Abstract
Epithelial metaplasia (EpM) is an acquired tissue abnormality resulting from the transformation of epithelium into another tissue with a different structure and function. This adaptative process is associated with an increased frequency of (pre)cancerous lesions. We propose that EpM is involved in cancer development by altering the expression of adhesion molecules important for cell-mediated antitumor immunity. Langerhans cells (LCs) are intraepithelial dendritic cells that initiate immune responses against viral or tumor antigens on both skin and mucosal surfaces. In the present study, we showed by immunohistology that the density of CD1a+ LCs is reduced in EpM of the uterine cervix compared with native squamous epithelium and that the low number of LCs observed in EpM correlates with the down-regulation of cell-surface E-cadherin. We also demonstrated that transforming growth factor-β1 is not only overexpressed in metaplastic tissues but also reduces E-cadherin expression in keratinocytes in vitro by inducing the promoter activity of Slug and Snail transcription factors. Finally, we showed that in vitro-generated LCs adhere poorly to keratinocytes transfected with either Slug or Snail DNA. These data suggest that transforming growth factor-β1 indirectly reduces antigen-presenting cell density in EpM by affecting E-cadherin expression, which might explain the increased susceptibility of abnormal tissue differentiation to the development of cancer by the establishment of local immunodeficiency responsible for EpM tumorigenesis.
Epithelial metaplasia (EpM) is initially an adaptative process that can occur in various organs in response to persistent injury. This epithelial tissue remodeling can be incomplete (immature) or complete (mature) depending on the persistence or not of the native epithelium. Although EpM is frequently observed in many organs such as the uterine cervix, bronchial tract, lower esophagus, and stomach, the phenomenon remains poorly understood. The process is usually associated with inflammation or proliferation of cells, such as that observed during tissue regeneration, and is likely connected to a modified expression of one or several genes at the level of multipotent stem cell cells.1,2
The metaplasia to dysplasia to cancer progression is frequently encountered,3,4 particularly in the uterine cervix where the substantial majority (87%) of (pre)cancerous lesions develop within a specific microenvironment, the transformation zone where a metaplastic process is observed.5 This implies that exogenous or endogenous factors specific to the anatomical milieu of the transformation zone may be conducive to oncogenic human papillomavirus (HPV) infection and cancer development. In a previous report, we proposed that a side effect of EpM is a deregulated production of immune factors important for the antitumor/antiviral immune response.6 Accordingly, epithelial cells can influence immune reactions in mucosal surfaces through the production of cytokines and/or chemokines or via cell-cell contact.7 For example, the intraepithelial expression of adhesion molecules, necessary to maintain a balanced turnover of immunocompetent cells, is probably influenced by the epithelial differentiation, which is altered in metaplastic areas.
The squamous epithelium is composed not only of keratinocytes but also of a type of immature dendritic cells (DCs), the Langerhans cells (LCs), which are important for the immunosurveillance of cutaneous or mucosal surfaces. Indeed, within the last several years, the importance of DC/LC intratumor infiltration has been emphasized. In particular, in the uterine cervix, the regression of HPV-related lesions has been shown to be associated with an increased intraepithelial infiltration of DCs/LCs.8 Several studies indicate that alterations in antigen presentation might occur in metaplasia. For example, previous works have demonstrated that metaplastic areas are associated with a lower density of LCs.9,10 Besides the communication of epithelial and immune cells by soluble factors, there also exist important cell-cell interactions. For example, the protein E-cadherin, which is critical for intercellular adhesiveness and maintenance of normal epithelial tissue architecture, is also involved in the homophilic and heterotypic interactions between epithelial cells and diverse cell types of the immune response, including LCs11 and intraepithelial T lymphocytes (via αEβ7 integrin).12 Furthermore, it is well known that the loss of E-cadherin expression is associated with the malignant transformation of metaplastic areas,13,14,15 metastatic dissemination, and unfavorable tumor prognosis. The mechanisms involved in the regulation of E-cadherin expression are either the hypermethylation of the CDH1 (E-cadherin) gene16,17 or the activity of transcription factors, such as Snail or Slug, previously described as strong repressors of E-cadherin expression.18,19,20,21 These zinc-finger transcription factors bind to E-boxes in the CDH1 promoter and their expression has been correlated to invasiveness of several human tumors derived from epithelial tissues.22,23,24,25 The expression of Snail and Slug factors can be particularly induced by transforming growth factor-β1 (TGF-β1),26,27,28 which is highly expressed in several human pathogenic processes such as fibrosis, inflammation, and cancer development.29,30
In this study, we postulated that inflammatory or immunomodulatory mediators, such as TGF-β1, could contribute to the malignant transformation of EpM by negatively interfering with E-cadherin expression and intraepithelial adhesion of antigen-presenting cells. We showed that the reduced density of intraepithelial LCs in areas of mature and immature EpM is correlated with a lower expression of E-cadherin and that TGF-β1 inhibits E-cadherin expression in keratinocytes by inducing the activity of Slug and Snail promoters.
Materials and Methods
Cervical Biopsy Specimens
Fifty-one paraffin-embedded cervical specimens from women who underwent total hysterectomy for noncervical benign uterine disease were retrieved from the Tumor Bank of the University of Liege. The mean age was 41.2 years (range, 35 to 47 years) and only four patients were menopausal. These tissue samples included both normal exocervical epithelium and areas of EpM (mature and/or immature). Forty frozen cervical biopsies taken from nonmenopausal women were also selected and contained either exocervical tissues (n = 12), mature metaplasia (n = 14), or immature metaplasia (n = 14). DNA was prepared from all cervical specimens with the QIAamp DNA mini kit (Qiagen, Valencia, CA) and tested, by PCR, for the presence of HPV DNA sequences using previously described protocols31 and consensus primers (GP5+/GP6).32,33 The protocol was approved by the Ethics Committee of the University Hospital of Liege.
Cell Culture
Human skin keratinocytes (HaCaT cells) were grown in a 3:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium containing 10% fetal calf serum (Gibco, Invitrogen Corp., Carlsbad, CA) and supplied with 1% nonessential amino acid (Gibco) and 1% sodium pyruvate (Gibco). The cells were incubated at 37°C in a humidified CO2 atmosphere until a 50 to 60% confluence was reached. For some experiments, HaCaT cells were cultivated in a serum deprivation medium (0.2% fetal calf serum) for 24 hours before stimulation with 10 ng/ml of TGF-β1 (Prepro Tech, Rocky Hill, NJ).34
Immunostaining
Serial sections of cervical biopsy specimens underwent immunostaining using antibodies directed against E-cadherin (clone HECD-1; Zymed, San Francisco, CA), CD1a (clone MTB1; Novocastra, Newcastle, UK), involucrin (clone SY5; Novocastra), TGF-β1 (clone TB21; Santa Cruz Biotechnology, Santa Cruz, CA), Snail (clone E-18; Santa Cruz Biotechnology), and Slug (clone D-19; Santa Cruz Biotechnology). Anti-E-cadherin, CD1a, involucrin, and TGF-β1 immunostaining was performed on paraffin sections whereas anti-Snail and Slug antibodies were used on frozen tissues. Sections were incubated with the primary antibodies for 1 hour at room temperature (E-cadherin, CD1a, involucrin, TGF-β1) or overnight at 4°C (Snail, Slug). The revelation was performed with the Envision kit (DAKO, Glostrup, Denmark) (TGF-β1), with the peroxidase LSAB2 system (DAKO) (E-cadherin, CD1a, involucrin) or with a conjugated anti-goat secondary antibody (Alexa Fluor 488, Invitrogen) (Slug, Snail), according to the supplier’s recommendations.
For the immunostaining of cell monolayers, the cells were grown on glass slides and treated as previously indicated. The slides were quickly washed with phosphate-buffered saline (PBS) followed by a fixation in 4% paraformaldehyde for 20 minutes. After incubation in 100% cold methanol for 5 minutes, the slides were incubated with a fluorescein isothiocyanate-conjugated anti-E-cadherin primary antibody (clone 36; BD Transduction Laboratories, Franklin Lakes, NJ). The nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Cells were observed at timely intervals for 72 hours and pictures were obtained using an epifluorescence microscope (Carl Zeiss Inc., Oberkochen, Germany).
Immunostaining Assessment
The E-cadherin immunostaining was evaluated, in hysterectomy specimens, by using a semiquantitative score of the intensity and extent of the staining according to an arbitrary scale. For staining intensity, 0 represented samples in which membrane E-cadherin expression was undetectable, whereas 1, 2, and 3 denoted samples with, respectively, a low, moderate, and strong staining. For staining extent, 0, 1, 2, and 3 represented samples in which E-cadherin expression was detectable, respectively, in <5%, 6 to 25%, 26 to 75%, and >75% of the epithelium. The same staining extent evaluation was used to assess Slug and Snail immunoreactivity. To provide a global score for each case, the results obtained with the two scales were multiplied, yielding a single scale with steps of 0 to 9.35,36 This scoring system was also used to evaluate the TGF-β1 immunostaining. A similar scoring system was used for CD1a evaluation, with the modification that the staining intensity was replaced by the density of CD1a+ LCs [low (1), moderate (2), and high (3)]. This method was validated using a computerized image analysis system (CAS; Becton Dickinson, Erembodegen, Belgium) following a method described previously.37
Western Blotting Analysis
Cells were lysed in a buffer containing 50 mmol/L Tris, pH 7.5, 300 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1% Igepal CA-630 (Sigma, St. Louis, MO), 1 mmol/L phenylmethyl sulfonyl fluoride (Sigma), and protease inhibitors (Roche, Bale, Switzerland). After quantification (BCA protein assay; Pierce, Rockford, IL), 25 μg of proteins were separated by electrophoresis on 4 to 12% NuPAGE polyacrylamide gels (Invitrogen) and transferred onto polyvinylidene difluoride membranes. The membranes were subsequently blocked with 5% skim milk for 30 minutes and incubated overnight at 4°C with anti-β-actin (Sigma-Aldrich, St. Louis, MO), anti-E-cadherin (BD Transduction Laboratories), anti-Snail (Santa Cruz Biotechnology), and anti-Slug (Santa Cruz Biotechnology) antibodies. The membranes were then washed with TBS-T and incubated with appropriated secondary antibodies. After washings, the protein bands were detected using an enhanced chemiluminescence system (ECL Plus; Amersham Biosciences, Piscataway, NJ).
Semiquantitative Reverse Transcriptase (RT)-PCR Analysis
One μg of total RNA extracted from cell cultures (RNeasy mini kit, Qiagen) and quantified with a ND-1000 spectrophotometer (NanoDrop, Wilmington, DE) was reverse-transcribed using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. The reactions were performed at 42°C for 50 minutes, followed by inactivation of the enzyme at 75°C for 15 minutes. The cDNA was stored at −20°C. RT-PCR reactions were performed using the following primer sequences: E-cadherin: forward: 5′-TATTCCTCCCATCAGCTGCCC-3′; reverse: 5′-CAATGCGTTCTCTATCCAGAGG-3′; Snail: forward: 5′-AATCGGAAGCCTAACTACAGCGAG-3′; reverse: 5′-CCTTCCCACTGTCCTCATCTGACA-3′; Slug: forward: 5′-CCTTCCTGGTCAAGAAGCATTTCA-3′; reverse: 5′-AGGCTCACATATTCCTTGTCACAG-3′; HPRT: forward: 5′-TTGGATATAAGCCAGACTTTGTTG-3′; reverse: 5′-AGATGTTTCCAAACTCAACTTGAA-3′. Samples were run on 1.8% agarose gels containing ethidium bromide and visualized with an UV transilluminator. mRNA levels were determined by densitometric analysis (Quantity One Software; Bio-Rad, Hercules, CA). HPRT was used as an internal control and the mRNA levels were normalized to this housekeeping gene.
Methylation-Specific PCR
DNA methylation status of CpG island at the 5′ end of CDH1 (E-cadherin) gene was determined by methylation-specific PCR. This method is based on cleavage by the methylation-sensitive endonuclease HpaII and subsequently amplification of a gene fragment by PCR using primers specific to sequences flanking the restrictive enzyme cut sites. One hundred ng of DNA extracted from frozen tissues sections were cleaved overnight at 37°C using the restriction enzyme HpaII (10 U) (Fermentas, Burlington, Canada). After a thermal treatment to distort the enzyme, PCR was then performed using the following primer pairs: forward: 5′-GGGGGGCGGTGCTCCGG-3′; reverse: 5′-ATGGCTGGCCGGGGACGC-3′. These primers allowed amplification of a part of CDH1 promoter containing six potentially methylated CpG islands. Positive and negative controls were performed using genomic DNA lacking enzymatic digestion and DNA treated with methylation-unsensitive endonuclease MspI, respectively.
siRNA Transfection and Gene Silencing
The day before transfection, 1.5 × 105 HaCaT cells per well of a six-well plate were seeded in 3 ml of appropriate growth medium. For each transfection, 50 ng of small interfering RNA (siRNA) and 3 μl of Transfectin (Bio-Rad) were diluted in 1 ml of Optimem (Invitrogen). The mixture was then incubated at room temperature for 20 minutes to allow the formation of siRNA-liposome complexes. Growth medium was aspirated from the cells and the transfecting solution was added drop by drop. The cells were incubated with the complexes for 4 hours at 37°C in a CO2 incubator. After incubation, 1 ml of growth medium (containing 20% of serum) was added without removing the transfection mixture. Twenty-four hours after transfection, the medium was replaced with normal growth medium. High-efficiency siRNA transfections were obtained in growing HaCaT cells using this protocol (>90% cells). The efficiency of transfection in RNA interfering experiments was monitored by using labeled 3′ATTO 647N-negative control siRNA (Eurogentec, Seraing, Belgium). The anti-human Snail and Slug siRNAs used were, respectively, designed by Jorda and colleagues38 and by Tripathi and colleagues.39
Plasmid Transfection and Heterotypic Cell Adhesion Assay
pcDNA3.1 Zeo expression vector (Invitrogen) containing full-length human Slug sequence and pEF6/Myc-His version A expression vector (Invitrogen) containing human Snail sequence were transfected into HaCaT cells plated in two-well Lab-Tek chamber slide (Nunc, Roskilde, Denmark) with 1.5 μl of Transfectin (Bio-Rad). The transfection protocol used was similar to that previously described for siRNA. Forty-eight hours after the start of transfection, 10 × 105 LCs generated as previously described40 and stained with the lipophilic fluorescent marker CM-DIL (Molecular Probes, Invitrogen) were plated in each well. After 1 day of co-culture, the slides were washed with PBS and fixed in 4% paraformaldehyde for 20 minutes. The nuclei were revealed with DAPI and finally, the number of LCs observed by field (magnification, ×200) was determined by using a epifluorescence microscope (Carl Zeiss Inc.) and by counting cells in 10 microscopic fields.
Statistical Analysis
Statistical analysis was performed with the Instat 3 software (Graph-Pad Software, San Diego, CA). The Spearman correlation test was used to establish the correlation between E-cadherin expression and LC density in the different histological tissues. The Kruskal-Wallis test was used to assess the difference between E-cadherin expression and LC density in the cervical specimens and to estimate the difference of LC number by microscopical field in the heterotypic cell adhesion assay. Correlations and differences were considered as statistically significant when P values were less than 0.05.
Results
The Density of CD1a-Positive LCs Is Correlated in Vivo with the Expression of Cell-Surface E-Cadherin
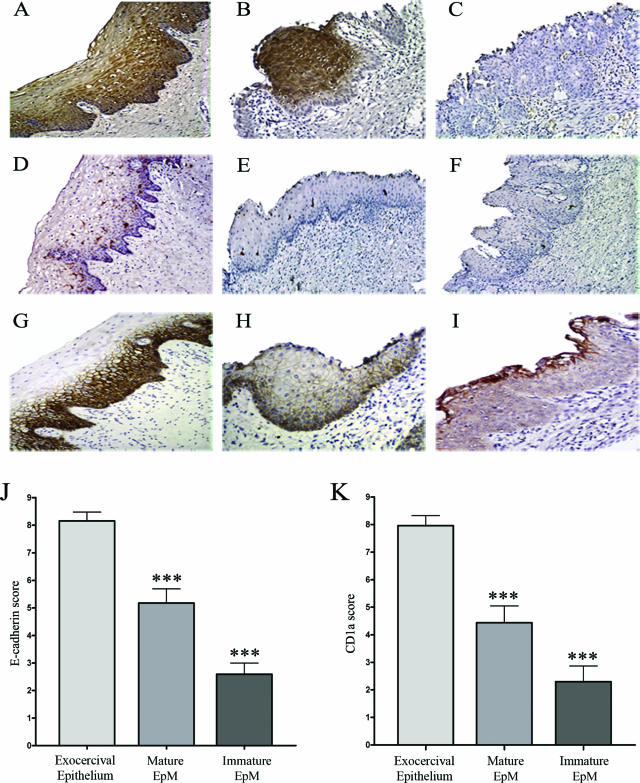
E-cadherin expression and CD1a+ cells were studied in 51 hysterectomy specimens. The exocervical squamous epithelium was tested as controls. Twenty-three samples show mature EpM and 39 immature EpM. Among the 23 mature EpM cases, 11 were adjacent to immature EpM. All these tissue specimens were negative for HPV. The immunostaining results are shown in Figure 1. Squamous exocervical epithelium (Figure 1A) as well as mature EpM (Figure 1B) showed strongly positive involucrin immunoreactivity whereas no or a low expression of this protein was found in immature EpM (Figure 1C). This marker for epithelial differentiation was therefore used to easily distinguish between mature and immature EpM. In the normal exocervical epithelium, CD1a+ cells were intermingled with keratinocytes in the (para)basal and intermediate cell layers (Figure 1D). In contrast, the density of these cells was significantly lower in areas of mature (Figure 1E) and immature (Figure 1F) EpM than in the squamous exocervical epithelium. This latter one was strongly positive for E-cadherin (Figure 1G). Positive cells were observed mostly in (para)basal and intermediate cell layers. In contrast to exocervical epithelium, all of the mature (Figure 1H) and immature (Figure 1I) EpM showed a lower anti-E-cadherin cell-surface immunoreactivity. However, in immature EpM, the glandular epithelium that persists at the surface of metaplastic keratinocytes showed a positive E-cadherin staining as the normal epithelium of the endocervix (data not shown). The density of CD1a+ cells and the E-cadherin immunoreactivity were statistically higher in the normal exocervical epithelium than in mature and immature EpM (P < 0001), as demonstrated by the semiquantitative evaluation of E-cadherin (Figure 1J) and CD1a (Figure 1K) intraepithelial expression. Moreover, a Spearman correlation between CD1a and E-cadherin scores was also observed in normal exocervical epithelium (Spearman, r = 0.3973; P value = 0.005), in mature (Spearman, r = 0.4538; P value = 0.0296), and in immature (Spearman, r = 0.4448; P value = 0.0046) EpM.
Figure 1.
Correlation between the density of CD1a+ LCs and the E-cadherin expression in squamous exocervical epithelium and in EpM (mature and immature). A–C: Involucrin expression in normal squamous epithelium and in areas of mature and immature EpM. Compared with exocervical epithelium (A) and mature EpM (B), the involucrin immunoreactivity is strongly decreased in immature EpM (C). D–F: Density of CD1a+ cells in normal squamous epithelium and in EpM. D: The normal squamous epithelium shows a high density of CD1a+ cells in the basal and suprabasal cell layers. Mature (E) and immature (F) EpM are infiltrated by a low density of CD1a+ cells. G–I: E-cadherin expression in normal squamous epithelium and in EpM. G: The normal squamous epithelium shows typical cell-surface E-cadherin staining of basal and intermediate keratinocytes. The E-cadherin immunoreactivity was intermediate and low in mature (H) and immature (I) EpM, respectively. J and K: Semiquantitative evaluation of E-cadherin and CD1a expression, respectively, in normal exocervix (n = 51), mature (n = 23), and immature (n = 39) areas of EpM. Asterisks indicate statistically significant differences (***P < 0.001). Original magnifications: ×100 (A, D–G); ×200 (B, C, H, I).
The Majority of Metaplastic and Exocervical Biopsies Are Unmethylated for CDH1 Gene
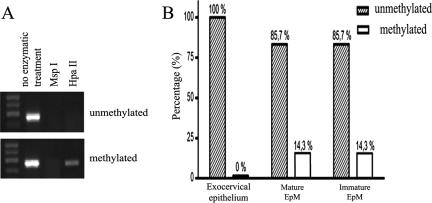
To determine the mechanism(s) responsible for the decreased expression of E-cadherin in the EpM, we first analyzed the methylation status of a panel of CpG islands associated with the promoter of E-cadherin (CDH1) gene by a restriction enzyme PCR. After a treatment with the methylation-sensitive endonuclease HpaII, the methylation level of CDH1 gene was established by PCR. DNA is not cleaved when enzymatic recognition sites are methylated and can be amplified by PCR. In contrast, when the target DNA sequence is unmethylated, its cleavage by HpaII prevents the DNA amplification by PCR. Untreated DNA and DNA treated with methylation-unsensitive endonuclease MspI were the positive and negative controls, respectively (Figure 2A). With this technique, methylated CDH1 gene was found in 0% (0 of 12) of exocervical biopsies and in 14.3% (2 of 14) of specimens containing mature or immature EpM (Figure 2B). No statistical difference in the methylation level of CDH1 gene was observed between exocervical and metaplastic specimens.
Figure 2.
The methylation status of CDH1 (E-cadherin) gene in exocervical and metaplastic tissues. A: Representative examples of PCR results for methylated and unmethylated CDH1 gene. After a treatment with the restriction enzyme HpaII, a PCR product is visualized under UV illumination for methylated tissue samples. Genomic DNA without enzyme digestion and DNA treated with methylation-unsensitive endonuclease MspI represent positive and negative control, respectively. B: Methylation analysis of CDH1 gene in cervical tissues with exocervical epithelium (n = 12), mature (n = 14), or immature EpM (n = 14).
TGF-β1, Slug, and Snail Transcription Factors Are Overexpressed in Areas of Mature and Immature EpM
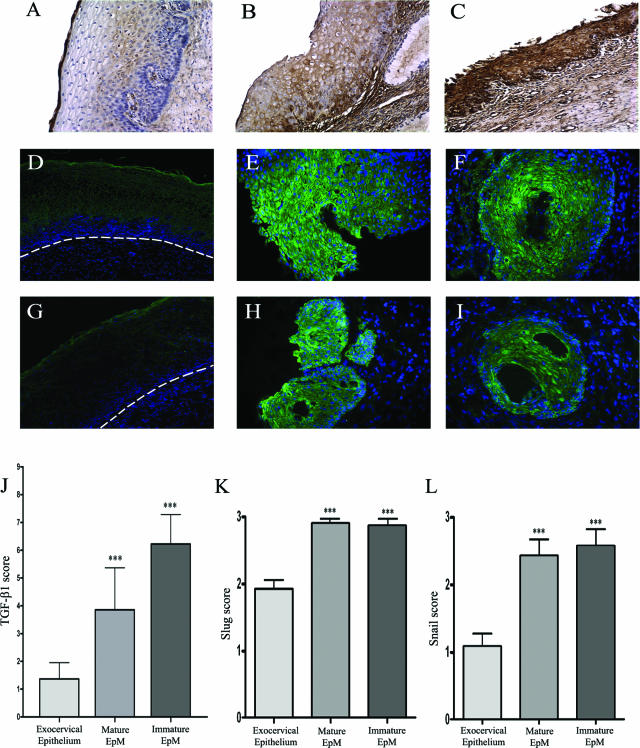
Next, we investigated the possible role of TGF-β1 and Slug/Snail transcription factors in the down-regulation of E-cadherin in EpM. By using immunohistochemistry in cervical biopsies, we showed that, compared to the exocervical epithelium (Figure 3A), EpM expressed significantly more TGF-β1 (P < 0.001; Figure 3, B and C). Furthermore, TGF-β1 staining was higher in immature (Figure 3C) than in mature (Figure 3B) EpM. Slug (Figure 3, E and F) and Snail (Figure 3, H and I) transcription factors were found to be highly expressed in mature and immature EpM. The staining was observed both in the cytoplasm and the nucleus of (para)basal and apical metaplastic cells. In contrast, the immunoreactivity was only detected in the upper epithelial cell layers (Figure 3, D and G), and the expression of these proteins was significantly lower in the exocervical epithelium than in EpM (P < 0.001; Figure 3, J–L).
Figure 3.
TGF-β1 (A–C), Slug (D–F), and Snail (G–I) immunostaining in cervical biopsy specimens. The exocervical epithelium shows a low TGF-β1 staining (A) whereas mature (B) and immature (C) EpM demonstrate, respectively, an intermediate and high expression of TGF-β1. The normal squamous exocervical epithelium shows a medium expression of Slug (D) and a low expression of Snail (G) transcription factors only in upper cell layers. The dashed line delineates the epithelium from the stroma. In contrast, mature (E and H) and immature (F and I) EpM demonstrates a strong Slug (E and F) and Snail (H and I) immunoreactivity. J: Semiquantitative evaluation of TGF-β1 expression, respectively, in normal exocervix (n = 51), mature (n = 23), and immature (n = 39) areas of EpM. Semiquantitative evaluation of Slug (K) and Snail (L) expression, respectively, in normal exocervix (n = 12), mature (n = 14), and immature (n = 14) areas of EpM. Asterisks indicate statistically significant differences (***P < 0.001). Original magnifications: ×100 (A–C, D, G); ×200 (E, F, H, I).
TGF-β1 Induces Slug and Snail Expression and Represses E-Cadherin in Keratinocytes
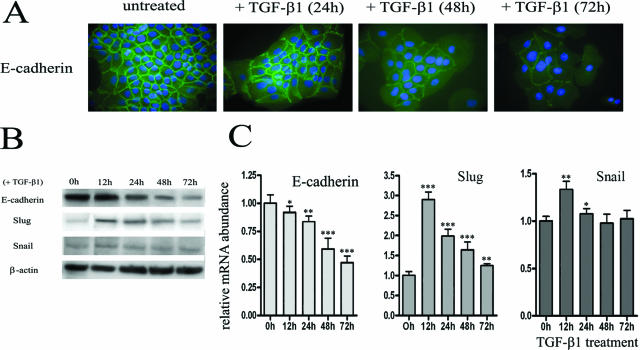
To determine in vitro the potential implication of TGF-β1 in the E-cadherin down-regulation in metaplastic keratinocytes, HaCaT cells were treated with TGF-β1 (10 ng/ml) for 24, 48, and 72 hours. Immunostaining demonstrated that E-cadherin was weakly detected in the cell junctions after 48 and 72 hours of incubation with TGF-β1 (Figure 4A). This decreased expression of E-cadherin in the presence of TGF-β1 was confirmed by Western blot (Figure 4B) and RT-PCR (Figure 4C). In addition, we found that TGF-β1 strongly increased the Slug transcription factor expression (Figure 4C). The maximum RNA level was obtained 12 hours after exposure to TGF-β1 and was followed by a steady decline in expression. However, the expression of Slug remained greater than the basal level at least up to 72 hours of TGF-β1 treatment. In contrast, the expression of Snail transcription factor was weakly induced by TGF-β1. Similar results were obtained at the protein level (Figure 4B).
Figure 4.
TGF-β1 inhibits E-cadherin and induces Slug and Snail expression in keratinocytes. A: The immunofluorescence demonstrates a diminished expression of E-cadherin (green) at the cell membrane after incubation with TGF-β1 during 48 hours. The nuclei were stained with DAPI (blue). Western blot (B) and RT-PCR (C) confirm the down-regulation of E-cadherin and show the increased expression of Slug and, at a lower level, of Snail transcription factors after TGF-β1 treatment. Results are representative of three independent experiments performed in duplicates. The mean ± SD is shown. Asterisks indicate statistically significant differences (*P < 0.05; **P < 0.01; ***P < 0.001). Original magnifications, ×630.
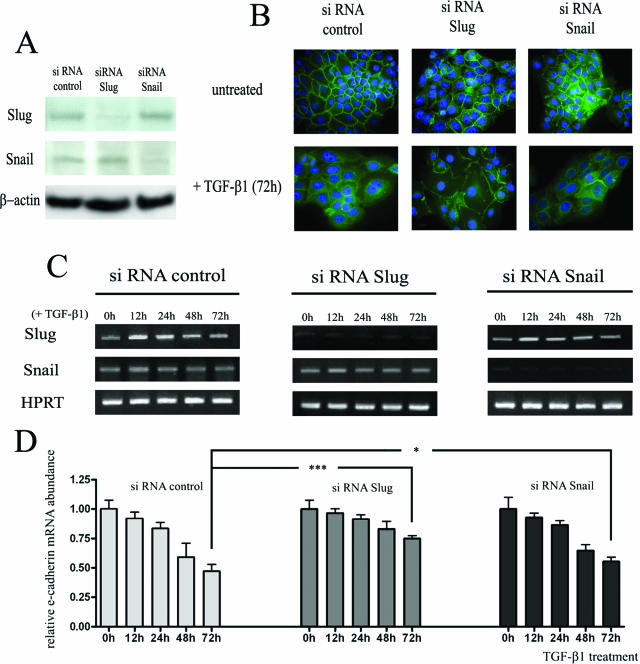
Slug and Snail Silencing Attenuates the Down-Regulation of E-Cadherin Caused by TGF-β1 in Keratinocytes
We next determined whether Slug and/or Snail transcription factors are required for the decreased expression of E-cadherin induced by TGF-β1 by examining the effects of Slug and Snail silencing on TGF-β1-induced down-regulation of E-cadherin in keratinocytes. Gene silencing efficiency was analyzed by Western blot (Figure 5A) and RT-PCR (Figure 5C). Immunocytology (Figure 5B) and RT-PCR (Figure 5D) showed that the down-regulation of E-cadherin induced by TGF-β1 was partially suppressed in Slug- and Snail-silenced HaCaT cells. RT-PCR analysis showed that after 72 hours of TGF-β1 treatment, E-cadherin mRNA was present in HaCaT-control siRNA at a level corresponding to 49% of that detected in untreated cells, compared to 77% (P < 0.001) and 56% (P < 0.05) in HaCaT cells transfected, respectively, with Slug and Snail siRNA. No synergistic effect was observed when keratinocytes were transfected with siRNAs against both Slug and Snail (data not shown). These data suggest that Slug and, at a lower extent, Snail transcription factors are, at least in part, necessary for the down-regulation of E-cadherin induced by TGF-β1 in keratinocytes.
Figure 5.
The decrease in E-cadherin expression caused by TGF-β1 is partially attenuated by Slug and Snail silencing. A: Efficiency of Slug and Snail silencing was demonstrated by Western blot 48 hours after siRNA transfection. B: Immunofluorescence analysis and subcellular localization of E-cadherin (green) in HaCaT-siRNA control, in HaCaT-siRNA Slug and in HaCaT-siRNA Snail stimulated or not with TGF-β1 for 72 hours. The nuclei are stained with DAPI (blue). The images are representative of results obtained in three different experiments. C: Expression of Slug and Snail in TGF-β1-treated cells was controlled by RT-PCR for the indicated times after siRNA transfection. D: Quantification of RT-PCR data demonstrated that the decrease in E-cadherin expression is greater in HaCaT transfected with siRNA control than in HaCaT transfected with siRNA Slug or siRNA Snail. Results are representative of three independent transfection experiments performed in duplicates. The mean ± SD is shown. Asterisks indicate statistically significant differences (*P < 0.05; ***P < 0.001). Original magnifications, ×630.
The Interactions between LCs and Keratinocytes Are Affected by the Down-Regulation of E-Cadherin Caused by Slug and Snail Transcription Factors
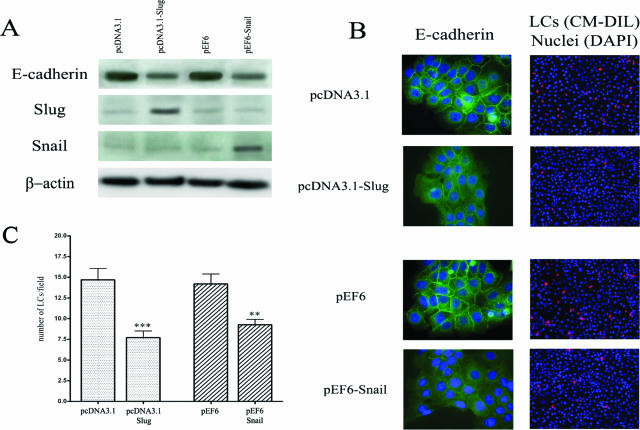
We finally investigated whether the reduction of E-cadherin expression caused by Slug and Snail transcription factors affects the adhesion of LCs to keratinocytes. We transiently transfected keratinocytes with Snail and Slug expression vectors and showed that Slug and Snail proteins levels were higher in transfected cells (Figure 6A). RT-PCR analysis showed similar results (data not shown). In addition, we confirmed by immunofluorescence (Figure 6B) and Western blot (Figure 6A) the down-regulation of E-cadherin induced by these two transcription factors. Forty-eight hours after transfection, a heterotypic cell adhesion assay was performed by using fluorescent labeled LCs that were incubated with keratinocytes transfected with the different vectors and we demonstrated that LCs poorly adhere to Slug- and Snail-transfected keratinocytes compared to cells transfected with empty vectors (Figure 6C). Similar results were obtained when the expression of E-cadherin in keratinocytes was reduced by a pretreatment with TGF-β1 for 72 hours (data not shown).
Figure 6.
The decrease in E-cadherin expression induced by Slug and Snail transcription factors affects the interactions between LCs and keratinocytes. A: Seventy-two hours after transfection with Slug and Snail transcription factors, the expression of E-cadherin, Slug and Snail was assessed by Western blot. B: Immunofluorescence analysis and subcellular localization of E-cadherin (green) in human Slug or Snail transfected keratinocytes. Transfections with corresponding empty expression vectors were used as controls. For each condition of transfection, a representative example of LC (red) density observed by field in the heterotypic cell adhesion assay is shown. The nuclei are stained with DAPI (blue). C: Graphic representation of the mean number ± SD of LCs observed by field (original magnification ×200) in the co-culture experiments. For each condition, three independent experiments were performed. The adhesion of LCs to human Slug- or Snail-transfected keratinocytes was significantly lower compared to cells transfected with empty vectors. Asterisks indicate statistically significant differences (**P < 0.01; ***P < 0.001). Original magnifications: ×630 (B, left); ×200 [B (right)].
Discussion
Although the precise mechanisms underlying induction of EpM are still obscure, it has been known for many years that specific metaplastic sites are at higher risk of developing cancer compared with the adjacent native epithelium.5,41,42 The increased risk of malignant transformation within the metaplastic epithelium could result from the accumulation of somatic gene mutations directly caused by factors responsible for EpM.4,43 However, intrinsic immune features altered in the metaplastic epithelium could also contribute to cancer development by preventing an efficient antitumor immune response.6 This effect could be mediated by a differential expression of soluble and/or membrane-associated factors. Consistent with this hypothesis, an altered expression of several soluble factors such as TGF-β, tumor necrosis factor (TNF)-α, and interleukin (IL)-10 has been observed in esophageal and cervical EpM.10,44,45 Similarly, the expression of E-cadherin, which is necessary for the retention of antigen-presenting cells in epithelial tissues,36 is decreased in gastric and esophageal areas of intestinal metaplasia compared to normal epithelium.14,46
LCs are antigen-presenting cells that play a key role in the immunosurveillance of epidermis and mucosal surfaces. LCs are required for the initiation of cellular immune responses to pathogens and the infiltration of tumors by antigen-presenting cells has been correlated with a better prognosis.47,48,49 In the present study, we demonstrated that the immunosurveillance represented by the density of CD1a+ LCs is decreased both in mature and immature cervical EpM compared with the normal squamous epithelium and is correlated with a lower intraepithelial expression of E-cadherin. These findings are in agreement with previous works reporting a reduced LC density in the cervical transformation zone.9,10 The observed correlation between E-cadherin expression on keratinocytes and LC density in normal and metaplastic epithelium suggests an important role of the heterotypic E-cadherin-mediated interaction between keratinocytes and LCs for the LC retention in the squamous epithelium. A similar correlation has been previously performed in HPV16-infected skin in which E6 viral oncoprotein inhibits E-cadherin expression.50 However, in contrast to cervical (pre)neoplasic lesions that are associated with HPV infection,51,52,53 the biopsies selected in this study were HPV-negative, suggesting that E6 viral oncoprotein is not involved in the down-regulation of E-cadherin in HPV-negative EpM.
To determine the mechanism by which E-cadherin expression is modulated in EpM, the methylation level of CDH1 (E-cadherin) promoter was studied by using a methylation-specific PCR. We found that the majority of analyzed cervical metaplastic samples are unmethylated. As already shown in intestinal metaplasia areas in the lower esophagus54 and the stomach,55 the frequency of hypermethylation was less than 40%, suggesting that other mechanisms are responsible for the down-regulation of E-cadherin observed in every EpM.
Slug and Snail are transcription factors that negatively regulate the expression of E-cadherin.18,19,20,21 In the present study, we found, by immunostaining, that these proteins are strongly expressed in the entire thickness of EpM. In contrast, these two proteins were only weakly detected in the upper epithelial cell layers of the normal exocervical epithelium and their expression was inversely correlated with that of E-cadherin that was mainly present in the (para)basal and intermediate cells of the squamous epithelium. As previously observed in pancreatic and esophageal cancer cells,56,57 Slug and Snail were observed in the cytoplasm as well as in the nucleus of keratinocytes. Interestingly, TGF-β1 was also expressed, at higher levels, in EpM compared with the adjacent native epithelium. TGF-β1 has been shown to induce Snail and/or Slug transcription factors and to down-regulate E-cadherin in several cell lines.26,27,28,58,59
To determine whether TGF-β1 is responsible for the induction of Slug and Snail transcription factors and for the reduction of E-cadherin expression observed in EpM, we treated HaCaT cells with TGF-β1 and observed a down-regulation of E-cadherin expression with a high and low induction of Slug and Snail transcription factors, respectively. Because similar results were obtained by RT-PCR and Western blot techniques, we concluded that TGF-β1 induces an increased expression of Slug and Snail transcription factor and not an inhibition of protein degradation. However, compared with previous data obtained with other cell lines,26,27,28,58,59 TGF-β1 differentially modified Slug and Snail expression in HaCaT keratinocytes. Zavadil and colleagues34 have previously shown that the patterns of activation of Slug and Snail by TGF-β1 were mutually exclusive and cell-type-dependent. Moreover, the difference in Snail expression observed in vivo (strong) and in vitro (low) is probably related to the fact that TGF-β1 is not the only soluble factor that can induce Snail expression. For example, the prostaglandin E2 (PGE2) can also up-regulate Snail60 and then could explain the high expression of Snail observed in vivo. Accordingly, PGE2 synthase was found to be highly expressed in mature and immature EpM compared to exocervical epithelium and a significantly increased Snail expression was observed in vitro in keratinocytes treated with PGE2 (data not shown).
In addition, we showed that Slug or Snail silencing partially abrogated the down-regulation of E-cadherin induced by TGF-β1 in keratinocytes. These results are in agreement with those reported by Takano and colleagues58 and demonstrate that these transcription factors are implicated in the reduction of E-cadherin expression caused by TGF-β1. However, the restitution of E-cadherin expression was stronger for Slug as compared to Snail silencing, suggesting that TGF-β1 reduces the E-cadherin expression in keratinocytes, mainly via the Slug transcription factor.
Finally, to determine the significance of this down-regulation of E-cadherin expression observed in EpM in terms of LC adhesion to keratinocytes, the impact of E-cadherin under-expression was studied by using a relevant heterotypic cell adhesion assay. We showed that the adhesion between LCs and epithelial cells is altered when E-cadherin expression by keratinocytes is inhibited. The importance of homophilic E-cadherin-mediated interactions between LCs and epithelial cells has been previously reported by several studies.11,36 However, the reduction of E-cadherin was unable to completely abrogate the LCs/keratinocytes interactions. Although there is evidence that E-cadherin stimulates the adhesion of LCs/DCs directly, we cannot exclude, in our study, the role of other adhesion molecules such as α6 integrins, CD44, or CD47.61,62,63
In conclusion, we demonstrated that TGF-β1 can indirectly induce decreased antigen presentation functions in EpM by affecting E-cadherin expression. The inability of the local immune system to mount a cell-mediated immune response against pathogens or cells in transformation, because of a deficit of adhesion molecules necessary for cell-to-cell interactions, might play an important role in the susceptibility of EpM for developing cancer. The progressive alteration of E-cadherin expression that has been demonstrated in bronchial,15 esophageal,13 and gastric14 metaplasia-dysplasia-carcinoma sequences could not only be an early indication signaling the malignant transformation of metaplastic cells but might also constitute one of the major determinants for establishing local immunodeficiency responsible for EpM tumorigenesis.
Footnotes
Address reprint requests to Michael Herfs, Department of Pathology B35, CHU Sart Tilman, 4000 Liege, Belgium. E-mail: m.herfs@student.ulg.ac.be.
Supported by the Marshall Program of the Walloon Region (Neoangio no. 616476), the Belgian Fund for Medical Scientific Research, and the Centre Anti-Cancereux près l’Université de Liège. P.D. is a senior research associate and M.H. is a research fellow of the Belgian National Fund for Scientific Research.
M.H. and P.H. contributed equally to this study.
References
- Slack JM. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat Rev Mol Cell Biol. 2007;8:369–378. doi: 10.1038/nrm2146. [DOI] [PubMed] [Google Scholar]
- Tosh D, Slack JM. How cells change their phenotype. Nat Rev Mol Cell Biol. 2002;3:187–194. doi: 10.1038/nrm761. [DOI] [PubMed] [Google Scholar]
- Elson DA, Riley RR, Lacey A, Thordarson G, Talamantes FJ, Arbeit JM. Sensitivity of the cervical transformation zone to estrogen-induced squamous carcinogenesis. Cancer Res. 2000;60:1267–1275. [PubMed] [Google Scholar]
- Quinlan JM, Colleypriest BJ, Farrant M, Tosh D. Epithelial metaplasia and the development of cancer. Biochim Biophys Acta. 2007;1776:10–21. doi: 10.1016/j.bbcan.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Burghardt E, Ostor AG. Site and origin of squamous cervical cancer: a histomorphologic study. Obstet Gynecol. 1983;62:117–127. [PubMed] [Google Scholar]
- Delvenne P, Hubert P, Jacobs N. Epithelial metaplasia: an inadequate environment for antitumour immunity? Trends Immunol. 2004;25:169–173. doi: 10.1016/j.it.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Uchi H, Terao H, Koga T, Furue M. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(Suppl 1):S29–S38. doi: 10.1016/s0923-1811(00)00138-9. [DOI] [PubMed] [Google Scholar]
- Sikorski M, Bieda T, Bobek M, Zrubek H. Dynamics of cervical Langerhans cell counts in the course of HPV-positive CIN treatment with the use of human recombinant interferon gamma. Eur J Gynaecol Oncol. 2005;26:294–298. [PubMed] [Google Scholar]
- Al-Saleh W, Giannini SL, Jacobs N, Moutschen M, Doyen J, Boniver J, Delvenne P. Correlation of T-helper secretory differentiation and types of antigen-presenting cells in squamous intraepithelial lesions of the uterine cervix. J Pathol. 1998;184:283–290. doi: 10.1002/(SICI)1096-9896(199803)184:3<283::AID-PATH25>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Giannini SL, Hubert P, Doyen J, Boniver J, Delvenne P. Influence of the mucosal epithelium microenvironment on Langerhans cells: implications for the development of squamous intraepithelial lesions of the cervix. Int J Cancer. 2002;97:654–659. doi: 10.1002/ijc.10084. [DOI] [PubMed] [Google Scholar]
- Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993;361:82–85. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Bailey T, Biddlestone L, Shepherd N, Barr H, Warner P, Jankowski J. Altered cadherin and catenin complexes in the Barrett’s esophagus-dysplasia-adenocarcinoma sequence: correlation with disease progression and dedifferentiation. Am J Pathol. 1998;152:135–144. [PMC free article] [PubMed] [Google Scholar]
- Chan AO, Wong BC, Lan HY, Loke SL, Chan WK, Hui WM, Yuen YH, Ng I, Hou L, Wong WM, Yuen MF, Luk JM, Lam SK. Deregulation of E-cadherin-catenin complex in precancerous lesions of gastric adenocarcinoma. J Gastroenterol Hepatol. 2003;18:534–539. doi: 10.1046/j.1440-1746.2003.02998.x. [DOI] [PubMed] [Google Scholar]
- Kato Y, Hirano T, Yoshida K, Yashima K, Akimoto S, Tsuji K, Ohira T, Tsuboi M, Ikeda N, Ebihara Y, Kato H. Frequent loss of E-cadherin and/or catenins in intrabronchial lesions during carcinogenesis of the bronchial epithelium. Lung Cancer. 2005;48:323–330. doi: 10.1016/j.lungcan.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Graff JR, Herman JG, Lapidus RG, Chopra H, Xu R, Jarrard DF, Isaacs WB, Pitha PM, Davidson NE, Baylin SB. E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res. 1995;55:5195–5199. [PubMed] [Google Scholar]
- Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci USA. 1995;92:7416–7419. doi: 10.1073/pnas.92.16.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De HA. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Bolós V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- Miyoshi A, Kitajima Y, Sumi K, Sato K, Hagiwara A, Koga Y, Miyazaki K. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer. 2004;90:1265–1273. doi: 10.1038/sj.bjc.6601685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi A, Kitajima Y, Kido S, Shimonishi T, Matsuyama S, Kitahara K, Miyazaki K. Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. Br J Cancer. 2005;92:252–258. doi: 10.1038/sj.bjc.6602266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimachi K, Tanaka S, Kameyama T, Taguchi K, Aishima S, Shimada M, Sugimachi K, Tsuneyoshi M. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin Cancer Res. 2003;9:2657–2664. [PubMed] [Google Scholar]
- Hardy RG, Vicente-Duenas C, Gonzalez-Herrero I, Anderson C, Flores T, Hughes S, Tselepis C, Ross JA, Sanchez-Garcia I. Snail family transcription factors are implicated in thyroid carcinogenesis. Am J Pathol. 2007;171:1037–1046. doi: 10.2353/ajpath.2007.061211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Park SY, Joo CK. Transforming growth factor-beta1 represses E-cadherin production via slug expression in lens epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:2708–2718. doi: 10.1167/iovs.06-0639. [DOI] [PubMed] [Google Scholar]
- Medici D, Hay ED, Goodenough DA. Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial-mesenchymal transition. Mol Biol Cell. 2006;17:1871–1879. doi: 10.1091/mbc.E05-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Pallone F, MacDonald TT. Smad7 in TGF-beta-mediated negative regulation of gut inflammation. Trends Immunol. 2004;25:513–517. doi: 10.1016/j.it.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- Delvenne P, Fontaine MA, Delvenne C, Nikkels A, Boniver J. Detection of human papillomaviruses in paraffin-embedded biopsies of cervical intraepithelial lesions: analysis by immunohistochemistry, in situ hybridization, and the polymerase chain reaction. Mod Pathol. 1994;7:113–119. [PubMed] [Google Scholar]
- Qu W, Jiang G, Cruz Y, Chang CJ, Ho GY, Klein RS, Burk RD. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Microbiol. 1997;35:1304–1310. doi: 10.1128/jcm.35.6.1304-1310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders PJ, van den Brule AJ, Schrijnemakers HF, Snow G, Meijer CJ, Walboomers JM. The use of general primers in the polymerase chain reaction permits the detection of a broad spectrum of human papillomavirus genotypes. J Gen Virol. 1990;71:173–181. doi: 10.1099/0022-1317-71-1-173. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detry C, Waltregny D, Quatresooz P, Chaplet M, Kedzia W, Castronovo V, Delvenne P, Bellahcene A. Detection of bone sialoprotein in human (pre)neoplastic lesions of the uterine cervix. Calcif Tissue Int. 2003;73:9–14. doi: 10.1007/s00223-002-2108-0. [DOI] [PubMed] [Google Scholar]
- Hubert P, Caberg JH, Gilles C, Bousarghin L, Franzen-Detrooz E, Boniver J, Delvenne P. E-cadherin-dependent adhesion of dendritic and Langerhans cells to keratinocytes is defective in cervical human papillomavirus-associated (pre)neoplastic lesions. J Pathol. 2005;206:346–355. doi: 10.1002/path.1771. [DOI] [PubMed] [Google Scholar]
- Delvenne P, Al-Saleh W, Gilles C, Thiry A, Boniver J. Inhibition of growth of normal and human papillomavirus-transformed keratinocytes in monolayer and organotypic cultures by interferon-gamma and tumor necrosis factor-alpha. Am J Pathol. 1995;146:589–598. [PMC free article] [PubMed] [Google Scholar]
- Jordà M, Olmeda D, Vinyals A, Valero E, Cubillo E, Llorens A, Cano A, Fabra A. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J Cell Sci. 2005;118:3371–3385. doi: 10.1242/jcs.02465. [DOI] [PubMed] [Google Scholar]
- Tripathi MK, Misra S, Khedkar SV, Hamilton N, Irvin-Wilson C, Sharan C, Sealy L, Chaudhuri G. Regulation of BRCA2 gene expression by the SLUG repressor protein in human breast cells. J Biol Chem. 2005;280:17163–17171. doi: 10.1074/jbc.M501375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert P, Bousarghin L, Greimers R, Franzen-Detrooz E, Boniver J, Delvenne P. Production of large numbers of Langerhans’ cells with intraepithelial migration ability in vitro. Exp Dermatol. 2005;14:469–477. doi: 10.1111/j.0906-6705.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Cossentino MJ, Wong RK. Barrett’s esophagus and risk of esophageal adenocarcinoma. Semin Gastrointest Dis. 2003;14:128–135. [PubMed] [Google Scholar]
- Whiting JL, Sigurdsson A, Rowlands DC, Hallissey MT, Fielding JW. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut. 2002;50:378–381. doi: 10.1136/gut.50.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CP, Hardie LJ. Reflux. Barrett’s oesophagus and adenocarcinoma: burning questions. Nat Rev Cancer. 2003;3:676–684. doi: 10.1038/nrc1166. [DOI] [PubMed] [Google Scholar]
- Giannini SL, Al-Saleh W, Piron H, Jacobs N, Doyen J, Boniver J, Delvenne P. Cytokine expression in squamous intraepithelial lesions of the uterine cervix: implications for the generation of local immunosuppression. Clin Exp Immunol. 1998;113:183–189. doi: 10.1046/j.1365-2249.1998.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tselepis C, Perry I, Dawson C, Hardy R, Darnton SJ, McConkey C, Stuart RC, Wright N, Harrison R, Jankowski JA. Tumour necrosis factor-alpha in Barrett’s oesophagus: a potential novel mechanism of action. Oncogene. 2002;21:6071–6081. doi: 10.1038/sj.onc.1205731. [DOI] [PubMed] [Google Scholar]
- Swami S, Kumble S, Triadafilopoulos G. E-cadherin expression in gastroesophageal reflux disease. Barrett’s esophagus, and esophageal adenocarcinoma: an immunohistochemical and immunoblot study. Am J Gastroenterol. 1995;90:1808–1813. [PubMed] [Google Scholar]
- Barnetson RS, Satchell A, Zhuang L, Slade HB, Halliday GM. Imiquimod induced regression of clinically diagnosed superficial basal cell carcinoma is associated with early infiltration by CD4 T cells and dendritic cells. Clin Exp Dermatol. 2004;29:639–643. doi: 10.1111/j.1365-2230.2004.01614.x. [DOI] [PubMed] [Google Scholar]
- Inoshima N, Nakanishi Y, Minami T, Izumi M, Takayama K, Yoshino I, Hara N. The influence of dendritic cell infiltration and vascular endothelial growth factor expression on the prognosis of non-small cell lung cancer. Clin Cancer Res. 2002;8:3480–3486. [PubMed] [Google Scholar]
- Reichert TE, Scheuer C, Day R, Wagner W, Whiteside TL. The number of intratumoral dendritic cells and zeta-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer. 2001;91:2136–2147. [PubMed] [Google Scholar]
- Matthews K, Leong CM, Baxter L, Inglis E, Yun K, Backstrom BT, Doorbar J, Hibma M. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down regulation of E-cadherin. J Virol. 2003;77:8378–8385. doi: 10.1128/JVI.77.15.8378-8385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhmer G, van den Brule AJ, Brummer O, Meijer CL, Petry KU. No confirmed case of human papillomavirus DNA-negative cervical intraepithelial neoplasia grade 3 or invasive primary cancer of the uterine cervix among 511 patients. Am J Obstet Gynecol. 2003;189:118–120. doi: 10.1067/mob.2003.439. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Munoz N. The viral etiology of cervical cancer. Virus Res. 2002;89:183–190. doi: 10.1016/s0168-1702(02)00187-9. [DOI] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Eads CA, Lord RV, Wickramasinghe K, Long TI, Kurumboor SK, Bernstein L, Peters JH, DeMeester SR, Demeester TR, Skinner KA, Laird PW. Epigenetic patterns in the progression of esophageal adenocarcinoma. Cancer Res. 2001;61:3410–3418. [PubMed] [Google Scholar]
- To KF, Leung WK, Lee TL, Yu J, Tong JH, Chan MW, Ng EK, Chung SC, Sung JJ. Promoter hypermethylation of tumor-related genes in gastric intestinal metaplasia of patients with and without gastric cancer. Int J Cancer. 2002;102:623–628. doi: 10.1002/ijc.10783. [DOI] [PubMed] [Google Scholar]
- Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769–4776. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- Uchikado Y, Natsugoe S, Okumura H, Setoyama T, Matsumoto M, Ishigami S, Aikou T. Slug expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:1174–1180. [PubMed] [Google Scholar]
- Takano S, Kanai F, Jazag A, Ijichi H, Yao J, Ogawa H, Enomoto N, Omata M, Nakao A. Smad4 is essential for down-regulation of E-cadherin induced by TGF-beta in pancreatic cancer cell line PANC-1. J Biochem (Tokyo) 2007;141:345–351. doi: 10.1093/jb/mvm039. [DOI] [PubMed] [Google Scholar]
- Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174:175–183. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohadwala M, Yang SC, Luo J, Sharma S, Batra RK, Huang M, Lin Y, Goodglick L, Krysan K, Fishbein MC, Hong L, Lai C, Cameron RB, Gemmill RM, Drabkin HA, Dubinett SM. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006;66:5338–5345. doi: 10.1158/0008-5472.CAN-05-3635. [DOI] [PubMed] [Google Scholar]
- Fukunaga A, Nagai H, Noguchi T, Okazawa H, Matozaki T, Yu X, Lagenaur CF, Honma N, Ichihashi M, Kasuga M, Nishigori C, Horikawa T. Src homology 2 domain-containing protein tyrosine phosphatase substrate 1 regulates the migration of Langerhans cells from the epidermis to draining lymph nodes. J Immunol. 2004;172:4091–4099. doi: 10.4049/jimmunol.172.7.4091. [DOI] [PubMed] [Google Scholar]
- Price AA, Cumberbatch M, Kimber I, Ager A. Alpha 6 integrins are required for Langerhans cell migration from the epidermis. J Exp Med. 1997;186:1725–1735. doi: 10.1084/jem.186.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staquet MJ. Adhesion and migration of epidermal dendritic cells. Pathol Biol (Paris) 1995;43:858–862. [PubMed] [Google Scholar]