Abstract
The superoxide dismutase (SOD) family is a major antioxidant system, and deficiency of Cu,Zn-superoxide dismutase (SOD1) in mice leads to many different phenotypes that resemble accelerated aging. The purpose of this study was to examine the morphology and physiology of the sensory retina in Sod1−/− mice. The amplitudes of the a- and b-waves of electroretinograms elicited by stimuli of different intensity were reduced in senescent Sod1−/− mice, and this reduction in amplitude was more pronounced with increasing age. Retinal morphometric analyses showed a reduced number of nuclei in both the inner nuclear cell layer and outer nuclear cell layer. Electron microscopy revealed swollen cells and degenerated mitochondria in the inner nuclear cell and outer nuclear cell layer of senescent Sod1−/− mice indicating necrotic cell death. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling revealed no significant differences in the number of apoptotic cells between Sod1−/− and wild-type mice, and activated caspase-3 could not be detected in the retina of Sod1−/− mice. In addition to the age-related macular degeneration-like phenotypes previously reported, Sod1−/− mice also present progressive retinal degeneration. Our results indicate that Sod1−/− mice may be a good model system in which to study the mechanism of reactive oxygen species-mediated retinal degeneration.
The superoxide dismutase (SOD) family is one of the main antioxidant systems in the body and is made up of the three SOD isoenzymes; Cu,Zn-superoxide dismutase (SOD1) exists in the cytoplasm, SOD2 or Mn-SOD in the mitochondrial matrix, and SOD3 or extracellular SOD in the interstitium of tissues as the secreted form.1 SOD1 catalyzes superoxide radical dismutation and is distributed throughout the body,1 and among the three isozymes, it is the most abundant in the retina.2 This suggests that SOD1 may be important in protecting the sensory retina from the reactive oxygen species (ROS)-mediated retinal damage.
Many models of retinal degeneration have been studied3,4; however, the mechanism of retinal cell deaths in some of these models has still not been determined. Cell death can be either necrotic or apoptotic, and necrotic cell death is characterized by a swelling of the cell membranes and organelles that leads to a disruption of the cell membranes and lysis. These alterations subsequently lead to inflammatory responses.5 Apoptosis is a process of programmed cell death, and involves an orchestrated series of biochemical events leading to characteristic cell morphology and death. In some models of retinal degeneration, the death of the neurons has been shown to be attributable to apoptosis.6,7,8
SOD1 deficiency (Sod1−/−) leads to many different phenotypes resembling aging, and the changes are attributable to an elevation of ROS.9,10 We recently reported that senescent Sod1−/− mice had features of age-related macular degeneration (AMD) including the presence of drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction.11 Because SOD1 is expressed throughout the retina and not only in the retinal pigment epithelium,11 we have hypothesized that the SOD1 deficiency will alter the morphology and physiology of the retina of Sod1−/− mice. To examine this hypothesis, we investigated the morphology of the retina of Sod1−/− mice of different ages by morphometric methods and the retinal function by electroretinography. We shall show that the amplitudes of the electroretinograms (ERGs) elicited by different light stimuli were reduced in senescent Sod1−/− mice. Morphometric analyses of the retina of Sod1−/− mice of different ages showed evidence of necrotic cell death in the retinal cell layers.
Materials and Methods
Animals
Sod1−/− mice that were backcrossed to C57BL/6 and wild-type (WT) mice were studied.11 All animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
ERGs
ERGs were recorded as described in detail.12,13,14 Briefly, animals were dark-adapted for at least 12 hours and prepared under dim red illumination. A contact lens electrode carrying light-emitting diodes (WLS-20; Mayo Corporation, Inazawa, Japan) was used for the recordings and stimulations. The ERGS were differentially amplified and filtered with the band pass filters set from 0.3 to 500 Hz for the a- and b-waves. White light pulses of 0.009, 3.0, and 65.0 cd*sm−2 were obtained from the light-emitting diodes embedded in the contact lens electrode. The stimulus duration was 0.03, 3.0, and 5.0 ms for 0.009, 3.0, and 65.0 cd*sm−2, respectively. The interstimulus interval was 2 seconds for 0.009 cd*sm−2 and 15 seconds for the two higher intensities.
To isolate the cone pathway, mice were exposed to a white adapting field of 25 cd*m−2 for 10 minutes, and the photopic and flicker ERGs were elicited by white light pulses of 3 ms delivered at a frequency of 1 and 10 Hz. The stimuli were presented on the white adapting field. Five to fifteen responses were averaged for the single-flash ERGs, and 15 responses for the 10-Hz flicker ERGs. The amplitude of the a-wave was measured from baseline to the trough of the a-wave, and the b-wave was measured from the trough of the a-wave to the peak of the b-wave. The implicit time was measured from the onset of the stimulus to the peak of the a- or b-waves.
Retinal Morphometry and Electron Microscopy
The enucleated eyes were immediately embedded in optimal cutting temperature (OCT) compound (Lab-Tek Products, Naperville, IL) and frozen in dry ice-ethanol for cryosectioning. The sections were stained with hematoxylin and eosin. Horizontal sections were cut through the optic nerve, and sections were obtained from WT and Sod1−/− mice (KO) at 10, 30, and 50 weeks of age (n = 10, each group). Sections with at least 20 rows of inner nuclear cell layer (INL) and outer nuclear cell layer (ONL) were selected, and the number of nuclei/column was calculated as described with slight modifications.15 The ratios of the number of nuclei in the Sod1−/− to WT (Sod1−/−/WT) numbers were used for the statistical analyses. For transmission electron microscopy, tissues were fixed, prepared, and observed as previously reported.11
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling (TUNEL) Staining
TUNEL was performed to detect apoptosis in the retina with the in situ cell death detection kit, POD (Roche Molecular Biochemicals, Indianapolis, IN). Briefly, frozen sections were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) and incubated in 0.1% Triton X-100 and 0.1% sodium citrate for 2 minutes at 4°C, rinsed twice in PBS, followed by incubation in 0.3% H2O2-containing methanol for 30 minutes at room temperature to block the endogenous POD activity. After washing in PBS, sections were incubated with the TUNEL reaction mixture containing terminal deoxynucleotidyl transferase in a moist chamber overnight at 4°C, washed again with PBS, and incubated with TUNEL POD for 1 hour at room temperature. To make the apoptotic cells visible, sections were incubated with 3′-diaminobenzidine as a chromogen and counter stained with hematoxylin.
Western Blots and Immunohistochemistry for Caspase
Western blot analysis for procaspase-3 and cleaved caspase-3 was performed with retina lysates. Briefly, retinas were sonicated in 0.5% Nonidet P-40, 10 mmol/L Tris-HCl (pH 7.5), and 150 mmol/L NaCl containing Complete Protease Inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). Tissue homogenates were centrifuged at 15,000 × g for 30 minutes, and equal amounts (20 μg) of tissue extract were added to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto an Immobilon-P membrane (Millipore, Bedford, MA). The membranes were blocked in 5% skim milk in Tris-buffered saline and probed with antibodies against caspase-3 (1:1000, 8G10; Cell Signaling, Danvers, MA). Immunoreactive procaspase-3 and cleaved caspase-3 were made visible with the ECL system (Amersham Biosciences, Piscataway, NJ) and luminoimage analyzer LAS-3000 (Fuji Film, Tokyo, Japan). For control, confluent NIH 3T3 cells were treated and incubated with 1 μmol/L staurosporine for 8 hours, and cell lysates were analyzed. Immunohistochemistry with anti-cleaved caspase-3 antibody (no. 9661, Cell Signaling) were performed for WT and KO with different ages (10 weeks, 30 weeks, and 50 weeks), following the manufacturer’s protocol. Nuclei were counterstained with 4,6-diamidino-2-phenylindole. Sections were prepared from mammary tissues from 2-week-old mice after weaning to obtain positive controls.
Statistical Analyses
Data are presented as the means ± SE of the means (SEMs), and analyzed with Student’s t-tests. For the thickness of the ONL and INL, Kruskal-Wallis test followed by Bonferroni post hoc test was performed to evaluate differences at all periods. A P value <0.05 was considered statistically significant.
Results
ERGs
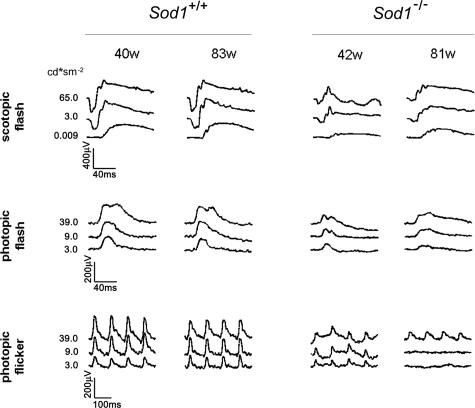
Representative scotopic, photopic, and 10-Hz flicker ERGs recorded from a 42-week-old mouse and an 81-week-old Sod1−/− mouse, and a 40-week-old mouse and an 83-week-old WT mouse are shown Figure 1. The ERGs were elicited by different stimulus intensities. At present, it is known that the scotopic a-wave originates from the activity of rods, and the scotopic and photopic b-waves from a depolarization of the on-bipolar cells and potassium efflux from the Mueller cells. The photopic ERGs and the 10-Hz flicker responses originate from the cones and cone-driven bipolar cells.
Figure 1.
ERGs recorded from age-matched WT and Sod1−/− mice. Data of two representative cases of the WT and two Sod1−/− mice are shown. ERGs were recorded under three different stimulus conditions (scotopic flash, photopic flash, photopic flicker). Stimulus intensities are shown at the left.
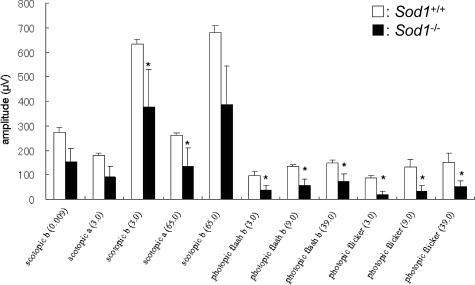
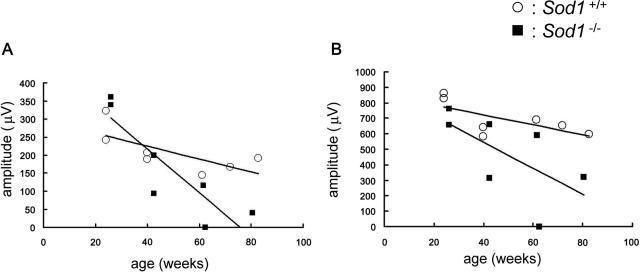
Our recordings showed that both the a- and b-waves were reduced at the age of 40 weeks. The reduction in the a- and b-waves in Sod1−/− mice was found at all stimulus intensities (Figure 2). The ages of the mice in each group were as follows [mean ± SE (range)]: 59.1 ± 7.6 (39.9 to 82.6) weeks in WT, and 57.9 ± 6.4 (42.4 to 80. 5) weeks in KO. At younger ages, ie, <20 weeks, the amplitudes of the ERGs in the two groups were not significantly different. However, the amplitudes of the scotopic a-waves (Figure 3A) and b-waves (Figure 3B) gradually decreased with increasing age in the Sod1−/− mice. Therefore, senescent Sod1−/− mice exhibited functional disturbances of both the outer and inner sensory retina. It is interesting that the oscillatory potentials were preserved even in a very old Sod1−/− mouse showing decreased amplitudes of a- and b-waves (Figure 1).
Figure 2.
Mean amplitudes of a- and b-waves of the ERGs. ERGs were recorded from age-matched WT and Sod1−/− mice >40 weeks of age (n = 5, each group). Asterisk indicates P < 0.05. White box, WT; black box, Sod1−/−.
Figure 3.
Age-related changes of ERGs. A: Age-related changes of the amplitudes of the scotopic a-wave. The amplitudes decrease with increasing age in Sod1−/− mice (black squares), in contrast to those of the WT (white circles). B: Age-related changes of the scotopic b-wave. Stimulus intensity was 65.0 cd*sm−2.
Retinal Histology and Ultrastructure
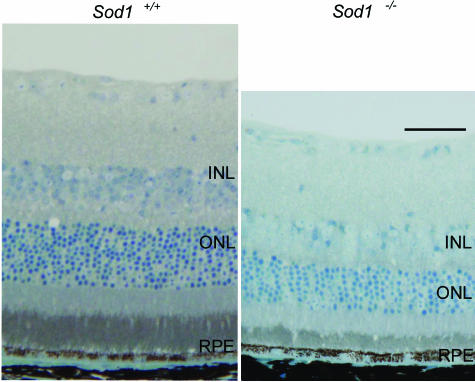
The thicknesses of the retinal layers of WT and the Sod1−/− mice at 10, 30, and 50 weeks of age were measured. The differences in the thicknesses of the INL and ONL were not significant for the two types of mice up to the age of ∼10 weeks. Thereafter, the thickness of the ONL and INL in Sod1−/− mice gradually decreased (Figure 4). The ratios (KO/WT) of the numbers of nuclei per row were 100.4%, 97.9%, and 81.4% in the INL, and 93.6%, 85.2%, and 71.9% in the ONL at ages 10, 30, and 50 weeks, respectively. The significant decrease in the thickness started at 50 weeks in the INL (P < 0.01) and 30 weeks in the ONL (P < 0.05), indicating that the photoreceptors, horizontal, bipolar, amacrine, and Mueller cells were most likely damaged in the senescent Sod1−/− mice.
Figure 4.
Retinal thinning of Sod1−/− mice. Toluidine blue-stained retina Quetol 812 sections (2 μm) of a 15-month-old WT mouse and an age-matched Sod1−/− mouse. Thinning of the INL and ONL is apparent. Scale bar = 50 μm.
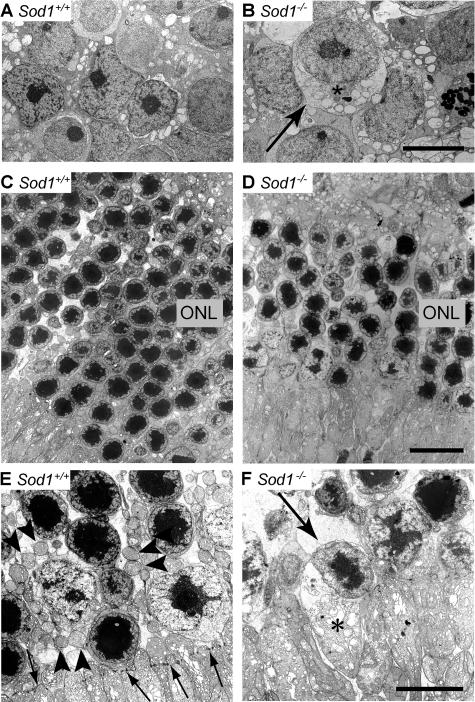
Ultrastructural examination showed a disorganized INL and ONL in 15-month-old senescent Sod1−/− mice (Figure 5, A–F). The nuclei were swollen, the cytoplasm was vacuolized, and the plasma and nuclear membranes in the INL were disrupted in a Sod1−/− mouse at 15 months of age (Figure 5, A and B). The ONL was also disorganized in contrast to the orderly alignment of each column of nuclei in the WT, and the density also decreased in the Sod1−/− mice (Figure 5, C and D). The mitochondria in the ONL were damaged, and the cell bodies were swollen. Necrotic cells were also observed (Figure 5F). We rarely observed retinal cells showing chromatin condensation, a morphological hallmark of apoptotic cell. These findings suggest that the death of the neurons in Sod1−/− mice was attributable to necrosis possibly because of elevated ROS.
Figure 5.
Ultrastructure of sensory retina of the senescent WT and age-matched Sod1−/− mice. A: INL of the WT of 15 months. B: Swollen nuclei in the INL of age-matched Sod1−/− mice (asterisk). Vacuolization and destruction of plasma and nuclear membranes can be seen (arrow). C: ONL of a WT mouse at 15 months of age. D: ONL of an age-matched Sod1−/− mouse. Cell layers are disorganized, and the cell density is decreased. The number of nuclei in the ONL is decreased in Sod1−/− mice. Higher magnification of ONL. WT (E), and Sod1−/− mice (F). Mitochondria in photoreceptors of ONL distinguished in Sod1−/− mice, as compared with the WT (arrowheads in E). External limiting membrane in the WT (arrows in E) is not distinct in a 15-month-old Sod1−/− mouse. Plasma membranes are not clear in a damaged cell of the Sod1−/− mouse (arrow in F). Swelling of the inner segments of the photoreceptors can be seen (asterisk). Scale bars: 2 μm (B, F); 5 μm (D).
Cell Deaths in Retinas of Senescent Sod1−/− Mice Is Not Caspase-3-Dependent
To explore the mechanism of cell death, we first examined if the apoptotic pathways were activated in retinas of Sod1−/− mice at ages 30 and 50 weeks. TUNEL staining showed that the difference between the WT and Sod1−/− mice was not significant (data not shown). Next, we performed Western blot analysis for the detection of the active form of caspase, however the activated form of caspase-3 was not apparent in the Sod1−/− mice. Immunohistochemistry with anti-cleaved caspase-3 antibody did not show apparent positive signals in the sensory retinas of KO and WT mice as compared with a positive control (data not shown). Therefore, the retinal degeneration of Sod1−/− mice appears not to be dependent mainly on apoptosis as in some other models of retinal degeneration.6,7,8 Considering that SOD1 is the most abundant antioxidant that scavenges superoxide in retina, cell deaths in ONL of Sod1−/− mice might be caused by the ROS-mediated necrotic pathway, rather than by the apoptotic pathways, as was compatible with the ultrastructural findings. Because the presence of inflammatory reactions is a hallmark of necrosis, diffuse deposition of immunoglobulins in retina-retinal pigment epithelium complex in Sod1−/− mice11 might be attributable to necrotic reactions. However, we are aware of the possibility that very slow apoptotic cell deaths could contribute to the retinal dysfunction in these mice.
Discussion
Our results showed that retina of SOD1-deficient mice had morphological and functional alterations that increased with increasing age. When mice were young (<5 months), the retinas appeared not significantly different from those of WT mice when examined by ophthalmoscopy.11
Different Roles of SOD1 and SOD2 in Retina
SOD1 and SOD2 are two major intracellular SODs and both are ubiquitously distributed in the sensory retina.11 The retinas of Sod2−/− mice are thinner than the same age WT mice even at birth.16 Because Sod2−/− mice die soon after birth by dilated cardiomyopathy,17 Sod2−/− mice are not suitable for the examination of age-related retinal dysfunction attributable to accumulated ROS. Current technical modalities for conditional knockout of SOD2 would be expected to overcome this problem.18 In contrast to the acute and severe retinal degeneration in Sod2−/− mice, the degeneration in Sod1−/− mice is mild and slowly progressive. Because the amounts of SOD2 and SOD3 proteins are approximately the same in WT and Sod1−/− mice,11 other oxidative stress scavenger systems such as SOD2 may at least partially protect the retina against ROS-mediated retinal damages in Sod1−/− mice in vivo.
SOD1 Is Necessary to Prevent Age-Related Degeneration of Sensory Retina
In addition to the retinal changes, the Sod1−/− mice have a short lifespan,9 ovarian dysfunction,19 liver tumors,9 anemia,10 fatty liver,20 muscle weakness,21 and hearing loss.22,23 In addition to the AMD-like phenotypes,11 we found a novel phenotype of the sensory retina of Sod1−/− mice. The slowly progressive degeneration of the retina appears to resemble the phenotype of the auditory system of Sod1−/− mice. Both the a- and b-waves of the ERGs were reduced in aged Sod1−/− mice. These results are compatible with the retinal histology showing the thinning of the INL and ONL. Because SOD1 is distributed throughout all retinal cell layers,11 it is not surprising that SOD1 deficiency affected both the INL and ONL.
Recently, a relationship between oxidative stress and retinal degeneration has been reported in animal models. Antioxidants reduce the number of cone deaths in a model of retinitis pigmentosa,24 and photo-oxidative stress causes retinal degeneration that can be rescued by antioxidants.25 Our results provide additional evidence that antioxidants may be potentially protective for some types of age-related retinal degeneration. Administration of N-acetylcysteine (NAC) can rescue Sod1−/− mice against the effect of anemia by reversing the production of ROS in their erythrocytes.10 It would be interesting to determine whether the retinal pathologies in Sod1−/− mice can be rescued by antioxidants including NAC, vitamins, or carotenoids. Our results showed no detectable differences in the apoptotic cell death between the retinas of Sod1−/− mice and the WT in the specimens examined. The nuclei of the retinal cells in senescent Sod1−/− mice were swollen suggesting that cell death may be caused by necrosis subsequent to elevated ROS attributable to the absence of SOD1.
Comparison with Other Models of Retinal Degeneration
Many animal models of retinal degeneration have been investigated.3,4 Apoptosis is observed in some inherited retinal diseases such as retinitis pigmentosa and in other pathological retinal conditions such as hypoxia and oxidative stresses.26,27 Several genetically mutated models of retinitis pigmentosa have been extensively investigated, including the RCS rat and rd mice. The retinal degeneration in the RCS rat is caused by an impairment of retinal pigmented epithelium because of mutation of receptor tyrosine kinase with subsequent photoreceptor cell death. The retinal cell death in RCS rat results from a caspase-1- and -2-dependent apoptosis.7,27,28,29,30 rd mice have a recessive mutation of the gene encoding rod cGMP phosphodiesterase β-subunit, and photoreceptor apoptosis also occurs in a caspase-dependent manner.6 A model of ROS-related retinal degeneration, mice deficient of uchl3, has been reported, and they show photoreceptor degeneration with TUNEL-positive cells, which begins at 2 to 3 weeks of age.8
These animal models of retinal degeneration differ from Sod1−/− mice; 1) Sod1−/− mice show very slow progressive degeneration, 2) the a- and b-waves are simultaneously affected, and 3) the death of the retinal cells appear to be caused mainly by necrosis rather than apoptosis. Because the Sod2−/− mice are not suitable for long-term follow-up studies,17 Sod1−/− mice seem to be a good model to study ROS-mediated retinal degeneration. Recently, an intraocular injection of paraquat, a strong pro-oxidant, was reported to induce more severe retinal damage to the retina of Sod1−/− mice than that in the WT. This would indicate that SOD1 may be protective against excessive amounts of exogenous oxidative stress.31 However, this study did not report the spontaneous retinal changes with age in Sod1−/− mice.
Relevance to Human Retinal Diseases
Oxidative stress is closely associated with retinal degeneration in humans.32,33 Retinal degeneration is caused by ROS because of iron overload, ie, ocular siderosis.32 Moreover, AMD is at present considered to be caused by elevated oxidative damages in retina because supplementation by high-dose vitamins and minerals can slow the progression of AMD.33 Patients with an early stage of AMD have decreased ERGs,34,35,36,37,38,39 therefore, Sod1−/− mice may mimic the early retinal dysfunction attributable to aging. Excess light to the retina causes retinal degeneration by elevated production of ROS in retina. Therefore, we consider that our findings may help in the understanding of the pathophysiology of the ROS-mediated retinal degeneration in humans. Because nutritional intervention including vitamin A palmitate and ω-3-rich fish slow the progression of disease in many retinitis pigmentosa patients,40 our findings support the hypothesis that antioxidants may be an additional interventional modality for some types of retinal degeneration.
In summary, we have demonstrated that SOD1 deficiency leads to retinal dysfunction and degenerative changes of retinal cell layers that are progressive. Because senescent Sod1−/− mice have choroidal neovascularization, a hallmark of wet-type AMD, Sod1−/− mice may exhibit features of both wet- and dry-type AMD. This implies that the etiology of these two clinically distinct entities in humans may be genetically identical.
Acknowledgments
We thank Satoshi Uchiyama, Ichie Kawamori, Yuka Kondo, and Chizuru Tsuda for technical assistance; Ei-ichiro Nagasaka and Masao Yoshikawa for ERG recording; Junko Moriya and Yumi Takanashi for histology; and Hisao Ohde for helpful discussion.
Footnotes
Address reprint requests to Yutaka Imamura, M.D., Department of Ophthalmology, Inaida Laboratory, Keio University School of Medicine, 35-Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan. E-mail: imamura@sc.itc.keio.ac.jp.
K.H. and M.H. contributed equally to this study.
Present address of T.S.: Department of Aging Control Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan.
References
- Valentine JS, Doucette PA, Potter SZ. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- Behndig A, Svensson B, Marklund SL, Karlsson K. Superoxide dismutase isoenzymes in the human eye. Invest Ophthalmol Vis Sci. 1998;39:471–475. [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- Dalke C, Graw J. Mouse mutants as models for congenital retinal disorders. Exp Eye Res. 2005;81:503–512. doi: 10.1016/j.exer.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Grooten JV, Goossens B, Vanhaesebroeck B, Fiers W. Cell membrane permeabilization and cellular collapse, followed by loss of dehydrogenase activity: early events in tumor necrosis factor-induced cytotoxicity. Cytokine. 1993;5:546–555. doi: 10.1016/s1043-4666(05)80003-1. [DOI] [PubMed] [Google Scholar]
- Hopp RMP, Ransom N, Hilsenbeck SG, Papermaster DS, Windle JJ. Apoptosis in the murine rd1 retinal degeneration is predominantly p53-independent. Mol Vis. 1998;4:5. [PubMed] [Google Scholar]
- Katai N, Kikuchi T, Shibuki H, Kuroiwa S, Arai J, Kurokawa T, Yoshimura N. Caspase-like proteases activated in apoptotic photoreceptors of Royal College of Surgeons rats. Invest Ophthalmol Vis Sci. 1999;40:1802–1807. [PubMed] [Google Scholar]
- Sano Y, Furuta A, Setuie R, Kikuchi H, Wang YL, Sakurai M, Kwon J, Noda M, Wada K. Photoreceptor cell apoptosis in the retinal degeneration of Uchl3-deficient mice. Am J Pathol. 2006;169:132–141. doi: 10.2353/ajpath.2006.060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- Iuchi Y, Okada F, Onuma K, Onoda T, Asano H, Kobayashi M, Fujii J. Elevated oxidative stress in erythrocytes due to a SOD1 deficiency causes anaemia and triggers autoantibody production. Biochem J. 2007;402:219–227. doi: 10.1042/BJ20061386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y, Noda S, Hashizume K, Shinoda K, Yamaguchi M, Uchiyama S, Shimizu T, Mizushima Y, Shirasawa T, Tsubota K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Natl Acad Sci USA. 2006;103:111282–111287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Peachey NS, Ripps H, Naash MI. Functional abnormalities in transgenic mice expressing a mutant rhodopsin gene. Invest Ophthalmol Vis Sci. 1995;36:62–71. [PubMed] [Google Scholar]
- Sieving PA, Murayama K, Naarendrop F. Push-pull model of primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci. 1994;11:519–532. doi: 10.1017/s0952523800002431. [DOI] [PubMed] [Google Scholar]
- Goto Y, Yasuda T, Tobimatsu S, Kato M. 20-Hz flicker stimulus can isolate the cone function in rat retina. Ophthalmic Res. 1998;30:368–373. doi: 10.1159/000055497. [DOI] [PubMed] [Google Scholar]
- Mori M, Metzqer D, Picaud S, Hindelang C, Simonutti M, Sahel J, Chambon P, Mark M. Retinal dystrophy resulting from ablation of RXR alpha in the mouse retinal pigment epithelium. Am J Pathol. 2004;164:701–710. doi: 10.1016/s0002-9440(10)63157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbach JM, Coscun PE, Grossniklaus HE, Kokoszka JE, Newman NJ, Wallace DC. Ocular pathology in mitochondrial superoxide dismutase (Sod2)-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:2173–2178. [PubMed] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berqer C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- Ikegami T, Suzuki Y, Shimizu T, Isono K, Koseki H, Shirasawa T. Model mice for tissue-specific deletion of the manganese superoxide dismutase (MnSOD) gene. Biochem Biophys Res Commun. 2002;296:729–736. doi: 10.1016/s0006-291x(02)00933-6. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–4011. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- Uchiyama S, Shimizu T, Shirasawa T. CuZn-SOD deficiency causes ApoB degradation and induces hepatic lipid accumulation by impaired lipoprotein secretion in mice. J Biol Chem. 2006;281:31713–31719. doi: 10.1074/jbc.M603422200. [DOI] [PubMed] [Google Scholar]
- Muller MF, Sonq W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, II, Csete M, Faulkner JA, Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- MacFadden SL, Ding D, Salvi R. Anatomical, metabolic and genetic aspects of age-related hearing loss in mice. Audiology. 2001;40:313–321. [PubMed] [Google Scholar]
- MacFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG, Salvi RJ. Cu/Zn deficiency potentiates hearing loss and cochlear pathology in aged 129.CD-1 mice. J Comp Neurol. 1999;413:101–112. [PubMed] [Google Scholar]
- Komeima K, Roqers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci USA. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanito M, Nishiyama A, Tanaka T, Masutani H, Nakamura H, Yodoi J, Ohira A. Change of redox status and modulation by thiol replenishment in retinal photooxidative damage. Invest Ophthalmol Vis Sci. 2002;43:2392–2400. [PubMed] [Google Scholar]
- Pacione LR, Szego MJ, Ikeda S, Nishina PM, McInnes RR. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu Rev Neurosci. 2003;26:657–700. doi: 10.1146/annurev.neuro.26.041002.131416. [DOI] [PubMed] [Google Scholar]
- Phelan JK, Bok D. A brief review of retinitis pigmentosa and the identified retinitis pigmentosa genes. Mol Vis. 2000;6:116–124. [PubMed] [Google Scholar]
- D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem. 2002;277:17016–17022. doi: 10.1074/jbc.M107876200. [DOI] [PubMed] [Google Scholar]
- Vollrath D, Feng W, Duncan JL, Yasumura D, D’Cruz PM, Chappelow A, Matthes MT, Kay MA, LaVail MM. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci USA. 2001;98:12584–12589. doi: 10.1073/pnas.221364198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong A, Shen J, Krause M, Akiyama H, Hackett AF, Lai H, Campochiaro PA. Superoxide dismutase 1 protects retinal cell from oxidative damage. J Cell Physiol. 2006;208:516–526. doi: 10.1002/jcp.20683. [DOI] [PubMed] [Google Scholar]
- Weiss MJ, Hofeldt AJ, Behrens M, Fisher K. Ocular siderosis. Diagnosis and management. Retina. 1997;17:105–108. doi: 10.1097/00006982-199703000-00004. [DOI] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119:1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RE, Chakravarthy U. Visual function and dysfunction in early and late age-related maculopathy. Prog Ret Eye Res. 2006;25:249–276. doi: 10.1016/j.preteyeres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Feigl B, Brown B, Lovie-Kitchin J, Swann P. The rod-mediated multifocal electroretinogram in aging and in early age-related maculopathy. Curr Eye Res. 2006;31:635–644. doi: 10.1080/02713680600762739. [DOI] [PubMed] [Google Scholar]
- Gerth C, Delahunt PB, Alam S, Morse LS, Werner JS. Cone-mediated multifocal electoretinogram in age-related macular degeneration: progression over a long-term follow-up. Arch Ophthalmol. 2006;124:345–352. doi: 10.1001/archopht.124.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GR, McGwin G, Jr, Phillips JM, Klein R, Owsley C. Impact of aging and age-related maculopathy on inactivation of the a-wave of the rod-mediated electroretinogram. Vision Res. 2006;46:1422–1431. doi: 10.1016/j.visres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Jackson GR, McGwin G, Jr, Phillips JM, Klein R, Owsley C. Impact of aging and age-related maculopathy on inactivation of the a-wave of the rod-mediated electroretinogram. Invest Ophthalmol Vis Sci. 2004;45:3271–3278. doi: 10.1167/iovs.04-0019. [DOI] [PubMed] [Google Scholar]
- Chen C, Wu L, Wu D, Huang S, Wen F, Luo G, Long S. The local cone and rod system function in early age-related macular degeneration. Doc Ophthalmol. 2004;109:1–8. doi: 10.1007/s10633-004-1041-0. [DOI] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]