming that the amplification efficiencies of GAPDH and the target mRNA messages were equal. Real-time PCR was performed in triplicate in 96-well MicroAmp optical reaction plates (Applied Biosystems). Each 25-μl reaction contained 1× 6-carboxylfluorescein-labeled Taqman Gene Expression Assay (for each target) or GAPDH endogenous control, Taqman Universal PCR Master Mix (Applied Biosystems) and cDNA diluted 1:25 in water. The PCR was performed using a 7500 Real-Time PCR System (Applied Biosystems) with an initial AmpErase uracil-N-glycosylase activation step conducted at 50°C for 2 minutes followed by a 10-minute incubation at 95°C to activate the Amplitaq Gold polymerase enzyme. This was followed by 40 cycles of denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 1 minute. The data were collected and analyzed using the ABI Sequence Detection Software, version 1.3.1 (Applied Biosystems).
Analysis of Placentas by Immunohistochemistry and Immunocytochemistry
Frozen tissue sections to be tested for BAFF-R were warmed to room temperature and fixed in cold acetone (−20°C) for 10 minutes, air dried, and then re-hydrated in PBS. The sections were blocked with 10% normal goat serum in antibody dilution buffer (1% bovine serum albumin in PBS, pH 8.0, containing 0.3% Tween 20) for 1 hour at room temperature. Following the block, tissues were incubated with mouse anti-human BAFF-R (Abcam, Inc., Cambridge, MA) or mouse isotype control IgG1 (Vector Laboratories, Burlingame, CA) at 10 μg/ml for 1 hour at 37°C or overnight at 4°C. Endogenous peroxidase activity was blocked with a peroxidase blocking reagent (Dako Corporation, Carpinteria, CA) for 15 minutes at room temperature before adding developing reagents. The tissue sections were washed three times with PBS, followed by the addition of biotin-labeled secondary antibody (Jackson ImmunoResearch, West Grove, PA) containing 5% human serum, for 25 minutes at room temperature. Binding of secondary reagents was identified using a streptavidin immunoperoxidase labeling kit from Zymed Laboratories, Inc. (South San Francisco, CA). The enzyme substrate was hydrogen peroxide containing 3-amino-9-ethylcarbazole chromogen (AEC kit, Zymed), which yields a red signal in positive cells. The sections were washed in water, counterstained with Mayer’s hematoxylin (Sigma-Aldrich, St. Louis, MO), overlaid with Crystal Mount (Biomeda Corp., Foster City, CA) overnight at room temperature, and mounted with Permount (Fisher Scientific, Pittsburgh, PA). The same procedure was used for immunocytochemistry, where anti-cytokeratin-7 defined this specific cell population in purified preparations of vCTB cells, and essentially the same strategy was used for paraformaldehyde-fixed, deparaffinized tissue sections tested with mouse anti-human TACI (R & D Systems, Minneapolis, MN) and rabbit anti-human BCMA (ProSci Incorporated, Poway, CA.) Appropriate serum controls were used.
Results
Characterization of Cells Harvested from Term Placentas
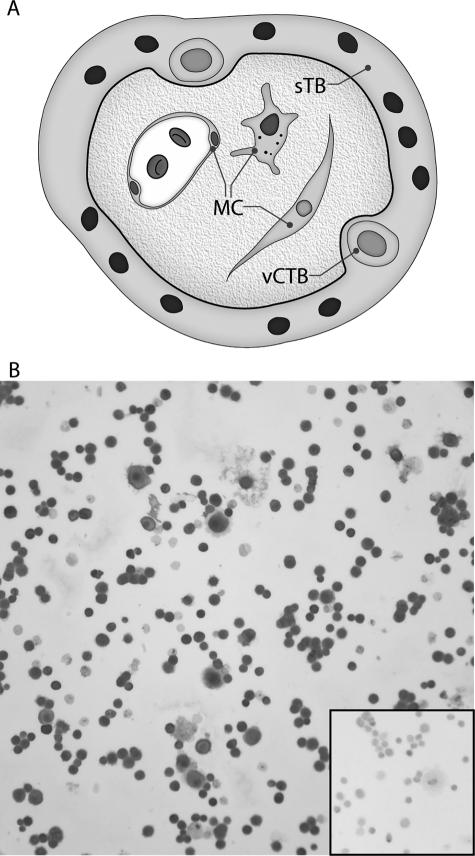
Our goal in the present experiments was to define the placental cell populations that could participate in autocrine and/or paracrine BAFF and APRIL signaling pathways in human placentas. Two embryologically distinct cellular constituents of human placental villi are shown in Figure 1A. Villous CTB cells underlie the continuous syncytial trophoblast layer; both trophoblast subpopulations arise from the trophectoderm layer of the blastocyst. By contrast, MCs are products of the inner cell mass of the blastocyst. Cells harvested from term placentas by density gradient centrifugation of enzyme-digested placental cells are enriched for vCTB cells, but the harvests also contain some MCs with similar densities. In these experiments, the two subpopulations were separated by magnetic bead affinity chromatography into HLA class I-negative vCTB cells and HLA class I-positive MCs, and then characterized for purity.
Figure 1.
Immunocytochemical identification of trophoblast cells in the vCTB cell preparations used in the experiments. A: Cartoon showing locations of vCTB and syncytiotrophoblast (sTB) cells and MCs in human term placental villus. B: Identification of cytokeratin-7-positive trophoblast cells. The percentage of trophoblast cells in the vCTB cell preparations was established by centrifuging the cells onto glass slides using a Shandon Cytospin (Shandon, Pittsburgh, PA) and immunostaining for cytokeratin-7 to reveal trophoblast cells. The cells were counterstained with Mayer’s hematoxylin. The inset shows failure of binding of the isotype control mAb. Original magnifications, ×200.
The flow-through cells from the affinity column averaged 90% to 95% vCTB cells by immunostaining with anti-cytokeratin-7 (Figure 1B). No signals were detected using the isotype-specific control mAb (inset to Figure 1B).
The cells remaining on the affinity columns were released and tested by flow cytometry to establish the content of CD14+ macrophages, which are abundant in the villous mesenchyme.25 In other contexts, macrophages are major BAFF- and APRIL-producing cells.4,5,6,7,8,9,10,11,12,13,14,15,16,17,18 At the same time, the preparations were tested for B-lymphocyte contamination using anti-CD19. In two experiments, CD14+ macrophages in non-lymphocyte-gated fractions of the MCs averaged 33%. The antibody-positive control, which consisted of anti-CD14-selected blood monocytes, was 100% positive. It should be noted that the intensity of anti-CD14 staining to placental macrophages was significantly lower (fluorescein isothiocyanate peak, 103) than the intensity of staining to peripheral blood mononuclear cells (fluorescein isothiocyanate peak, 104.8). CD19+ B lymphocytes comprised 0.4% of lymphocyte-gated MCs. Positive control experiments using peripheral blood mononuclear cells gave expected proportions of ∼30% CD19+ B lymphocytes.
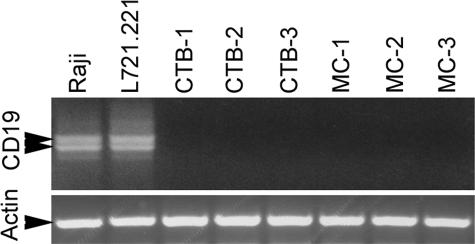
To exclude definitively the possibility that B lymphocytes contaminated the vCTB or MC preparations, semiquantitative RT-PCR was performed for CD19 mRNA. Figure 2 (top panel) shows that although both Raji cells and L721.221 cells exhibited the doublet predicted for CD19, neither vCTB cells nor MCs contained cells transcribing the CD19 gene. Amplicons from Raji and L721.221 cells were identified as authentic CD19 by sequence analysis. Loading controls (actin; Figure 2, bottom panel) showed that lanes contained essentially equal amounts of mRNA.
Figure 2.
Analysis of CD19 mRNA in cytotrophoblast cells (CTB) and mesenchymal cells (MC) by semiquantitative RT-PCR. Upper panel: The CD19 dimer is present in the positive control cells, Raji and L721.221, but absent in three preparations of CTB cells and matching MCs. Lower panel: Amplification of β-actin showed equal loading of samples onto the gel.
BAFF and APRIL mRNAs in Purified Subpopulations of Placental Cells Identified using Real-Time PCR
Having established that preparations of vCTB cells and MCs contained predominantly the desired study populations and no cells that could give artifactual results, real-time PCR was used to quantify BAFF and APRIL mRNAs in vCTB cells from three different placentas and matching MCs from the same three placentas. GAPDH was used as an endogenous control to normalize RNA input and HeLa cell cDNA was used as a calibrator against which levels of BAFF and APRIL transcripts in vCTB cells and MC were measured. An additional three harvests of term vCTB cells were tested for the ligand mRNAs using the same technique, with results essentially identical to those reported below.
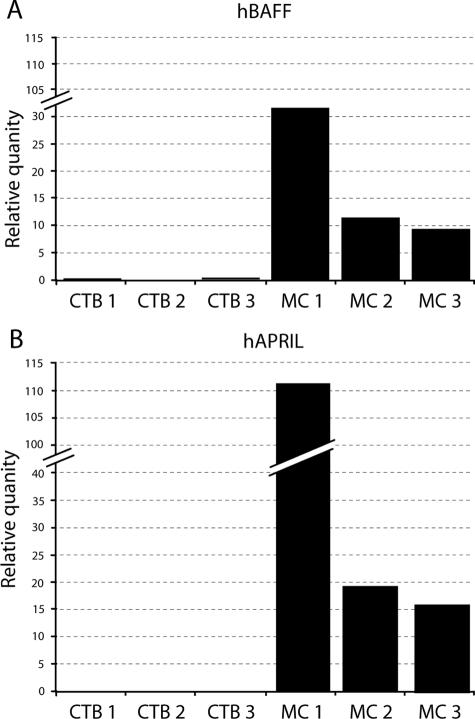
Figure 3A shows that both vCTB cell and MC preparations contained transcripts derived from the Baff gene. In comparison with the HeLa cells used as calibrators, ratios of levels of BAFF mRNA in the three preparations of vCTB cells averaged 0.2:1 (vCTB/HeLa) whereas, in contrast, MC mRNA averaged 19:1 (MC/HeLa) (Table 1). Thus, levels in vCTB cells were low in comparison to MC cell levels, with a ratio of 1:95.
Figure 3.
Analysis of BAFF (A) and APRIL (B) mRNA in three preparations of purified vCTB cells and matching MCs from the same placentas by real-time PCR. HeLa cells were used as calibrators.
Table 1.
Ligand (BAFF, APRIL) and receptor (BAFF-R, TACI, BCMA) mRNA levels in vCTB cells and MCs from term placentas assessed by real-time PCR
| mRNA | vCTB/calibrator | MC/calibrator | vCTB/MC |
|---|---|---|---|
| Ligand | |||
| BAFF | 0.2:1* | 19:1* | 1:95 |
| APRIL | 0.2:1* | 53:1* | 1:260 |
| Exclusive BAFF receptor | |||
| BAFF-R | 4.0:1* | 12:1* | 1:3 |
| Shared receptors | |||
| TACI | 0.5:1† | 11:1† | 1:22 |
| BCMA | 5.0:1† | 12:1† | 1:2 |
Values for vCTB cell mRNAs from three different placentas were averaged as were values for preparations of MCs taken from the same three placentas. The averaged values were used to establish ratios of levels of vCTB cells and MCs to the specific calibrator cell lines as well as the ratio of vCTB/MC.
Calibrator, HeLa cells.
Calibrator, L721.221 cells.
Figure 3B shows that levels of APRIL mRNA in the three preparations of vCTB cells relative to HeLa cells were identical to those of BAFF/HeLa (0.2:1) whereas in MC, APRIL/HeLa mRNA yielded an average ratio of 53:1 (Table 1). Thus, the differential between BAFF transcript levels in vCTB cells and MC was even greater for APRIL than for BAFF, with a vCTB cell/MC ratio of 1:260.
These experiments clearly identified placental MCs as a primary source of transcripts encoding BAFF and APRIL, with MC levels greatly exceeding vCTB cell levels in all samples tested. Although this was true in sum, ranges of individual values relative to HeLa cells were broad for MC (BAFF, range 15 to 38; APRIL, range 18 to 118). A previous study3 identified BAFF- and APRIL-specific proteins in term human placentas by Western blotting. Thus, the messages are known to be translated. Immunostains were not done because of the difficulty of identifying cell-specific production of soluble proteins by immunohistochemistry.
Exclusive Receptors for BAFF in Placental Cells Identified using Real-Time PCR and Immunohistochemistry
In the next group of studies the goal was to ascertain whether any term placental cells might receive BAFF signals. The same set of three harvests of MCs and matching preparations of vCTB cells shown above in Figure 3, A and B, were tested. As before, GAPDH was used as an endogenous control to normalize RNA input and cDNA from HeLa cells was used as a calibrator for BAFF-R.
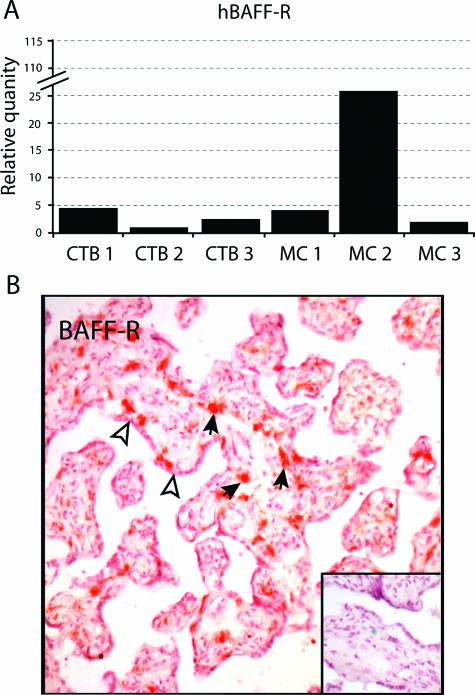
As shown in Figure 4A, both vCTB cells and MCs contained readily detectable BAFF-R mRNA. Levels were not dramatically different in the two embryologically distinct subpopulations of placental cells, with relative values of vCTB/HeLa cells of 4:1 and MC/HeLa cells of 12:1, and a vCTB/MC ratio of 1:3 (Table 1). As with production of ligands in MCs, variation was observed among samples in production of BAFF-R mRNAs by MCs, with one sample containing markedly higher levels of BAFF-R mRNA than the other two. Because BAFF, APRIL, and BAFF-R mRNAs were all tested against the same standard, HeLa cells, it could be concluded that levels of BAFF-R mRNA in both types of placental cells were usually low in comparison with BAFF mRNA levels.
Figure 4.
Analysis of BAFF-R in the three preparations of purified vCTB cells and matching MCs from the same placentas. A: BAFF-R mRNAs in vCTB cells and MCs using 721.221 cells as calibrators in real-time PCR experiments. B: BAFF-R proteins in placental villi identified by immunohistochemistry. Solid arrows point to positive mesenchymal cells; open arrowheads point to syncytiotrophoblast. The inset shows that no signals were obtained when isotype-specific mouse IgG was substituted for anti-BAFF-R. Original magnifications, ×200.
To determine whether BAFF-R mRNAs in placental cells were translated into protein, frozen sections of term placentas were tested by immunohistochemistry using an anti-BAFF-R mAb. Figure 4B shows that cells in the villous stroma, which resembled macrophages by morphological criteria (pleiomorphic cells with extended processes), density and distribution,25 were strongly BAFF-R positive. Weak staining was observed in syncytiotrophoblasts. Villous cytotrophoblast cells are sparse in term placentas and were not identified by marker expression. Positive cells beneath the syncytium that resembled vCTB cells by morphology (round cells with vesiculated nuclei) were observed in ∼10% of cross-sections of placental villi. The inset to Figure 4B shows that the isotype-specific control mAb did not bind.
Both the real-time PCR and immunohistochemical studies indicated that BAFF signaling through the specific BAFF-R may take place in two placental cell populations, trophoblast cells and MCs.
Shared BAFF and APRIL Receptors in vCTB Cells and MCs Identified using Real-Time PCR and Immunohistochemistry
Two additional genes encode receptors that are not exclusive to BAFF but are shared by BAFF and APRIL, Taci and Bcma. To determine whether these receptors might transduce BAFF and/or APRIL signals to vCTB cells and/or MC, real-time PCR was used to evaluate TACI and BCMA mRNAs in the three harvests of MCs and vCTB cells reported above. Glyceraldehyde-6-phosphate dehydrogenase was used as a loading control and L721.221 cells, a B-cell tumor line, was used for calibration since levels of TACI and BCMA transcripts were unsatisfactorily low in the HeLa cells used to calibrate BAFF, APRIL, and BAFF-R mRNAs.
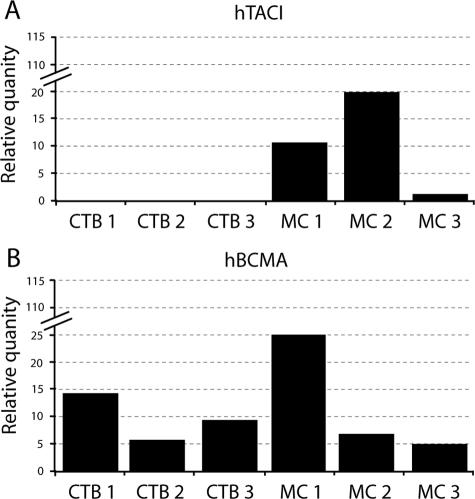
The results shown in Figure 5A demonstrate that vCTB cells contain barely detectable levels of TACI, whereas levels in matching MCs were readily identified. The ratio of relative quantities of TACI mRNAs to L721.221 cells in vCTB cells was 0.5:1 and 11:1 in MCs. This yielded a vCTB/MC ratio of 1:22.
Figure 5.
Analysis of TACI and BCMA mRNAs in the three preparations of purified vCTB cells and matching MCs from the same placentas. A: TACI mRNAs in vCTB cells and MCs using 721.221 cells as calibrators in real-time PCR experiments. B: BCMA mRNAs in vCTB cells and MCs using 721.221 cells as calibrators in real-time PCR experiments.
By contrast, as shown in Figure 5B, BCMA mRNAs in both vCTB cells and MCs were easily identified. The ratio of relative quantities of BCMA messages in vCTB:L721.221 cells was 5:1 and in MCs was 12:1. This yielded a vCTB/MC ratio of 1:2.
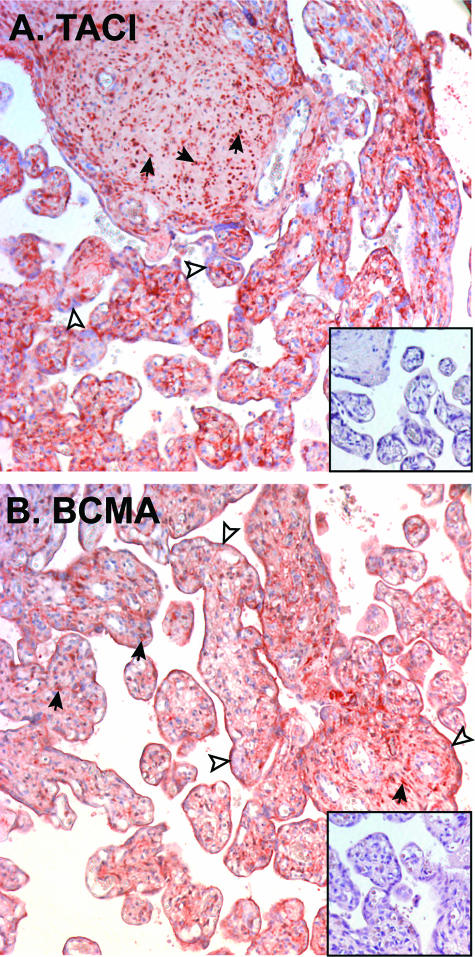
To ascertain whether or not the TACI and BCMA transcripts were translated into proteins, immunohistochemical studies were performed on fixed tissue sections of term placentas. Figure 6A shows TACI immunostaining in term placental villi. Consistent with the real-time PCR results, TACI immunostaining was weak to negative in syncytiotrophoblasts but was clearly identified in the MCs of placental villi. No signals were observed when nonspecific isotype control mAb was substituted for specific antibody (inset, Figure 6A).
Figure 6.
Immunohistochemical identification of TACI and BCMA in human term placentas. A: Anti-TACI immunostaining of term human placenta. Inset, isotype-matched negative control. B: Anti-BCMA immunostaining of human term placenta. Inset, negative control. Open arrowheads mark syncytiotrophoblast; solid arrows mark MCs. Original magnifications, ×200.
Again, consistent with the real-time PCR results, BCMA was clearly identified in both syncytiotrophoblasts and MCs of placental villi (Figure 6B). No signals were observed when nonspecific isotype control mAb was substituted for specific antibody (inset, Figure 6B).
Summary
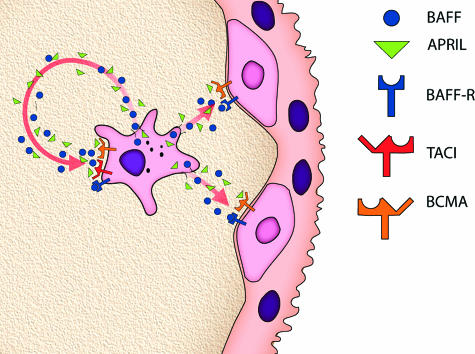
As illustrated in Figure 7, the major points regarding potential autocrine and paracrine pathways for BAFF and APRIL signaling in human term placentas identified in this study were: i) ligands originate with MCs; and ii) receptors are differentially distributed, with vCTB cells capable of receiving paracrine stimulation via BAFF-R and BCMA, whereas MCs are capable of serving as autocrine targets via signaling through all three receptors.
Figure 7.
Cartoon illustrating the finding that MCs in term placental villi are major sites of BAFF and APRIL ligand synthesis and that both vCTB cells and MCs may receive BAFF/APRIL signals. BAFF and APRIL may target in an autocrine manner to three receptors, BAFF-R, TACI, and BCMA, on MC and in a paracrine manner to two receptors, BAFF-R and BCMA, on vCTB cells.
Discussion
The results of this study indicate that two TNFSF ligands mainly associated with B-lymphocyte development and differentiation, BAFF and APRIL, may serve these and/or other functions in human placentas. Further, we show that MCs, not vCTB cells, are the major sources of BAFF and APRIL in term placental villi, and that signaling strategies will differ between vCTB cells and MCs due to differences in receptor expression.
The results reported here expand those in an earlier study in which transcription and translation of the Baff and April genes were identified in human placentas by semiquantitative PCR, immunoblotting, and immunohistochemistry.3 The current study contains more definitive and therefore more useful results, using affinity purification procedures to obtain enriched populations of placental cells as well as real-time PCR and immunohistochemical studies to ascertain cell-specific receptor message and protein expression. Our improved separation methods for vCTB cells permit us to state with some confidence that BAFF and APRIL messages previously thought to arise from vCTB cells3 were likely contributed by contaminating MCs. Regarding receptors, the earlier study did not investigate Baff-R, which was newly described at the time,16,19 or the cellular distribution of TACI and BCMA because tools for analysis of these receptors were as yet untested. We formally excluded the possibility that the messages identified in vCTB cells or MCs were due to B cell contamination by showing that none of the preparations exhibited CD19 messages.
The two ligands have been previously reported in placentas, but here we are able to report definitively that BAFF and APRIL are synthesized almost exclusively in MCs. This was not entirely unexpected because MC preparations derived from enzyme digests of term placental villi contain a high proportion of macrophages, which are prominent among the cell lineages known to produce BAFF and APRIL. Although some BAFF and APRIL mRNA was identified in vCTB cells, these RNAs are likely to have been contributed by the 2% to 5% contamination by MCs that takes place regardless of how carefully purification procedures are done. These real-time PCR experiments were of particular importance because BAFF and APRIL are soluble proteins and, consequently, specific cellular sites of protein production are difficult to identify in situ.
Unexpectedly, we learned in this set of experiments that human term placental cells transcribe and translate not only Baff and April ligand genes but also the genes encoding their exclusive and shared receptors. In contrast to the situation with the two ligands, which were clearly more prominent in MCs than in vCTB cells, both lineages were shown to be capable of receiving BAFF and APRIL signals. Mesenchymal cells likely transduce BAFF signals via all three BAFF receptors, BAFF-R, TACI, and BCMA, whereas vCTB cells are probably signaled exclusively through BAFF-R and BCMA. Collectively, the data suggest that the placenta may be a site of intensive autocrine and paracrine signaling to local placental cells.
These surprising observations raise the question of how BAFF and APRIL might function within the placenta. Our previous ideas were that placental BAFF and APRIL were exported to the mother, where the cytokines could buttress the mother’s production of antibodies for her own immunological defense, or to the fetus to boost the developing immune system.3 We here propose an entirely novel concept: perhaps placental BAFF and APRIL are used to drive placental cell maturation, development, and/or differentiation. As summarized recently by Treml et al,18 BAFF signaling through the BAFF-R regulates the primary B-cell pool, whereas BAFF and/or APRIL acting via the TACI receptor affect short-lived, proliferating B cells The same two cytokines signaling through the BCMA receptor govern memory B cells. The possibility that BAFF in the placenta may have similar actions, at least as regards placental macrophages, is supported by a recent study showing that monocytes exposed to BAFF respond with increased survival, activation, and differentiation.21 It will be of considerable interest to perform functional experiments.
Regarding increased survival, our data show that both vCTB cells and MCs have the potential to activate the BAFF:BAFF-R signaling pathway, which would interfere with apoptosis via implementation of both the alternative nuclear factor κB2 and classic nuclear factor κB1 pathways known to support innate immunity.26 It is tempting, further, to speculate that the ability of vCTB cells and MCs to proliferate may also be regulated by TACI, which is unexpressed in the former and highly expressed in the latter. BCMA, which is associated with terminal differentiation in B cells, was equally expressed in term placental vCTB cells and MCs. This suggests a mechanism by which further differentiation in these subpopulations is limited.
Whether any placental pathologies are due to over- or underproduction of BAFF, APRIL, and/or the three receptors remains to be discovered. Regarding overproduction, BAFF is excessive in autoimmune disorders27,28,29,30,31, and studies on endometriotic lesions indicate that areas of pathological cell growth are filled with plasma cells due to excessive production of BAFF by macrophages.32 If BAFF and/or APRIL is important to programming trophoblast functions, relationships between underproduction and failure of trophoblast migration such as occurs in pre-eclampsia could be envisioned. If important to programming placental macrophage functions, immunity in the placenta might be compromised, leading to infection-associated preterm labor and delivery.
In summary, the results of this study designed to dissect potential autocrine and paracrine pathways for BAFF and APRIL signaling in human term placentas achieved that goal but also produced evidence in support of an emerging concept regarding development and immune mediators. Certain cytokines and growth factors originally identified in the immune system are now known to influence reproduction and embryogenesis. This is the case with tumor necrosis factor α,32 two other TNFSF ligands, ie, LIGHT33,34 and TRAIL (tumor necrosis factor apoptosis-inducing ligand),35 and two unrelated cytokines, colony-stimulating factor-136 and transforming growth factor-β.37 The same is very likely also to be true of the B-cell regulators, BAFF and APRIL.
Acknowledgments
We appreciate assistance with the preparation of figures (S. Fernald, University of Kansas School of Medicine Image Center).
Footnotes
Address reprint requests to Joan S. Hunt, Ph.D., University Distinguished Professor, Department of Anatomy and Cell Biology, University of Kansas Medical Center, Mail Stop 3050, 3901 Rainbow Boulevard, Kansas City, KS 66160-7400. E-mail: jhunt@kumc.edu.
Supported by a National Institutes of Health grant (HD24212) to J.S.H.
References
- Hunt JS, Chen HL, Miller L. Tumor necrosis factors: pivotal factors in pregnancy? Biol Reprod. 1996;54:554–562. doi: 10.1095/biolreprod54.3.554. [DOI] [PubMed] [Google Scholar]
- Phillips TA, Ni J, Hunt JS. Death-inducing tumor necrosis factor (TNF) superfamily ligands and receptors are transcribed in human placentas. CTBs, placental macrophages and placental cell lines. Placenta. 2001;22:663–672. doi: 10.1053/plac.2001.0703. [DOI] [PubMed] [Google Scholar]
- Phillips TA, Ni J, Hunt JS. Cell-specific expression of B lymphocyte- (APRIL. BLyS) and Th2- (CD30L/CD153) promoting tumor necrosis factor superfamily ligands in human placentas. J Leukocyte Biol. 2003;74:81–87. doi: 10.1189/jlb.0103033. [DOI] [PubMed] [Google Scholar]
- Moore PA, Belvedere O, Orr A, Pier K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Boone T, Delaney J, Hawkins N, Kelly M, Ramakrishnan M, McCabe S, Qiu W, Kornuc M, Xia XZ, Guo J, Stolina M, Boyle WJ, Sarosi I, Hsu H, Senaldi G, Theill LE. APRIL and TALL-1 and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol. 2000;1:252–256. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- Rolink AG, Melchers F. BAFFled B cells survive and thrive: roles of BAFF in B-cell development. Curr Opin Immunol. 2002;14:266–275. doi: 10.1016/s0952-7915(02)00332-1. [DOI] [PubMed] [Google Scholar]
- Rolink AG, Tschopp J, Schneider P, Melchers P. BAFF is a survival and maturation factor for mouse B cells. Eur J Immunol. 2002;32:2004–2010. doi: 10.1002/1521-4141(200207)32:7<2004::AID-IMMU2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- So T, Lee SW, Croft M. Tumor necrosis factor/tumor necrosis factor receptor family members that positively regulate immunity. Int J Hematol. 2005;83:1–11. doi: 10.1532/IJH97.05120. [DOI] [PubMed] [Google Scholar]
- Salzer U, Grimbacher B. TACItly changing tunes: farewell to a yin and yang of BAFF receptor and TACI in humoral immunity? New genetic defects in common variable immunodeficiency. Curr Opin Allergy Clin Immunol. 2005;5:496–503. doi: 10.1097/01.all.0000191887.89773.cc. [DOI] [PubMed] [Google Scholar]
- Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–289. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Miller JP, Stadanlick JE, Cancro MP. Space, selection and surveillance: setting boundaries with BLyS. J Immunol. 2006;176:6405–6410. doi: 10.4049/jimmunol.176.11.6405. [DOI] [PubMed] [Google Scholar]
- Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006;18:263–275. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Tangye SG, Bryant VL, Cuss AK, Good KL. BAFF, APRIL and human B cell disorders. Semin Immunol. 2006;18:305–317. doi: 10.1016/j.smim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Mackay F, Leung H. The role of the BAFF/APRIL system on T cell function. Semin Immunol. 2006;18:284–289. doi: 10.1016/j.smim.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Kalled S. Impact of the BAFF/BR3 axis on B cell survival, germinal center maintenance and antibody production. Semin Immunol. 2006;18:290–296. doi: 10.1016/j.smim.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Woodland RT, Schmidt MR, Thompson CB. BLyS and B cell homeostasis. Semin Immunol. 2006;18:318–326. doi: 10.1016/j.smim.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Treml LS, Crowley JE, Cancro MP. BLyS receptor signatures resolve homeostatically independent compartments among naïve and antigen-experienced B cells. Semin Immunol. 2006;18:261–262. doi: 10.1016/j.smim.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- Moreaux J, Cremer FW, Reme T, Raab M, Mahtouk K, Kaukel P, Pantesco V, De Vos J, Jourdan E, Jauch A, Legouffe E, Moos M, Fiol G, Goldschmidt H, Rossi JF, Hose D, Klein B. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood. 2005;106:1021–1030. doi: 10.1182/blood-2004-11-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SK, Arendt BK, Darce JR, Wu X, Jelinek DF. A role for BLyS in the activation of innate immune cells. Blood. 2006;108:2687–2694. doi: 10.1182/blood-2005-12-017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace JL, Morales PJ, Phillips TA, Hunt JS. Analysis of soluble isoforms of HLA-G. Methods Mol Med. 2006;122:181–203. doi: 10.1385/1-59259-989-3:181. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Phillips TA, Ka H, Pace JL, Hunt JS. Isolation and culture of term human trophoblast cells. Methods Mol Med. 2006;121:203–217. doi: 10.1385/1-59259-983-4:201. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens: new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Vince GS, Johnson PM. Immunobiology of human uteroplacental macrophages—friend or foe? Placenta. 1996;17:191–199. doi: 10.1016/s0143-4004(96)90038-7. [DOI] [PubMed] [Google Scholar]
- Enzler T, Bonizzi G, Silverman BJ, Otero DC, Widhopf GF, Anzelon-Mills A, Rickert RC, Karin M. Alternative and classical NF-κB signaling retains autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25:403–415. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway J, Mauri D, Cavill D, Gordon TP, Mackay CR, Mackay F. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szodoray P, Jonsson R. The BAFF/APRIL system in systemic autoimmune diseases with a special emphasis on Sjogren’s syndrome. Scand J Immunol. 2005;62:421–428. doi: 10.1111/j.1365-3083.2005.01688.x. [DOI] [PubMed] [Google Scholar]
- Mackay F, Sierro F, Grey ST, Gordon TP. The BAFF/APRIL system: an important player in systemic rheumatic diseases. Curr Dir Autoimmun. 2005;8:243–65. doi: 10.1159/000082106. [DOI] [PubMed] [Google Scholar]
- Dorner T, Lipsky PE. Signalling pathways in B cells: implications for autoimmunity. Curr Top Microbiol Immunol. 2006;305:213–240. doi: 10.1007/3-540-29714-6_11. [DOI] [PubMed] [Google Scholar]
- Hever A, Roth RB, Hevezi P, Marin ME, Acosta JA, Acosta H, Rojas J, Herrera R, Grigoriadis D, White E, Conion PJ, Maki RA, Zlotnik A. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc Natnl Acad Sci USA. 2007;104:12451–12456. doi: 10.1073/pnas.0703451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RM, Ni J, Hunt JS. Differential expression of LIGHT and its receptors in human placentas and amniochorion membranes. Am J Pathol. 2002;161:2011–2017. doi: 10.1016/S0002-9440(10)64479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill RM, Hunt JS. Soluble receptor (DcR3) and cellular inhibitor of apoptosis-2 (cIAP-2) protect human cytotrophoblast cells against LIGHT-mediated apoptosis. Am J Pathol. 2004;165:309–317. doi: 10.1016/S0002-9440(10)63298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh RJ, Harris LK, Freeman A, Baker PN, Aplin JD, Whitley GS, Cartwright JE. Fetal-derived trophoblast use the apoptotic cytokine tumor necrosis factor-alpha-related apoptosis-inducing ligand to induce smooth muscle cell death. Circ Res. 2007;100:834–841. doi: 10.1161/01.RES.0000261352.81736.37. [DOI] [PubMed] [Google Scholar]
- Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000;6:589–593. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- Lysiak JJ, Hunt J, Pringle GA, Lala PK. Localization of transforming growth factor beta and its natural inhibitor decorin in the human placenta and decidua throughout gestation. Placenta. 1995;3:221–231. doi: 10.1016/0143-4004(95)90110-8. [DOI] [PubMed] [Google Scholar]