Abstract
Bile acids (BAs) facilitate emulsification, absorption and transport of fats and sterols in the intestine and liver and are essential for normal digestion. However, accumulation of BAs in the intestine can result in damage to the intestinal epithelium. Using the neonatal rat model of NEC, we have recently shown that BAs accumulate in both the ileal lumen and enterocytes of neonatal rats with NEC and the increased BA levels are positively correlated with disease severity. Importantly, when BAs are not allowed to accumulate, neonatal rat pups develop significantly less disease. In addition, BA transporters are altered during disease development. These data indicate that BAs play an important role in the development of experimental NEC, and suggest that the inability of neonatal rats to adequately regulate BA transporters may be a mechanism by which ileal damage occurs.
Index Words: necrotizing enterocolitis, bile acids, enterohepatic circulation
Introduction
Necrotizing enterocolitis (NEC) affects thousands of newborns each year in the United States alone, and with a mortality rate ranging from 10–50%, this disease remains a major cause of morbidity and mortality in prematurely born infants 1–3. Severe NEC is characterized by an extensive hemorrhagic inflammatory necrosis of the distal ileum and proximal colon 4; the disease can be mild to severe with clinical presentation ranging from abdominal distension, pneumatosis intestinalis, occult or frank blood in stools, intestinal gangrene, bowel perforation, sepsis and shock 5–7. Survivors of a severe episode of NEC frequently suffer the effects of short bowel syndrome 3, 8, 9, resulting in prolonged medical expenses and chronic gastrointestinal difficulties 10. While prematurity, enteral feeding, intestinal hypoxia-ischemia, and bacterial colonization are considered major risk factors for development of NEC, the pathophysiology of this disease remains poorly understood and no predictive tests are currently available.
Bile acids (BAs) are physiologic compounds that facilitate emulsification, absorption and transport of fats and sterols in the intestine and liver. Enterohepatic circulation of BAs is an essential process involving coordinated regulation of BA synthesis in the liver, transport of BAs from the liver to the intestine, and transport of BAs back to the liver. When enterohepatic circulation is altered, accumulation of hydrophobic BAs in the intestine can result in damage to the intestinal epithelium 11–13. Importantly, when exogenous BAs are introduced into the GI tract of neonatal rats via intragastric gavage, histologic damage to ileal architecture is similar to what is observed in animals with experimental NEC (Figure 1) 14.
Figure 1. Ileal damage in BA gavaged rats resembles damage in experimental NEC.
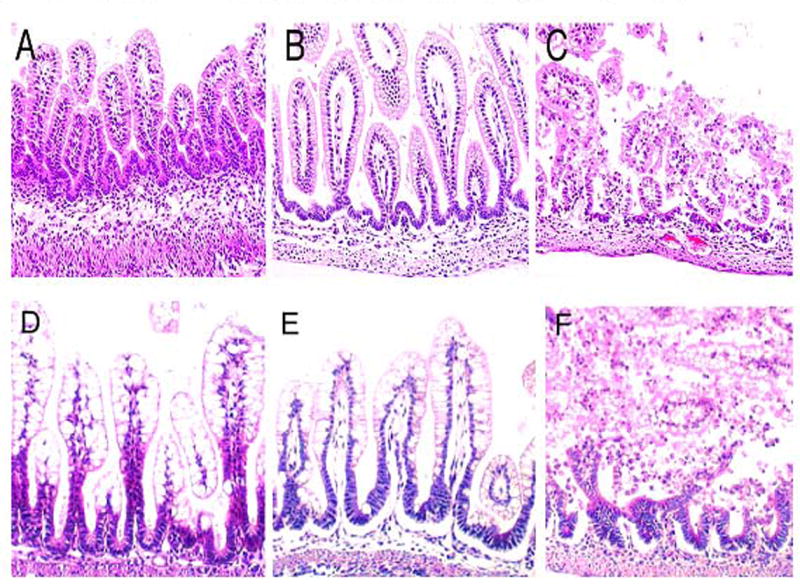
In the upper panels, representative slides from neonatal rats gavaged with (A) PBS, (B) one dose of 50 mM sodium deoxycholic acid, and (C) two doses of 50 mM sodium deoxycholic acid. In the lower panels, representative slides from (D) DF, histologic score = 0; (E) NEC, histologic score +2; (F) NEC animals with full necrosis, histologic score +4. In contrast to the ileal tissue shown in (A), the large enterocytes visible in the DF ileum (D) are seen because these animals were not fasted prior to removal of ileal tissue. Magnification 100X. Taken from 14.
During gestation, biliary BA levels are markedly increased 15, and meconium contains significant levels of BAs. Total BA levels in feces decreases between birth and day 7, then begins to increase 16. In addition, the composition of BAs changes after birth and in the months to follow 17–20. The bile salt pool size in premature infants is significantly reduced compared to term infants, but bile salt synthesis is high 19. Studies in animals have shown that many of the key processes of BA homeostasis are immature in newborns 21–27 and reach maturity by the time of weaning. It has been proposed that breast-fed newborns may not have need for high levels of BAs, as human milk fat is digested differently than solid foods or cow’s milk-based formula 22, 28–32. Diet can also affect levels and composition of BAs. Fecal deoxycholic acid (DCA) and lithocholic acid (LCA) were significantly lower in breast-fed infants than in formula-fed infants and the percent of secondary BAs excreted in feces was significantly higher in the formula-fed group by 11 months of age 33. Significantly, the incidence of NEC is 6–10 times higher in formula-fed infants compared to those that are breast-fed 34.
Ileal BAs in Experimental NEC
To determine if BA play a role in NEC pathogenesis, total BA levels were evaluated in ileal luminal flushes in neonatal rats using the neonatal rat model of NEC. This model, developed by Barlow 35, 36 and modified by Caplan 37, reproduces the major risk factors for NEC – prematurity of the gastrointestinal tract, enteral formula feeding, hypoxia/ischemia and bacterial colonization. NEC is developed by feeding prematurely born, never suckled rats with cow’s milk-based formula coupled with exposure to asphyxia and cold stress. Dam-fed (DF) pups undergoing the same schedule of asphyxia and cold stress do not develop NEC and are utilized as controls. In the neonatal rat model, many clinical and pathological changes are similar to those found in humans: the abdomen is distended, blood is detected in stool, and the ileum and proximal colon are the most affected parts of the intestine. In addition, the key risk factors for human NEC (intestinal immaturity, enteral formula feeding, bacterial colonization and hypoxia/ischemia) are essential factors to develop disease 37–39. When neonatal rats were subjected to the NEC protocol, total BAs were significantly increased in the ileal luminal contents compared to DF controls (Table 1) and these elevated values were positively correlated to disease severity 14. If intestinal BAs are required for development of NEC injury, then reduction of BAs should result in decreased incidence and severity of NEC. Cholestyramine (Chol) binds BA in the intestine and the resulting Chol/BA complex passes out of the body without being absorbed 40–42. When neonatal rats subjected to the NEC protocol were given Chol, both the incidence and severity of ileal damage was significantly decreased. In addition, pharmacologic sequestration of BAs with Chol resulted in increased survival during NEC development 14.
Table 1.
Ileal Luminal BAs in Experimental NEC.
| Group | Total BA (uM/L) | CA (ug/ml) | DCA (ug/ml) |
|---|---|---|---|
| DF | 148 ± 54 | 0.14 ± 0.02 | Not Detected |
| NEC* | 722 ± 143# | 1.07 ± 0.41* | 0.37 ± 0.002* |
Total BA levels were determined from ileal luminal flushes using the Diazyme Total BA kit; BA composition of luminal flushes was determined using LC/MS/MS.
p ≤ 0.01;
p ≤ 0.05.
Data taken from 14.
In the classic BA synthetic pathway, the primary BAs cholic acid (CA) and chenodeoxycholic acid (CDCA) are synthesized from cholesterol in the liver by the enzyme CYP7A1 43. After synthesis, CA and CDCA are secreted into bile via the bile salt excretory pump (BSEP). BAs can be deconjugated by bacteria in the small intestine to form secondary BAs, principally DCA and LCA. Bile acids can be further characterized as either hydrophilic or hydrophobic. The hydrophobic BAs (e.g. CA and DCA acid) are considered toxic when they accumulate in the intestine 11–13. During experimental NEC, CA and DCA acid levels are significantly increased in the luminal contents of neonatal rats with NEC (Table 1), indicating ileal luminal BAs consist mainly of the more toxic, hydrophobic BAs 14. These data are also consistent with our understanding that intestinal microflora are essential for the development of NEC as the formation of DCA requires the presence of intestinal bacteria.
These data strongly suggest that BAs play a critical role in the development of experimental NEC. However, a basic question remained - does BA accumulation in the ileum cause ileal injury or is it a consequence of damage to ileal tissue from other mediators? Neonatal rats subjected to the NEC protocol were sacrificed post-birth at 24, 48, 72 or 96 hours. A section of distal ileum from each animal was flushed to evaluate total luminal BA levels and an adjacent section of distal ileum was evaluated for histological damage by a blinded observer. 38, 39, 44–46. By 48 hours, ileal luminal BA levels were elevated in animals with NEC, but histological damage did not significantly increase until the 72 hour observation point (Figure 2A). Similar results were also obtained when intra-enterocyte BA levels were compared to histologic damage over the course of disease development (Figure 2B) 47. Thus, it appears that BA levels increase prior to ileal damage further supporting the concept that BAs play a crucial role in disease development.
Figure 2. Temporal relationship between BAs and ileal injury.
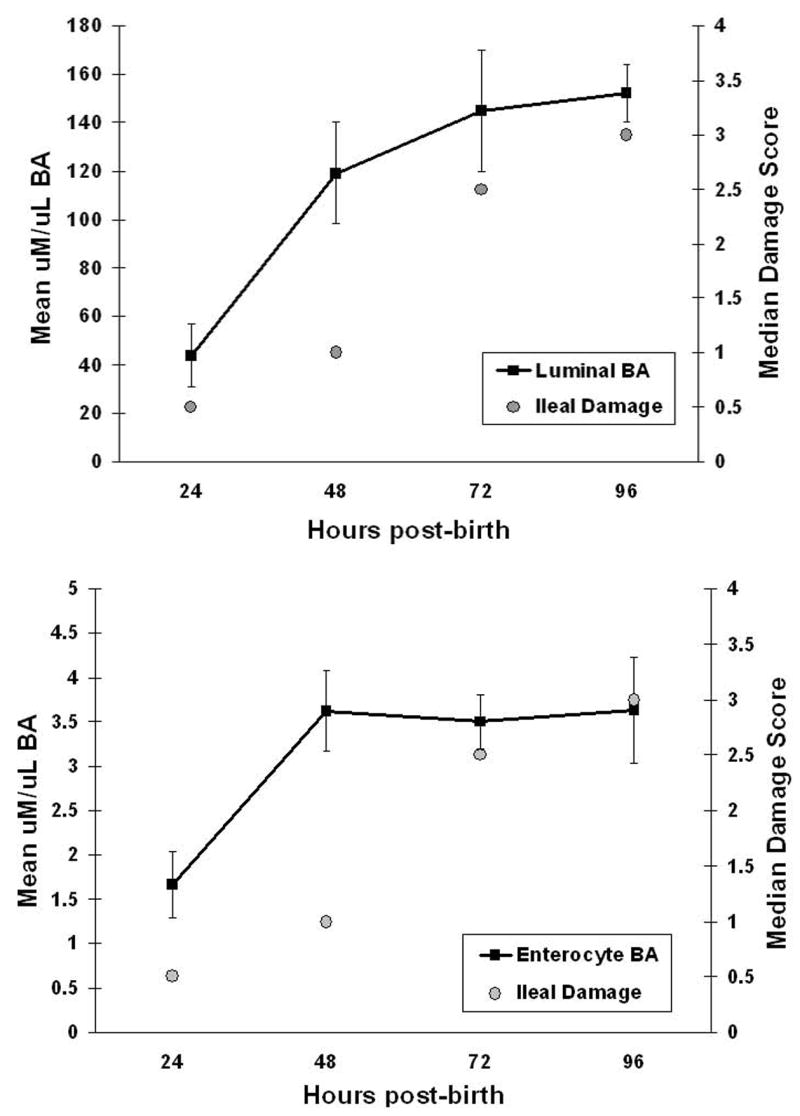
To determine if BA dysregulation causes or is a consequence of ileal injury, never-suckled, prematurely born rats were hand-fed with cow’s milk-based formula and exposed to asphyxia and cold stress twice a day to develop NEC. Animals were sacrificed post-birth at 24 (n=6), 48 (n=6), 72 (n=6) or 96 hours (n=5). A 3 cm section of distal ileum from each animal was flushed with 400ul PBS to evaluate total luminal BA levels (A). After flushing with additional PBS, this section of ileum was homogenized in 400ul PBS to determine intra-enterocyte BA levels (B). Total BA levels were determined using the Diazyme Total BA Kit. An adjacent section of distal ileum was evaluated for histological damage by a blinded observer using our previously published NEC scoring system where 0 is normal and 4 indicates full necrosis.
Ileal BA transporters in NEC
Both primary and secondary BAs are reclaimed in the distal ileum - the site of injury during NEC - via the apical sodium-dependent bile acid transporter (ASBT) 48, 49. BAs are transported into the enterocyte via ASBT and are thought to be bound to the ileal bile acid binding protein (IBABP) 50. At the basolateral surface of enterocytes, the heteromeric organic solute transporter (OSTα-β) removes BAs from the cell and transports them into portal circulation 51. The transport of BA from the ileal lumen to portal circulation is crucial for BA homeostasis and to insure that BAs do not accumulate in the enterocytes. In the intestine, ASBT, IBABP and OSTα-β are expressed predominantly in the terminal ileum 51–54. In rat ileum, Asbt, Ibabp and OSTα-β undergo biphasic regulation; they are expressed in the fetus, down-regulated in the neonate, then up-regulated at weaning 21, 48, 50, 55–58. These variations likely reflect the changing BA levels described during fetal development, the neonatal period and weaning.
Asbt, Ibabp, Ostα-β protein levels from distal ileum were evaluated during the development of experimental NEC. As expected, Asbt was not present in most 4 day-old, DF neonates. However, Asbt levels in animals with NEC were significantly increased (Figure 3A) and decreased when luminal BA levels were reduced with Chol 14. While Asbt protein levels in neonatal rats with NEC were only approximately 10% of that seen in weanling rats 14, the Asbt appears to be functional, as intra-enterocyte levels of BAs are also significantly elevated compared to control pups 47. These data also suggest that intra-enterocyte rather than luminal BA accumulation contributes to ileal injury during NEC pathogenesis.
Figure 3. Ileal Asbt, Ibabp and Ost-β.
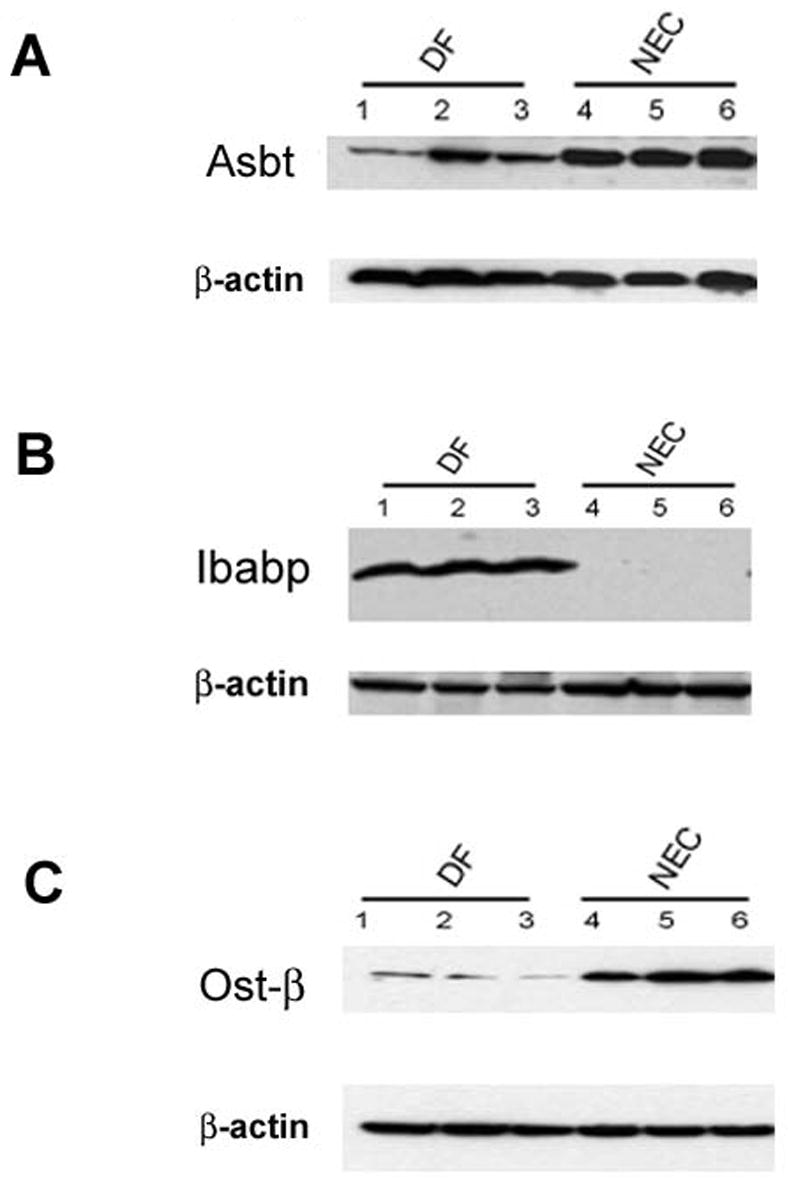
A: Representative ileal protein samples from DF and NEC groups subjected to Western blotting to detect Asbt. B: Representative ileal protein samples from DF and NEC groups subjected to Western blotting to detect Ibabp. C: Representative ileal protein samples from DF and NEC groups were subjected to Western blotting to detect Ost-β. Data taken from 14.
In contrast to Asbt, Ibabp was consistently detected in the DF group only and was produced only minimally in animals with NEC (Figure 3B) 14. For Ostα-β, similar protein levels were observed in both DF and NEC groups (Figure 3C) but its was found primarily in the crypts and not in the upper villi as seen in weanling animals 14. This suggests a possible mechanism by which BAs accumulate in enterocytes during experimental NEC; elevated luminal BAs are transported into the enterocytes by Asbt, but cannot be moved through the cell to be transported into portal circulation. The observation that portal BA levels are decreased in neonatal rats with NEC supports this hypothesis 14.
Inflammatory Mediators and Hepatic BA Transporters
There are mechanisms by which BAs affect and are affected during the development of intestinal inflammation that are not directly related to alterations in enterohepatic circulation of BAs. TNF-α, a pro-inflammatory cytokine which is known to affect hepatic BA transporters 59–61, can alter BA uptake in hepatoma cell lines 62, and is a crucial component of NEC pathogenesis 46, 63, 64. Further, TNF-α is known to contribute to compromised epithelial barrier functions and affect intestinal permeability 65–67. Thus, inflammatory mediators could affect BA levels during NEC by influencing BA transport or altering intestinal permeability. If inflammation causes elevation of BAs, then infiltration of macrophages - a hallmark of inflammation in experimental NEC - should be apparent prior to elevation of BAs. Intra-enterocyte BA levels increase prior to ileal macrophage infiltration during the development of experimental NEC 47; data that strongly suggest that inflammation does not cause elevation in BA levels.
Completion of enterohepatic circulation of BAs is mediated primarily by the sodium-dependent taurocholate co-transporting polypeptide (NTCP)68 which is located on the basolateral membrane of hepatocytes. Like the ileal BA transporters, NTCP displays ontogenic regulation ; in rats, Ntcp mRNA is expressed at adult levels by day 7 post birth 69, 70, but it functions at only 75% of the adult rate until approximately 4 weeks of age. Ntcp is significantly decreased in rats with NEC compared to DF controls 71. It has not been established if the decrease in Ntcp is specific for this disease process, or if it is simply in response to lower levels of BAs re-circulating through portal circulation. However, TNF-α has been shown to down regulate Ntcp and hepatic TNF-α is elevated during experimental NEC 46, 63. Neonatal rats subjected to the NEC protocol were either injected with anti-TNF-α or vehicle. Ntcp mRNA was significantly increased in pups with NEC given anti-TNF-α (Figure 4). Thus, elevated hepatic TNF-α in NEC may contribute to the down-regulation of Ntcp 71. In the rat, sodium-independent uptake of BAs is mediated by members of the organic anion-transporting polypeptide (Oatp) family, Oatp1, Oatp2 and Oatp4 and dianionic conjugated BAs are secreted into bile via the multi-drug-resistance-associated protein 2 (Mrp2). It has been shown that TNF-α can down-regulate both Oatp4 and Mrp2 mRNA 72–74. To determine if Oatp and/or Mrp2 expression is altered during NEC pathogenesis, we examined mRNA in 4 day-old DF, NEC and NEC + anti-TNF-α groups. Oatp4 and Mrp2 mRNA were significantly decreased in NEC and NEC + anti-TNF-α groups compared to DFs. There was no difference in Oatp2 expression between groups (Figure 4). Thus, down-regulation of Oatp4 and Mrp2 in NEC does not appear to be a consequence of elevated TNF-α and suggests that another factor may down-regulate these transporters in NEC 71.
Figure 4. Hepatic BA transporter regulation in NEC.
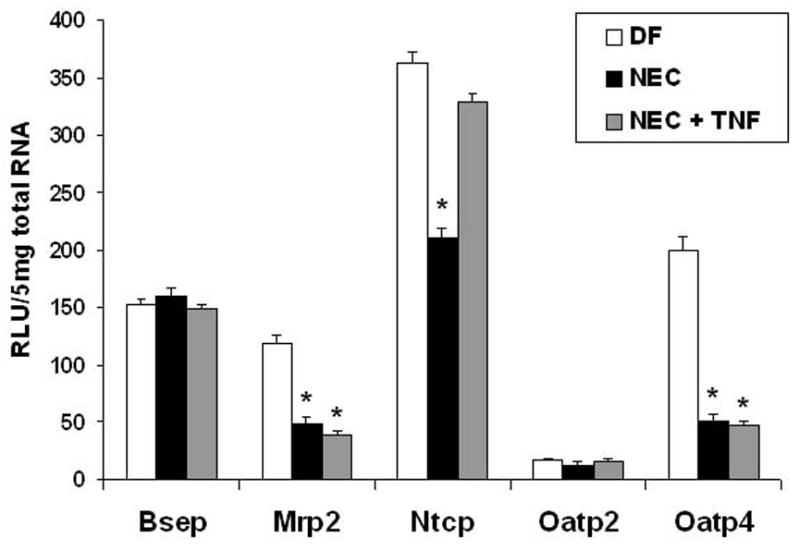
Hepatic mRNA expression of Bsep, Ntcp, Mrp2, Oatp2 and Oatp4 were assessed from DF, NEC and NEC + anti-TNF groups using the Quantigenetm signal amplification assay (because Oatp1 is not expressed until postnatal day 15, Oatp1 expression was not evaluated). * p ≤ 0.01 versus DF.
The decreased levels of BAs returned to the liver via portal circulation and/or because of decreased Ntcp should result in continued production of BAs. Because direct cannulation of the bile duct is not possible in 4 day-old rats, total BA levels were evaluated in proximal jejunal contents to indirectly determine BA production from the liver. Not unexpectedly, the overall levels of jejunal BA were similar between DF control animals and those with NEC 14. In addition, levels of Bsep, the transporter responsible for exporting BAs from the liver, were unchanged (Figure 4).
Nuclear Receptors and BA Accumulation
Regulation of BA metabolism involves multiple steps and the nuclear receptors farnesoid X receptor (FXR) 75 and liver X receptor (LXR) 76 are essential components that regulate BA and cholesterol. Oxidized metabolites of cholesterol are involved in positive feed-forward pathways involved in cholesterol homeostasis via LXR along with and the retinoid X receptor (RXR)76. FXR, for which BAs are natural ligands, operates in an opposing fashion to LXR to repress BA synthesis 75. BA-activated FXR inhibits CYP7A1 transcription in the liver (via the small heterodimer partner, SHP77) and activated FXR can also repress NTCP (via SHP)77, but can up-regulate BSEP expression 78. In enterocytes, BA bound to ileal FXR induces expression of IBABP 79, 80. Previous research has shown that unlike rabbits, mice and humans, the rat Asbt gene is not regulated via a negative feedback mechanism by BAs 81–85. BA responsiveness of ASBT is mediated by FXR-dependent activation of SHP and subsequent inhibition of LRH-1 (liver receptor homologue-1) 84, 85. The rat Asbt promoter lacks a functional LRH-1 cis-acting element, and rat ileum does not express the LRH-1 protein 86. The genes encoding human OSTα-β have also been shown to be induced by BA and FXR 87, 88. Thus, BA responsiveness can be regulated by positive and negative feedback mechanisms via interactions between BAs and FXR 89, 90. Ileal FXR mRNA levels were examined in DF and NEC groups and there were no differences observed. However FXR expression was significantly lower than what was observed in weanling rats 14. In contrast, hepatic FXR mRNA was significantly lower in animals with NEC. Further studies to evaluate the contribution of FXR and to assess if the low levels of ileal FXR are sufficient to regulate BA transporters during the development of disease are crucial to understanding the overall process of BA homeostasis during NEC.
Tying Together the Complex Regulation of BAs in NEC
Our working paradigm for the role of BAs in NEC pathogenesis is as follows (Figure 5): In dam-fed (DF) neonatal rats, low levels of ileal BA transporters can effectively transport naturally low levels of hydrophilic, luminal BAs through the enterocyte and out into portal circulation. During NEC, toxic, hydrophobic ileal luminal BA levels are significantly increased and Asbt and Ost α-β are precociously up-regulated, perhaps in an attempt to deal with elevated luminal and intra-enterocyte BAs, respectively. However, Ibabp is significantly decreased. Our data suggests that without adequate levels of Ibabp, BAs are not efficiently transported into portal circulation, and may accumulate in the enterocytes where they contribute to ileal damage. Weanling animals express high levels of Asbt, Ibabp, and Ostα-β compared to neonatal animals, and efficiently transfer BAs from the lumen into portal blood. Decreased portal blood BA levels in NEC result in diminished levels of Ntcp, with less BAs extracted from portal circulation. This leads to continued production of BAs which contributes to more accumulation of BAs in the intestine.
Figure 5. Working Paradigm for BA dysregulation during NEC Pathogenesis.
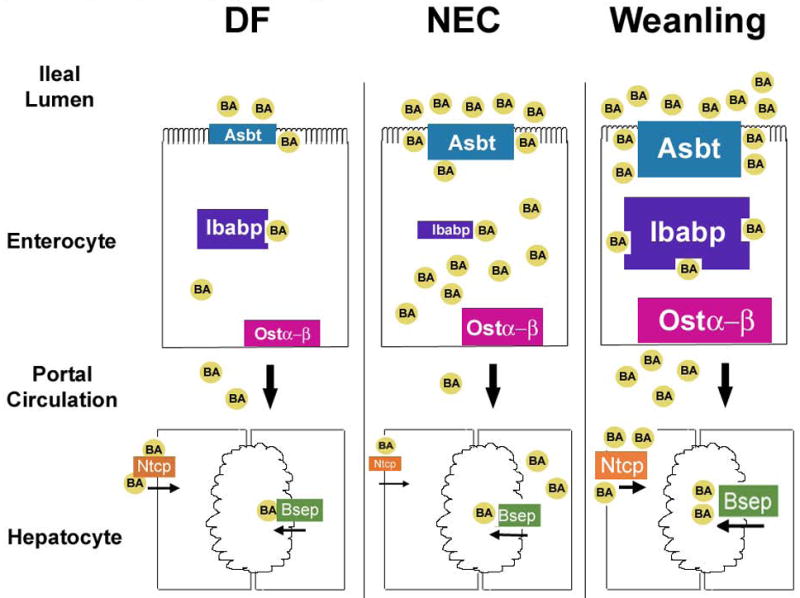
During development of experimental NEC, BAs in the terminal ileum increase which leads to precocious expression of Asbt, the BA transporter responsible for moving BAs from the ileal lumen into enterocytes. This allows BAs to be transported across the apical surface of enterocytes, but transport is compromised because Ibabp is not sufficiently induced in the neonatal intestine. BAs accumulate within the enterocytes and the unsuccessful transport of BAs is reflected in the diminished levels of BAs in portal circulation. The accumulation of toxic BA promotes accelerated cellular damage to the terminal ileum. Diminished BAs reclaimed by the liver promotes continued production of BAs, which exacerbates ileal BA accumulation. In normal, DF animals, BA levels remain low because they consume mother’s milk and low levels of ileal BA transporters are adequate to effectively transport these BAs maintaining physiologic BA metabolism. When weaned, rats produce larger amounts of BAs but BA homeostasis is maintained because ileal BA transport accommodates the increased levels of BAs.
The contribution of BAs to NEC pathogenesis represents a shift in the way this disease has traditionally been viewed. While further study is essential, there is strong evidence in animal models that BAs play a critical role in the initiation of ileal damage during disease development. The inability of neonatal intestine to adequately regulate ileal BA transport may be a mechanism by which BAs accumulate and could help explain why the disease predominates in premature infants. This paradigm can also encompass two other important risk factors for NEC, formula feeding and bacterial colonization: formula feeding elicits more toxic, potentially tissue damaging BAs than breast feeding 33 and the formation of secondary BAs, which require bacterial-induced deconjugation, occurs only in animals with NEC 14. Additional studies will clarify the role of BAs in both experimental and human disease.
Acknowledgments
Supported by HD54485 (M.D.H.) and HD39657 (B.D.) from the National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stoll BJ. Epidemiology of Necrortizing Enterocollitis. Clinics in Perinatology. 1994;21:205–218. doi: 10.1016/S0095-5108(18)30341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kafetzis DA, Skevaki C, Costalos C. Neonatal necrotizing enterocolitis: an overview. Curr Opin Infect Dis. 2003;16:349–55. doi: 10.1097/00001432-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Caplan MS, MacKendrick W. Necrotizing enterocolitis: a review of pathogenetic mechanisms and implications for prevention. Pediatr Pathol. 1993;13:357–69. doi: 10.3109/15513819309048223. [DOI] [PubMed] [Google Scholar]
- 4.Israel EJ. Neonatal necrotizing enterocolitis, a disease of the immature intestinal mucosal barrier. Acta Paediatr Suppl. 1994;396:27–32. doi: 10.1111/j.1651-2227.1994.tb13238.x. [DOI] [PubMed] [Google Scholar]
- 5.Neu J. Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am. 1996;43:409–32. doi: 10.1016/S0031-3955(05)70413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballance WA, Dahms BB, Shenker N, et al. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr. 1990;117:S6–13. doi: 10.1016/s0022-3476(05)81124-2. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence G, Bates J, Gaul A. Pathogenesis of neonatal necrotising enterocolitis. Lancet. 1982;1:137–9. doi: 10.1016/s0140-6736(82)90383-x. [DOI] [PubMed] [Google Scholar]
- 8.Kliegman RM, Fanaroff AA. Neonatal necrotizing enterocolitis: a nine-year experience. Am J Dis Child. 1981;135:603–7. doi: 10.1001/archpedi.1981.02130310009005. [DOI] [PubMed] [Google Scholar]
- 9.Kliegman RM, Fanaroff AA. Neonatal necrotizing enterocolitis: a nine-year experience. II Outcome assessment. Am J Dis Child. 1981;135:608–11. doi: 10.1001/archpedi.1981.02130310014006. [DOI] [PubMed] [Google Scholar]
- 10.Weimer J. The Economic benefits of Breastfeeding: A Review and Analysis. Food and Rural Economics Division, Economic Research Service; U.S. Department of Agriculture: 2001. [Google Scholar]
- 11.Craven PA, Pfanstiel J, Saito R, et al. Relationship between loss of rat colonic surface epithelium induced by deoxycholate and initiation of the subsequent proliferative response. Cancer Res. 1986;46:5754–9. [PubMed] [Google Scholar]
- 12.Craven PA, DeRubertis FR. Role of activation of protein kinase C in the stimulation of colonic epithelial proliferation by unsaturated fatty acids. Gastroenterology. 1988;95:676–85. doi: 10.1016/s0016-5085(88)80014-3. [DOI] [PubMed] [Google Scholar]
- 13.Milovic V, Teller IC, Faust D, et al. Effects of deoxycholate on human colon cancer cells: apoptosis or proliferation. Eur J Clin Invest. 2002;32:29–34. doi: 10.1046/j.0014-2972.2001.00938.x. [DOI] [PubMed] [Google Scholar]
- 14.Halpern MD, Holubec H, Saunders TA, et al. Bile Acids Induce Ileal Damage During Experimental Necrotizing Enterocolitis. Gastroenterology. 2006;130:359–372. doi: 10.1053/j.gastro.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colombo C, Zuliani G, Ronchi M, et al. Biliary bile acid composition of the human fetus in early gestation. Pediatr Res. 1987;21:197–200. doi: 10.1203/00006450-198702000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Kimura A, Yamakawa R, Ushijima K, et al. Fetal bile acid metabolism during infancy: analysis of 1 beta-hydroxylated bile acids in urine, meconium and feces. Hepatology. 1994;20:819–24. doi: 10.1002/hep.1840200408. [DOI] [PubMed] [Google Scholar]
- 17.Boehm G, Braun W, Moro G, et al. Bile acid concentrations in serum and duodenal aspirates of healthy preterm infants: effects of gestational and postnatal age. Biol Neonate. 1997;71:207–14. doi: 10.1159/000244419. [DOI] [PubMed] [Google Scholar]
- 18.Watkins JB, Ingall D, Szczepanik P, et al. Bile-salt metabolism in the newborn. Measurement of pool size and synthesis by stable isotope technic. N Engl J Med. 1973;288:431–4. doi: 10.1056/NEJM197303012880902. [DOI] [PubMed] [Google Scholar]
- 19.Watkins JB, Szczepanik P, Gould JB, et al. Bile salt metabolism in the human premature infant. Preliminary observations of pool size and synthesis rate following prenatal administration of dexamethasone and phenobarbital. Gastroenterology. 1975;69:706–13. [PubMed] [Google Scholar]
- 20.Yousef IM, Tuchweber B. Bile acid composition in neonatal life in rats. Biol Neonate. 1982;42:105–12. doi: 10.1159/000241583. [DOI] [PubMed] [Google Scholar]
- 21.Shneider BL, Setchell KD, Crossman MW. Fetal and neonatal expression of the apical sodium-dependent bile acid transporter in the rat ileum and kidney. Pediatr Res. 1997;42:189–94. doi: 10.1203/00006450-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Hwang ST, Henning SJ. Ontogenic regulation of components of ileal bile acid absorption. Exp Biol Med (Maywood) 2001;226:674–80. doi: 10.1177/153537020222600713. [DOI] [PubMed] [Google Scholar]
- 23.Suchy FJ, Balistreri WF, Heubi JE, et al. Physiologic cholestasis: elevation of the primary serum bile acid concentrations in normal infants. Gastroenterology. 1981;80:1037–41. [PubMed] [Google Scholar]
- 24.Suchy FJ, Balistreri WF. Uptake of taurocholate by hepatocytes isolated from developing rats. Pediatr Res. 1982;16:282–5. doi: 10.1203/00006450-198204000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Suchy FJ, Courchene SM, Balistreri WF. Ontogeny of hepatic bile acid conjugation in the rat. Pediatr Res. 1985;19:97–101. doi: 10.1203/00006450-198501000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Balistreri WF, Heubi JE, Suchy FJ. Immaturity of the enterohepatic circulation in early life: factors predisposing to "physiologic" maldigestion and cholestasis. J Pediatr Gastroenterol Nutr. 1983;2:346–54. [PubMed] [Google Scholar]
- 27.Heubi JE, Balistreri WF, Suchy FJ. Bile salt metabolism in the first year of life. J Lab Clin Med. 1982;100:127–36. [PubMed] [Google Scholar]
- 28.Hamosh M. Digestion in the premature infant: the effects of human milk. Seminars in Perinatology. 1994;18:485–94. [PubMed] [Google Scholar]
- 29.Hamosh M. Digestion in the newborn. Clinics in Perinatology. 1996;23:191–209. [PubMed] [Google Scholar]
- 30.Hamosh M, Iverson SJ, Kirk CL, et al. Milk lipids and neonatal fat digestion: relationship between fatty acid composition, endogenous and exogenous digestive enzymes and digestion of milk fat. World Review of Nutrition & Dietetics. 1994;75:86–91. doi: 10.1159/000423556. [DOI] [PubMed] [Google Scholar]
- 31.Armand M, Hamosh M, Mehta NR, et al. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatric Research. 1996;40:429–37. doi: 10.1203/00006450-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Lien EL. The role of fatty acid composition and positional distribution in fat absorption in infants. J Pediatr. 1994;125:S62–8. doi: 10.1016/s0022-3476(06)80738-9. [DOI] [PubMed] [Google Scholar]
- 33.Hammons JL, Jordan WE, Stewart RL, et al. Age and diet effects on fecal bile acids in infants. J Pediatr Gastroenterol Nutr. 1988;7:30–8. doi: 10.1097/00005176-198801000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–23. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 35.Barlow B, Santulli TV, Heird WC, et al. An experimental study of acute neonatal enterocolitis--the importance of breast milk. J Pediatr Surg. 1974;9:587–95. doi: 10.1016/0022-3468(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 36.Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery. 1975;77:687–90. [PubMed] [Google Scholar]
- 37.Caplan MS, Hedlund E, Adler L, et al. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol. 1994;14:1017–28. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 38.Dvorak B, Halpern MD, Holubec H, et al. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol. 2002;282:G156–64. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 39.Dvorak B, Halpern MD, Holubec H, et al. Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model. Pediatr Res. 2003;53:426–33. doi: 10.1203/01.PDR.0000050657.56817.E0. [DOI] [PubMed] [Google Scholar]
- 40.Grundy SM, Ahrens EH, Jr, Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971;78:94–121. [PubMed] [Google Scholar]
- 41.Huff JW, Gilfillan JL, Hunt VM. Effect of Cholestyramine, a Bile Acid-Binding Polymer on Plasma Cholesterol and Fecal Bile Acid Excretion in the Rat. Proc Soc Exp Biol Med. 1963;114:352–5. doi: 10.3181/00379727-114-28674. [DOI] [PubMed] [Google Scholar]
- 42.Shepherd J, Packard CJ, Bicker S, et al. Effect of cholestyramine on low-density lipoproteins. N Engl J Med. 1980;303:943–4. doi: 10.1056/nejm198010163031620. [DOI] [PubMed] [Google Scholar]
- 43.Heuman DM, Hylemon PB, Vlahcevic ZR. Regulation of bile acid synthesis. III Correlation between biliary bile salt hydrophobicity index and the activities of enzymes regulating cholesterol and bile acid synthesis in the rat. J Lipid Res. 1989;30:1161–71. [PubMed] [Google Scholar]
- 44.Halpern MD, Holubec H, Dominguez JA, et al. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res. 2002;51:733–9. doi: 10.1203/00006450-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Halpern MD, Dominguez JA, Dvorakova K, et al. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr. 2003;36:126–33. doi: 10.1097/00005176-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 46.Halpern MD, Holubec H, Dominguez JA, et al. Hepatic inflammatory mediators contribute to intestinal damage in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G695–702. doi: 10.1152/ajpgi.00353.2002. [DOI] [PubMed] [Google Scholar]
- 47.Halpern MD, Khailova L, Molla-Hosseini D, et al. Elevated Bile Acids Precede Ileal Damage and Inflammation During the Development of Necrotizing Enterocolitis. Gastroenterology. 2007;132:A1–2. [Google Scholar]
- 48.Shneider BL, Dawson PA, Christie DM, et al. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J Clin Invest. 1995;95:745–54. doi: 10.1172/JCI117722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilchrist W, Burkhalter E, Eaton C, et al. The effect of indomethacin on the secretion of human salivary epidermal growth factor. Am J Gastroenterol. 1994;89:97–100. [PubMed] [Google Scholar]
- 50.Sacchettini JC, Hauft SM, Van Camp SL, et al. Developmental and structural studies of an intracellular lipid binding protein expressed in the ileal epithelium. J Biol Chem. 1990;265:19199–207. [PubMed] [Google Scholar]
- 51.Dawson PA, Hubbert M, Haywood J, et al. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–8. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crossman MW, Hauft SM, Gordon JI. The mouse ileal lipid-binding protein gene: a model for studying axial patterning during gut morphogenesis. J Cell Biol. 1994;126:1547–64. doi: 10.1083/jcb.126.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coppola CP, Gosche JR, Arrese M, et al. Molecular analysis of the adaptive response of intestinal bile acid transport after ileal resection in the rat. Gastroenterology. 1998;115:1172–8. doi: 10.1016/s0016-5085(98)70088-5. [DOI] [PubMed] [Google Scholar]
- 54.Stelzner M, Hoagland V, Somasundaram S. Distribution of bile acid absorption and bile acid transporter gene message in the hamster ileum. Pflugers Arch. 2000;440:157–62. doi: 10.1007/s004240000281. [DOI] [PubMed] [Google Scholar]
- 55.Christie DM, Dawson PA, Thevananther S, et al. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol. 1996;271:G377–85. doi: 10.1152/ajpgi.1996.271.2.G377. [DOI] [PubMed] [Google Scholar]
- 56.Hwang ST, Henning SJ. Hormonal regulation of expression of ileal bile acid binding protein in suckling rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1555–63. doi: 10.1152/ajpregu.2000.278.6.R1555. [DOI] [PubMed] [Google Scholar]
- 57.Hwang ST, Urizar NL, Moore DD, et al. Bile acids regulate the ontogenic expression of ileal bile acid binding protein in the rat via the farnesoid X receptor. Gastroenterology. 2002;122:1483–92. doi: 10.1053/gast.2002.32982. [DOI] [PubMed] [Google Scholar]
- 58.Halpern MD, Ballatori N, Clark J, et al. Ontogenic Regulation of the Rat Heteromeric Organic Solute Transporter. Gastroenterology. 2006;130(4 Suppl 2):374. [Google Scholar]
- 59.Green RM, Beier D, Gollan JL. Regulation of hepatocyte bile salt transporters by endotoxin and inflammatory cytokines in rodents. Gastroenterology. 1996;111:193–8. doi: 10.1053/gast.1996.v111.pm8698199. [DOI] [PubMed] [Google Scholar]
- 60.Bohan A, Chen WS, Denson LA, et al. Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3(Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem. 2003;278:36688–98. doi: 10.1074/jbc.M304011200. [DOI] [PubMed] [Google Scholar]
- 61.Whiting JF, Green RM, Rosenbluth AB, et al. Tumor necrosis factor-alpha decreases hepatocyte bile salt uptake and mediates endotoxin-induced cholestasis. Hepatology. 1995;22:1273–8. doi: 10.1016/0270-9139(95)90639-8. [DOI] [PubMed] [Google Scholar]
- 62.Sturm E, Zimmerman TL, Crawford AR, et al. Endotoxin-stimulated macrophages decrease bile acid uptake in WIF-B cells, a rat hepatoma hybrid cell line. Hepatology. 2000;31:124–30. doi: 10.1002/hep.510310120. [DOI] [PubMed] [Google Scholar]
- 63.Halpern MD, Clark JA, Saunders TA, et al. Reduction of Experimental Necrotizing Enterocolitis with Anti-TNF-{alpha} Am J Physiol Gastrointest Liver Physiol. 2006;290:G757–G764. doi: 10.1152/ajpgi.00408.2005. [DOI] [PubMed] [Google Scholar]
- 64.Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics. 1999;103:766–71. doi: 10.1542/peds.103.4.766. [DOI] [PubMed] [Google Scholar]
- 65.Abreu MT, Palladino AA, Arnold ET, et al. Modulation of barrier function during Fas-mediated apoptosis in human intestinal epithelial cells. Gastroenterology. 2000;119:1524–36. doi: 10.1053/gast.2000.20232. [DOI] [PubMed] [Google Scholar]
- 66.Ruemmele FM, Russo P, Beaulieu J, et al. Susceptibility to FAS-induced apoptosis in human nontumoral enterocytes: role of costimulatory factors. J Cell Physiol. 1999;181:45–54. doi: 10.1002/(SICI)1097-4652(199910)181:1<45::AID-JCP5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 67.Hanada S, Harada M, Koga H, et al. Tumor necrosis factor-alpha and interferon-gamma directly impair epithelial barrier function in cultured mouse cholangiocytes. Liver Int. 2003;23:3–11. doi: 10.1034/j.1600-0676.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 68.Takikawa H. Hepatobiliary transport of bile acids and organic anions. J Hepatobiliary Pancreat Surg. 2002;9:443–7. doi: 10.1007/s005340200055. [DOI] [PubMed] [Google Scholar]
- 69.Hardikar W, Ananthanarayanan M, Suchy FJ. Differential ontogenic regulation of basolateral and canalicular bile acid transport proteins in rat liver. J Biol Chem. 1995;270:20841–6. doi: 10.1074/jbc.270.35.20841. [DOI] [PubMed] [Google Scholar]
- 70.Arrese M, Trauner M, Ananthanarayanan M, et al. Maternal cholestasis does not affect the ontogenic pattern of expression of the Na+/taurocholate cotransporting polypeptide (ntcp) in the fetal and neonatal rat liver. Hepatology. 1998;28:789–95. doi: 10.1002/hep.510280328. [DOI] [PubMed] [Google Scholar]
- 71.Khailova L, Cherrington N, Molla-Hosseini D, et al. Hepatic Bile Acid Transporters are Down Regulated in Experimental Necrotizing Enterocolitis. Gastroenterology. 2007;132:A455. [Google Scholar]
- 72.Cherrington NJ, Slitt AL, Li N, et al. Lipopolysaccharide-mediated regulation of hepatic transporter mRNA levels in rats. Drug Metab Dispos. 2004;32:734–41. doi: 10.1124/dmd.32.7.734. [DOI] [PubMed] [Google Scholar]
- 73.Geier A, Dietrich CG, Voigt S, et al. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology. 2003;38:345–54. doi: 10.1053/jhep.2003.50317. [DOI] [PubMed] [Google Scholar]
- 74.Hartmann G, Cheung AK, Piquette-Miller M. Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia. J Pharmacol Exp Ther. 2002;303:273–81. doi: 10.1124/jpet.102.039404. [DOI] [PubMed] [Google Scholar]
- 75.Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–5. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 76.Chen D, Wang W, Wang J. Influence of anti-TNF alpha monocolonal antibody on intestinal barrier in rats with acute pancreatitis. Chin Med Sci J. 2000;15:257. [PubMed] [Google Scholar]
- 77.Denson LA, Sturm E, Echevarria W, et al. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121:140–7. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- 78.Ananthanarayanan M, Balasubramanian N, Makishima M, et al. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276:28857–65. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 79.Chung HL, Hwang JB, Park JJ, et al. Expression of transforming growth factor beta1, transforming growth factor type I and II receptors, and TNF-alpha in the mucosa of the small intestine in infants with food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2002;109:150–4. doi: 10.1067/mai.2002.120562. [DOI] [PubMed] [Google Scholar]
- 80.Nakahara M, Furuya N, Takagaki K, et al. Ileal bile acid-binding protein functionally associated with the farnesoid X receptor or Ileal bile acid transporter regulates bile acid activity in the small intestine. J Biol Chem. 2005 doi: 10.1074/jbc.M507454200. [DOI] [PubMed] [Google Scholar]
- 81.Neimark E, Chen F, Li X, et al. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology. 2004;40:149–56. doi: 10.1002/hep.20295. [DOI] [PubMed] [Google Scholar]
- 82.Li H, Chen F, Shang Q, et al. FXR-activating ligands inhibit rabbit ASBT expression via FXR-SHP-FTF cascade. Am J Physiol Gastrointest Liver Physiol. 2005;288:G60–6. doi: 10.1152/ajpgi.00170.2004. [DOI] [PubMed] [Google Scholar]
- 83.Arrese M, Trauner M, Sacchiero RJ, et al. Neither intestinal sequestration of bile acids nor common bile duct ligation modulate the expression and function of the rat ileal bile acid transporter. Hepatology. 1998;28:1081–7. doi: 10.1002/hep.510280424. [DOI] [PubMed] [Google Scholar]
- 84.Lu TT, Makishima M, Repa JJ, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–15. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 85.Goodwin B, Jones SA, Price RR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–26. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 86.Chen F, Ma L, Dawson PA, et al. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem. 2003;278:19909–16. doi: 10.1074/jbc.M207903200. [DOI] [PubMed] [Google Scholar]
- 87.Landrier JF, Eloranta JJ, Vavricka SR, et al. Am J Physiol Gastrointest Liver Physiol. 2005. The Nuclear Receptor for Bile Acids, FXR, Transactivates the Human Organic Solute Transporter -{alpha} and -{beta} Genes. [DOI] [PubMed] [Google Scholar]
- 88.Lee H, Zhang Y, Lee FY, et al. FXR regulates organic solute transporter alpha and beta in the adrenal gland, kidney and intestine. J Lipid Res. 2005 doi: 10.1194/jlr.M500417-JLR200. [DOI] [PubMed] [Google Scholar]
- 89.Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–53. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 90.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]


