Abstract
Retinal microvascular cell loss plays a critical role in the pathogenesis of diabetic retinopathy. To examine this further, type 1 streptozotocin-induced diabetic rats and type 2 Zucker diabetic fatty rats were treated by intravitreal injection of the tumor necrosis factor-specific inhibitor pegsunercept, and the impact was measured by analysis of retinal trypsin digests. For type 2 diabetic rats, the number of endothelial cells and pericytes positive for diabetes-enhanced activated caspase-3 decreased by 81% and 86%, respectively, when treated with pegsunercept (P < 0.05). Similarly, the number of diabetes-enhanced terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling-positive endothelial cells and pericytes decreased by 81% and 67% respectively when treated with pegsunercept (P < 0.05). Diabetes-increased activated caspase-3- and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling-positive microvascular cell numbers were both reduced by 81% and 80%, respectively, in pegsunercept-treated type 1 diabetic rats (P < 0.05). Inhibition of tumor necrosis factor reduced type 1 diabetes-enhanced pericyte ghost formation by 87% and the number of type 2 diabetes-enhanced pericyte ghosts by 62% (P < 0.05). Similarly, increased acellular capillary formation caused by type 1 and type 2 diabetes was reduced by 68% and 67%, respectively, when treated with pegsunercept (P < 0.05). These results demonstrate a previously unrecognized role of tumor necrosis factor-α in promoting the early pathogenesis of diabetic retinopathy leading to loss of retinal microvascular cells and demonstrate the potential therapeutic benefit of modulating its activity.
Diabetes mellitus is the most common metabolic disease worldwide. There are currently 21 million people with diabetes in the United States and ∼655,000 new cases diagnosed each year, with more than 90% having type 2 diabetes.1 Diabetes is the leading cause of blindness among adults in the 20 to 74 year age bracket,2 renal failure, and lower limb amputations, and is a major risk factor for cardiovascular disease, stroke, neuropathy, and periodontitis.1,3 Twenty years after the onset of diabetes, almost all patients with type 1 diabetes and over 60% of patients with type 2 diabetes will have some degree of retinopathy.2 Because of its prevalence, the impact of diabetes on medical costs associated with diagnosis and treatment of diabetes in general, and diabetic retinopathy in particular, is large.1
Early microvascular changes with diabetes can be seen in both experimental animal models and humans, including thickening of capillary basement membranes, apoptosis of microvascular cells, loss of pericytes, and acellular capillary formation.4,5 Several human and animal studies indicate that microvascular cell apoptosis plays a crucial role in the development of early lesions.6,7 Diabetic animals exhibit a 3- to 10-fold increase in terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)-positive microvascular cells and twofold increase in acellular capillary formation in microvascular cells examined in retinal trypsin digest, as compared with normoglycemic animals.7,8,9 Acellular capillary development is problematic because it promotes tissue nonperfusion, which stimulates the production of angiogenic factors and subsequent proliferation of vessels.10 The latter is a hallmark of proliferative diabetic retinopathy, the second major stage.
Tumor necrosis factor (TNF)-α is a pleiotropic cytokine implicated for early inflammatory changes seen in the diabetic retina. In the diabetic retina, astrocytes and Muller cells are potential source of TNF-α.11 In addition, TNF-α is found in the extracellular matrix, endothelium, and vessel walls of fibrovascular tissue and is elevated in the vitreous of eyes with diabetic retinopathy.12,13 In a study of STZ-induced diabetes in Brown Norway rats, diabetes of even 2 weeks duration increased TNF-α level by more than twofold.14 In humans, TNF-α immunoreactivity is seen in the majority of retinal specimens obtained from patients with diabetic retinopathy.12 Although the first generation TNF inhibitor etanercept has been shown to reduce intercellular adhesion molecule 1 levels, endothelial nitric oxide synthase gene expression and nuclear factor kappa B (NF-κB) activity in diabetic retina,11 there are no reports on the effect of TNF on the loss of microvascular cells and the formation of lesions associated with early diabetic retinopathy.
Despite progress in understanding the pathogenesis of diabetic retinopathy, the molecular mechanisms leading to enhanced loss of critical microvascular cells in the early stages of this complication are not well understood. This is particularly true for type 2 diabetes, which is the most prevalent form yet has been studied far less often than type 1 models of experimental diabetic retinopathy. To investigate the loss of microvascular cells in diabetic retinopathy, we examined the role of TNF, demonstrating for the first time that diabetes enhances TNF plays a prominent role in microvascular cell death in both type 1 and type 2 diabetic retinas. The data suggest a potential therapeutic benefit of inhibiting TNF-α activity in preventing the progression of early diabetic retinopathy, for which there is currently no effective preventive treatment.
Materials and Methods
Animal Studies
To study the role of TNF inhibition in type 1 diabetes, we used a well accepted streptozotocin model of experimental retinopathy, which exhibits early changes seen in human diabetic retinopathy.8 Intraperitoneal injection of streptozotocin (55 mg/kg) in 0.05 M citrate buffer was performed in ∼8-week-old Sprague-Dawley rats purchased from Charles River Laboratories (Wilmington, MA). Control animals were given intraperitoneal injection of vehicle alone. Animals were considered diabetic when the blood glucose levels were >250 mg/dl. One to five units of isophane insulin was subcutaneously injected as needed to maintain serum glucose levels ∼300 mg/dl, zero to three times per week. All animals had free access to food and water and were maintained under a 14-hour on/10-hour off light cycle. To study the effect of TNF-α in a type 2 model of diabetes, Zucker diabetic fatty rats (fa/fa) (ZDF) and genetically matched lean controls (fa/+) were purchased from Charles River Laboratories. For each group, n = 6 except for diabetic rats treated with vehicle alone (n = 12). The diabetic status was monitored by blood glucose levels, weight measurements and at the time of euthanasia, glycosylated hemoglobin levels were measured by affinity chromatography (Glyco-tek affinity column kit; Helena Laboratories, Beaumont, TX). Animal experiments were approved by the Institutional Animal Care and Use Committee at the Boston University Medical Center. Unless otherwise noted, rats were euthanized ∼6 months after the spontaneous onset of hyperglycemia, when their age was typically 33 to 34 weeks.
Application of Inhibitors
To study the impact of TNF inhibition on retinal microvascular cell loss in diabetic animals, pegsunercept was used. Pegsunercept is a second generation TNF inhibitor that consists of a recombinant soluble TNF-receptor-1 which has been modified by attachment of polyethylene glycol to extend its biological half-life. Pegsunercept was used since a related inhibitor, etanercept, had proven effective in blocking ocular inflammation11,15,16 and had been shown to have a favorable pharmokinetics profile on intravitreal injection.17 In type 1 diabetic animals, pegsunercept (peg-sTNFR1, 50 μg in 10 μl sterilized phosphate-buffered saline [PBS]), generously provided by Amgen (Thousand Oaks, CA) was applied by intravitreal injection 6, 12, and 18 weeks after the onset of hyperglycemia as described.18 Normoglycemic and diabetic control rats received vehicle injection alone. In type 2 diabetic animals, pegsunercept was applied by intravitreal injection at 12 weeks and again at 18 weeks after the onset of hyperglycemia. Normoglycemic littermates and diabetic control rats received vehicle injection alone (10 μl of sterilized PBS). The dose and time of TNF inhibitor was based on a pilot study.17 Intravitreal injection was used as the route of administration because the vitreous chamber is a closed compartment in which an inhibitor such as pegsunercept would have a prolonged half-life.18 Like etanercept, pegsunercept blocks TNF-α from binding to its receptor, thereby blocking TNF signaling and reducing TNF stimulated inflammation.19 Preliminary studies indicated that treatment with two injections of pegsunercept, 4 weeks apart, reduced TNF-α levels demonstrating efficacy. No ocular toxicity was observed with up to three injections of pegsunercept or vehicle alone, sterile PBS.
Molecular Analysis
In type 1 diabetic studies, eyes were enucleated from the animals that had been diabetic for 12 weeks, and retinas were isolated and snap-frozen in liquid nitrogen. Total mRNA was isolated and quantified as described previously.20 TNF-α was quantified by quantitative real time-PCR (qrt-PCR) as described previously using TaqMan primers and probes (Applied Biosystems, Foster City, CA).20 In type 2 diabetic studies, eyes were enucleated from the animals that had been hyperglycemic for 8 to 10 weeks or matched normoglycemic animals, and retinas were isolated and snap-frozen in liquid nitrogen. Protein was isolated using a protein isolation kit along with protease inhibitor and phosphatase inhibitor cocktails from Pierce Biotechnology (Rockford, IL) according to manufacturer’s instructions and quantified using a bicinchoninic acid kit (Pierce). TNF-α levels were measured from extracted proteins with a rat TNF-α enzyme-linked immunosorbent assay kit (Quantikine, R&D Systems, Minneapolis, MN) as per the manufacturer’s instructions.
Apoptosis, Acellular Capillaries, and Pericyte Ghosts Counts
Apoptosis was investigated in retinal microvascular cells in retinal trypsin digests (RTDs) by the TUNEL assay using the DeadEnd fluorometric kit (Promega, San Luis Obispo, CA) and by immunohistochemistry using an antibody specific for activated caspase-3 (Cell Signaling Technology, Danvers, MA). Before assay RTDs were rehydrated in PBS and permeabilized with 0.5% Triton X-100 in PBS for 1 hour at room temperature. In some cases, a negative control was performed in which the TUNEL assay was performed without the terminal transferase, and a positive control was performed consisting of RTDs exposed to DNase I (Promega; 500 U/ml in 40 mmol/L Tris-HCl, 6 mmol/L MgCl2 buffer, pH 7.5) for 10 minutes at room temperature.
For immunohistochemistry RTDs were incubated in blocking agent (Chemicon, Temecula, CA) followed by overnight incubation with primary or matched control antibody, which was localized by incubation with a biotinylated secondary antibody and fluorescein avidin (Vector Laboratories, Inc). Mounting media contained nuclear stain 4′, 6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA). The entire specimen was surveyed for TUNEL-positive or caspase-3-positive cells by fluorescence microscopy. Fluorescent, DAPI, and phase contrast images (original magnification ×400) of the same field were digitally captured. Data are presented as number of positive (TUNEL, caspase-3, or DAPI) cells per RTD.
For histomorphometric analysis such as acellular capillary counts and pericyte ghost counts, retinal trypsin digests were stained with periodic acid-Schiff (PAS)-hematoxylin, and examined by light microscopy as described previously.5 Captured PAS-stained images of 13 to 15 fields in the mid-retina were examined by a blinded observer to count the number of acellular capillaries as described previously and shown by boxes in Figure 1a.8 Data are presented as number of acellular capillaries per mm2 of the RTD. Pericyte ghosts were counted as intramural pockets lacking normal cell contents and marked by faint basement membrane out pouching in capillaries.21,22 Data are presented as number of pericyte ghosts/1000 microvascular cells. Counts were confirmed by a second blinded independent examiner.
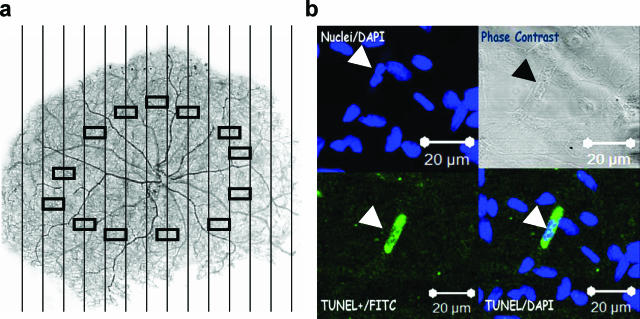
Figure 1.
Diabetes increases retinal microvascular cell apoptosis. a: A low power image of a typical retinal trypsin digest of the retinal microvasculature is shown. Vertical lines represent the boundaries of contiguous fields in which the entire retina was examined for the presence of TUNEL- or cleaved caspase-3-positive cells. Rectangles in the mid-retinal area represent the areas examined for acellular capillaries and pericyte ghosts. Original magnification ×20. b: Representative image of a TUNEL-positive retinal microvascular cell in a diabetic specimen. Upper left panel, DAPI staining; lower left panel, TUNEL staining; upper right panel, phase contrast image of the corresponding field; lower right panel, merged TUNEL/DAPI images. Arrow points to a TUNEL-positive microvascular cells. The bar represents 20 μm at original magnification ×400.
Statistical Analysis
Statistical significance was determined by one-way analysis of variance with Tukey’s posthoc test for comparisons between multiple groups at the P < 0.05 level.
Results
Type 1 diabetic rats weighed less and had significantly higher glycosylated hemoglobin levels compared to normoglycemic rats, typical of type 1 diabetes (Table 1). Type 2 diabetic rats weighed more and had significantly higher glycosylated hemoglobin levels as compared with normoglycemic littermates, typical of type 2 diabetes (Table 1). Treatment of the diabetic rats with TNF inhibitor pegsunercept had no effect on weight or glycosylated hemoglobin levels in both models (Table 1).
Table 1.
Body Weight and Glycosylated Hemoglobin Levels in the Type 1 and Type 2 Models of Diabetes
| Phenotype | Body weight (g) | Gly.HbA1c (% total) |
|---|---|---|
| Type 1 model | ||
| Control | 616 ± 11 | 4.3 ± 0.2 |
| Diabetic | 389 ± 13* | 10.3 ± 0.8* |
| Diabetic + pegsunercept | 377 ± 16* | 11.1 ± 0.7* |
| Type 2 model | ||
| Control | 407.0 ± 21.0 | 6.3 ± 0.7 |
| Diabetic | 590.0 ± 67.0* | 14.1 ± 1.4* |
| Diabetic + pegsunercept | 530.0 ± 43.0* | 13.0 ± 1.0* |
Data represent measurements taken at the time of euthanasia and are presented as mean ± SD.
Significance when compared to control rats.
To establish that TNF is elevated, TNF-α mRNA levels in retinas of the type 1 diabetic model and TNF-α levels in retinal protein extracts of type 2 diabetic model were determined. TNF-α mRNA levels determined by real-time PCR were significantly elevated 61-fold (P < 0.05) in type 1 diabetic rats with diabetes of 12 weeks duration when compared to age-matched normoglycemic animals (1 in normoglycemic rats to 61.2 + 9.1 in diabetic rats; n = 6; P < 0.05). In type 2 diabetic animals, TNF-α protein levels were increased by threefold when compared to normoglycemic littermates (21.3 ± 1.4 pg/mg in normoglycemic rats to 68.7 ± 6.5 pg/mg in diabetic rats; n = 6; P < 0.05).
TUNEL- and cleaved caspase-3-positive cells were measured in contiguous non-overlapping fields in the entire rat retina in RTDs following the vertical columns as shown in Figure 1a. A representative TUNEL-positive microvascular cell, DAPI-stained nuclei, merged image, and corresponding phase contrast image are shown in Figure 1b. As a control, immunohistochemistry experiments were performed with antibody to CD18 demonstrating that there were very few immune cells trapped within the microvasculature in the retinal trypsin digests, and none of these were apoptotic when immunohistochemistry with CD18 was combined with the TUNEL assay (data not shown).
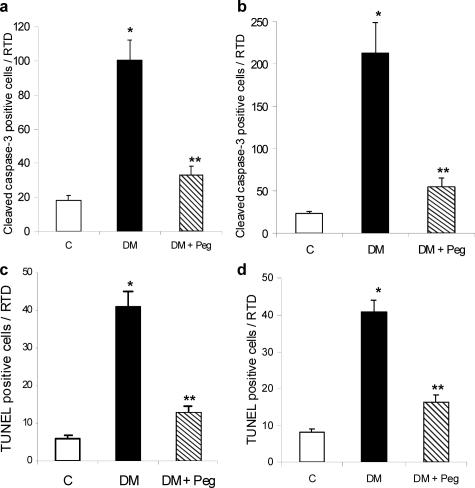
To investigate the impact of elevated TNF-α levels on the loss of microvascular cells, rats were treated by intravitreal injections of pegsunercept in both type 1 and type 2 diabetic models and compared to diabetic rats injected with vehicle alone. Treatment with pegsunercept for the last 8 weeks of the 12 weeks of type 1 diabetes reduced the increase caused by diabetes in retinal TNF-α mRNA by more than 80% (P < 0.05). Since caspase-3 cleavage represents an important pathway through which apoptosis takes place, we measured the number of cleaved caspase-3-positive microvascular cells in retinal trypsin digests. In the type 1 diabetic animals this number increased 5.5 fold (P < 0.05) in diabetic retinas compared to matched normoglycemic controls (Figure 2a). TNF inhibition reduced this increase by 82% (P < 0.05) (Figure 2a). Similar experiments were performed with ZDF and matched control rats. Type 2 diabetes increased the number of caspase-3-positive microvascular cells by 10-fold. Application of the TNF inhibitor reduced this increase by 83% (P < 0.05) (Figure 2b). Thus, pegsunercept reduced diabetes-enhanced caspase-3-positive microvascular cells by more than 80% in type 1 and type 2 diabetic retinas.
Figure 2.
TNF inhibition confers protection in both type 1 and type 2 diabetic animals against diabetes-induced microvascular cell apoptosis. a: The mean number of cleaved caspase-3-positive cells was determined in normoglycemic control rats (C), streptozotocin-induced diabetic rats (DM), and streptozotocin-induced diabetic rats treated with pegsunercept (DM + Peg). Data represent the mean ± SEM (n = 5). b: The mean number of cleaved caspase-3-positive cells was determined in normoglycemic control rats (C), ZDF diabetic rats (DM) and ZDF diabetic rats treated with pegsunercept (DM + Peg). Data represent the mean ± SEM (n = 5). c: The mean number of TUNEL-positive cells in type 1 diabetic model was determined in normoglycemic control rats (C), streptozotocin-induced diabetic rats (DM) and streptozotocin-induced diabetic rats treated with pegsunercept (DM + Peg). Data represent the mean ± SEM (n = 6). d: The mean number of TUNEL-positive microvascular cells was determined in normoglycemic control rats (C), ZDF diabetic rats (DM) and ZDF diabetic rats treated with pegsunercept (DM + Peg). Data represent the mean ± SEM (n = 6). *Statistically significant compared to control (P < 0.05). **Statistically significant as compared with diabetic (P < 0.05).
To assess apoptosis the TUNEL assay was performed in retinal trypsin digests. In the type 1 diabetic model, there was a 6.8-fold increase in TUNEL-positive microvascular cells as compared with matched normoglycemic controls. This increase was reduced by 80% when diabetic rats were treated with pegsunercept (P < 0.05) (Figure 2c). In the type 2 diabetic model a fivefold increase in TUNEL-positive microvascular cells was observed, which was reduced by 76% when TNF was inhibited with pegsunercept (P < 0.05) (Figure 2d). Thus, for both type 1 and type 2 diabetic models TNF inhibition reduced diabetes-enhanced apoptosis by 76 to 80%.
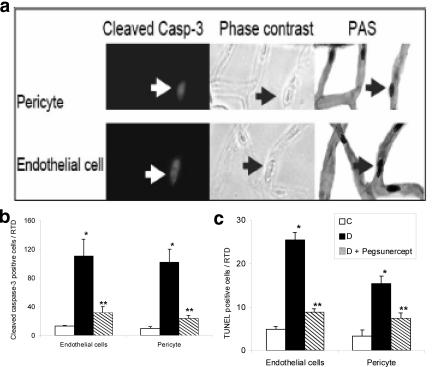
In the type 2 diabetic model, we further subdivided the apoptotic microvascular cells into endothelial cells and pericytes (Figure 3). Representative images of cleaved caspase-3-positive pericytes and endothelial cells and phase contrast and corresponding PAS-stained images are shown in Figure 3a. Cleaved caspase-3-positive endothelial cells increased 10-fold and pericytes eightfold compared to normoglycemic littermates (Figure 3b). The number of TUNEL-positive microvascular pericytes and endothelial cells both increased fivefold in the ZDF diabetic compared to normoglycemic rats (Figure 3c). Pegsunercept significantly reduced the increased numbers of caspase-3-positive endothelial cells and pericytes by 81% and 86% (P < 0.05), respectively (Figure 3b). Similarly TNF inhibition with pegsunercept significantly reduced diabetes-enhanced TUNEL-positive endothelial cells by 81% and pericytes by 67% (P < 0.05) (Figure 3c).
Figure 3.
TNF inhibition reduces pericyte and endothelial cell apoptosis in type 2 diabetic retina. a: Representative image of a cleaved caspase-3-positive pericyte (upper left panel) and endothelial cell (lower left panel), phase contrast (central panel), and PAS staining (right panel). Arrow points to a cleaved caspase-3-positive microvascular cells in a diabetic specimen. b: The mean number of cleaved caspase-3-positive endothelial cells and pericytes was determined in normoglycemic rats (C), Zucker diabetic fatty rats (DM) and Zucker diabetic fatty rats treated with pegsunercept (DM + Peg). Data represent the mean ± SEM (n = 5). c: The mean number of TUNEL-positive retinal endothelial cells and pericytes was determined in normoglycemic control rats (C), ZDF diabetic rats (DM), and ZDF diabetic rats treated with pegsunercept (DM + Peg). Data represent the mean ± SEM (n = 6). *Statistically significant compared to control (P < 0.05). **Statistically significant as compared with diabetic (P < 0.05).
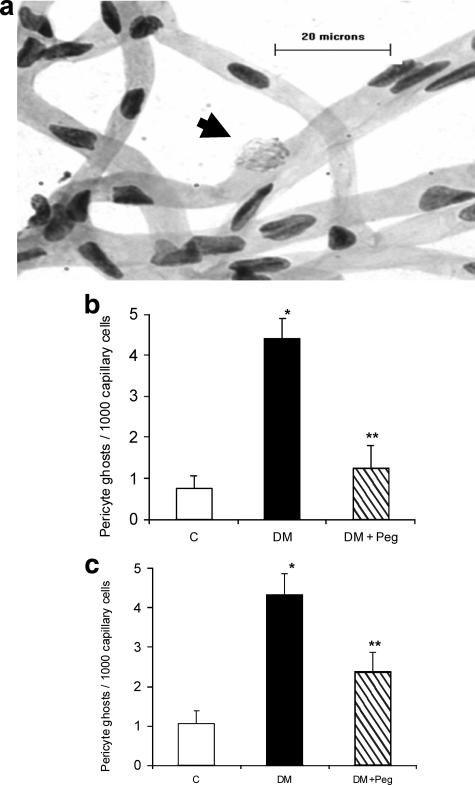
The ultimate effect of apoptosis is the formation of pericyte ghosts and acellular capillaries, which represent an important histological finding that is characteristic of the early lesions of diabetic retinopathy.7 A representative figure showing a pericyte ghost denoted by an out-pouching of the microvascular cell with pericyte remnants is shown in Figure 4a. Type 1 diabetes increased the numbers of pericyte ghosts 5.8-fold (Figure 4b). TNF inhibition reduced this increase by 87% (P < 0.05) (Figure 4b). When similar counts were performed in a type 2 diabetic model, diabetes increased the number of pericyte ghosts fourfold (Figure 4c). TNF inhibition reduced this increase by 62% (P < 0.05) (Figure 4c).
Figure 4.
TNF inhibition reduces pericyte ghost formation in both type 1 and type 2 diabetic animals. a: RTDs was prepared from a diabetic specimen and stained with PAS-hematoxylin. Arrow points to a pericyte ghost. The bar represents 20 μm at original magnification, ×400. b: The mean number of pericyte ghosts was determined in normoglycemic control rats (C), streptozotocin-induced diabetic rats (DM), and streptozotocin-induced diabetic rats treated with pegsunercept (DM + Peg). Data represent the mean ± SEM (n = 5). c: The mean number of pericyte ghosts was determined in normoglycemic control rats (C), ZDF diabetic rats (DM) and ZDF diabetic rats treated with pegsunercept (DM + Peg). Data represent the mean ± SEM (n = 5). *Statistically significant as compared with control (P < 0.05). **Statistically significant as compared with diabetic (P < 0.05).
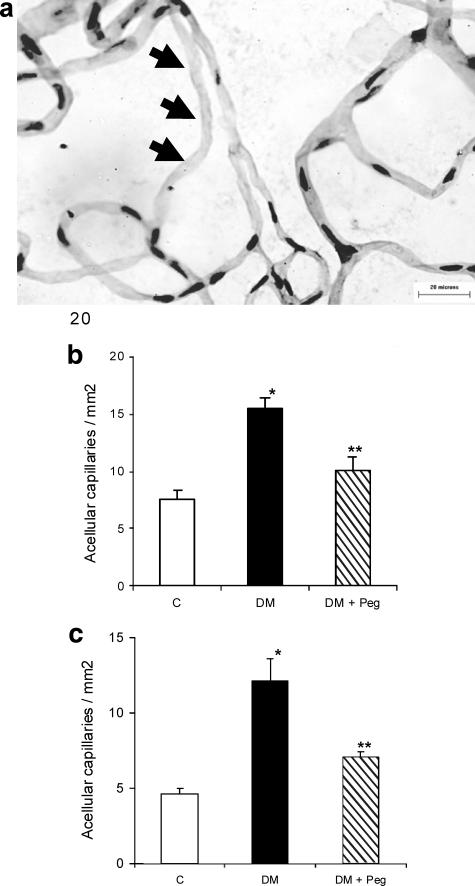
A representative figure showing an acellular capillary characterized by lack of both endothelial cells and pericytes is shown in Figure 5a. In the type 1 diabetic model, the formation of acellular capillaries was increased in diabetic rats by 2.0-fold compared to normoglycemic rats (P < 0.05) (Figure 5b). Treatment with pegsunercept reduced this increase by 68% (P < 0.05) (Figure 5b). When similar experiments were conducted in a type 2 diabetic model, diabetes increased acellular capillary formation by 2.6-fold, and TNF inhibition prevented diabetes-induced acellular capillary formation by 67% (P < 0.05) (Figure 5c).
Figure 5.
TNF inhibition reduces acellular capillary in both type 1 and type 2 diabetic animals. a: PAS-hematoxylin-stained RTDs from a diabetic retina. Arrows point to an acellular capillary. The bar represents 20 μm at original magnification, ×400. b: The mean number of acellular capillaries was determined in normoglycemic control rats (C), streptozotocin-induced diabetic rats (DM) and streptozotocin-induced diabetic rats treated with pegsunercept (DM + Peg). Data represent the mean ± SEM (n = 6). c: The mean number of acellular capillaries was determined in normoglycemic control rats (C), ZDF diabetic rats (DM), and ZDF diabetic rats treated with pegsunercept (DM + Peg). Data represent the mean ± SEM (n = 6). *Statistically significant compared to control (P < 0.05). **Statistically significant compared to diabetic (P < 0.05).
Discussion
Elevated levels of TNF-α have been associated with the early events of diabetic retinopathy including the expression of adhesion molecules,12 and TNF inhibition has been shown to inhibit leukostasis.16 However, there have been no previous reports that have linked the elevated expression of TNF in diabetic retinas to pathological changes. To investigate the role of diabetes-enhanced TNF-α expression in microvascular apoptosis, both type 1 and type 2 diabetic rats were treated with the pegsunercept. Inhibition of TNF-α caused approximately 76 to 80% reduction in the number of microvascular cells that expressed activated caspase-3 and were TUNEL-positive. Thus, TNF plays a significant role in diabetes-enhanced microvascular cell apoptosis. Longer term studies were also performed to investigate the consequence of TNF-stimulated apoptosis. Pegsunercept significantly reduced the increase in pericyte ghost formation and acellular capillary formation caused by diabetes. These results demonstrate for the first time that TNF-α plays a major role in the loss of microvascular cells. The higher rates of endothelial cell apoptosis observed do not necessarily lead to faster loss of these cells compared to pericytes cells since circulating endothelial progenitor cells may replace some of the apoptotic endothelial cells, though this does not occur for pericytes.23
There have been relatively few studies comparing the development of retinopathy in type 1 and type 2 diabetic patients. However, it is possible that there are subtle differences between them.24 We observed that both type 1 and type 2 diabetic models exhibited relatively similar increases in the number of microvascular cells that were apoptotic as assessed by the TUNEL assay. Both type 1 and type 2 diabetic models exhibited increases in pericyte ghost formation and acellular capillary development that were relatively consistent. The results with the TUNEL assay and acellular capillary formation are similar with another type 2 diabetic rat, the Goto-Kakizaki rat.6 When TNF was inhibited by treatment with pegsunercept, it was striking that the reduction in apoptosis and loss of microvascular cells was similar in both type 1 and type 2 diabetic models.
The protective effect of TNF inhibition may be explained by direct effect of TNF-α on microvascular cells as it is a pro-apoptotic cytokine.25 Since TNF-α inhibition has been shown to inhibit intercellular adhesion molecule 1 levels and NF-κB activity in diabetic retina, an alternate approach of identification in which direct stimulation of rat microvascular endothelial cells with TNF-α could induce several genes responsible for endothelial cell activation and apoptosis has also been used (Supplemental Table 1,26,27,28 available at http://ajp.amjpathol.org). Alternatively, TNF could enhance apoptosis through indirect mechanisms. For example, TNF-α inhibition could reduce the expression of other pro-apoptotic factors29 or reduce leukostasis that can promote endothelial injury through various pathways.16 However, these mechanisms are mutually compatible and may occur simultaneously. Overall, the effect of TNF inhibition on reducing endothelial and pericyte apoptosis is consistent with findings that TNF-α directly stimulates microvascular endothelial cell30 and pericyte apoptosis (unpublished data).
Hyperglycemia leads to retinal biochemical alteration in a manner dependent on genetics and/or environmental factors. Retinal biochemical alterations include activation of polyol pathway, advanced glycation endproducts accumulation and diacylglycerol/protein kinase C activation, leading to reactive oxygen species formation.31,32 This biochemical alteration may lead to inadvertently increased expression of various growth factor and cytokines (TNF-α). Hyperglycemia per se or through TNF up-regulation activates target transcription factors such as NF-κB.33 Increased activation of transcription factor NF-κB can lead to increased expression of pro-inflammatory mediators such as TNF-α, interleukins 1β and 6, which has been also implicated in the pathogenesis of diabetic retinopathy. Increased TNF-α expression may further drive NF-κB-dependent gene transcription.34 Thus inhibition of TNF-α by pegsunercept disrupts this feedback loop leading to inhibition of several inflammatory mediators.
Increased apoptosis is implicated in several other diabetic complications such as neuronal apoptosis in neuropathy, cardiomyocyte apoptosis in cardiomyopathy, and mesangial cell apoptosis in nephropathy. Thus, it is possible that TNF-α up-regulation may contribute to-enhanced apoptosis observed in other diabetes-associated complications, and TNF-α inhibition may prove beneficial. Overall the data presented in this paper suggest that diabetic retinopathy, a chronic inflammatory condition, is characterized by early TNF-α up-regulation in both type 1 and type 2 diabetes. This up-regulation leads to the loss of microvascular cells a critical early event in the etiology of diabetic retinopathy. TNF inhibition by soluble TNF-α receptor molecules results in protection of retinal microvasculature.
Footnotes
Address reprint requests to Dana T. Graves, Boston University School of Dental Medicine, 700 Albany St. W-202 D, Boston, MA 02118. E-mail: dgraves@bu.edu.
Supported by NIH grants RO1DE07559 and R01EY014702, and, in part, by a departmental grant from the Massachusetts Lions Eye Research Organization.
References
- Engelgau M, Giess L, Saaddine J, Boyle J, Benjamin S, Gregg E, Tierney E, Rios-Burrows N, Mokdad A, Ford E, Imperatore G, Narayan K. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 2003;26:226–229. doi: 10.2337/diacare.26.1.226. [DOI] [PubMed] [Google Scholar]
- Graves DT, Liu R, Alikhani M, Al-Mashat H, Trackman PC. Diabetes-enhanced inflammation and apoptosis–impact on periodontal pathology. J Dent Res. 2006;85:15–21. doi: 10.1177/154405910608500103. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J, Bernardes R. Nonproliferative retinopathy in diabetes type 2. Initial stages and characterization of phenotypes, Prog Retin Eye Res. 2005;24:355–377. doi: 10.1016/j.preteyeres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Roy S, Sato T, Paryani G, Kao R. Downregulation of fibronectin overexpression reduces basement membrane thickening and vascular lesions in retinas of galactose-fed rats. Diabetes. 2003;52:1229–1234. doi: 10.2337/diabetes.52.5.1229. [DOI] [PubMed] [Google Scholar]
- Yatoh S, Mizutani M, Yokoo T, Kozawa T, Sone H, Toyoshima H, Suzuki S, Shimano H, Kawakami Y, Okuda Y, Yamada N. Antioxidants and an inhibitor of advanced glycation ameliorate death of retinal microvascular cells in diabetic retinopathy. Diabetes Metab Res Rev. 2006;22:38–45. doi: 10.1002/dmrr.562. [DOI] [PubMed] [Google Scholar]
- Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy, Horm Metab Res. 2005;37 Suppl 1:39–43. doi: 10.1055/s-2005-861361. [DOI] [PubMed] [Google Scholar]
- Sun W, Gerhardinger C, Dagher Z, Hoehn T, Lorenzi M. Aspirin at low-intermediate concentrations protects retinal vessels in experimental diabetic retinopathy through non-platelet-mediated effects. Diabetes. 2005;54:3418–3426. doi: 10.2337/diabetes.54.12.3418. [DOI] [PubMed] [Google Scholar]
- Kern TS, Tang J, Mizutani M, Kowluru RA, Nagaraj RH, Romeo G, Podesta F, Lorenzi M. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000;41:3972–3978. [PubMed] [Google Scholar]
- Ljubimov AV, Caballero S, Aoki AM, Pinna LA, Grant MB, Castellon R. Involvement of protein kinase CK2 in angiogenesis and retinal neovascularization. Invest Ophthalmol Vis Sci. 2004;45:4583–4591. doi: 10.1167/iovs.04-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16:438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- Limb GA, Chignell AH, Green W, LeRoy F, Dumonde DC. Distribution of TNF alpha and its reactive vascular adhesion molecules in fibrovascular membranes of proliferative diabetic retinopathy. Br J Ophthalmol. 1996;80:168–173. doi: 10.1136/bjo.80.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy, Eye. 2006;20:1366–1369. doi: 10.1038/sj.eye.6702138. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006;20:323–325. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- Falappone PC, Iannone F, Scioscia C, Grattagliano V, Covelli M, Lapadula G. [The treatment of recurrent uveitis with TNF-alpha inhibitors]. Reumatismo. 2004;56:185–189. [PubMed] [Google Scholar]
- Koizumi K, Poulaki V, Doehmen S, Welsandt G, Radetzky S, Lappas A, Kociok N, Kirchhof B, Joussen AM. Contribution of TNF-alpha to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Invest Ophthalmol Vis Sci. 2003;44:2184–2191. doi: 10.1167/iovs.02-0589. [DOI] [PubMed] [Google Scholar]
- Fauser S, Kalbacher H, Alteheld N, Koizumi K, Krohne TU, Joussen AM. Pharmacokinetics and safety of intravitreally delivered etanercept. Graefes Arch Clin Exp Ophthalmol. 2004;242:582–586. doi: 10.1007/s00417-004-0895-x. [DOI] [PubMed] [Google Scholar]
- Oshitari T, Polewski P, Chadda M, Li A-F, Sato T, Roy S. Effect of combined antisense oligonucleotides against high-glucose-and diabetes-induced overexpression of etracellular mtrix cmponents and icreased vscular prmeability. Diabetes. 2006;55:86–92. [PubMed] [Google Scholar]
- Furst DE, Fleischmann R, Kopp E, Schiff M, Edwards C, 3rd, Solinger A, Macri M. A phase 2 dose-finding study of PEGylated recombinant methionyl human soluble tumor necrosis factor type I in patients with rheumatoid arthritis. J Rheumatol. 2005;32:2303–2310. [PubMed] [Google Scholar]
- Al-Mashat HA, Kandru S, Liu R, Behl Y, Desta T, Graves DT. Diabetes enhances mRNA levels of proapoptotic genes and caspase activity which contribute to impaired healing, Diabetes. 2006;55:487–495. doi: 10.2337/diabetes.55.02.06.db05-1201. [DOI] [PubMed] [Google Scholar]
- Kern TS, Engerman RL. Comparison of retinal lesions in alloxan-diabetic rats and galactose-fed rats. Curr Eye Res. 1994;13:863–867. doi: 10.3109/02713689409015087. [DOI] [PubMed] [Google Scholar]
- Kowluru RA. Retinal metabolic abnormalities in diabetic mouse: comparison with diabetic rat. Curr Eye Res. 2002;24:123–128. doi: 10.1076/ceyr.24.2.123.8158. [DOI] [PubMed] [Google Scholar]
- Otani A, Dorrell MI, Kinder K, Moreno SK, Nusinowitz S, Banin E, Heckenlively J, Friedlander M. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest. 2004;114:765–774. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE. Visual impairment in diabetes. Ophthalmology. 1984;91:1–9. [PubMed] [Google Scholar]
- Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1168–L1178. doi: 10.1152/ajplung.2001.280.6.L1168. [DOI] [PubMed] [Google Scholar]
- Alikhani M, Alikhani Z, He H, Liu R, Popek BI, Graves DT. Lipopolysaccharides indirectly stimulate apoptosis and global induction of apoptotic genes in fibroblasts. J Biol Chem. 2003;278:52901–52908. doi: 10.1074/jbc.M307638200. [DOI] [PubMed] [Google Scholar]
- Miyahara T, Kikuchi T, Akimoto M, Kurokawa T, Shibuki H, Yoshimura N. Gene microarray analysis of experimental glaucomatous retina from cynomologous monkey. Invest Ophthalmol Vis Sci. 2003;44:4347–4356. doi: 10.1167/iovs.02-1032. [DOI] [PubMed] [Google Scholar]
- Olivero OA, Tejera AM, Fernandez JJ, Taylor BJ, Das S, Divi RL, Poirier MC. Zidovudine induces S-phase arrest and cell cycle gene expression changes in human cells. Mutagenesis. 2005;20:139–146. doi: 10.1093/mutage/gei019. [DOI] [PubMed] [Google Scholar]
- Alikhani M, Alikhani Z, Graves DT. FOXO1 functions as a master switch that regulates gene expression necessary for tumor necrosis factor-induced fibroblast apoptosis. J Biol Chem. 2005;280:12096–12102. doi: 10.1074/jbc.M412171200. [DOI] [PubMed] [Google Scholar]
- Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H. HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol. 2006;291:C897–C908. doi: 10.1152/ajpcell.00032.2006. [DOI] [PubMed] [Google Scholar]
- Kaji Y, Usui T, Ishida S, Yamashiro K, Moore TCB, Moore J, Yamamoto Y, Yamamoto H, Adamis AP. Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Invest Ophthalmol Vis Sci. 2007;48:858–865. doi: 10.1167/iovs.06-0495. [DOI] [PubMed] [Google Scholar]
- Miranda M, Muriach M, Roma J, Bosch-Morell F, Genovés JM, Barcia J, Araiz J, Díaz-Llospis M, Romero FJ. [Oxidative stress in a model of experimental diabetic retinopathy: the utility of peroxinytrite scavengers.]. Arch Soc Esp Oftalmol. 2006;81:27–32. doi: 10.4321/s0365-66912006000100007. [DOI] [PubMed] [Google Scholar]
- Kowluru RA. Effect of advanced glycation end products on accelerated apoptosis of retinal capillary cells under in vitro conditions. Life Sci. 2005;76:1051–1060. doi: 10.1016/j.lfs.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Song HY, Regnier CH, Kirschning CJ, Goeddel DV, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2, Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]