Abstract
Expression profiling is a well established tool for the genome-wide analysis of human cancers. However, the high sensitivity of this approach combined with the well known cellular and molecular heterogeneity of cancer often result in extremely complex expression signatures that are difficult to interpret functionally. The majority of sporadic colorectal cancers are triggered by mutations in the adenomatous polyposis coli (APC) tumor suppressor gene, leading to the constitutive activation of the Wnt/β-catenin signaling pathway and formation of adenomas. Despite this common genetic basis, colorectal cancers are very heterogeneous in their degree of differentiation, growth rate, and malignancy potential. Here, we applied a cross-species comparison of expression profiles of intestinal polyps derived from hereditary colorectal cancer patients carrying APC germline mutations and from mice carrying a targeted inactivating mutation in the mouse homologue Apc. This comparative approach resulted in the establishment of a conserved signature of 166 genes that were differentially expressed between adenomas and normal intestinal mucosa in both species. Functional analyses of the conserved genes revealed a general increase in cell proliferation and the activation of the Wnt/β-catenin signaling pathway. Moreover, the conserved signature was able to resolve expression profiles from hereditary polyposis patients carrying APC germline mutations from those with bi-allelic inactivation of the MYH gene, supporting the usefulness of such comparisons to discriminate among patients with distinct genetic defects.
Colorectal cancer (CRC) is one of the major causes of morbidity and mortality among Western societies. Although the vast majority of CRC cases are sporadic, a considerable fraction has been attributed to hereditary and familial factors.1 Hereditary CRC syndromes have served as unique models to elucidate the molecular and cellular mechanisms underlying colorectal tumor initiation and progression to malignancy, as the same genes mutated in the germline of affected individuals are also known to play rate-limiting roles in the majority of the sporadic cases.2 This is certainly the case of the adenomatous polyposis coli (APC) tumor suppressor gene, known to be mutated in the germline of individuals affected by familial adenomatous polyposis (FAP)3,4,5,6 and in the majority of the sporadic CRC cases.7,8,9 In fact, loss of APC function appears to play a rate-limiting and initiating role in the adenoma-carcinoma sequence.10 Among the multiple functional domains characterized in its coding sequence, APC’s ability to bind and down-regulate β-catenin, the main signaling molecule in the canonical Wnt pathway, is generally regarded as its main tumor-suppressing activity.10 Loss of APC function or oncogenic activation of β-catenin, as observed in the vast majority of the sporadic CRC cases, leads to intracellular accumulation of β-catenin and its translocation to the nucleus, where it binds to transcription factors of the T cell factor/lymphoid enhancer-binding factor (TCF/LEF) family and modulates transcription of a broad spectrum of downstream target genes.11,12 The vast majority (70 to 90%) of FAP patients have been shown to carry germline APC mutations.13 More recently, biallelic mutations in the base excision repair gene MYH were found in a subset of polyposis families with attenuated clinical presentation and an autosomal recessive inheritance pattern, often referred to as MAP (MYH-associated polyposis).14
In view of its initiating role in intestinal cancer, several preclinical models carrying germline mutations in the endogenous mouse Apc tumor supressor gene have been generated, and their phenotype has been characterized.15 The predisposition of these mouse models to multiple intestinal adenomas closely resembles the FAP phenotype at the molecular, cellular, and phenotypic level.15 One exception to the latter is represented by the proximal localization and distribution of adenomas along the gastrointestinal (GI) tract of Apc-mutant mouse models when compared with the colorectal clustering of polyps among FAP patients.15
Expression profiling by cDNA and oligonucleotide microarrays represents a powerful tool for genome-wide transcriptional analysis. Several studies in the literature have reported on the comparison of expression profiles from colorectal tumors and normal intestinal mucosa in an attempt to identify differentially expressed genes, predict, whenever feasible, clinical outcome, and elucidate the molecular and cellular mechanisms underlying colorectal tumorigenesis.16 However, the different lists of genes differentially expressed in CRC are often very extensive and only partially overlapping among independent studies, possibly reflecting differences in the methodologies and in cohorts used.16 To pinpoint conserved and functionally relevant genes differentially expressed between normal and malignant tissues, cross-species comparison have been successfully applied by comparing expression signatures of hepatocellular carcinoma, and prostate and lung cancer derived from human patients and mouse models.17,18,19,20
Here, we report the cross-species comparison of expression profiles of intestinal adenomas from FAP patients with established APC germline mutations and from Apc+/1638N, a mouse model for familial polyposis previously developed in our laboratory and characterized by the development of an average of five tumors in the upper GI tract, together with other extra-intestinal manifestations characteristic of FAP patients, such as epidermal cysts and desmoids.21,22 A total of 166 genes were found to be highly conserved between the two species and are likely to play important roles in the cellular and molecular mechanisms underlying adenoma formation in the gastrointestinal tract. Among these, several Wnt downstream target genes are included, as also expected from the selection of APC-mutant mouse and human adenomas. Notably, the conserved APC signature also made it possible to distinguish FAP tumors from MAP ones in an unsupervised fashion.
Materials and Methods
Patients and Tumor Samples
Colorectal adenomatous polyps were obtained from a total of 13 patients from the Department of Surgery, Heinrich Heine University (Dusseldorf, Germany); the Institute of Medical Genetics, Cardiff University (Cardiff, UK); the Department of Pathology, Leiden University Medical Center (Leiden, The Netherlands); and the Department of Surgery, Erasmus Medical Center (Rotterdam, The Netherlands). Of the 13 polyposis patients, 8 carried truncating APC mutations, whereas 5 were found to carry biallelic MYH mutations. Detailed characterization of the polyposis patients carrying biallelic MYH or monoallelic APC germline mutations and of the corresponding tumor samples used in the present study has been reported elsewhere.23 Normal epithelial mucosa from 3 healthy individuals was also collected (control samples NC1 to NC3). All tissue samples were snap-frozen, embedded in OCT medium, and stored at −80°C. Detailed sample processing procedures were as previously described.24 All of the analyzed adenomatous lesions were matched for histology (low-grade dysplasia) and anatomical location (left-sided colon or rectum). Two to six polyps were analyzed for each individual patient.
Mouse Strains and Material
All wild-type and Apc+/1638N mice used in this study were inbred C57BL6/J, maintained under SPF conditions and fed ad libitum. Duodenal adenomas and normal mucosa samples were collected from 9-month-old males, briefly washed in PBS, and snap-frozen in OCT medium. Hematoxylin and eosin (H&E) staining of all tissues was performed to determine histology and tumor content.
Laser Capture Microdissection and RNA Isolation
Sample preparation and laser capture microdissection (LCM) were performed as previously described using a PALM MicroBeam microscope system (P.A.L.M. Microlaser Technologies, Bernereid, Germany).23 In short, 10-μm cryosections were mounted on microscope slides with a polyethylene naphthalate membrane and stained by H&E to allow histological identification of the desired normal and tumor cells. Approximately 1000 to 2000 cells, corresponding to 600,000 (human samples)- and 1,200,000 (mouse specimens)-μm2 areas, were microdissected.
RNA was isolated from the LCM samples by Mini RNeasy columns (QIAGEN, Valencia, CA), according to the manufacturer’s instructions, including a DNase step on the column. Quality of the isolated RNA was checked with RNA 6000 Pico LabChip kit (Agilent Technologies, Palo Alto, CA).
Expression Profiling and Data Analysis
Human Adenoma Samples
Each RNA sample that passed the quality controls was linearly amplified with two rounds of amplification using the MessageAmp kit (Ambion, Huntingdon, UK), according to the manufacturer’s protocol. Quality and quantity of each amplified RNA sample was again evaluated by Nano Lab-on-Chip (Agilent Technologies). One-μg aliquots of target and reference amplified RNA were labeled with Cy5-dUTP and Cy3-dUTP, respectively (Amersham Biosciences, Amersham, UK) by reverse transcription using the CyScribe First Strand cDNA Labeling kit (Amersham Biosciences). Each labeling reaction was further purified with the CyScribe GFX purification kit (Amersham Biosciences). Subsequently, both labeled cDNAs were hybridized on a human 18K cDNA microarray encompassing 19,200 spots (representing 18,432 independent cDNAs) produced and obtained from The Netherlands Cancer Institute Microarray Facility (Amsterdam, The Netherlands). The cDNAs spotted in this array platform were PCR-amplified from a clone set purchased from Research Genetics (Huntsville, AL). Hybridization and washing procedures were performed according to the manufacturer’s (The Netherlands Cancer Institute Microarray Facility) protocol. Sixteen-bit fluorescent images from the expression arrays were acquired with an Agilent DNA Microarray Scanner (Agilent Technologies), and the resulting TIFF images were analyzed with the software GenePix Pro 4.0 (Axon Instruments, Union City, CA). For each array, a GenePix results file (.gpr) with the extracted Cy3 and Cy5 spot and background raw intensities was generated.
Expression data analysis was performed with a set of functions implemented in R (http://www.R-project.org/25). Briefly, .gpr files from the array platforms were directly loaded into the R environment using the marray package to extract the background-corrected Cy3 and Cy5 median raw intensity per spot. Intensity data from both platforms were normalized with the variance stabilization and normalization function implemented in the vsn package.26
To find genes that could better characterize the histological and mutational status of the analyzed samples, we have used “mixed-effects” regression models. Briefly, we fitted the following model: Y(i) = α + β∗histology + γ∗mutation + δ∗patient + error(i), where Y represents the log-ratio of the expression value for clone i; histology and mutation are categorical variables represented as stages (normal or adenoma) and (APC or MYH), respectively, whereas α represents the baseline expression level of clone i. A patient effect must be included in the model to correctly handle multiple samples derived from the same patient. Although both histology and mutation are assumed to have fixed effects, the patient effect is assumed to be random, as the patients included in this study represent the heterogeneous (outbred) population of familial CRC patients. This model was fitted to the data using the MAANOVA package.27 Moderated F-test statistics, which take advantage of the large number of genes being analyzed simultaneously to yield more reliable variance estimates, were extracted corresponding to histology and mutation effects. Their P values were subsequently corrected for multiple testing using Benjamini and Hochberg’s false discovery rate (FDR) method.28
Mouse Adenoma Samples
RNA samples were submitted to a double round of amplification according to the Small Sample Labeling Protocol vII (Affymetrix, Inc, Santa Clara, CA). Quality of synthesized cRNA was checked using the RNA 6000 Nano LabChip kit (Agilent Technologies). Labeled cRNA products were hybridized to mouse arrays MOE430A (Affymetrix, Inc) according to the manufacturer’s instructions. Data analysis was performed using R Statistical Computing software v2.4.125 complemented with BioConductor Packages affy,29 limma,30,31 and vsn.26 Cel files were uploaded and summarized using the affy package and normalized with vsn at the probe level. Using an empirical-Bayesian linear model (implemented in the package limma), we identified genes that were differentially expressed, with multiple testing correction performed using Benjamini and Hochberg’s FDR step-up method.28 Hierarchical clustering (Euclidean similarity measure) was performed on vsn-normalized data for all probe identifications (IDs) using Spotfire DecisionSite 9.0. (http:www.spotfire.com).
To test and/or confirm that a set of genes yielded a differential expression signature between groups of samples, the globaltest32 of vsn-normalized data was performed using R Statistical Computing software v2.4.125 complemented with the BioConductor Package globaltest.32
Functional Annotation Analysis
GenBank accession numbers or Affymetrix probe set IDs were assigned to biological process using the GO (Gene Ontology) chart feature offered by the Database for Annotation Visualization and Integrated Discovery (DAVID) 2006 (http://david.abcc.ncifcrf.gov/home.jsp). Individual genes from selected expression signatures were also placed in the context of their molecular and functional interactions by using Ingenuity Pathways Analysis (IPA) tools according to the manufacturer’s instructions (Ingenuity Systems, Redwood City, CA).
Data Integration
The cross-species comparison was performed using the Sequence Retrieval System (SRS).33 Both sets were first annotated using Unigene; next, the SRS system was used to pair the two species’ Unigene entries based on Homologene. The MOE430A array includes a total of 22,626 probes, whereas the human cDNA array encompasses 19,200 probes. Of the 22,626 mouse probes in the mouse Affymetrix platform, the SRS system retrieved a total of 12,083 homologous genes encompassed by the human array. Due to probe multiplicity in both platforms, the total overlap between the platforms consists of 18,369 entries.
From the two original data sets (mouse adenomas versus normal mucosa and human adenomas versus normal mucosa), probe sets with the same direction of differential expression were selected, adding to a total of 9495 probes. Further selection was applied based on the statistical significance according to the following thresholds: mouse data FDR <5% and human data FDR <0.5% (n = 234 probes). To exclude the possibility that the observed overlap resulted by sheer chance, we have performed a χ2 test with the selected probes and reached a highly significant P value (P = 0.007).
Immunohistochemistry
Immunohistochemistry of mouse and human normal and adenomatous intestinal sections was performed according to standard protocols. The following antibodies were used and optimized for human and mouse tissues: MacMarcks (Calbiochem cat.442708; EMD Biosciences, San Diego, CA), CyclinA (cat.GTX27956; Genetex, San Antonio, TX), AnnexinI (cat.71-3400; Zymed, South San Francisco, CA). Signal detection of these antibodies was obtained by the Rabbit Envision+ System-HRP (cat.K4011; DakoCytomation, Carpinteria, CA). CD44 (cat.553131; BD Pharmingen, San Diego, CA) was detected using a secondary antibody goat anti-rat, HRP labeled.
Results
The overall rationale and strategy of the present study was to attempt the comparative expression profiling of intestinal adenomas derived from FAP patients carrying APC germline mutations23 and from the Apc+/1638N mouse model.21,22 In both cases, we aimed at using tumor samples in which the initiating and rate-limiting mutation event is represented by loss of APC tumor-suppressing function to gain insight into conserved molecular and cellular pathways relevant for intestinal tumor initiation. Furthermore, we explored the ability of the conserved gene signature to discriminate between adenomas from polyposis patients carrying germline APC mutations from those with different genetic defects.
Expression Profiling Analysis of Familial Adenomatous Polyps
Colorectal adenomatous polyps (n = 42) have been obtained from a total of eight unrelated FAP patients carrying previously identified germline APC mutations.23 To obtain expression signatures exclusively derived from parenchymal cells and avoid the confounding effects of infiltrating and adjacent stromal cells, dysplastic tumor cells were collected by laser capture microdissection (LCM). Control LCM samples were obtained from the intestinal mucosa of three individuals with no CRC history. RNA was extracted from the microdissected tumor and normal specimens and subsequently used for expression profiling by hybridization to human 18K cDNA arrays generated at The Netherlands Cancer Institute Microarray Facility (see Materials and Methods). The expression profiling data have been deposited at the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo) and is accessible through GEO Series accession number GSE9689.
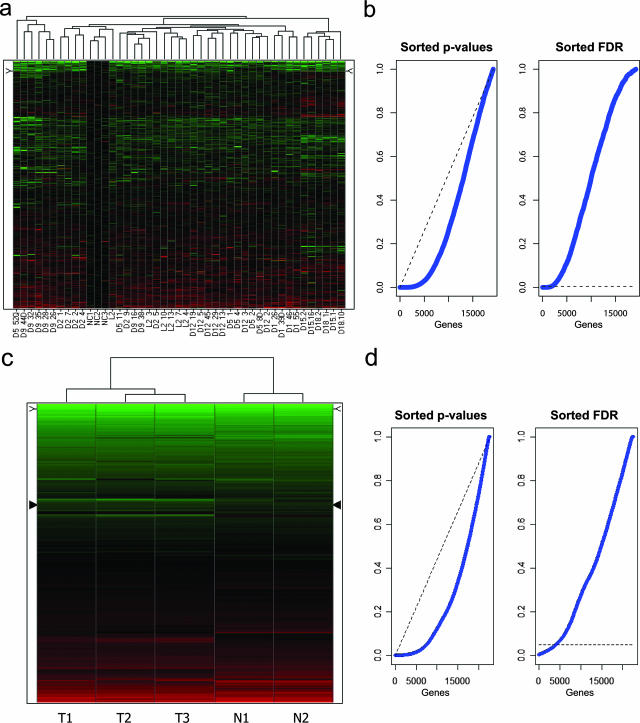
Two-dimensional hierarchical clustering was first applied to all 19,200 array probes to generate an overview, without any prior filtering, of the global gene expression differences among all samples (Figure 1a). Notably, the three normal colon mucosa specimens (NC1-3) do not cluster separately from the adenoma samples. Also, several samples from the same individual are often observed to cluster together, indicative of a patient effect. In view of the latter, a statistical approach using a mixed-effects regression model34 was applied to all samples to determine whether specific patterns of gene expression could be associated with the adenomatous polyps. The linear mixed-effects model was fitted considering histology (adenoma versus normal mucosa) as having a fixed effect and patient as having a random effect. This procedure not only allowed us to calculate P values for all genes, but also to control the variance component associated with random patient-specific genetic variation, ie, the variability in gene expression related to the genetic background of each individual patient. With an FDR set to 0.5%, a total of 1859 probes appeared to be differentially expressed between normal colonic epithelium and adenomas (see Supplemental Tables S1 and S2 at http://ajp.amjpathol.org). The relatively high number of differentially expressed genes even under highly stringent conditions clearly illustrates the strong effect of histology on global gene expression. This is also further illustrated by the empirical cumulative P values distribution function, which is clearly distinct from what would be expected if no effect was detectable (Figure 1b). Of the 1859 probes, 839 (45%) and 1020 (55%) were, respectively, up- and down-regulated in tumor cells when compared with normal mucosa (see Supplemental Tables S1 and S2 at http://ajp.amjpathol.org).
Figure 1.
Unsupervised hierarchical cluster analysis of expression profiles from human and mouse intestinal polyps, without preliminary gene selection. Up- and down-regulated probes are depicted in red and green, respectively. a: Unsupervised hierarchical clustering analysis of 42 colorectal adenomatous polyps (obtained from eight unrelated FAP patients with previously identified germline APC mutations23) and 3 control normal mucosa samples (labeled as NC1-3) obtained from individuals with no history of CRC. b: Distribution of P values (left plot) relative to the comparison of patient-derived colorectal adenomas versus normal mucosa samples, sorted in ascending order (blue line). The dashed (black) line represents what would be expected if no effect was detectable. In the right plot, FDR-adjusted sorted P values are shown. The dashed line represents the FDR threshold used in our study to select the differentially expressed genes in the human set that led to the selection of 1859 probes. c: Unsupervised hierarchical clustering analysis of duodenal adenomas (n = 3, labeled T1–T3) and normal mucosa (n = 2, N1–N2) samples obtained from inbred C57BL6/J Apc+/1638N and Apc+/+ mice, respectively. d: Distribution of P values (left plot) relative to the comparison of mouse duodenal adenomas versus normal tissue samples, sorted in ascending order (blue line). The dashed (black) line represents what would be expected if no effect was detectable. In the right plot, FDR-adjusted sorted P values are shown. The dashed line represents the FDR threshold used in our study to select the differentially expressed genes in the mouse set that led to the selection of 4137 probes.
To gain insight into the biological relevance of the newly generated list of genes differentially expressed in adenomatous polyps, we used the GO-based bioinformatics tool DAVID35 (see Materials and Methods). An overview of the functional categories represented by the genes differentially expressed among APC-mutant adenomatous polyps when compared with normal colonic mucosa reveals a very broad spectrum of biological processes, ranging from different aspects of cellular metabolism to apoptosis, cell migration, and immune response (data not shown). The broadness of the transcriptional profiles of the colorectal polyps when compared with normal mucosa is likely to reflect the heterogeneity of these benign tumors even at this very early stage of the adenoma-carcinoma sequence.
Expression Profiling Analysis of Apc+/1638N Mouse Intestinal Adenomas
Duodenal adenomas and normal mucosal samples from age- and sex-matched C57BL6/J Apc+/1638N mice (n = 3) and Apc+/+ controls (n = 2) were collected and snap-frozen as for the above human polyps. Histological analysis of these lesions confirmed their benign adenomatous nature (not shown). Also in these cases, dysplastic epithelial cells were collected by LCM. Control expression signatures were obtained from wild-type C57BL6/J epithelial cells microdissected from the same anatomical location. Total RNA was extracted from normal and tumor samples and hybridized to Affymetrix MOE430A arrays. The corresponding data have been deposited in National Center for Biotechnology Information’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE9580. Unsupervised two-dimensional hierarchical clustering was able to discriminate and correctly cluster tumor from normal samples (Figure 1c). Empirical-Bayesian linear regression analysis allowed the identification of statistically significant differences between normal and tumor samples.30,31 Notwithstanding the admittedly limited sample size, a strong gene expression signature of the tumor samples is detected as illustrated by the empirical cumulative distribution function of the P values, which is clearly distinct from what would be expected if no effect was detectable (Figure 1d). Such a strong histology-specific gene expression signature, despite the small sample size, may result from the use of inbred animals in which genetic background is identical among unrelated tumor-bearing mice. An FDR threshold of 5% resulted in the identification of as many as 4137 probes differentially regulated between normal and tumor tissue, and 2163 (52%) and 1974 (48%) up- and down-regulated, respectively (see Supplemental Tables S3 and S4 at http://ajp.amjpathol.org). As for the human gene list, annotation analysis of the mouse genes by DAVID revealed a very broad and partially overlapping spectrum of cellular functions (data not shown).
Cross-Species Comparison
As shown above, expression profiling analysis of human and mouse intestinal adenomas and their normal tissue counterparts resulted in very extensive lists of differentially expressed genes even when stringent parameters were used. We postulated that the cross-species comparison of the genes differentially expressed between the two independent data sets would limit bystander and adaptation effects and help in narrowing down the list to conserved transcripts more likely to play relevant roles in the tumorigenic process. As the two profiling data sets were generated with different microarray platforms (cDNA and oligonucleotide arrays for the human and mouse tumors, respectively), the comparative analysis was performed exclusively on the probes present in both platforms (see Materials and Methods).
Conserved probes were selected by applying the following inclusion criteria: FDR <5% for the mouse set (n = 4137 probes) and FDR <0.5% for the human set (n = 1859 probes). Further filtering was performed to eliminate probes with discordant transcriptional directions (eg, up- vs. down-regulated probes) between the two species. Following this procedure, a total of 234 probes representative of 166 genes were selected, 100 and 66 of which were up- and down-regulated, respectively (Tables 1 and 2). In those cases in which a gene is represented by more than one probe in the array platform, the probe ID associated with the lowest FDR value was selected.
Table 1.
The Cross-Species Conserved 166-Gene Signature: Up-Regulated Genes
| Mouse
|
Human
|
||||
|---|---|---|---|---|---|
| Probe ID | Unigene* | Gene symbol | Unigene† | Gene symbol | Gene description |
| 1448213_at | Mm.248360 | Anxa1 | Hs.494173 | ANXA1 | Annexin A1 |
| 1424460_s_at | Mm.284649 | Aytl2 | Hs.368853 | AYTL2 | Acyltransferase like 2 |
| 1424278_a_at | Mm.8552 | Birc5 | Hs.514527 | BIRC5 | Baculoviral IAP repeat-containing 5 (survivin) |
| 1416815_s_at | Mm.927 | Bub3 | Hs.418533 | BUB3 | BUB3 budding uninhibited by benzimidazoles 3 homolog (yeast) |
| 1455356_at | Mm.36834 | Camsap1 | Hs.522493 | CAMSAP1 | Calmodulin-regulated spectrin-associated protein 1 |
| 1416884_at | Mm.280968 | Cbx3 | Hs.381189 | CBX3 | Chromobox homolog 3 (HP1 gamma homolog, Drosophila) |
| 1425616_a_at | Mm.36697 | Ccdc23 | Hs.113919 | CCDC23 | Coiled-coil domain containing 23 |
| 1427031_s_at | Mm.24035 | Ccdc52 | Hs.477144 | CCDC52 | Coiled-coil domain containing 52 |
| 1417911_at | Mm.4189 | Ccna2 | Hs.58974 | CCNA2 | Cyclin A2 |
| 1417419_at | Mm.273049 | Ccnd1 | Hs.523852 | CCND1 | Cyclin D1 |
| 1438560_x_at | Mm.296985 | Cct4 | Hs.421509 | CCT4 | Chaperonin containing TCP1, subunit 4 (delta) |
| 1417258_at | Mm.282158 | Cct5 | Hs.1600 | CCT5 | Chaperonin containing TCP1, subunit 5 (epsilon) |
| 1423760_at | Mm.423621 | Cd44 | Hs.502328 | CD44 | CD44 molecule (Indian blood group) |
| 1452242_at | Mm.9916 | Cep55 | Hs.14559 | CEP55 | Centrosomal protein 55 kDa |
| 1417457_at | Mm.222228 | Cks2 | Hs.83758 | CKS2 | CDC28 protein kinase regulatory subunit 2 |
| 1449300_at | Mm.200327 | Cttnbp2 nl | Hs.485899 | CTTNBP2NL | CTTNBP2 N-terminal like |
| 1454268_a_at | Mm.271671 | Cyba | Hs.513803 | CYBA | Cytochrome b-245, alpha polypeptide |
| 1419275_at | Mm.148693 | Dazap1 | Hs.222510 | DAZAP1 | DAZ associated protein 1 |
| 1424198_at | Mm.68971 | Dlg5 | Hs.500245 | DLG5 | Discs, large homolog 5 (Drosophila) |
| 1435122_x_at | Mm.128580 | Dnmt1 | Hs.202672 | DNMT1 | DNA (cytosine-5-)-methyltransferase 1 |
| 1452052_s_at | Mm.27695 | Eif3s1 | Hs.404056 | EIF3S1 | Eukaryotic translation initiation factor 3, subunit 1 alpha |
| 1426674_at | Mm.21671 | Eif3s9 | Hs.371001 | EIF3S9 | Eukaryotic translation initiation factor 3, subunit 9 eta |
| 1448797_at | Mm.4454 | Elk3 | Hs.591015 | ELK3 | ETS-domain protein (SRF accessory protein 2) |
| 1437211_x_at | Mm.427018 | Elovl5 | Hs.520189 | ELOVL5 | ELOVL family member 5, elongation of long chain fatty acids (FEN1/Elo2, SUR4/Elo3-like, yeast) |
| 1420965_a_at | Mm.241073 | Enc1 | Hs.104925 | ENC1 | Ectodermal-neural cortex (with BTB-like domain) |
| 1451550_at | Mm.6972 | Ephb3 | Hs.2913 | EPHB3 | EPH receptor B3 |
| 1417301_at | Mm.4769 | Fzd6 | Hs.591863 | FZD6 | Frizzled homolog 6 (Drosophila) |
| 1419595_a_at | Mm.20461 | Ggh | Hs.78619 | GGH | Gamma-glutamyl hydrolase (conjugase, folylpolygammaglutamyl hydrolase) |
| 1419205_x_at | Mm.46029 | Gpatc4 | Hs.193832 | GPATC4 | G patch domain containing 4 |
| 1433736_at | Mm.248353 | Hcfc1 | Hs.83634 | HCFC1 | Host cell factor C1 (VP16-accessory protein) |
| 1423051_at | Mm.426956 | Hnrpu | Hs.166463 | HNRPU | Heterogeneous nuclear ribonucleoprotein U (scaffold attachment factor A) |
| 1426705_s_at | Mm.21118 | Iars | Hs.445403 | IARS | Isoleucine-tRNA synthetase |
| 1422546_at | Mm.440026 | Ilf3 | Hs.465885 | ILF3 | Interleukin enhancer binding factor 3 |
| 1456097_a_at | Mm.257094 | Itgb3bp | Hs.166539 | ITGB3BP | Integrin beta 3 binding protein (beta3-endonexin) |
| 1421344_a_at | Mm.100253 | Jub | Hs.655832 | JUB | ajuba homolog (Xenopus laevis) |
| 1452118_at | Mm.102761 | 2600005C20Rik | Hs.129621 | KIAA0179 | KIAA0179 |
| 1427080_at | Mm.29068 | 2610036D13Rik | Hs.370118 | KIAA0406 | KIAA0406 |
| 1448169_at | Mm.22479 | Krt18 | Hs.406013 | KRT18 | Keratin 18 |
| 1416621_at | Mm.285453 | Llgl1 | Hs.513983 | LLGL1 | Lethal giant larvae homolog 1 (Drosophila) |
| 1434210_s_at | Mm.245210 | Lrig1 | Hs.518055 | LRIG1 | Leucine-rich repeats and immunoglobulin-like domains 1 |
| 1417511_at | Mm.28560 | Lyar | Hs.425427 | LYAR | Hypothetical protein FLJ20425 |
| 1439426_x_at | Mm.177539 | Lzp-s | Hs.524579 | LYZ | Lysozyme (renal amyloidosis) |
| 1455941_s_at | Mm.325746 | Map2k5 | Hs.114198 | MAP2K5 | Mitogen-activated protein kinase kinase 5 |
| 1437226_x_at | Mm.424974 | Marcksl1 | Hs.75061 | MARCKSL1 | MARCKS-like 1 |
| 1439081_at | Mm.122725 | Mgea5 | Hs.500842 | MGEA5 | Meningioma expressed antigen 5 (hyaluronidase) |
| 1424001_at | Mm.280311 | Mki67ip | Hs.367842 | MKI67IP | MKI67 (FHA domain) interacting nucleolar phosphoprotein |
| 1449478_at | Mm.4825 | Mmp7 | Hs.2256 | MMP7 | Matrix metallopeptidase 7 (matrilysin, uterine) |
| 1455129_at | Mm.130883 | Mtdh | Hs.377155 | MTDH | Metadherin |
| 1419254_at | Mm.443 | Mthfd2 | Hs.469030 | MTHFD2 | Methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 2, methenyltetrahydrofolate cyclohydrolase |
| 1452778_x_at | Mm.290407 | Nap1l1 | Hs.524599 | NAP1L1 | Nucleosome assembly protein 1-like 1 |
| 1423046_s_at | Mm.290027 | Ncbp2 | Hs.591671 | NCBP2 | Nuclear cap binding protein subunit 2 |
| 1455035_s_at | Mm.29363 | Nol5a | Hs.376064 | NOL5A | Nucleolar protein 5A (56 kDa with KKE/D repeat) |
| 1416606_s_at | Mm.28203 | Nola2 | Hs.27222 | NOLA2 | Nucleolar protein family A, member 2 (H/ACA small nucleolar RNPs) |
| 1449140_at | Mm.276504 | Nudcd2 | Hs.140443 | NUDCD2 | NudC domain containing 2 |
| 1428277_at | Mm.387724 | Otud6b | Hs.30532 | OTUD6B | OTU domain containing 6B |
| 1435368_a_at | Mm.277779 | Parp1 | Hs.177766 | PARP1 | poly-(ADP-ribose) polymerase family, member 1 |
| 1452620_at | Mm.29856 | Pck2 | Hs.75812 | PCK2 | Phosphoenolpyruvate carboxykinase 2 (mitochondrial) |
| 1426838_at | Mm.37562 | Pold3 | Hs.82502 | POLD3 | Polymerase (DNA-directed), delta 3, accessory subunit |
| 1427094_at | Mm.9199 | Pole2 | Hs.162777 | POLE2 | Polymerase (DNA directed), epsilon 2 (p59 subunit) |
| 1449648_s_at | Mm.3458 | Rpo1–1 | Hs.584839 | POLR1C | polymerase (RNA) I polypeptide C |
| 1433552_a_at | Mm.273217 | Polr2b | Hs.602757 | POLR2B | polymerase (RNA) II (DNA directed) polypeptide B |
| 1436505_at | Mm.11815 | Ppig | Hs.470544 | PPIG | Peptidylprolyl isomerase G (cyclophilin G) |
| 1428265_at | Mm.7726 | Ppp2r1b | Hs.546276 | PPP2R1B | protein phosphatase 2 (formerly 2A), regulatory subunit A, beta isoform |
| 1423775_s_at | Mm.227274 | Prc1 | Hs.567385 | PRC1 | protein regulator of cytokinesis 1 |
| 1452032_at | Mm.30039 | Prkar1a | Hs.280342 | PRKAR1A | Protein kinase, cAMP-dependent, regulatory, type I, alpha (tissue-specific extinguisher 1) |
| 1451576_at | Mm.71 | Prkdc | Hs.491682 | PRKDC | Protein kinase, DNA-activated, catalytic polypeptide |
| 1435859_x_at | Mm.2462 | Psmc2 | Hs.437366 | PSMC2 | Proteasome (prosome, macropain) 26S subunit, ATPase, 2 |
| 1426631_at | Mm.58660 | Pus7 | Hs.520619 | PUS7 | Pseudouridylate synthase 7 homolog (S. cerevisiae) |
| 1448899_s_at | Mm.204634 | Rad51ap1 | Hs.591046 | RAD51AP1 | RAD51-associated protein 1 |
| 1423700_at | Mm.12553 | Rfc3 | Hs.115474 | RFC3 | Replication factor C (activator 1) 3 |
| 1456375_x_at | Mm.314056 | Trim27 | Hs.440382 | RFP | Tripartite motif-containing 27 |
| 1439403_x_at | Mm.435574 | Rnf12 | Hs.653288 | RNF12 | Ring finger protein 12 |
| 1437309_a_at | Mm.180734 | Rpa1 | Hs.461925 | RPA1 | Replication protein A1 |
| 1453362_x_at | Mm.16775 | Rps24 | Hs.356794 | RPS24 | Ribosomal protein S24 |
| 1416276_a_at | Mm.66 | Rps4x | Hs.446628 | RPS4X | Ribosomal protein S4, X-linked |
| 1416120_at | Mm.99 | Rrm2 | Hs.226390 | RRM2 | Ribonucleotide reductase M2 polypeptide |
| 1422864_at | Mm.4081 | Runx1 | Hs.149261 | RUNX1 | Runt-related transcription factor 1 (acute myeloid leukemia 1; aml1 oncogene) |
| 1420824_at | Mm.33903 | Sema4 d | Hs.655281 | SEMA4D | Sema domain, immunoglobulin domain (Ig), transmembrane domain (TM) and short cytoplasmic domain, (semaphorin) 4D |
| 1434972_x_at | Mm.391719 | Sfrs1 | Hs.68714 | SFRS1 | Splicing factor, arginine/serine-rich 1 (splicing factor 2, alternate splicing factor) |
| 1417623_at | Mm.399997 | Slc12a2 | Hs.162585 | SLC12A2 | Solute carrier family 12 (sodium/potassium/chloride transporters), member 2 |
| 1418326_at | Mm.27943 | Slc7a5 | Hs.513797 | SLC7A5 | Solute carrier family 7 (cationic amino acid transporter, y+ system), member 5 |
| 1422771_at | Mm.325757 | Smad6 | Hs.153863 | SMAD6 | SMAD family member 6 |
| 1424206_at | Mm.246803 | Smarca5 | Hs.589489 | SMARCA5 | SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 5 |
| 1452422_a_at | Mm.1323 | Snrpb2 | Hs.280378 | SNRPB2 | Small nuclear ribonucleoprotein polypeptide B |
| 1419156_at | Mm.240627 | Sox4 | Hs.643910 | SOX4 | SRY (sex determining region Y)-box 4 |
| 1433502_s_at | Mm.220843 | Tsr1 | Hs.388170 | SRR | TSR1, 20S rRNA accumulation, homolog (S. cerevisiae) |
| 1415849_s_at | Mm.378957 | Stmn1 | Hs.693592 | STMN1 | Stathmin 1/oncoprotein 18 |
| 1450743_s_at | Mm.260545 | Syncrip | Hs.571177 | SYNCRIP | Synaptotagmin binding, cytoplasmic RNA interacting protein |
| 1423601_s_at | Mm.2215 | Tcof1 | Hs.519672 | TCOF1 | Treacher Collins-Franceschetti syndrome 1 |
| 1416358_at | Mm.34564 | 0610009O03Rik | Hs.632581 | TETRAN | Tetracycline transporter-like protein (TETRAN) |
| 1434317_s_at | Mm.272025 | Tex10 | Hs.494648 | TEX10 | Testis expressed sequence 10 |
| 1426397_at | Mm.172346 | Tgfbr2 | Hs.82028 | TGFBR2 | Transforming growth factor, beta receptor II |
| 1424641_a_at | Mm.219648 | Thoc1 | Hs.654460 | THOC1 | THO complex 1 |
| 1427318_s_at | Mm.34674 | Fer1l3 | Hs.500572 | FER1L3 | Fer-1-like 3, myoferlin (C. elegans) |
| 1449041_a_at | Mm.27063 | Trip6 | Hs.534360 | TRIP6 | Thyroid hormone receptor interactor 6 |
| 1437906_x_at | Mm.19169 | Txnl1 | Hs.114412 | TXNL1 | Thioredoxin-like 1 |
| 1422842_at | Mm.3065 | Xrn2 | Hs.255932 | XRN2 | 5′-3′ exoribonuclease 2 |
| 1448363_at | Mm.221992 | Yap1 | Hs.503692 | YAP1 | Yes-associated protein 1 |
| 1427208_at | Mm.289103 | Zfp451 | Hs.485628 | ZNF451 | Zinc finger protein 451 |
| 1416757_at | Mm.335237 | Zwilch | Hs.21331 | ZWILCH | Zwilch, kinetochore associated, homolog (Drosophila) |
For simplicity, the gene description is only given for the human entry.
Unigene build 163;
Unigene build 202.
Table 2.
The Cross-Species Conserved 166-Gene Signature: Down-Regulated Genes
| Mouse
|
Human
|
||||
|---|---|---|---|---|---|
| Probe ID | Unigene* | Gene | Unigene† | Gene | Description |
| 1424600_at | Mm.213898 | Abp1 | Hs.647097 | ABP1 | Amiloride binding protein 1 [amine oxidase (copper-containing)] |
| 1427034_at | Mm.754 | Ace | Hs.298469 | ACE | Angiotensin I-converting enzyme (peptidyldipeptidase A) 1 |
| 1418553_at | Mm.170461 | Arhgef18 | Hs.465761 | ARHGEF18 | Rho/rac guanine nucleotide exchange factor (GEF) 18 |
| 1459924_at | Mm.340818 | Atp6v0a1 | Hs.463074 | ATP6V0A1 | ATPase, H+ transporting, lysosomal V0 subunit a1 |
| 1416582_a_at | Mm.4387 | Bad | Hs.370254 | BAD | BCL2-antagonist of cell death |
| 1423635_at | Mm.103205 | Bmp2 | Hs.73853 | BMP2 | Bone morphogenetic protein 2 |
| 1456616_a_at | Mm.726 | Bsg | Hs.591382 | BSG | BSG: Basigin (Ok blood group) |
| 1424226_at | Mm.218590 | 9030617O03Rik | Hs.309849 | C14orf159 | Chromosome 14 open reading frame 159 |
| 1427944_at | Mm.150568 | C1qdc1 | Hs.234355 | C1QDC1 | C1q domain containing 1 |
| 1449248_at | Mm.177761 | Clcn2 | Hs.436847 | CLCN2 | Chloride channel 2 |
| 1416565_at | Mm.400 | Cox6b1 | Hs.431668 | COX6B1 | Cytochrome c oxidase subunit Vib polypeptide 1 (ubiquitous) |
| 1420617_at | Mm.339792 | Cpeb4 | Hs.127126 | CPEB4 | Cytoplasmic polyadenylation element binding protein 4 |
| 1415677_at | Mm.21623 | Dhrs1 | Hs.348350 | DHRS1 | Dehydrogenase/reductase (SDR family) member 1 |
| 1416697_at | Mm.1151 | Dpp4 | Hs.368912 | DPP4 | Dipeptidylpeptidase 4 (CD26, adenosine deaminase complexing protein 2) |
| 1450314_at | Mm.140332 | Dqx1 | Hs.191705 | DQX1 | DEAQ box polypeptide 1 (RNA-dependent ATPase) |
| 1421136_at | Mm.9478 | Edn3 | Hs.1408 | EDN3 | Endothelin 3 |
| 1423005_a_at | Mm.264215 | Espn | Hs.147953 | ESPN | Espin |
| 1421969_a_at | Mm.256025 | Faah | Hs.528334 | FAAH | Fatty acid amide hydrolase |
| 1452117_a_at | Mm.170905 | Fyb | Hs.370503 | FYB | FYN binding protein (FYB-120/130) |
| 1436889_at | Mm.338713 | Gabra1 | Hs.175934 | GABRA1 | γ -Aminobutyric acid (GABA) A receptor, alpha 1 |
| 1423236_at | Mm.30249 | Galnt1 | Hs.514806 | GALNT1 | UDP-N-acetyl-α -d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 1 (GalNAc-T1) |
| 1418863_at | Mm.247669 | Gata4 | Hs.243987 | GATA4 | GATA binding protein 4 |
| 1429076_a_at | Mm.283495 | Gdpd2 | Hs.438712 | GDPD2 | Glycerophosphodiester phosphodiesterase domain containing 2 |
| 1449144_at | Mm.260925 | Gna11 | Hs.654784 | GNA11 | Guanine nucleotide binding protein (G protein), alpha 11 (Gq class) |
| 1419371_s_at | Mm.195451 | Gosr2 | Hs.463278 | GOSR2 | Golgi SNAP receptor complex member 2 |
| 1416416_x_at | Mm.37199 | Gstm1 | Hs.75652 | GSTM5 | Glutathione S-transferase M5 |
| 1425343_at | Mm.41506 | Hdhd3 | Hs.7739 | HDHD3 | Haloacid dehalogenase-like hydrolase domain containing 3 |
| 1422527_at | Mm.16373 | H2-DMa | Hs.351279 | HLA-DMA | HLA-DMA: Major histocompatibility complex, class II, DM alpha |
| 1419455_at | Mm.4154 | Il10rb | Hs.418291 | IL10RB | Interleukin 10 receptor, beta |
| 1418265_s_at | Mm.1149 | Irf2 | Hs.374097 | IRF2 | Interferon regulatory factor 2 |
| 1433775_at | Mm.331907 | C77080 | Hs.591502 | KIAA1522 | KIAA1522 |
| 1425547_a_at | Mm.279599 | Klc4 | Hs.408062 | KLC4 | Kinesin light chain 4 |
| 1451322_at | Mm.28108 | Cmbl | Hs.192586 | CMBL | Carboxymethylenebutenolidase homolog (Pseudomonas) |
| 1425780_a_at | Mm.241387 | Tmem167 | Hs.355606 | TMEM167 | Transmembrane protein 167 |
| 1425704_at | Mm.192213 | BC022224 | Hs.462859 | MGC4172 | Short-chain dehydrogenase/reductase |
| 1425930_a_at | Mm.628 | Mlx | Hs.383019 | MLX | MAX-like protein X |
| 1450376_at | Mm.2154 | Mxi1 | Hs.501023 | MXI1 | MAX interactor 1 |
| 1425230_at | Mm.31686 | Nags | Hs.8876 | NAGS | N-Acetylglutamate synthase |
| 1448331_at | Mm.29683 | Ndufb7 | Hs.532853 | NDUFB7 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 7 |
| 1415821_at | Mm.15125 | Nptn | Hs.187866 | NPTN | Neuroplastin |
| 1451274_at | Mm.276348 | Ogdh | Hs.488181 | OGDH | Oxoglutarate (α- ketoglutarate) dehydrogenase (lipoamide) |
| 1417677_at | Mm.32744 | Opn3 | Hs.534399 | OPN3 | Opsin 3 |
| 1449330_at | Mm.29872 | Pdzd3 | Hs.374726 | PDZD3 | PDZ domain containing 3 |
| 1435872_at | Mm.328931 | Pim1 | Hs.81170 | PIM1 | Pim-1 oncogene |
| 1425542_a_at | Mm.240396 | Ppp2r5c | Hs.368264 | PPP2R5C | Protein phosphatase 2, regulatory subunit B′, gamma isoform |
| 1422847_a_at | Mm.2314 | Prkcd | Hs.155342 | PRKCD | Protein kinase C, delta |
| 1424456_at | Mm.4341 | Pvrl2 | Hs.655455 | PVRL2 | Poliovirus receptor-related 2 (herpesvirus entry mediator B) |
| 1430527_a_at | Mm.261818 | Rnf167 | Hs.7158 | RNF167 | Ring finger protein 167 |
| 1448704_s_at | Mm.22362 | H47 | Hs.32148 | SELS | Selenoprotein S |
| 1448299_at | Mm.246670 | Slc1a1 | Hs.444915 | SLC1A1 | Solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1 |
| 1424441_at | Mm.330113 | Slc27a4 | Hs.656699 | SLC27A4 | Solute carrier family 27 (fatty acid transporter), member 4 |
| 1433595_at | Mm.281800 | Slc35d1 | Hs.213642 | SLC35D1 | Solute carrier family 35 (UDP-glucuronic acid/UDP-N-acetylgalactosamine dual transporter), member D1 |
| 1421225_a_at | Mm.41044 | Slc4a4 | Hs.5462 | SLC4A4 | Solute carrier family 4, sodium bicarbonate cotransporter, member 4 |
| 1448783_at | Mm.45874 | Slc7a9 | Hs.408567 | SLC7A9 | Solute carrier family 7 (cationic amino acid transporter, y+ system), member 9 |
| 1436797_a_at | Mm.300594 | Surf4 | Hs.512465 | SURF4 | Surfeit 4 |
| 1428095_a_at | Mm.33869 | Tmem24 | Hs.587176 | TMEM24 | Transmembrane protein 24 |
| 1417895_a_at | Mm.25295 | Tmem54 | Hs.534521 | TMEM54 | Transmembrane protein 54 |
| 1434553_at | Mm.26088 | Tmem56 | Hs.483512 | TMEM56 | Transmembrane protein 56 |
| 1420412_at | Mm.1062 | Tnfsf10 | Hs.478275 | TNFSF10 | Tumor necrosis factor (ligand) superfamily, member 10 |
| 1428327_at | Mm.305318 | Trak1 | Hs.535711 | TRAK1 | Trafficking protein, kinesin binding 1 |
| 1448737_at | Mm.18590 | Tspan7 | Hs.441664 | TSPAN7 | Tetraspanin 7 |
| 1448782_at | Mm.291015 | Txndc11 | Hs.313847 | TXNDC11 | Thioredoxin domain containing 11 |
| 1435110_at | Mm.290433 | Unc5b | Hs.585457 | UNC5B | Unc-5 homolog B |
| 1426399_at | Mm.26515 | Vwa1 | Hs.449009 | VWA1 | Von Willebrand factor A domain containing 1 |
| 1436953_at | Mm.223504 | Wipf1 | Hs.654521 | WASPIP | WAS/WASL interacting protein family, member 1 |
| 1416545_at | Mm.240076 | Zdhhc7 | Hs.592065 | ZDHHC7 | Zinc finger, DHHC-type containing 7 |
For simplicity, the gene description is only given for the human entry.
Unigene build 163;
Unigene build 202.
Bioinformatic analysis of the 166 genes was performed by assigning them to functional groups based on their GO classification in addition to other information from the scientific literature (Table 3). Overall, up-regulation of genes with functions related to cell division was observed: DNA replication and repair, cell cycle regulation, and the maintenance of genomic integrity. Also, the transcriptional and translational machinery was up-regulated when compared with normal tissues.
Table 3.
GO Annotations of the Cross-Species Conserved Genes
| Cellular function | Direction | Genes |
|---|---|---|
| Cell cycle | Up | CCND1, CCNA2, CKS2*, CEP55 |
| DNA replication | Up | RRM2, RFC3, PUS7*, POLE2, RPA1, NAP1L1 |
| DNA repair | Up | XRN2*, PUS7*, POLD3, PARP1, RAD51AP1 |
| Apoptosis | Up | DLG5, BIRC5 |
| Down | BAD, IRF2*, TNFSF10*, UNC5B, PIM1 | |
| RNA and protein biogenesis, processing and transport | Up | RPS4X, NOLA2, CCT5, ILF3, NCBP2, TCOF1, MKI67IP, THOC1, EIF3S9, EIF3S1, POLR2B, SYNCRIP, IARS, SNRPB2, RPS24, NOLA5A, HNRPU, XRN2*, POLR1C, SFRS1, PPIG, CCT4 |
| Down | ZDHHC7, GOSR2, GALNT1, TRAK1 | |
| TGFβ | Up | TGFBR2, SMAD6 |
| Down | BMP2 | |
| Chromatin remodelization | Up | CBX3, SMARCA5, DNMT1 |
| Cytoskeleton organization | Up | LLGL1, KRT18, CTTNBP2NL |
| Down | ARHGEF18, KLC4, WASPIP*, ESPN* | |
| Genome integrity (mitotic | Up | STMN1, ZWILCH, BUB3, CKS2*, PRC1, BIRC5*, PARP1*, PRKDC |
| checkpoint and telomere maintenance) | Down | ESPN* |
| Cell adhesion and migration | Up | SEMA4D, JUB, CD44, TRIP6, DLG5, MAP2K5 |
| Down | NPTN, PVRL2, TSPAN7, WASPIP* | |
| Transcription factors | Up | SOX4, RUNX1, YAP1, MTDH, ITGB3BP, ZFP451, RFP, RNF12 |
| Down | MLX, MXI1, GATA4, IRF2 | |
| G protein signaling | Up | PRKAR1A |
| Down | OPN3, PRKCD, GNA11 | |
| Immune response | Up | ANXA1 |
| Down | IRF2*, TNFSF10*, IL10RB, HLA-DMA, BSG, SELS | |
| Chromatin remodelization | Up | CBX3, SMARCA5, DNMT1 |
| Metabolism | Up | SLC7A5, MTHFD2, GGH, AYTL2, MGEA5, PCK2 |
| Down | DHRS1, GSTM1, DGAT1, SLC27A4, NAGS, HDHD3, GDPD2, SLC7A9, OGDH | |
| Proteolysis | Up | ENC1, PSMC2, MMP7 |
| Down | DPP4, ACE, RNF167 |
Functional annotations were retrieved from GO and the scientific literature. Genes belonging to more than one functional category are marked with*. Gene symbols refer to the human annotation.
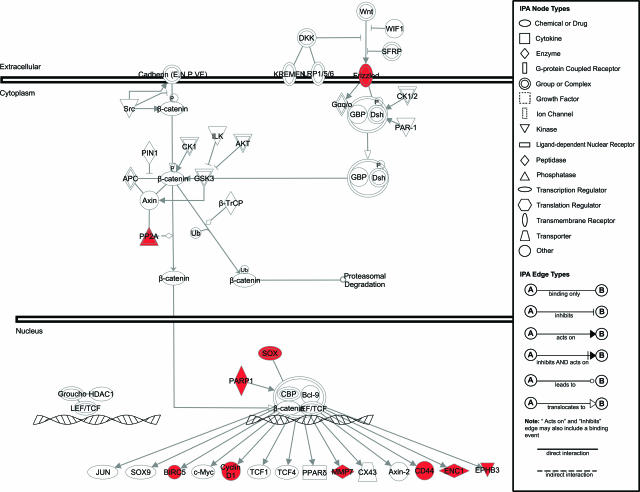
To map the conserved genes to existing signaling and cellular pathways, we used the web-based software application IPA (Ingenuity Systems). As expected from our selection of human and mouse intestinal tumors arising from APC mutations, IPA revealed several differentially expressed genes encoding for members of the Wnt signal transduction pathway (Figure 2). Of the other pathways included in the IPA database, only the extracellular signal-regulated kinase/mitogen-activated protein kinase signaling network encompassed more than two differentially expressed genes in the conserved signature, namely PKA, PKC, PP1/PP2A, and ELK3 (not shown).
Figure 2.
IPA of the genes encompassed by the conserved 166 signatures and belonging to the Wnt signal transduction pathway. The canonical Wnt pathway from the IPA database was slightly modified to accommodate additional Wnt target genes.11,12 The signaling network is represented graphically as nodes (symbols representing genes) and lines/arrows (biological relationship between the genes according to the legend). Red and green gene symbols denote up- and down-regulated genes, respectively. White symbols denote genes not differentially expressed in the conserved signature.
Immunohistochemical Validation of Conserved Targets
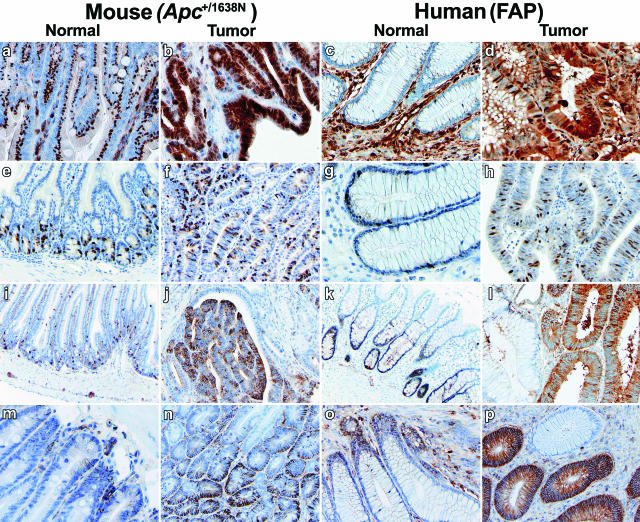
To validate the results of our comparative cross-species expression analysis, we have performed immunohistochemistry (IHC) on mouse and human intestinal tissues with antibodies directed against proteins encoded by differentially expressed genes from the list reported in Table 1. Enhanced expression of the cell surface glycoprotein CD44 is an early event in the adenoma-carcinoma sequence both in mouse and human,36,37 and is thought to result from direct CD44 transcriptional up-regulation by Wnt/β-catenin signaling.37 Accordingly, CD44 was found to be conserved in our cross-species analysis and was used as an internal positive control for the IHC analysis (Figure 3, m–p).
Figure 3.
Immunohistochemistry validation analysis of cross-species conserved genes. Human (colorectal polyps and normal mucosa from FAP patients carrying germline APC mutations) and mouse (duodenal adenomas and normal mucosa from inbred C57BL6/J Apc+/1638N and Apc+/+ mice) tissue sections were analyzed with specific antibodies (see Materials and Methods) for expression of the following proteins: ANXA1 (a–d), CCNA2 (e–h), MARCKSL1 (i–l), and CD44 (m–p).
The annexin A1 (ANXA1) gene, up-regulated in both human and mouse APC/Apc-mutant adenomas (Table 1), encodes for annexin A1, an anti-inflammatory protein induced by glucocorticoids and overexpressed in colitis in both human and rat.38,39,40 Annexin A1 IHC analysis reveals a distinct perinuclear localization in normal intestinal mucosa, possibly in association with the endoplasmic reticulum (Figure 3, a and b). In Apc+/1638N and FAP intestinal tumors, cytoplasmic accumulation of annexin A1 is observed concomitantly with loss of the perinuclear localization (Figure 3, c and d). In distinct tumor areas, nuclear localization was also observed, possibly suggestive of mitogenic stimulation as previously reported.41 Also, annexin A1 expression appears not to be confined to the intestinal epithelium, but also to extend to the stromal compartment42 (Figure 3, a–d).
Cyclin A2 (CCNA2) is a ubiquitously expressed regulator of cell cycle progression known to promote G1/S and G2/M transitions.43 In normal mouse (upper GI) and human (colon) intestinal mucosa, CCNA2 IHC analysis shows nuclear expression mainly restricted to the crypts (Figure 3, e–h). CCNA2 up-regulation in both mouse and human intestinal adenomas (Table 1) is reflected by an increase in the relative number of cells with nuclear CCNA2 staining spread throughout the tumors. The latter is indicative of enhanced cell proliferation of APC-mutant tumor cells, as was also confirmed by the GO analysis of the conserved gene list (Table 3).
To date, the function of Marks-like protein 1 (MARCKSL1) is not fully elucidated, although evidence in the literature indicates that it might be involved in the regulation of intracellular Ca2+ levels under the control of protein kinase C.44 Up-regulation of this protein has been previously reported in prostate cancer,45 and is here found in both FAP and Apc+/1638N adenomas (Table 1). Similar to what was observed for CCCNA2, MARCKSL1 expression is limited to a specific subset of cells within the normal human (colon) and mouse (upper GI) intestinal crypts. Likewise, a pronounced increase in cytoplasmic expression is observed in the vast majority of tumor cells (Figure 3, i–l). Overall, the above IHC results validate the cross-species expression profiling data for genes belonging to distinct GO and functional categories.
The Conserved Cross-Species Signature As a Tool to Differentiate Hereditary Polyposis Syndromes
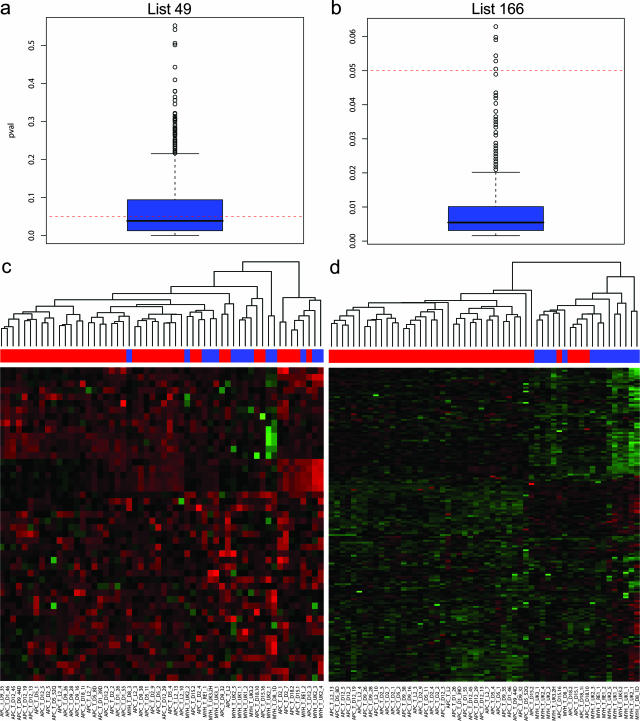
Apart from its implications for the understanding of the molecular and cellular mechanisms underlying APC-driven colorectal tumorigenesis, the conserved cross-species signature may represent a useful tool to discriminate among adenomas from hereditary patients with different genetic syndromes, namely APC- and MYH-associated polyposis. To this aim, an additional 14 adenomas have been obtained from five unrelated patients with pathologically confirmed polyposis of the colon and carrying biallelic MYH germline mutations.23 As for the APC-mutant polyps, RNA was extracted from the microdissected MAP adenomas and subsequently used for expression profiling. First, unsupervised hierarchical clustering was applied to all 56 profiles (from both the APC- and MYH-mutant patients) without any prior filtering to generate an overview of the global gene expression differences among all samples. Overall, we could not observe any clear association with mutation status (data not shown). The mixed-effects regression model34 was again applied, this time fitted to consider mutation (APC and MYH germline mutation carriers) as having a fixed effect and patient as having a random effect. With an FDR set to 0.5%, we were able to select 49 genes differentially expressed between FAP and MAP adenomas (Table 4). To investigate further whether the 49-gene signature as a whole can predict the underlying APC or MYH gene defect, we applied the previously described globaltest for the analysis of microarray data.32 This test assesses whether the global expression pattern of a group of genes is significantly related to any given parameter. It should be noted that, when applying the globaltest, the patient effect cannot be regarded as random and therefore be controlled by its inclusion in the model as a confounder, as it would also represent the genotype effect (all samples from a patient belong to the same genotype). We circumvented this problem by first selecting at random one sample at a time from each patient and then applying the globaltest. After repeating this process for 1000 random combinations of patients’ samples, 57% of the computed P values were found to be below the 0.05 threshold, whereas the maximum value is close to 0.5 (Figure 4a). Next, we repeated this procedure with the conserved 166-gene signature. In sharp contrast with the previous result, 99.4% of the computed P values are below the 0.05 threshold with a maximum of 0.06 (Figure 4b). Accordingly, two-dimensional hierarchical clustering analysis of the expression profiles obtained from all of the FAP and MAP polyps with the 49- and 166-gene signatures confirms that the latter is considerably more discriminative than the former in resolving tumors from carriers of APC germline mutations from those derived from MAP patients (Figure 4, c and d).
Table 4.
The 49-Gene Signature Based on Statistically Significant Differences (FDR = 0.5%) Between Expression Profiles of FAP- and MAP-Derived Adenomatous Polyps, After Implementation of the Mixed-Effect Regression Model34 Fitted Considering Mutation (APC vs. MYH) as Having Fixed Effect and Patient as Having a Random Effect
| GenBank | Gene symbol | Gene description |
|---|---|---|
| H41285 | GDPD2 | Glycerophosphodiester phosphodiesterase domain containing 2 |
| T46878 | EIF3S1 | Eukaryotic translation initiation factor 3, subunit 1 alpha |
| AA479795 | ISG20 | Interferon-stimulated exonuclease gene |
| AA151214 | G3BP2 | Ras-GTPase activating protein SH3 domain-binding protein 2 |
| H28091 | PMP22 | Peripheral myelin protein 22 |
| N59330 | NUP35 | Nucleoporin |
| H19333 | LOC285550 | Hypothetical protein LOC285550 |
| AA449688 | FLJ32065 | Hypothetical protein FLJ32065 |
| N/A | N/A | — |
| AA977417 | AA977417 | — |
| N50636 | RAP1GDS1 | RAP1, GTP-GDP dissociation stimulator 1 |
| T61866 | IPO7 | Importin 7 |
| AA453435 | LTV1 | LTV1 homolog (S. cerevisiae) |
| N91962 | EEF1E1 | Eukaryotic translation elongation factor 1 epsilon 1 |
| H77636 | CD68 | CD68 antigen |
| AI308916 | PRSS3 | Protease, serine, 3 (mesotrypsin) |
| AA478589 | APOE | Apolipoprotein E |
| AA459401 | KLK10 | Kallikrein 10 |
| AA625765 | DDA1 | DDA1 |
| AA205665 | SET | SET translocation (myeloid leukemia-associated) |
| AA707453 | FLJ43855 | Similar to sodium- and chloride-dependent creatine transporter |
| AA464147 | CARS | Cysteinyl-tRNA synthetase |
| AA456630 | ARHGEF18 | Rho/rac guanine nucleotide exchange factor (GEF) 18 |
| N20475 | CTSD | Similar to RIKEN cDNA 6330512M04 gene (mouse) |
| N31935 | ANGPTL1 | Angiopoietin-like 1 |
| R77512 | PCDH1 | Protocadherin 1 (cadherin-like 1) |
| N31492 | FMO4 | Topoisomerase (DNA) I pseudogene 1 |
| N45236 | KIAA0114 | KIAA0114 gene product |
| H60549 | CD59 | CD59 antigen, complement regulatory protein |
| AA907626 | KIF26B | Kinesin family member 26B |
| AA917374 | TIMP2 | TIMP metallopeptidase inhibitor 2 |
| AA983530 | VNN1 | Vanin 1 |
| N90109 | NCL | U23 small nucleolar RNA |
| H15431 | POLR2D | Polymerase (RNA) II (DNA directed) polypeptide D |
| H52673 | BAK1 | BCL2-antagonist/killer 1 |
| T72259 | CYP2A6 | Cytochrome P450, family 2, subfamily A, polypeptide 6 |
| AA455910 | F2R | Coagulation factor II (thrombin) receptor |
| AA775840 | C9orf123 | Chromosome 9 open reading frame 123 |
| AA464566 | LRP1 | Low density lipoprotein-related protein 1 |
| AA489640 | IFIT1 | Interferon-induced protein with tetratricopeptide repeats 1 |
| AA962541 | LOC286167 | Hypothetical protein LOC286167 |
| AA464421 | PCGF2 | Polycomb group ring finger 2 |
| H25761 AI668603 | — | — |
| AA447748 | DLD | Dihydrolipoamide dehydrogenase |
| AA278755 | CEP27 | Centrosomal protein |
| AI261833 | SLC7A9 | Solute carrier family 7 (cationic amino acid transporter, y+ system) member 9 |
| H72802 | ESPN | Espin |
| AA608713 | C1QDC1 | C1q domain containing 1 |
| AA047465 | SLC6A8 | Solute carrier family 6 (neurotransmitter transporter, creatine), member 8 |
Figure 4.
Analysis of the cross-species conserved signature as a tool to separate hereditary polyposis syndromes due to APC (FAP) or MYH (MAP) germline mutations. The globaltest32 was performed with the 49 (a)- and 166 (b)-gene signature and graphically represented by box plots of the P values generated after 1000 iterations in which only one random sample from each patient was used at a time. Box plots were generated using the standard settings present in R2.4.1. The filled blue boxes encompass the range of P values representative of 50% of the data points, whereas the central line represents the median. two-dimensional hierarchical clustering analysis was performed with the 49 (c)- and 166 (d)-gene signature, respectively, on the expression profiles obtained from all 56 colorectal adenomas (42 from APC and 14 from MYH gene mutation carriers). The colored bar above the heat map represents the mutation status of the corresponding polyp samples: red, polyps from patients carrying germline APC mutations; blue, polyps from patients carrying bi-allelic germline MYH mutations.
Discussion
Expression profiling by oligonucleotide and cDNA microarray platforms has rapidly become a commonly used tool for the qualitative and quantitative evaluation of the genome-wide transcriptional activity of human cancers. However, the outcomes of expression profiling of cancers are often very complex as they reflect the heterogeneity of cell types and biological activities present within the neoplastic mass, thus making their functional interpretation a difficult task. This is certainly the case for the expression (and genomic) profiles obtained to date from colorectal cancers. Although several studies have been published in the scientific literature, the degree of overlap between independent data sets is limited, possibly also as a consequence of differences in patient cohorts and methodologies used.16 Cross-species comparison of cancer profiling data represents a valuable approach to i) decrease the complexity of omics signatures, ii) pinpoint conserved target genes more likely to play rate-limiting functional roles in tumor initiation and progression to malignancy, and iii) accelerate the development of tailor-made anticancer therapies.46,47
Notwithstanding the above-mentioned heterogeneity, colorectal cancer represents, at least from a genetic perspective, a relatively homogeneous disease as the vast majority of the sporadic cases is known to be triggered by somatic mutations at the APC or CTNNB1 (β-catenin) genes, leading to the constitutive activation of the canonical Wnt signaling pathway.10 These mutations are known to initiate the formation of aberrant crypt foci and adenomatous polyps, the earliest benign precursors of the adenoma-carcinoma sequence. Also, germline APC mutations underlie FAP, an autosomal dominant predisposition to the development of multiple adenomatous polyps throughout the colon-rectum.48 The availability of a unique collection of adenomatous polyps obtained from FAP patients carrying germline APC mutations and from a mouse model, Apc+/1638N, carrying a targeted mutation in the endogenous Apc gene allowed us to perform the cross-species computational comparison of their gene expression profiles and derive a conserved 166-gene signature. It should be noted that whereas FAP patients mainly develop polyps in the colon-rectum, Apc mouse models are characterized by adenomas clustering in the upper gastrointestinal tract, mainly in the duodenum. This anatomical difference between the mouse and human adenomas used for the cross-species comparison may exert a confounding effect in our computational analysis as duodenum and colon-rectum represent distinct organs. However, it may also confer an additional advantage to our approach as tissue-specific differences between the two GI tracts are likely to be filtered out, thus retaining only those conserved differentially expressed genes more likely to play functional roles in intestinal tumor formation, regardless of anatomical sub-location. The same holds true for our methodological approach: different microarray platforms were used to derive the human (cDNA arrays) and mouse (oligonucleotide arrays) gene profiles. In both cases, laser-guided microdissection was used to enrich in tumor cells without the confounding effects of contaminating stromal cells. Overall, our IHC analysis of a subset of proteins encoded by the conserved genes confirmed their differential expression between normal tissue and adenomas in both species (Figure 3), thus validating our methodological strategy.
The significance thresholds used to generate the differentially expressed lists of genes for the human (approximately 10% of the represented genes, with FDR = 0.5%) and mouse (approximately 18% of the represented genes, with FDR = 5%) studies are admittedly arbitrary. They were chosen using two generic criteria: i) the gene lists would be representative of the differential signature without encompassing an excessive percentage of false positives, and ii) the resulting conserved list of differentially expressed genes would be sufficiently large to enable pathway analysis. The presence in our cross-species signature of several genes known to be differentially expressed in sporadic colorectal cancers16 also represents an indirect confirmation of the general validity of our computational approach.
GO-based functional analysis of the 166 conserved genes reveals a general increase in cell division as shown by the up-regulation of genes related to DNA replication and repair, cell cycle regulation, and the maintenance of genomic integrity (Table 3). Notably, exclusively up-regulated genes were encompassed within these categories, indicative of the increased proliferation rate of tumor cells when compared with normal ones. Genes belonging to the transcriptional and translational machinery were also up-regulated when compared with normal tissues. These included genes involved in ribosome biogenesis, mRNA synthesis and maturation, and protein synthesis and folding (Table 3).
As expected from our selection of adenomas from APC-mutant patients and mouse models, several members of the Wnt signal transduction pathway are included among the conserved 166 differentially expressed genes (Figure 2). These included the Frizzled receptor homolog FZD6, the protein phosphatase type 2A (PP2A), the HMG box transcription factor SOX4,49 and several Wnt downstream transcriptional targets, namely, the matrix metallopeptidase matrilysin (MMP7),36,37,50 CD44,36 ENC1 (ectodermal-neural cortex 1)51, ephrin receptor B3 (EPHB3),52 cyclin D1 (CCND1),53,54 and the apoptosis inhibitor survivin (BIRC5)55,56 (Figure 2). However, AXIN2, a well known downstream Wnt target gene, is not included in this list simply because its probe is not encompassed by the human cDNA array. Other known Wnt target genes such as EPHB2, SOX9, and MYC were excluded because of the high stringency of the statistical thresholds used or to their absence in one of the platforms. Recently, an “intestinal Wnt/TCF4 signature” was obtained by integrating expression profiling data from CRC cell lines engineered with an inducible block of Wnt signaling and from sporadic human adenomas and carcinomas.11 Comparison of this 208-gene Wnt/TCF gene signature with our cross-species conserved list revealed 10 common entries, 4 of which belong to the Wnt/β-catenin signaling pathway (CD44, ENC1, EPHB3, and SOX4). The latter is not surprising in view of the different computational approaches and tumor cohorts used. Moreover, the use of CRC cell lines with dominant negative TCF4 constructs does not necessarily mimic the initial and rate-limiting loss of APC function characteristic of the mouse and human adenomas used in our cross-species analysis. Of more interest is the comparison with the study by Kaiser et al57 in which a cross-species comparison was performed among human and mouse intestinal tumors together with mouse embryonic stages of intestinal development. As depicted in Supplemental Table S5 (see http://ajp.amjpathol.org), the overlap between the two studies is high, with 46 of 166 differentially expressed genes shared between the data sets. Notably, the overlap is considerably higher with genes showing similar behavior in intestinal tumorigenesis and embryonic development.
Apoptosis inhibition in the adenomas, as suggested by BIRC5 up-regulation, is also strengthened by the conserved down-regulation of the BAD gene, encoding for a potent pro-apoptotic protein. BAD forms heterodimers with BCL2 and BCL-XL, thus repressing their anti-apoptotic function.58,59
Two members of the TGF-β signaling pathways are up-regulated among the cross-species conserved genes, namely SMAD6 and TGFBR2. The TGF-β ligand mediates its effects through the transmembrane type I (TGFBR1) and type II receptor subunits (TGFBR2), and in the cytoplasm through stimulatory and inhibitory SMADs. The up-regulation of the TGFBR2 gene encoding for the type II receptor is remarkable in view of its frequent mutational inactivation in a substantial proportion of sporadic colon cancers.60 The SMAD6 gene encodes for an inhibitory SMAD protein that becomes up-regulated as the result of a negative feedback loop. SMAD6 is thought to represent a key component in the integration of signals from different pathways and was shown to exert BMP inhibitory activity.61 Down-regulation of the bone morphogenetic BMP2 gene apparently confirms the inhibition of this TGF-β-related pathway. Although its role in tumorigenesis is yet unclear, SMAD6 up-regulation has been reported in other tumor types.62 Overall, the conserved gene signature is indicative of the activation of TGF-β and inhibition of BMP signaling at early stages of intestinal tumorigenesis. However, this observation needs to be validated by additional expression and reporter assays.
Among the many genes encompassed by the cross-species conserved signature, the up-regulation of ANXA1 is of interest in view of its phospholipase A2 (PPA2) inhibitory activity, an enzyme involved in the synthesis of prostaglandins during inflammation.42 Antibodies against annexin A1 have been found in patients with inflammatory bowel disorders.38 Also, its up-regulation was shown to occur in mitogenically stimulated cells in a PKC phosphorylation-dependent fashion, accompanied by its translocation from the cytoplasm to the nucleus.41 Notably, changes in ANXA1 subcellular localization were also observed in our IHC validation analysis (Figure 3).
Apart from its implications for the understanding and elucidation of the molecular and cellular mechanisms underlying APC-driven intestinal tumor formation, the cross-species conserved gene signature may also represent a useful tool to discriminate among hereditary polyposis patients with distinct genetic defects. Expression profiling analysis of the additional set of 14 colorectal adenomas obtained from patients carrying bi-allelic mutations at the MYH gene showed a high degree of similarity with the APC profiles. This could be explained by previous observations, according to which the APC gene is a preferential target for somatic mutations in colorectal adenomas from carriers of bi-allelic MYH germline mutations.63 The observed high degree of similarity between expression profiles from FAP and MAP polyps could then be explained provided that the somatic APC mutation does represent the initiating event in MYH-associated polyp formation. Alternatively, human adenoma profiles may be similar notwithstanding the initiating genetic defect, as indicated by our own most recent results with the expression analysis of three polyposis patients of unknown genetic basis (and no germline mutations found after sequencing of the MYH and APC genes). Also in these cases, the resulting profiles were virtually indistinguishable from those derived from MYH- and APC-mutant polyps (data not shown).
Nevertheless, by applying an FDR threshold of 0.5%, we could generate a 49-gene signature based on differences between MYH- and APC-mutant human polyps. Yet, both globaltest and two-dimensional hierarchical clustering analyses showed that the conserved 166-signature clusters more accurately the expression profile data from FAP and MAP patients than does the 49-gene signature (Figure 4).
In conclusion, cross-species comparison of expression profiles of intestinal adenomas obtained from hereditary polyposis patients and mouse models carrying germline APC mutations resulted in a signature of 166 differentially expressed genes. Functional annotation of the conserved genes indicates an overall increase in cell division and the up-regulation of the Wnt/β-catenin signaling pathway. These main cellular and molecular changes are accompanied by a plethora of gene-specific changes yet to be tested by functional assays to determine their relative contribution to intestinal tumor formation. Additional validation on independent polyp cohorts and further fine-tuning of the conserved gene signature are needed toward the development of an expression-based assay to classify hereditary polyposis syndromes.
Acknowledgments
We thank Dr. Guido Jenster and Dr. Don de Lange for granting access and helping with the Sequence Retrieval System (SRS), and for fruitful discussions; Dr. Bruce J. Aronow for providing the detailed list of genes from his group’s microarray results used here for a comparison; and Mr. Frank van der Panne for his assistance with the artwork.
Footnotes
Address reprint requests to Riccardo Fodde, Ph.D., Dept. of Pathology, Erasmus MC, PO Box 2040, 3000CA Rotterdam, The Netherlands. E-mail: r.fodde@erasmusmc.nl.
These studies were supported by grants from the Dutch Cancer Society (EMCR 2001-2482), The Netherlands Organisation for Scientific Research (NWO/Vici 016.036.636), the BSIK program of the Dutch Government grant 03038, the EU FP6 (MCSCs), “Deutsche Krebshilfe, Verbundprojekt familiarer Darmkrebs”, and the Centre for Medical Systems Biology (CMSB).
C.G. and J.C. equally contributed to the study.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- de la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer. 2004;4:769–780. doi: 10.1038/nrc1453. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hughes J, Warrington J, McPherson J, Wasmuth J, LePaslier D, Abderrahim H, Cohen D, Leppert M, White R. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Joslyn G, Carlson M, Thliveris A, Albertsen H, Gelbert L, Samowitz W, Groden J, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hughes JP, Warrington J, McPherson J, Wasmuth J, Le Paslier D, Abderrahim H, Cohen D, Leppert M, White R. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991;66:601–613. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hamilton SR, Hedge P, Markham A, Carlson M, Joslyn G, Groden J, White R, Miki Y, Miyoshi Y, Nishisho I, Nakamura Y. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991;251:1366–1370. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Johnson KA, Bryan TM, Hill DE, Markowitz S, Willson JK, Paraskeva C, Petersen GM, Hamilton SR, Vogelstein B, Kinzler KW. The APC gene product in normal and tumor cells. Proc Natl Acad Sci USA. 1993;90:2846–2850. doi: 10.1073/pnas.90.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H. The intestinal Wnt/TCF signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Vlad A, Rohrs S, Klein-Hitpass L, Muller O. The first five years of the Wnt targetome, Cell Signal. 2008;20:795–802. doi: 10.1016/j.cellsig.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Lipton L, Tomlinson I. The genetics of FAP and FAP-like syndromes. Fam Cancer. 2006;5:221–226. doi: 10.1007/s10689-005-5673-3. [DOI] [PubMed] [Google Scholar]
- Cheadle JP, Sampson JR. Exposing the MYtH about base excision repair and human inherited disease. Hum Mol Genet. 2003;12 Spec No 2:R159–R165. doi: 10.1093/hmg/ddg259. [DOI] [PubMed] [Google Scholar]
- Fodde R, Smits R. Disease model: familial adenomatous polyposis. Trends Mol Med. 2001;7:369–373. doi: 10.1016/s1471-4914(01)02050-0. [DOI] [PubMed] [Google Scholar]
- Cardoso J, Boer J, Morreau H, Fodde R. Expression and genomic profiling of colorectal cancer. Biochim Biophys Acta. 2007;1775:103–137. doi: 10.1016/j.bbcan.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sweet-Cordero A, Mukherjee S, Subramanian A, You H, Roix JJ, Ladd-Acosta C, Mesirov J, Golub TR, Jacks T. An oncogenic KRAS2 expression signature identified by cross-species gene-expression analysis. Nat Genet. 2005;37:48–55. doi: 10.1038/ng1490. [DOI] [PubMed] [Google Scholar]
- Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Lee JS, Chu IS, Mikaelyan A, Calvisi DF, Heo J, Reddy JK, Thorgeirsson SS. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36:1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- Lee JS, Grisham JW, Thorgeirsson SS. Comparative functional genomics for identifying models of human cancer. Carcinogenesis. 2005;26:1013–1020. doi: 10.1093/carcin/bgi030. [DOI] [PubMed] [Google Scholar]
- Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B, Breukel C, Alt E, Lipkin M, Khan PM, Kucherlapati R. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci USA. 1994;91:8969–8973. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits R, van der Houven van Oordt W, Luz A, Zurcher C, Jagmohan-Changur S, Breukel C, Khan PM, Fodde R. Apc1638N: a mouse model for familial adenomatous polyposis-associated desmoid tumors and cutaneous cysts. Gastroenterology. 1998;114:275–283. doi: 10.1016/s0016-5085(98)70478-0. [DOI] [PubMed] [Google Scholar]
- Cardoso J, Molenaar L, de Menezes RX, van Leerdam M, Rosenberg C, Moslein G, Sampson J, Morreau H, Boer JM, Fodde R. Chromosomal instability in MYH- and APC-mutant adenomatous polyps. Cancer Res. 2006;66:2514–2519. doi: 10.1158/0008-5472.CAN-05-2407. [DOI] [PubMed] [Google Scholar]
- Cardoso J, Molenaar L, de Menezes RX, Rosenberg C, Morreau H, Moslein G, Fodde R, Boer JM. Genomic profiling by DNA amplification of laser capture microdissected tissues and array CGH. Nucleic Acids Res. 2004;32:e146. doi: 10.1093/nar/gnh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. R: A Language for Data Analysis and Graphics. Journal of Computational and Graphical Statistics. 1996;Vol 5:299–314. [Google Scholar]
- Huber W, von Heydebreck A, Sultmann H, Poustka A, Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics. 2002;18(Suppl 1):S96–S104. doi: 10.1093/bioinformatics/18.suppl_1.s96. [DOI] [PubMed] [Google Scholar]
- Wu H, Kerr MK, Cui X, Gary A. New York: Churchill; MAANOVAA Software Package for the Analysis of Spotted cDNA Microarray Experiments. 2005:p 314–341. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc B Met. 1995:289–300. [Google Scholar]
- Gautier L, Cope L, Bolstad BM, Irizarry RA. affy-analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article 3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Limma: linear models for microarray data. Gentleman R, Cary V, Dudoit S, Irizarry R, Huber W, editors. New York: Springer,; Bioinformatics and Computational Biology Solutions using R and Bioconductor. 2005:pp 397–420. [Google Scholar]
- Goeman JJ, van de Geer SA, de Kort F, van Houwelingen HC. A global test for groups of genes: testing association with a clinical outcome. Bioinformatics. 2004;20:93–99. doi: 10.1093/bioinformatics/btg382. [DOI] [PubMed] [Google Scholar]
- Veldhoven A, de Lange D, Smid M, de Jager V, Kors JA, Jenster G. Storing, linking, and mining microarray databases using SRS. BMC Bioinformatics. 2005;6:192. doi: 10.1186/1471-2105-6-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MK, Martin M, Churchill GA. Analysis of variance for gene expression microarray data. J Comput Biol. 2000;7:819–837. doi: 10.1089/10665270050514954. [DOI] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- Abbasi AM, Chester KA, Talbot IC, Macpherson AS, Boxer G, Forbes A, Malcolm AD, Begent RH. CD44 is associated with proliferation in normal and neoplastic human colorectal epithelial cells. Eur J Cancer. 1993;29A:1995–2002. doi: 10.1016/0959-8049(93)90461-n. [DOI] [PubMed] [Google Scholar]
- Wielenga VJ, Smits R, Korinek V, Smit L, Kielman M, Fodde R, Clevers H, Pals ST. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol. 1999;154:515–523. doi: 10.1016/S0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Gavins FN. Annexin 1: an endogenous anti-inflammatory protein. News Physiol Sci. 2003;18:60–64. doi: 10.1152/nips.01424.2002. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Pages P, Guimbaud R, Chaussade S, Bueno L, Escourrou J, Comera C. Annexin 1 is secreted in situ during ulcerative colitis in humans. Inflamm Bowel Dis. 2004;10:584–592. doi: 10.1097/00054725-200409000-00013. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Comera C, Bueno L. Annexin 1 is overexpressed and specifically secreted during experimentally induced colitis in rats. Eur J Biochem. 1995;232:603–610. doi: 10.1111/j.1432-1033.1995.tb20850.x. [DOI] [PubMed] [Google Scholar]
- Kim YS, Ko J, Kim IS, Jang SW, Sung HJ, Lee HJ, Lee SY, Kim Y, Na DS. PKCdelta-dependent cleavage and nuclear translocation of annexin A1 by phorbol 12-myristate 13-acetate. Eur J Biochem. 2003;270:4089–4094. doi: 10.1046/j.1432-1033.2003.03800.x. [DOI] [PubMed] [Google Scholar]
- Pepinsky RB, Sinclair LK, Browning JL, Mattaliano RJ, Smart JE, Chow EP, Falbel T, Ribolini A, Garwin JL, Wallner BP. Purification and partial sequence analysis of a 37-kDa protein that inhibits phospholipase A2 activity from rat peritoneal exudates. J Biol Chem. 1986;261:4239–4246. [PubMed] [Google Scholar]
- Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raufman JP, Malhotra R, Xie Q, Raffaniello RD. Expression and phosphorylation of a MARCKS-like protein in gastric chief cells: further evidence for modulation of pepsinogen secretion by interaction of Ca2+/calmodulin with protein kinase C. J Cell Biochem. 1997;64:514–523. [PubMed] [Google Scholar]
- Chaib H, Cockrell EK, Rubin MA, Macoska JA. Profiling and verification of gene expression patterns in normal and malignant human prostate tissues by cDNA microarray analysis. Neoplasia. 2001;3:43–52. doi: 10.1038/sj.neo.7900126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber TG, Sawyers CL. Cross-species comparisons of cancer signaling. Nat Genet. 2005;37:7–8. doi: 10.1038/ng0105-7. [DOI] [PubMed] [Google Scholar]
- Peeper D, Berns A. Cross-species oncogenomics in cancer gene identification. Cell. 2006;125:1230–1233. doi: 10.1016/j.cell.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Fodde R, Khan PM. Genotype-phenotype correlations at the adenomatous polyposis coli (APC) gene. Crit Rev Oncog. 1995;6:291–303. doi: 10.1615/critrevoncog.v6.i3-6.60. [DOI] [PubMed] [Google Scholar]
- Reichling T, Goss KH, Carson DJ, Holdcraft RW, Ley-Ebert C, Witte D, Aronow BJ, Groden J. Transcriptional profiles of intestinal tumors in Apc(Min) mice are unique from those of embryonic intestine and identify novel gene targets dysregulated in human colorectal tumors. Cancer Res. 2005;65:166–176. [PubMed] [Google Scholar]
- Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- Fujita M, Furukawa Y, Tsunoda T, Tanaka T, Ogawa M, Nakamura Y. Up-regulation of the ectodermal-neural cortex 1 (ENC1) gene, a downstream target of the beta-catenin/T-cell factor complex, in colorectal carcinomas. Cancer Res. 2001;61:7722–7726. [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway, Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Fields JZ, Ehrlich SM, Boman BM. The chemopreventive agent sulindac attenuates expression of the antiapoptotic protein survivin in colorectal carcinoma cells. J Pharmacol Exp Ther. 2004;308:434–437. doi: 10.1124/jpet.103.059378. [DOI] [PubMed] [Google Scholar]
- Zhu HX, Zhang G, Wang YH, Zhou CQ, Bai JF, Xu NZ. [Indomethacin induces apoptosis through inhibition of survivin regulated by beta-catenin/TCF4 in human colorectal cancer cells]. Ai Zheng. 2004;23:737–741. [PubMed] [Google Scholar]
- Kaiser S, Park YK, Franklin JL, Halberg RB, Yu M, Jessen WJ, Freudenberg J, Chen X, Haigis K, Jegga AG, Kong S, Sakthivel B, Xu H, Reichling T, Azhar M, Boivin GP, Roberts RB, Bissahoyo AC, Gonzales F, Bloom GC, Eschrich S, Carter SL, Aronow JE, Kleimeyer J, Kleimeyer M, Ramaswamy V, Settle SH, Boone B, Levy S, Graff JM, Doetschman T, Groden J, Dove WF, Threadgill DW, Yeatman TJ, Coffey RJ, Jr, Aronow BJ. Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol. 2007;8:R131. doi: 10.1186/gb-2007-8-7-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelekar A, Chang BS, Harlan JE, Fesik SW, Thompson CB. Bad is a BH3 domain-containing protein that forms an inactivating dimer with Bcl-XL. Mol Cell Biol. 1997;17:7040–7046. doi: 10.1128/mcb.17.12.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, Brattain M, Willson JKV. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- Hata A, Lagna G, Massague J, Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12:186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH. Fine tuning and cross-talking of TGF-beta signal by inhibitory Smads. J Biochem Mol Biol. 2005;38:9–16. doi: 10.5483/bmbrep.2005.38.1.009. [DOI] [PubMed] [Google Scholar]
- Al-Tassan N, Chmiel NH, Maynard J, Fleming N, Livingston AL, Williams GT, Hodges AK, Davies DR, David SS, Sampson JR, Cheadle JP. Inherited variants of MYH associated with somatic G: C→T:A mutations in colorectal tumors. Nat Genet. 2002;30:227–232. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]