Oxidized fatty acids, termed oxylipins, are an important class of signaling molecule in plants, especially related to plant stress responses and innate immunity. The best-characterized oxylipins are jasmonic acid (JA) and its immediate precursor 12-oxo-phytodienoic acid (OPDA), which are formed enzymatically and accumulate in response to various stresses, in particular wounding and pathogen infection (Block et al., 2005). In addition, a number of biologically active oxylipins are formed nonenzymatically via the action of reactive oxygen species (ROS), which also accumulate in response to pathogen infection, heavy metal uptake, and other stresses. There is growing evidence that nonenzymatically formed oxylipins, including hydroxy fatty acids and phytoprostanes, play important signaling roles in plant stress responses (Sattler et al., 2006).
Recent work with JA and OPDA has shown that both are active as signaling molecules and induce the expression of overlapping but distinct sets of genes. JA signaling leads to the interaction of the F-box ubiquitin ligase CORONATINE-INSENSITIVE1 (COI1) with JAZ transcriptional repressors, mediating degradation of these repressors of downstream JA-induced genes, many of which are dependent on the key transcription factor MYC2/JIN1 (Chini et al., 2007; reviewed in Santner and Estelle, 2007). Whereas JA induces a set of COI1-dependent genes, OPDA has been found to induce a set of largely COI1-independent genes (Stintzi et al., 2001; Taki et al., 2005). Phytoprostanes have also been shown to activate the expression of stress response genes, leading to enhanced protection from subsequent oxidative stress (Thoma et al., 2003; Loeffler et al., 2005), but little is known about phytoprostane signal transduction.
Phytoprostanes are grouped into a number of classes, depending on the ring and side chain structures, and plants are known to produce a number of different types (Thoma et al., 2004). The A1-type (PPA1) and the deoxy-J1-type phytoprostanes, as well as OPDA, belong to a subgroup of oxylipins known as reactive electrophile species (RES) because they contain a reactive α,β-unsaturated carbonyl structure that may contribute to their biological activity. RES, for example, have the ability to modify proteins directly by binding to free thiol groups, a property that is not shared by weak electrophilic oxylipins, including JA and the B1-type phytoprostanes (PPB1). It has been suggested that RES, including those produced nonenzymatically, induce a common set of defense genes (Almeras et al., 2003; Weber et al., 2004; Farmer and Davoine, 2007).
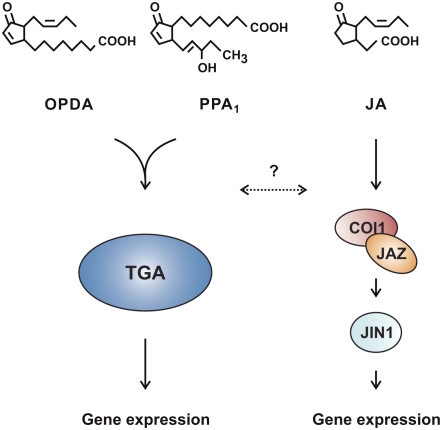
In this issue of The Plant Cell, Mueller et al. (pages 768–785) examine the effects of OPDA and phytoprostanes on gene expression and plant growth and development in Arabidopsis and show that these oxylipins share similar biological activity that appears to differ considerably from that of JA. In addition, phytoprostanes might have unique activities and targets not shared by OPDA or JA. The authors employ whole-genome microarray analysis, liquid chromatography–tandem mass spectrometry analysis, and enzymatic assays to investigate the biological activity of phytoprostanes in relation to OPDA and JA. Differences in gene induction between JA and OPDA/phytoprostanes suggest the existence of multiple oxylipin signal transduction pathways. Interestingly, many of the genes induced by phytoprostanes and OPDA were found to be dependent on the TGA transcription factors TGA2, TGA5, and TGA6, demonstrating that these factors play an important role in oxylipin signaling (see figure). In addition, it was shown that some of the genes induced by OPDA and phytoprostanes encode enzymes involved in their metabolism (conjugation and/or detoxification). This work shows that nonenzymatically produced oxylipins likely constitute an important component of oxylipin signaling in plant stress responses.
Figure 1.
Oxylipin Signaling Pathways.
JA signaling is to some extent dependent on the key transcriptional activator MYC2/JIN1, which is regulated by COI1-dependent degradation of JAZ repressors (Chini et al., 2007). The work of Mueller et al. (2008) suggests a model wherein TGA transcription factors contribute to phytoprostane and OPDA signaling. Although not shown here, phytoprostanes and OPDA also appear to have their own unique responses.
PHYTOPROSTANES AND OPDA HAVE OVERLAPPING AND INDEPENDENT FUNCTIONS IN ARABIDOPSIS
The authors first conducted microarray analysis of gene expression in Arabidopsis cell cultures after treatment with A1-type phytoprostanes (PPA1). In response to PPA1, 926 genes showed statistically significant up- or downregulation greater than twofold, and many of the induced genes were classified as putatively involved in stress responses, such as cytochrome P450 enzymes, UDP-glucuronyl/-glycosyltransferases, glutathione S-transferases (GSTs), ABC transporters, and heat shock proteins. Repressed genes included those involved in cell cycle regulation and cell growth.
Further RNA gel blot analysis of the expression of 13 of the genes induced by PPA1 showed that most also were strongly induced by OPDA, as well as dJ1-type and B1-type phytoprostanes, but exhibited more moderate or no induction in response to JA. These results showed that biological activities of JA, OPDA, and various phytoprostanes partially overlap, and PPA1 appears to have activity very similar to that of OPDA, which differs considerably from that of JA. However, the activities of all species did not correlate strictly with electrophilicity (e.g., the weak electrophile PPB1 appeared to show more similarity to the RES than to JA for the genes tested).
Further experiments conducted in plants offered confirmation that PPA1 and OPDA share similar biological activities. Microarray analysis using whole plants and comparisons with publicly available microarray data through Genevestigator (www.genevestigator.ethz.ch; Zimmermann et al., 2004) showed that 42% of the PPA1-induced genes were upregulated more than threefold by both PPA1 and OPDA, whereas only 7% of PPA1-induced genes were also induced by JA. The set of genes upregulated by PPA1 also showed considerable overlap with genes induced by pathogens, such as Pseudomonas syringae. These data support the notion that pathogen infection leads to enhanced production of ROS that can trigger the formation of nonenzymatic oxylipins, as also shown by Grun et al. (2007). It is also important to note that, although there was considerable overlap between PPA1 and OPDA, PPA1 appeared to have a unique signature. The authors speculate that phytoprostanes may have a particular function in detoxification of xenobiotic compounds (various chemicals and pathogen infection situations that elicit the accumulation of ROS/RES).
TGA TRANSCRIPTION FACTORS PLAY A ROLE IN OXYLIPIN SIGNALING
Mueller et al. next searched the promoters of genes induced by PPA1 for a high abundance of specific binding sites to identify transcription factors potentially involved in the response to phytoprostanes. They found that approximately half of the PPA1-induced genes in both cell culture and whole-plant experiments contain a TGA-motif (TGACG) in the first 500 bp upstream of the start codon. They conducted microarray gene expression analysis using a tga2 tga5 tga6 triple mutant of Arabidopsis and found that 60% of the genes upregulated by PPA1 in the wild type (247 of 411) were not induced in the triple mutant, providing confirmation that these TGA transcription factors are involved in mediating gene regulation by PPA1.
These TGA transcription factors were also found to be important in OPDA-mediated regulation of gene expression. In response to OPDA, 30% of the genes induced in the wild type (225 of 760) were not induced in the tga2 tga5 tga6 triple mutant. Although similar in number, the sets of genes induced in a TGA-dependent manner by PPA1 (247) and by OPDA (225) contained just 48 genes in common (i.e., expression of 48 genes was increased by PPA1 and OPDA in a TGA-dependent manner). This could reflect some real differences in the response to PPA1 versus OPDA but could partly be due to limitations of the microarray analysis. The take-home message is that a large percentage of genes regulated by OPDA and PPA1 (30 to 60%) is dependent on TGA transcription factors. The microarray data further showed that a relatively large number of genes (>200) were upregulated by PPA1 and OPDA in the tga2 tga5 tga6 triple mutant but were not induced by these oxylipins in the wild type, suggesting that TGA transcription factors might negatively regulate the expression of some genes.
These results build upon previous information on the role of TGA factors in stress responses. For example, TGA2, TGA5, and TGA6 are known to interact with NPR1, a key factor required for salicylic acid–dependent induction of systemic acquired resistance in Arabidopsis (Zhang et al., 1999). Previous analysis of the tga2 tga5 tga6 triple mutant has also shown that these factors play overlapping and essential roles in this process in vivo (Zhang et al., 2003). Ndamukong et al. (2007) found that these TGA factors, together with salicylic acid–responsive glutaredoxin, also mediate suppression of the JA-responsive gene PDF1.2. Future work will focus on identifying other TGA-interacting partners that may be involved in the response to oxylipins and to what extent the JA response differs from that of OPDA and phytoprostanes.
METABOLISM OF REACTIVE OXYLIPINS
Because of the highly reactive nature of OPDA and PPA1 oxylipins, Mueller et al. investigated whether they induce genes encoding enzymes capable of detoxifying them or metabolizing them into less reactive compounds. Two types of enzymes that might reduce the reactive cyclopentenone ring to an unreactive ring are GSTs and OPDA-reductases (OPRs). The gene expression analyses showed that OPDA and PPA1 induce several genes encoding OPR and GST enzymes. Using enzymatic assays conducted with purified heterologous proteins produced in Escherichia coli, the authors further demonstrate that OPDA and PPA1 are in vitro substrates of two strongly induced enzymes, OPR1 and GST6. In addition, the authors were able to measure an accumulation of PPA1- and OPDA-glutathione conjugates in Arabidopsis leaves following infection with P. syringae.
The work of Mueller et al. presents convincing data that nonenzymatically formed oxylipins, such as phytoprostanes, contribute significantly to plant response to stress and impact signaling pathways that regulate gene expression. In addition, they demonstrate that phytoprostanes are metabolized and converted to less active compounds by induced OPR and GST enzymes. Although there appears to be significant overlap between phytoprostane and OPDA activity, the responses were not identical, suggesting that phytoprostanes might also have a unique set of responses. Phytoprostanes therefore constitute another class of oxylipin in plants (in addition to JA and OPDA) that must be taken into account in considering oxylipin signaling.
References
- Almeras, E., Stolz, S., Vollenweider, S., Reymond, P., Mene-Saffrane, L., and Farmer, E.E. (2003). Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 34 205–216. [DOI] [PubMed] [Google Scholar]
- Block, A., Schmelz, E., Jones, J.B., and Klee, H.J. (2005). Coronatine and salicylic acid: The battle between Arabidopsis and Pseudomonas for phytohormone control. Mol. Plant Pathol. 6 79–83. [DOI] [PubMed] [Google Scholar]
- Chini, A., Fonseca, S., Fernandez, G., Adie, B., Chico, J.M., Lorenzo, O., Garcia-Casado, G., Lopez-Vidriero, I., Lozano, F.M., Ponce, M.R., Micol, J.L., and Solano, R. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448 666–671. [DOI] [PubMed] [Google Scholar]
- Farmer, E.E., and Davoine, C. (2007). Reactive electrophile species. Curr. Opin. Plant Biol. 10 380–386. [DOI] [PubMed] [Google Scholar]
- Grun, G., Berger, S., Matthes, D., and Mueller, M.J. (2007). Early accumulation of non-enzymatically synthesized oxylipins in Arabidopsis thaliana after infection with Pseudomonas syringae. Funct. Plant Biol. 34 65–71. [DOI] [PubMed] [Google Scholar]
- Loeffler, C., Berger, S., Guy, A., Durand, T., Bringmann, G., Dreyer, M., von Rad, U., Durner, J., and Mueller, M.J. (2005). B1-phytoprostanes trigger plant defense and detoxification responses. Plant Physiol. 137 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, S., Hilbert, B., Dueckershoff, K., Roitsch, T., Krischke, M., Muelle, M.J., and Berger, S. (2008). General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 20 768–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndamukong, I., Abdallat, A.A., Thurow, C., Fode, B., Zander, M., Weigel, R., and Gatz, C. (2007). SA-inducible Arabidopsis glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1.2 transcription. Plant J. 50 128–139. [DOI] [PubMed] [Google Scholar]
- Santner, A., and Estelle, M. (2007). The JAZ proteins link jasmonate perception with transcriptional changes. Plant Cell 19 3839–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler, S.E., Mene-Saffrane, L., Farmer, E.E., Krischke, M., Mueller, M.J., and DellaPenna, D. (2006). Nonenzymatic lipid peroxidation reprograms gene expression and activates defense markers in Arabidopsis tocopherol-deficient mutants. Plant Cell 18 3706–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi, A., Weber, H., Reymond, P., Browse, J., and Farmer, E.E. (2001). Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc. Natl. Acad. Sci. USA 98 12837–12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki, N., et al. (2005). 12-Oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 139 1268–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma, I., Krischke, M., Loeffler, C., and Mueller, M.J. (2004). The isoprostanoid pathway in plants. Chem. Phys. Lipids 128 135–148. [DOI] [PubMed] [Google Scholar]
- Thoma, I., Loeffler, C., Sinha, A.K., Gupta, M., Krischke, M., Steffan, B., Roitsch, T., and Mueller, M.J. (2003). Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J. 34 363–375. [DOI] [PubMed] [Google Scholar]
- Weber, H., Chételat, A., Reymond, P., and Farmer, E.E. (2004). Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J. 37 877–888. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Tessaro, M.J., Lassner, M., and Li, X. (2003). Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 15 2647–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y.L., Fan, W.H., Kinkema, M., Li, X., and Dong, X. (1999). Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc. Natl. Acad. Sci. USA 96 6523–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, P., Hirsch-Hoffmann, M., Hennig, L., and Gruissem, W. (2004). GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136 2621–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]