Abstract
12-oxo-phytodienoic acid and several phytoprostanes are cyclopentenone oxylipins that are formed via the enzymatic jasmonate pathway and a nonenzymatic, free radical–catalyzed pathway, respectively. Both types of cyclopentenone oxylipins induce the expression of genes related to detoxification, stress responses, and secondary metabolism, a profile clearly distinct from that of the cyclopentanone jasmonic acid. Microarray analyses revealed that 60% of the induction by phytoprostanes and 30% of the induction by 12-oxo-phytodienoic acid was dependent on the TGA transcription factors TGA2, TGA5, and TGA6. Moreover, treatment with phytoprostanes and 12-oxo-phytodienoic acid inhibited cell division and root growth, a property also shared by jasmonic acid. Besides being potent signals, cyclopentenones and other lipid peroxidation products are reactive electrophiles that can covalently bind to and damage proteins. To this end, we show that at least two of the induced detoxification enzymes efficiently metabolize cyclopentenones in vitro. Accumulation of two of these metabolites was detectable during Pseudomonas infection. The cyclopentenone oxylipin gene induction profile resembles the defense response induced by a variety of lipophilic xenobiotics. Hence, oxidized lipids may activate chemosensory mechanisms of a general broad-spectrum detoxification network involving TGA transcription factors.
INTRODUCTION
Oxylipins can be formed by enzymatic and nonenzymatic pathways. Important enzymatically formed oxylipins are 12-oxo-phytodienoic acid (OPDA) and jasmonic acid (JA). These compounds act as signals regulating plant development and plant stress responses. The enzymes catalyzing most of the biosynthetic steps are known (Delker et al., 2006). OPDA and its metabolite JA accumulate in response to stress stimuli such as wounding and pathogen infection (Block et al., 2005). This accumulation correlates with the increased expression of several biosynthetic enzymes, thereby increasing the capacity to synthesize OPDA and JA in response to stress. In addition, nonenzymatic pathways are triggered by free radicals as well as reactive oxygen species (ROS) and lead to the simultaneous formation of an array of oxidized lipids, including hydroxy fatty acids and phytoprostanes (Imbusch and Mueller, 2000; Gobel et al., 2002). Nonenzymatically formed oxylipins accumulate during a variety of stresses; this has been attributed to an increase in ROS (i.e., after pathogen infection or heavy metal intoxication) (Thoma et al., 2003; Montillet et al., 2005; Grun et al., 2007).
Exogenous application of oxylipins induces a variety of plant responses. For instance, several oxylipins were found to affect root growth and development (Vellosillo et al., 2007). Recently, the analysis and comparison of gene regulation by OPDA and JA greatly increased our knowledge (Stintzi et al., 2001; Taki et al., 2005). The use of a mutant defective in OPDA reductase3 (OPR3) that cannot convert OPDA to JA allowed the separation of effects mediated by OPDA directly from effects that occur in response to OPDA after its conversion to JA. Gene regulation by both compounds overlaps, but there are also distinct effects. In an analysis of 21,500 genes, OPDA induced the expression of 157 genes that were not regulated by JA (Taki et al., 2005). The majority of these genes encoded proteins involved in stress responses, such as heat shock proteins and glutathione S-transferases (GSTs), or proteins involved in signaling, such as transcription factors and kinases. About half of the genes upregulated by OPDA were also induced by wounding. In comparison, genes induced by JA were classified mainly as being involved in JA biosynthesis, ascorbate and GSH metabolism, and indole glucosinolate synthesis (Sasaki-Sekimoto et al., 2005). For several of the JA-inducible genes, it has been shown that the regulation of expression requires COI1 (for CORONATINE-INSENSITIVE1), an F-box protein (Xie et al., 1998; Devoto et al., 2005) that targets regulators of the signaling pathway such as the JASMONATE ZIM DOMAIN (JAZ) proteins for ubiquitin-dependent proteolysis (Chini et al., 2007; Thines et al., 2007; Yan et al., 2007). By contrast, the expression of several target genes responsive to OPDA (but not to JA) is independent of the JA-COI1 pathway. These results led to the model that JA induces a set of COI1-dependent genes and OPDA induces a set of largely COI1-independent genes (Stintzi et al., 2001; Taki et al., 2005). Genes that are induced by both substances seem to be at least partly COI1-dependent. This suggests that signaling pathways besides the COI1-dependent pathway exist that have not been identified yet and that mediate the effects of OPDA.
Phytoprostanes are structurally highly similar to OPDA and are also biologically active. These compounds induce the accumulation of secondary metabolites in different plant systems, activate the expression of genes related to stress responses, increase the activity of mitogen-activated protein kinases, and protect cells from subsequent oxidative stress (Thoma et al., 2003; Loeffler et al., 2005). OPDA and several classes of phytoprostanes, but not JA, are cyclopentenones and contain a reactive α,β-unsaturated carbonyl structure, classifying them as reactive electrophilic species (RES). It has been proposed that the cyclopentenone ring is a critical feature determining biological activity (Almeras et al., 2003). According to this hypothesis, OPDA and phytoprostanes are expected to display similar biological effects and possibly functions. However, phytoprostanes and OPDA share similarities with JA, since both classes of substances induce the accumulation of secondary metabolites. One goal of this work was to elucidate whether phytoprostanes are more similar to OPDA or to JA with respect to their biological activities.
Levels of signaling molecules are regulated by both biosynthesis and metabolism. Metabolic pathways for JA include β-oxidation, reduction of the keto group followed by glycosylation, carboxy group modification by methylation, and conjugation to sugars or amino acids. Interestingly, most of these metabolites also display biological activities (Wasternack, 2007). The metabolism of OPDA and cyclopentenone phytoprostanes has not been investigated in detail. Most of these molecules are found esterified in complex membrane lipids that are thought to represent biologically inactive storage forms. The release of oxylipins from membranes results in biologically active compounds. It has been postulated that the α,β-unsaturated carbonyl group of the cyclopentenone ring system of free cyclopentenones is essential for both the signaling function and the chemical reactivity, which may lead to the covalent modification of polypeptides (Farmer and Davoine, 2007). There are at least two groups of enzymes that may reduce the reactive cyclopentenone ring to an unreactive cyclopentanone ring system and, hence, alter reactivity and signal function: GSTs and OPRs. Both groups of proteins comprise enzymes that display low substrate specificity and are thought to play a role in the detoxification of reactive lipophilic xenobiotics and endogenous reactive lipid oxidation products.
To elucidate the biological activities of phytoprostanes in comparison with OPDA, we performed genome-wide expression analysis and also showed that enzymes induced by phytoprostanes are capable of metabolizing these compounds in vitro. In addition, we provide evidence for the contribution of TGA transcription factors to oxylipin signaling.
RESULTS
Nonenzymatically Formed Phytoprostanes Regulate Gene Expression
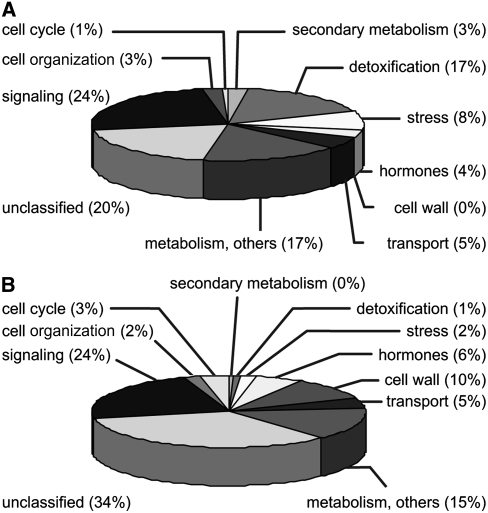
In order to get a comprehensive view of the biological activity of nonenzymatically formed reactive oxylipins, the regulation of gene expression in Arabidopsis thaliana was analyzed using the Affymetrix ATH1 chip representing 22,500 genes. A mixotrophic cell culture of Arabidopsis was treated with 75 μM A1-phytoprostanes (PPA1) and harvested after 4 h. Three independent replicates of each treatment were analyzed, and the fold change of normalized signals from PPA1-treated cell culture versus control cell culture was calculated. In response to PPA1, 926 genes showed a greater than twofold upregulation or downregulation with P < 0.05, representing 4% of the genes analyzed (see Supplemental Tables 1 and 2 online). For further analysis, genes exhibiting a differential expression of greater than threefold were used. Genes regulated by PPA1 were assigned to functional categories using the NetAFFX analysis center and MapMan (www.affymetrix.com/analysis/index.affx and http://gabi.rzpd.de/projects/MapMan) (Thimm et al., 2004; Usadel et al., 2005). The classification of genes regulated by a factor of >3 is depicted in Figure 1. The expression of 157 genes was induced, and the expression of 211 genes was repressed. Approximately 20% of the upregulated genes and 34% of the downregulated genes were classified as putative, hypothetical, or expressed proteins. Five percent of induced and repressed genes were related to transport, and 24% were related to signal transduction, including transcription factors, phosphatases, and kinases. Differences in the classification profile between induced and repressed proteins were also obvious. Seventeen percent of the upregulated genes encoded proteins that are putatively involved in detoxification (Table 1), such as cytochrome P450 enzymes, UDP-glucuronyl/glycosyltransferases, GSTs, and ATP binding cassette (ABC) transporters. In addition, several genes related to stress responses were induced, including heat shock factors, heat shock proteins, and an alternative oxidase (Table 1). By contrast, only a small number of detoxification- and stress-related genes were downregulated. Classification categories represented in the repressed gene fraction comprised regulators of the cell cycle such as cyclins and regulators of cell growth such as cell wall biosynthetic enzymes and proteins mediating auxin responses (Table 2).
Figure 1.
Regulation of Gene Expression by PPA1 in Arabidopsis.
(A) Classification of genes with at least threefold higher expression in Arabidopsis cell cultures at 4 h after treatment with 75 μM PPA1 relative to controls.
(B) Classification of genes with at least threefold lower expression in Arabidopsis cell cultures at 4 h after treatment with 75 μM PPA1 relative to controls.
Table 1.
Genes Upregulated by PPA1 Treatment
| Gene Locus | Fold Induction | P | Description | TGACG Present |
|---|---|---|---|---|
| At3g28740 | 205.2 | 0.003 | Cytochrome P450 family protein (CYP81D11) | + |
| At3g14660 | 46.4 | 0.015 | Cytochrome P450, putative | + |
| At3g14650 | − | |||
| At1g13080 | 11.4 | 0.045 | Cytochrome P450 family protein | − |
| At3g14690 | 11.1 | 0.012 | Cytochrome P450, putative | + |
| At3g14650 | − | |||
| At3g14640 | − | |||
| At3g14620 | 8.2 | 0.018 | Cytochrome P450, putative | − |
| At3g03470 | 3.1 | 0.025 | Cytochrome P450, putative | + |
| At4g34131 | 105.4 | 0.005 | UDP-glucoronosyl/UDP-glucosyl transferase family (UGT73B2) | + |
| At4g34135 | + | |||
| At1g05560 | 15.5 | 0.010 | UDP-glucose transferase (UGT75B2) | − |
| At4g01070 | 4.2 | 0.038 | UDP-glucoronosyl/UDP-glucosyl transferase family | − |
| At2g30140 | 3.7 | 0.005 | UDP-glucoronosyl/UDP-glucosyl transferase family | − |
| At1g17170 | 61.7 | 0.021 | Glutathione S-transferase, putative (GSTU24) | − |
| At2g47730 | 22.6 | 0.020 | Glutathione S-transferase6 (GST6) | + |
| At1g17180 | 17.0 | 0.041 | Glutathione S-transferase, putative | − |
| At1g74590 | 12.9 | 0.006 | Glutathione S-transferase, putative | − |
| At1g78340 | 6.5 | 0.024 | Glutathione S-transferase, putative | + |
| At2g29420 | 4.8 | 0.010 | Glutathione S-transferase, putative | + |
| At2g29460 | 3.7 | 0.050 | Glutathione S-transferase, putative | − |
| At1g78380 | 3.0 | 0.007 | Glutathione S-transferase, putative (GSTU19) | + |
| At1g76680 | 3.3 | 0.011 | 12-Oxo-phytodienoate reductase (OPR1/OPR2) | + |
| At1g76690 | + | |||
| At1g15520 | 24.5 | 0.006 | ABC transporter family protein (PDR12) | + |
| At1g02530 | 10.5 | 0.017 | Multidrug resistance P-glycoprotein, putative | − |
| At1g02520 | − | |||
| At3g47780 | 9.6 | 0.019 | ABC transporter family protein | + |
| At2g47000 | 8.7 | 0.037 | Multidrug-resistant ABC transporter, putative | − |
| At3g47730 | 8.2 | 0.018 | ABC transporter family protein | − |
| At2g34660 | 6.9 | 0.006 | Glutathione S-conjugate ABC transporter (MRP2) | + |
| At1g30400 | 5.9 | 0.011 | glutathione S-conjugate ABC transporter (MRP1) | + |
| At3g55090 | 3.7 | 0.050 | ABC transporter family protein | − |
| At3g21250 | 3.3 | 0.030 | ABC transporter family protein | + |
| At2g29500 | 57.8 | 0.005 | 17.6-kD class I small heat shock protein (HSP17.6B-CI) | − |
| At4g36990 | 12.3 | 0.005 | Heat shock factor protein 4 (HSF4) | − |
| At3g12580 | 5.4 | 0.049 | Heat shock protein 70, putative/HSP70, putative | − |
| At3g23990 | 3.5 | 0.025 | Chaperonin (CPN60) (HSP60) | + |
| At1g56300 | 3.5 | 0.043 | DNAJ heat shock N-terminal domain–containing protein | − |
| At4g20860 | 25.7 | 0.003 | FAD binding domain–containing protein | + |
| At1g32350 | 22.1 | 0.017 | Alternative oxidase, putative (AOX3) | + |
| At4g01870 | 20.1 | 0.006 | TOLB protein–related | + |
| At4g37990 | 15.0 | 0.012 | Mannitol dehydrogenase, putative (ELI3-2) | − |
| At5g16980 | 9.5 | 0.008 | NADP-dependent oxidoreductase, putative | + |
| At2g39200 | 7.1 | 0.009 | Seven transmembrane MLO family protein/(MLO12) | − |
| At1g75280 | 5.5 | 0.009 | Isoflavone reductase, putative | − |
| At3g05360 | 4.9 | 0.006 | Disease resistance family protein/LRR family protein | − |
| At3g13610 | 4.3 | 0.023 | Oxidoreductase, 2OG-Fe(II) oxygenase family protein | − |
| At5g15870 | 3.7 | 0.040 | Glycosyl hydrolase family 81 protein | − |
| At5g16970 | 3.3 | 0.019 | NADP-dependent oxidoreductase, putative | + |
| At5g16980 | + | |||
| At5g16990 | − | |||
| At5g17000 | + | |||
| At5g37980 | 3.0 | 0.038 | NADP-dependent oxidoreductase, putative | + |
Shown are genes related to detoxification, secondary metabolism, and stress responses that are greater than threefold induced in PPA1-treated (75 μM) Arabidopsis cell cultures relative to controls. Microarray data are derived from three biologically independent experiments; details are given in Supplemental Table 1 online.
Table 2.
Genes Downregulated by PPA1 Treatment
| Gene Locus | Fold Induction | P | Description |
|---|---|---|---|
| At3g11700 | 5.1 | 0.026 | β-Ig-H3 domain–containing protein/fasciclin domain–containing protein |
| At1g44110 | 4.4 | 0.013 | Cyclin, putative |
| At1g08560 | 4.0 | 0.016 | Syntaxin-related protein KNOLLE (KN)/syntaxin 111 (SYP111) |
| At5g23860 | 3.8 | 0.025 | Tubulin β-8 chain (TUB8) (TUBB8) |
| At4g03620 | 3.7 | 0.018 | Myosin heavy chain–related |
| At3g12110 | 3.6 | 0.028 | Actin11 (ACT11) |
| At4g34160 | 3.5 | 0.031 | Cyclin delta-3 (CYCD3) |
| At1g18370 | 3.2 | 0.011 | Kinesin motor family protein (NACK1) |
| At1g76540 | 3.1 | 0.023 | Cell division control protein, putative |
| At3g11520 | 3.1 | 0.027 | Cyclin, putative (CYC2) |
| At5g09870 | 5.3 | 0.025 | Cellulose synthase, catalytic subunit, putative |
| At2g23130 | 5.2 | 0.022 | Arabinogalactan protein (AGP17) |
| At1g70710 | 5.1 | 0.045 | Endo-1,4-β-glucanase (EGASE)/cellulase |
| At4g12730 | 5.1 | 0.018 | Fasciclin-like arabinogalactan protein (FLA2) |
| At5g57560 | 5.1 | 0.023 | Endo-xyloglucan transferase (TCH4) |
| At4g28250 | 4.9 | 0.023 | β-Expansin, putative (EXPB3) |
| At3g02120 | 4.6 | 0.035 | Hyp-rich glycoprotein family protein |
| At3g62110 | 4.5 | 0.006 | Glycoside hydrolase family 28 protein/polygalacturonase (pectinase) family protein |
| At4g38400 | 4.4 | 0.015 | Expansin family protein (EXPL2) |
| At3g15720 | 4.4 | 0.028 | Glycoside hydrolase family 28 protein/polygalacturonase (pectinase) family protein |
| At3g06770 | 4.1 | 0.029 | Glycoside hydrolase family 28 protein/polygalacturonase (pectinase) family protein |
| At3g03050 | 4.0 | 0.027 | Cellulose synthase family protein (CslD3) |
| At1g02730 | 3.7 | 0.006 | Cellulose synthase family protein |
| At5g64740 | 3.1 | 0.046 | Cellulose synthase, catalytic subunit, putative |
| At1g15580 | 10.1 | 0.025 | Indoleacetic acid–induced protein 5 (IAA5)/auxin-induced protein (AUX2-27) |
| At1g23080 | 6.8 | 0.013 | Auxin efflux carrier protein, putative |
| At4g32280 | 5.2 | 0.013 | Auxin-responsive AUX/IAA family protein |
| At1g73590 | 4.3 | 0.049 | Auxin efflux carrier protein, putative (PIN1) |
| At5g65670 | 4.1 | 0.013 | Indoleacetic acid–induced protein 9 (IAA9) |
| At5g43700 | 3.8 | 0.016 | Indoleacetic acid–induced protein 4 (IAA4)/auxin-induced protein (AUX2-11) |
| At1g04240 | 3.4 | 0.024 | Indoleacetic acid–induced protein 3 (IAA3) |
| At2g33310 | 3.2 | 0.036 | Indoleacetic acid–induced protein 13 (IAA13) |
| At3g62100 | 3.2 | 0.028 | Auxin-responsive protein, putative |
| At4g28640 | 3.1 | 0.025 | Indoleacetic acid–induced protein 11 (IAA11) |
Shown are genes related to cell division/cell organization, cell wall metabolism, and the auxin pathway, which are 33% or less expressed in PPA1-treated (75 μM) Arabidopsis cell cultures relative to controls. Microarray data are derived from three biologically independent experiments; details are given in Supplemental Table 2 online.
Gene Regulation by PPA1 Is Similar to Regulation by OPDA and Pathogens but Not by JA
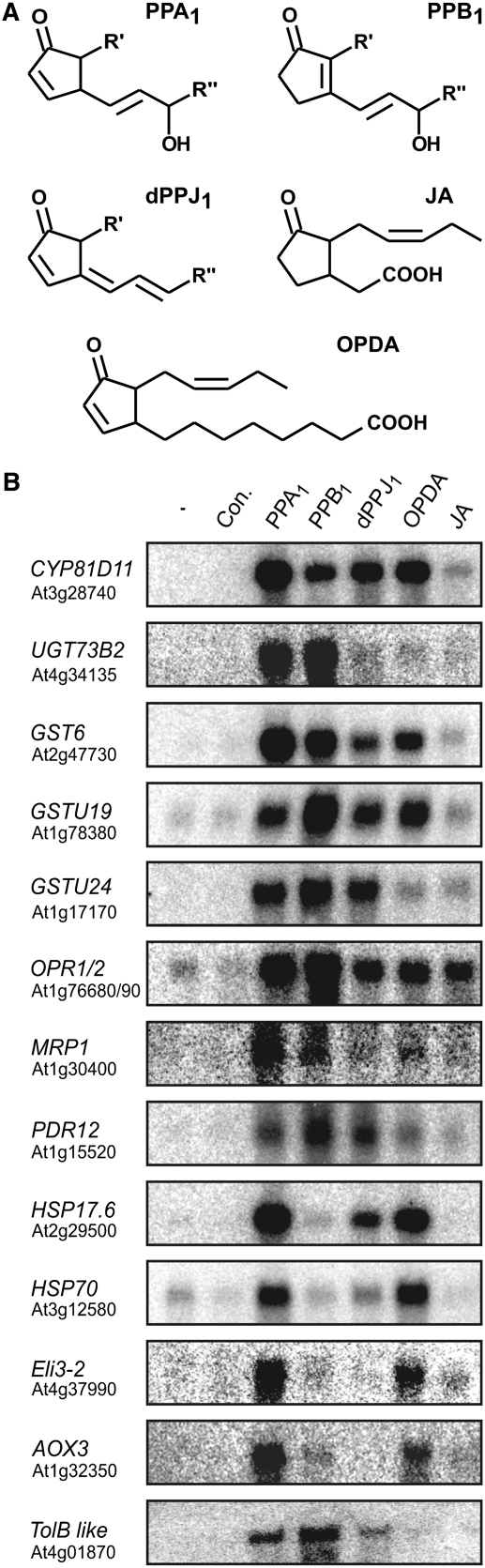
In order to verify the microarray data and compare the regulation of gene expression by different oxylipins, the expression of 13 genes in response to phytoprostanes of the A1-, B1-, and dJ1 type and to the enzymatically formed oxylipins OPDA and JA was studied by RNA gel blot analysis (Figure 2). Representative genes were chosen from each group of PPA1-responsive genes related to detoxification (cytochrome P450 enzymes, UDP-glucuronyl/glycosyltransferases, GSTs, OPR1/2, and ABC transporters) and stress responses (heat shock proteins, ELI3, alternative oxidase, and TOLB-related). Expression of all genes was induced by PPA1, confirming the microarray data. By contrast, JA did not induce or only slightly induced the expression of these genes, indicating differences in the effect of this C12-cyclopentanone to the C18-cyclopentenones. One exception was OPR1/2, which was clearly induced by JA, albeit more weakly than by PPA1. Gene expression was not specifically regulated by PPA1 but, in addition, all genes were also upregulated by one or more of the other oxylipins tested. Expression of most of the genes related to detoxification was also elevated in response to the other two phytoprostanes and to OPDA. Expression of the stress-related genes was also induced by OPDA; expression of both heat shock proteins was additionally induced by dJ1-phytoprostanes. Some of these genes showed the strongest induction with PPA1 (UGT73B2, GST6, HSP17.6, HSP70, and AOX3), while other genes exhibited the strongest increase in response to PPB1 (GST U19, OPR1/2, PDR12, and TOLB).
Figure 2.
Regulation of Gene Expression by Different Oxylipins in Arabidopsis.
(A) Chemical structures of the different oxylipins used. Phytoprostanes are composed of type I (R′ = C7H14COOH, R″ = C2H5) and type II (R′ = C2H5, R″ = C7H14COOH) stereoisomers.
(B) Expression of PPA1-responsive genes in Arabidopsis cell cultures in response to 75 μM OPDA, JA, and A1-, B1-, and dJ1-phytoprostanes or in cultures not treated (−) or treated with 0.5% methanol in water as control (Con.). Cell cultures were harvested at 4 h after treatments. The experiment was repeated at least three times; a representative RNA gel blot is shown. Eight micrograms of RNA was loaded per lane, and blots were hybridized with the indicated probes. Gel loading was monitored by ethidium bromide staining of the gel (see Supplemental Figure 1 online).
The Internet source Genevestigator (www.genevestigator.ethz.ch) (Zimmermann et al., 2004) was used to compare in silico the regulation of gene expression by different stimuli. The greatest overlap with the set of genes upregulated by PPA1 was evident with genes upregulated by Pseudomonas syringae (Table 3). This is in agreement with the model that pathogen treatment leads to enhanced production of ROS, which then trigger the formation of nonenzymatic oxylipins. In fact, it has been shown that treatment of Arabidopsis plants with P. syringae leads to an accumulation of nonenzymatically formed hydroxy fatty acids and phytoprostanes (Grun et al., 2007). Also in support of this model, high similarity to the set of PPA1-responsive genes was found with the genes induced after treatment with ozone, a common ROS. We compared gene induction by the oxylipins OPDA (39% based on the data of Taki et al. [2005]) and methyl jasmonate (MeJa) with gene induction by PPA1 and found high similarity with induction by OPDA but little similarity with induction by MeJa, in agreement with our RNA gel blot data (Figure 2).
Table 3.
Regulation of PPA1-Responsive Genes by Different Stimuli
| Treatment | Coregulated Genes (%) |
|---|---|
| Pseudomonas syringae | 50 |
| Botrytis cinerea | 38 |
| Benzoxazolin-2-one | 28 |
| Salicylic acid | 19 |
| Methyl jasmonate | 3 |
| Ozone | 42 |
Shown are percentages of PPA1-induced genes (at least threefold) that are also at least threefold increased by the treatments listed according to Genevestigator analysis and to Baerson et al. (2005) for the allelochemical benzoxazolin-2-one.
To test whether gene induction by PPA1 in whole plants is similar to induction in the cell culture system used to date, expression in response to PPA1 and OPDA was analyzed in plants by microarray analyses using the ATH1 chip. Similar to gene regulation in the cell culture system, treatment of plants with PPA1 changed the mRNA levels of 651 genes by greater than twofold, and a high proportion of the induced genes were related to detoxification and stress responses (see Supplemental Table 3 online). Thirty percent of the genes that were upregulated in the cell culture experiment were also induced in whole plants. This indicates that both systems are similar but also that differences in the physiological and morphological state between cell culture and plants can lead to dramatic differences in responsiveness. Treatment with OPDA altered the expression of 1221 genes by greater than twofold (see Supplemental Table 3 online). The list of OPDA-induced genes may also include genes that are responsive to JA rather than OPDA, since OPDA might have been converted to JA prior to gene induction. In agreement with in silico analysis, 42% of the PPA1-induced genes were upregulated by PPA1 and OPDA by greater than threefold (see Supplemental Table 4 online). Interestingly, the overlap of genes induced by PPA1 in plants with the set of JA-induced genes (threefold regulation) was higher (7%) than in the cell culture system (3%), again supporting differences between cell culture and whole plants. Expression of a subset of genes was verified using RNA gel blot analysis (see Supplemental Figure 3 online). Since the plant system has the advantage that mutants can be analyzed, this system was chosen for further experiments.
TGA Factors Are Involved in Gene Induction by Phytoprostanes and OPDA
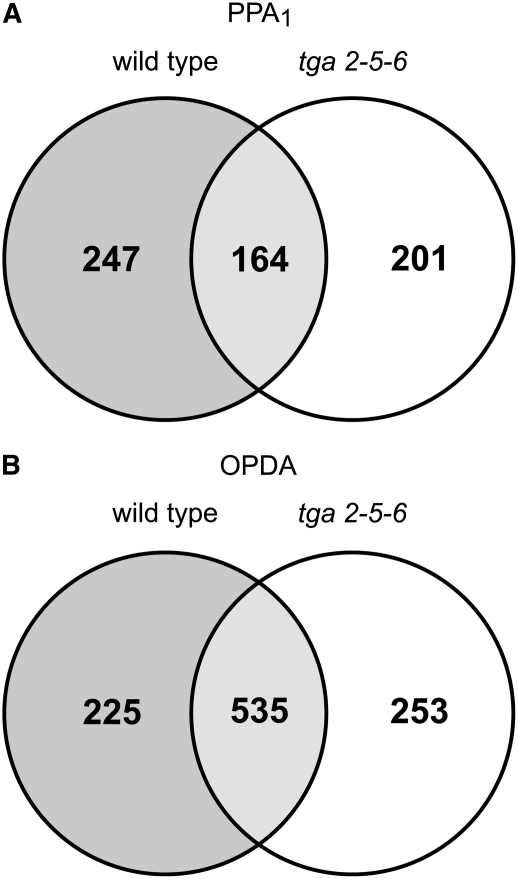
Little is known about signal transduction pathways mediating the response to OPDA and phytoprostanes. To find transcription factors potentially involved in the response to these oxylipins, the promoters of genes that show a greater than twofold induction by PPA1 were analyzed for a high abundance of specific binding sites using The Arabidopsis Information Resource Motif Analysis tool. In the cell culture system and in the plant system, 49% and 53%, respectively, of the promoters of PPA1-upregulated genes contain a TGA motif (TGACG) in the first 500 bp upstream of the start codon. These TGA motifs constitute putative binding sites for TGA transcription factors (Lam et al., 1989). To investigate whether TGA transcription factors play a role in the response to PPA1 and OPDA, gene regulation in a triple mutant defective in the expression of TGA2, TGA5, and TGA6 was analyzed using Affymetrix ATH1 chips. Of the 411 genes upregulated by PPA1 in the wild type, 247 (corresponding to 60%; Figure 3A) were not induced in the mutant tga2-5-6, indicating that indeed the transcription factors TGA2, TGA5, and TGA6 are involved in mediating gene regulation by PPA1. Analysis of the 500-bp upstream regions revealed that 42% of the genes induced by PPA1 in the wild type but not in tga2-5-6 contain TGACG motifs (included in Supplemental Table 3 online). This indicates that in 58% of TGA-dependent genes, either binding motifs are different from TGACG or these genes are indirectly regulated by TGA factors through other transcription factors that are responsive to TGA2, TGA5, and TGA6.
Figure 3.
Venn Diagram Comparing Genes Upregulated by Oxylipins in Wild-Type and tga2-5-6 Plants.
Expression was analyzed in wild-type and tga2-5-6 Arabidopsis plants at 4 h after treatment with 75 μM OPDA or PPA1 in comparison with controls.
(A) Number of genes with twofold or greater induction by PPA1 in wild-type and tga2-5-6 plants.
(B) Number of genes with twofold or greater induction by OPDA in wild-type and tga2-5-6 plants.
In the case of OPDA, 30% of the genes (225 of 760) induced in the wild type were not induced in tga2-5-6 (Figure 3B), indicating that these transcription factors are also involved in mediating gene regulation by OPDA. Expression of 48 genes was increased by PPA1 as well as OPDA in a TGA-dependent manner (Table 4).
Table 4.
Genes Upregulated by PPA1 and OPDA Treatment Dependent on the Presence of TGA2, TGA5, and TGA6
| Fold Induction
|
TGACG Present | P
|
||||
|---|---|---|---|---|---|---|
| Gene Locus | Description | PPA1 | OPDA | PPA1 | OPDA | |
| At5g13080 | WRKY family transcription factor | 10.4 | 4.4 | + | 0.009 | 0.006 |
| At3g26830 | Cytochrome P450 71B15, putative (CYP71B15) | 9.6 | 7.9 | − | 0.034 | 0.023 |
| At5g22300 | Nitrilase 4 (NIT4) | 9.3 | 6.6 | + | 0.021 | 0.013 |
| At2g43500 | RWP-RK domain–containing protein | 8.4 | 2.2 | + | 0.032 | 0.035 |
| At3g25190 | Nodulin, putative | 6.8 | 3.1 | − | 0.019 | 0.007 |
| At2g34660 | Glutathione S-conjugate ABC transporter (MRP2) | 6.6 | 2.4 | + | 0.016 | 0.003 |
| At5g52020 | AP2 domain–containing protein | 5.8 | 2.2 | − | 0.040 | 0.020 |
| At2g34500 | Cytochrome P450 family protein | 5.8 | 3.8 | − | 0.026 | 0.012 |
| At5g51500 | Pectinesterase family protein | 5.5 | 4.6 | + | 0.030 | 0.004 |
| At2g12190 | Cytochrome P450, putative | 5.4 | 3.3 | − | 0.021 | 0.013 |
| At1g64950 | − | |||||
| At1g64940 | − | |||||
| At1g64930 | − | |||||
| At5g02780 | In2-1 protein, putative | 5.2 | 3.0 | + | 0.019 | 0.021 |
| At3g10500 | No apical meristem (NAM) family protein | 4.7 | 2.1 | + | 0.006 | 0.007 |
| At2g37770 | Aldo/keto reductase family protein | 4.4 | 3.7 | + | 0.016 | 0.009 |
| At1g30400 | Glutathione S-conjugate ABC transporter (MRP1) | 4.1 | 2.1 | + | 0.005 | 0.007 |
| At3g59140 | ABC transporter family protein | 3.9 | 2.4 | − | 0.021 | 0.029 |
| At3g14620 | Cytochrome P450, putative | 3.8 | 2.7 | − | 0.013 | 0.032 |
| At1g17860 | Trypsin and protease inhibitor family protein/Kunitz family protein | 3.7 | 2.4 | + | 0.006 | 0.005 |
| At5g59510 | Expressed protein | 3.7 | 2.4 | − | 0.035 | 0.050 |
| At5g03490 | UDP-glucoronosyl/UDP-glucosyl transferase family protein | 3.7 | 2.5 | + | 0.019 | 0.034 |
| At3g63380 | Calcium-transporting ATPase, plasma membrane-type, putative/Ca2+-ATPase, putative (ACA12) | 3.5 | 5.9 | + | 0.025 | 0.009 |
| At3g09270 | Glutathione S-transferase, putative | 3.5 | 2.6 | − | 0.013 | 0.008 |
| At3g01420 | Pathogen-responsive α-dioxygenase, putative | 3.4 | 2.1 | − | 0.041 | 0.014 |
| At1g72680 | Cinnamyl alcohol dehydrogenase, putative | 3.3 | 2.0 | + | 0.011 | 0.004 |
| At1g72900 | Disease resistance protein (TIR-NBS class), putative | 3.3 | 3.7 | + | 0.034 | 0.028 |
| At3g48850 | Mitochondrial phosphate transporter, putative | 3.2 | 2.6 | + | 0.021 | 0.016 |
| At3g01970 | WRKY family transcription factor | 3.2 | 4.1 | − | 0.036 | 0.023 |
| At4g22070 | WRKY family transcription factor | 3.1 | 2.2 | + | 0.046 | 0.015 |
| At1g13990 | Expressed protein | 3.0 | 3.0 | + | 0.033 | 0.011 |
| At5g19440 | Cinnamyl alcohol dehydrogenase, putative (CAD) | 2.9 | 2.4 | − | 0.010 | 0.005 |
| At5g14570 | Transporter, putative | 2.8 | 2.1 | + | 0.040 | 0.008 |
| At1g64660 | Cys/Met metabolism pyridoxal-phosphate–dependent enzyme family protein | 2.8 | 6.5 | − | 0.018 | 0.019 |
| At3g21700 | Expressed protein | 2.7 | 2.3 | − | 0.016 | 0.011 |
| At5g22860 | Ser carboxypeptidase S28 family protein | 2.7 | 3.4 | + | 0.012 | 0.020 |
| At1g63340 | Flavin-containing monooxygenase–related/FMO-related flavin–containing monooxygenase family protein/FMO family protein | 2.7 | 2.6 | + | 0.036 | 0.025 |
| At1g62580 | + | |||||
| At2g21620 | Universal stress protein (USP) family protein/responsive to desiccation protein (RD2) | 2.7 | 2.1 | + | 0.025 | 0.031 |
| At1g23440 | Pyrrolidone-carboxylate peptidase family protein | 2.5 | 2.1 | + | 0.006 | 0.026 |
| At1g17020 | Oxidoreductase, 2OG-Fe(II) oxygenase family protein | 2.4 | 2.6 | − | 0.045 | 0.028 |
| At5g17860 | Cation exchanger, putative (CAX7) | 2.3 | 3.9 | 0.040 | 0.012 | |
| At2g43510 | Trypsin inhibitor, putative | 2.3 | 7.3 | − | 0.016 | 0.003 |
| At1g33590 | Disease resistance protein–related/LRR protein–related | 2.3 | 2.5 | + | 0.030 | 0.017 |
| At4g30490 | AFG1-like ATPase family protein | 2.2 | 2.2 | − | 0.034 | 0.009 |
| At4g37980 | Mannitol dehydrogenase, putative (ELI3-1) | 2.2 | 2.7 | − | 0.024 | 0.006 |
| At5g65300 | Expressed protein | 2.2 | 2.5 | + | 0.042 | 0.026 |
| At3g14680 | Cytochrome P450, putative | 2.1 | 2.4 | − | 0.041 | 0.016 |
| At1g06800 | Lipase class 3 family protein | 2.1 | 2.2 | − | 0.017 | 0.016 |
| At2g47800 | Glutathione conjugate transporter (MRP4) | 2.1 | 2.7 | + | 0.032 | 0.008 |
| At3g05880 | Hydrophobic protein (RCI2A)/low temperature– and salt-responsive protein (LTI6A) | 2.1 | 2.2 | + | 0.045 | 0.029 |
| At2g24180 | Cytochrome P450 family protein | 2.1 | 2.0 | − | 0.017 | 0.010 |
Shown are genes that show twofold or greater induction by PPA1 as well as OPDA (75 μM) in Arabidopsis wild-type plants relative to controls but no induction in tga2-5-6. Microarray data are derived from three biologically independent experiments; details are given in Supplemental Table 3 online.
Interestingly, the array data revealed that >200 genes were upregulated by PPA1 and OPDA in tga2-5-6 but not induced by these oxylipins in the wild type (Figure 3). This suggests that TGA transcription factors might also negatively regulate gene expression. In addition, 86 and 80 genes showed greater than twofold higher or lower expression in the wild-type control samples compared with tga2-5-6 control samples, respectively, indicating that the TGA transcription factors are also important for basal expression (see Supplemental Table 5 online). Several of the genes with lower basal expression in tga2-5-6 as well as with TGA-dependent induction were related to detoxification. Among the detoxification genes that were induced by PPA1 and OPDA, 50% showed induction or basal expression dependent on TGA2, TGA5, and TGA6. Additionally, several genes exhibited lower induction in tga2-5-6 than in the wild type, indicating partial dependence on TGA2, TGA5, and TGA6. One example is OPR1/2, which was 6-fold induced by PPA1 in the wild type and 4.7-fold induced in tga2-5-6. In agreement with the array data, lower expression and/or induction in tga2-5-6 was also found in RNA gel blot analysis for a subset of genes (see Supplemental Figure 4 online).
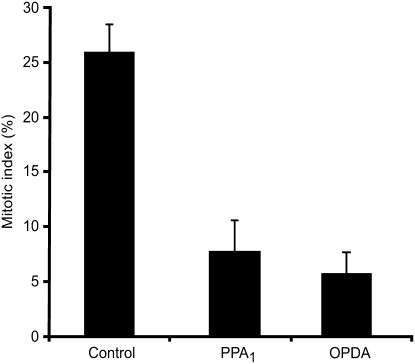
PPA1 and OPDA Inhibit Cell Cycle Progression and Root Growth
The gene expression data indicate that PPA1 may induce stress responses and inhibit growth and cell division. In order to prove that gene regulation reflects physiological processes occurring in the plant, we investigated whether PPA1 inhibits root growth and the progression of the cell cycle. The tobacco (Nicotiana tabacum) BY2 cell culture is an established system for the analysis of cell cycle regulation. For synchronization of the cell cycle, aphidicolin was used to arrest the cells at the G1/S phase transition. After removal of aphidicolin, cell cycle progression was monitored by determining the mitotic index. Nine hours after aphidicolin removal, 26% of the cells were in mitosis. Treatment with 15 μM OPDA or PPA1 led to a reduction of the mitotic index to 6% for OPDA and 7% for PPA1 (Figure 4). To exclude the possibility that these oxylipins simply increase cell death, which would also result in a decreased mitotic index, cells were stained with trypan blue and cell death was monitored. In all samples, cell viability was ∼80%, indicating that OPDA and PPA1 did not trigger cell death but indeed reduced the number of cells that enter mitosis.
Figure 4.
Inhibition of Cell Cycle Progression by OPDA and PPA1 in Tobacco.
Synchronized Bright Yellow 2 cells were treated with 15 μM OPDA or PPA1. The percentage of cells in mitosis was determined after 9 h (means ± sd; n = 3 independent experiments).
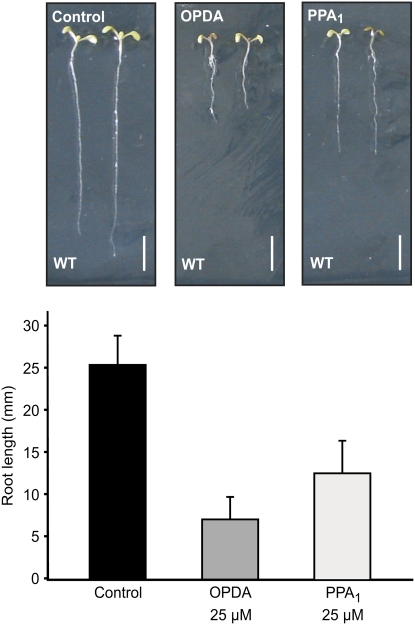
The effects of PPA1 and OPDA on root growth were tested by growing seedlings on vertical plates with solid medium containing 25 μM of either oxylipin. Overall growth of the seedlings, and particularly root growth, was reduced on oxylipin-containing medium. After 8 days, root length was 28 and 50% of control values on OPDA and PPA1 medium, respectively (Figure 5). In order to investigate whether OPDA or its metabolite JA is responsible for root growth inhibition, the opr3 mutant, which cannot convert OPDA to JA, was analyzed. This mutant also showed shorter roots on OPDA-containing medium, indicating that indeed OPDA inhibits root growth (see Supplemental Figure 5 online). However, inhibition of root growth by OPDA in opr3 was less than in the wild type, suggesting a contribution of JA to the observed inhibition effect. Inhibition by JA in opr3 was similar to that in the wild type.
Figure 5.
Inhibition of Root Growth by OPDA and PPA1 in Arabidopsis.
Seedlings were germinated on medium containing 25 μM OPDA or PPA1, and root growth was measured after 8 d (means ± sd; n = 20 seedlings). Three independent experiments were performed with similar results.
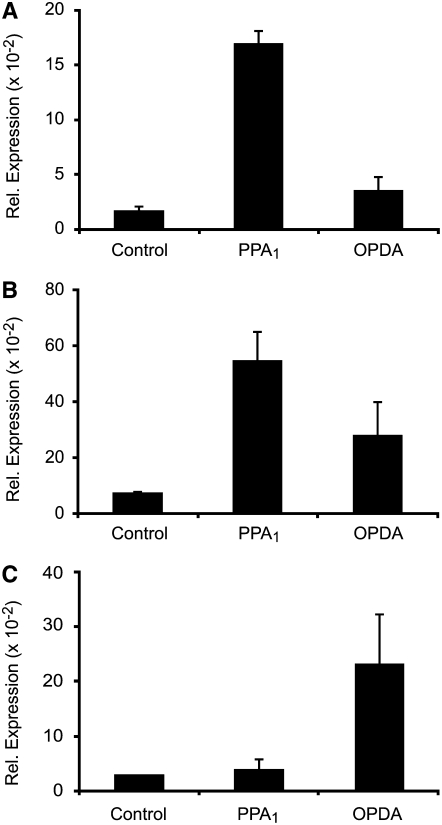
PPA1 and OPDA Can Be Metabolized by OPR1
PPA1 and OPDA are reactive molecules containing an α,β-unsaturated carbonyl structure that can bind to free thiol groups in proteins by Michael addition. The expression of a high number of genes related to detoxification was upregulated by PPA1. The proteins encoded by the induced genes could possibly be involved in the typically nonspecific metabolism of an array of reactive lipid peroxidation products (electrophiles), including OPDA and PPA1. Reduction of cyclopentenones to cyclopentanones is one metabolic pathway that results in the elimination of the chemically reactive, electrophilic α,β-unsaturated carbonyl structure. For OPDA, it is known that OPR3 catalyzes this reaction, leading to OPC8:0, which is a precursor in the biosynthesis of JA. In Arabidopsis, there are at least two additional genes coding for OPR-like proteins that are not involved in the jasmonate pathway (Schaller et al., 2000). As shown above, the expression of OPR1/2 is induced by OPDA and PPA1. Since the nucleotide sequences of the OPR1 and OPR2 cDNAs share 87% identity, the expression of both genes cannot be distinguished by the ATH1 chip or by RNA gel blot analysis. Therefore, the expression of OPR1 and OPR2 in response to OPDA and PPA1 was analyzed by real-time quantitative PCR experiments using primer pairs specific for OPR1, OPR2, and OPR3. As shown in Figure 6, mRNA levels of OPR1 and OPR2 increased strongly in response to PPA1 (10-fold and 8-fold for OPR1 and OPR2, respectively), while mRNA levels of OPR3, which is involved in the jasmonate pathway, were not elevated. By contrast, OPR3 mRNA levels were induced eightfold by OPDA, while OPR1 and OPR2 were only moderately induced (twofold and fourfold, respectively).
Figure 6.
Regulation of OPR1, OPR2, and OPR3 Expression by OPDA and PPA1.
Expression of OPR1 (A), OPR2 (B), and OPR3 (C) in response to OPDA and PPA1. Arabidopsis cell cultures were treated with 75 μM OPDA, 75 μM PPA1, or 0.5% methanol/water (Control) and harvested after 4 h. Relative levels of expression were determined by real-time quantitative RT-PCR. Values were normalized to the expression of Actin2/8 (means ± sd; n = 3 independent experiments).
To investigate whether PPA1 are substrates for OPR1 and OPR3, these proteins were expressed as (His)6-tagged fusion proteins in Escherichia coli and purified by affinity chromatography. The enzymatic activities with PPA1 as substrates and, for comparison, with OPDA were determined. The reaction was followed by monitoring the oxidation of the cosubstrate NADPH (see Supplemental Figure 6 online). The reaction products were identified by HPLC coupled to tandem mass spectrometry (HPLC-MS/MS; see Supplemental Figure 7 online). In agreement with published data, OPR3 catalyzed the reduction of OPDA much more effectively than OPR1 (Schaller et al., 2000). Specific activities for OPR1 and OPR3 were 0.7 and 2.9 nkat/mg protein, respectively. By contrast, PPA1 were better substrates for OPR1 (7.1 nkat/mg) than for OPR3 (2.9 nkat/mg). It was expected that the reduction of the electrophilic cyclopentenone ring system would dramatically alter the signaling potency. To test this, the biological activity of the enzymatic products of reduced OPDA (by OPR3) and PPA1 (by OPR1) on the induction of the OPDA- and PPA1-responsive GST6 promoter was analyzed using luciferase reporter plants. Activation of the GST6 promoter by the enzymatically converted oxylipins was clearly reduced in comparison with OPDA and PPA1 (see Supplemental Figure 8 online).
PPA1 and OPDA Are Substrates for GST6
Another commonly used cellular strategy for metabolizing reactive substances is to conjugate them to GSH by both nonenzymatic and enzymatic GST-catalyzed reactions. The microarray experiments showed the induction of eight GSTs by PPA1 (Table 1). From these GSTs, GST6 is strongly induced by PPA1 as well as OPDA and is predicted to be localized in the chloroplast, where the biosynthesis of OPDA takes place (Delker et al., 2006) and the majority of phytoprostanes are expected to be formed because of the predominant presence of the precursor linolenic acid in the plastid membrane. Therefore, PPA1 and OPDA were tested as potential substrates for GST6. The open reading frame of GST6 without the chloroplast target sequence and with a C-terminal His6 tag was expressed in E. coli. The protein was purified by affinity chromatography and incubated with GSH and PPA1 or OPDA. After different times, samples were subjected to HPLC-MS/MS analysis and levels of PPA1 or OPDA were quantified. In addition, the resulting oxylipin-GSH conjugates were identified by the detection of specific fragments generated by collision-induced dissociation (CID). Major fragments of the PPA1-GSH adduct (m/z 308, 293, 275, and 179) and the OPDA-GSH adduct (m/z 598, 308, 273, and 179) were used to monitor the reaction in the multiple reaction monitoring (MRM) mode (see Supplemental Figures 9 and 10 online). Under the incubation conditions used, nonenzymatic conjugation could not be observed. From the initial reaction kinetics, the specific activities for the different substrates were determined. Purified GST6 displayed a specific activity of 110 pkat/mg protein for OPDA and 20 pkat/mg protein for PPA1.
In Vivo Accumulation of PPA1-GSH and OPDA-GSH Conjugates in Arabidopsis after P. syringae Infection
In tomato (Solanum lycopersicum), it has been shown that basal levels of free PPA1 are rather low in comparison with the levels of OPDA (13 versus 212 ng/g dry weight, respectively). Levels of jasmonates, PPA1, and other phytoprostanes were found to accumulate after infection with Botrytis cinerea. For example, levels of JA and PPA1 both increased by a factor of 4 (Thoma et al., 2003). However, in Arabidopsis leaves, basal levels of free PPA1 were below the limit of detection of the HPLC-MS/MS system, while levels of OPDA in the untreated control were in the range of 0.31 ± 0.093 μg/g dry weight and those in the mock-infiltrated (partially wounded) leaves were in the range of 2.12 ± 0.12 μg/g dry weight. After infiltration of P. syringae into leaves, a dramatic accumulation of free OPDA was observed (175 ± 7.57 μg/g dry weight at 10 h after infection), while PPA1 was still undetectable.
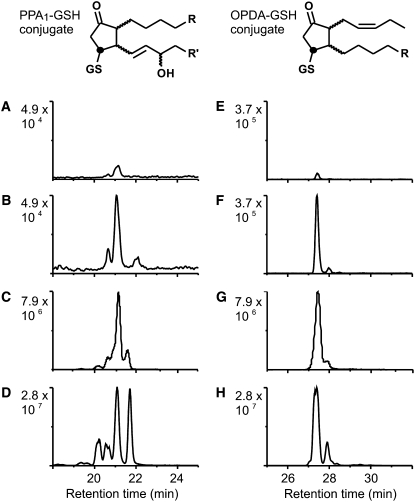
Typically, cyclopentenones are rapidly metabolized in both plants and animals. In untreated and mock-infiltrated leaves, OPR reduction metabolites of OPDA and PPA1 could not be detected. However, GSH adducts of both PPA1 and OPDA could be unequivocally identified by HPLC-MS/MS by their retention times and four characteristic MRM fragment ions (Figure 7; see Supplemental Figures 9 and 10 online). Due to the lack of appropriate isotopically labeled standards, the conjugates could not be quantified. Interestingly, the relative ratio of the OPDA-GSH adduct to the PPA1-GSH adduct was 2.3:1. After infiltration of P. syringae, the relative intensity of the peak area of both conjugates increased dramatically and the ratio of the OPDA to the PPA1 adduct increased slightly to 5.6:1. These results indicate that PPA1 are formed and rapidly metabolized in Arabidopsis leaves. Although there was a huge difference in the levels of free PPA1 and OPDA, the ratio of their GSH adducts was in the same range in mock-infiltrated and infected leaves. As shown in Figure 7, the isomer distribution of the PPA1-GSH adducts clearly indicates that the adducts in Arabidopsis leaves are enzymatic products and not formed by nonenzymatic conjugation to GSH.
Figure 7.
HPLC-MS/MS Chromatograms of PPA1 and OPDA Adducts to GSH.
Endogenous PPA1-GSH and OPDA-GSH adducts from Arabidopsis leaves and in vitro–synthesized adducts were analyzed and unequivocally identified by four characteristic MRM transitions (PPA1-GSH: m/z 616→598, 616→308, 616→273, and 616→179; OPDA-GSH: m/z 600→308, 600→293, 600→275, and 600→179). Chromatograms display the total ion current of the four transitions. Peaks represent different isomers of the conjugate.
(A) and (E) Relative levels of PPA1-GSH (A) and OPDA-GSH (B) at 10 h after mock infiltration of Arabidopsis leaves.
(B) and (F) Relative levels of PPA1-GSH (B) and OPDA-GSH (F) at 10 h after P. syringae infiltration of Arabidopsis leaves.
(C) and (G) PPA1-GSH (C) and OPDA-GSH (G) enzymatically synthesized using recombinant GST6 at pH 7.5.
(D) and (H) PPA1-GSH (D) and OPDA-GSH (H) adducts prepared by nonenzymatic conjugation at pH 10.
PPA1 are a mixture of two racemic regioisomers (in total, 12 stereoisomers), while synthetic OPDA comprises 4 stereoisomers. Stereoisomers are not completely resolved by the HPLC column. Enzymatic conjugation with GSH is expected to result in conjugates ([C] and [G]) with either R or S configuration at the labeled carbon. By nonenzymatic reaction with GSH, R- and S-configured conjugates are formed, which doubles the number of stereoisomers. The peak pattern of endogenously occurring conjugates suggests that they have been formed enzymatically by GST(s).
DISCUSSION
Biological Activities of Phytoprostanes, OPDA, and JA
The best studied oxylipins in plants and animals, jasmonates and prostaglandins, respectively, are enzymatically synthesized signaling molecules involved in development, reproduction, and host defense. In addition, all organisms that utilize polyunsaturated fatty acid substrates for oxylipin pathways also contain chemically oxidized lipids. Some oxidized lipids, such as the plant phytoprostanes and mammalian isoprostanes, are structural congeners of jasmonates and prostaglandins. Notably, phytoprostanes and isoprostanes comprise evolutionarily old classes of biologically active, oxidized lipids that were prevalent throughout the evolution of jasmonates in plants and prostaglandins in animals (Mueller, 2004). Moreover, the biological activities of several phytoprostanes appear to overlap at least partially with those of jasmonates (Thoma et al., 2003) and prostaglandins (Traidl-Hoffmann et al., 2005; Mariani et al., 2007). For instance, in plants, several classes of phytoprostanes as well as OPDA and JA trigger the synthesis of antimicrobial and/or antioxidative secondary metabolites (Iqbal et al., 2005; Loeffler et al., 2005). One of the first biological activities discovered for JA/MeJa was its retarding effect on root and seedling growth (Dathe et al., 1981; Yamane et al., 1981). More recently, the inhibition of cell cycle progression by JA was also reported (Swiatek et al., 2002). Both of these JA activities are shared by OPDA and PPA1 (Figures 4 and 5) and a variety of other oxylipins (Vellosillo et al., 2007), indicating that some biological effects can be displayed by structurally different enzymatically and chemically oxidized lipids. Potentially, common biological activities may indicate and represent evolutionarily conserved functions of oxidized lipids.
The full spectrum of biological activities of phytoprostanes remains to be uncovered and to be compared with that of jasmonates such as OPDA and JA. Using Affymetrix full genome arrays, we analyzed the impact of a cyclopentenone phytoprostane, PPA1, on gene expression in Arabidopsis cell cultures and plants. The results indicate that gene regulation by the two cyclopentenone compounds PPA1 and OPDA is to a large extent similar (93 genes were greater than threefold induced by OPDA and PPA1), while there is clearly less overlap with JA-responsive genes (16 genes are greater than threefold induced by MeJa and PPA1 in plants according to an analysis performed with the Genevestigator system).
The cyclopentenone oxylipin gene induction profile also shows similarities to the xenobiotic defense response in plants (Table 3) and animals. Molecules that trigger a xenobiotic defense response in Arabidopsis comprise a variety of structurally diverse, membrane-permeable lipophilic xenobiotics such as barbiturates, herbicides, benzenesulfonamide safeners, (chloro)phenols, and others (Baerson et al., 2005). Due to the broad spectrum of xenobiotic elicitors, it has been postulated that broad-specificity chemosensory mechanisms recognize xenobiotic lipids and coordinately upregulate batteries of detoxifying enzymes exhibiting broad substrate specificities (Baerson et al., 2005).
OPDA and Phytoprostane Signaling
It remains to be clarified how the phytoprostane and OPDA signal is perceived and transduced in plants. By contrast, several factors contributing to JA signaling are known. COI1 is a central regulator for JA responses. In addition, transcription factors involved in JA signaling have been identified. The first transcription factor thus described was ORCA3, regulating JA responses in Catharanthus roseus (van der Fits and Memelink, 2000). In Arabidopsis, the MYC transcription factor JIN1 was also identified as a downstream factor in JA signaling (Lorenzo et al., 2004). Recently, the JAZ family of proteins was discovered to be the missing link between COI1 and downstream transcription factors. The JAZ protein JAI3 is a target for COI1-dependent degradation and regulates the activity of JIN1 (Chini et al., 2007). Similar functions might be performed by the other members of the JAZ family. In addition, an interaction of COI1 and JAZ1 that is dependent on the presence of the Ile conjugate of JA has been reported, suggesting that this complex is responsible for JA conjugate perception (Thines et al., 2007). The differences in gene induction between JA and OPDA/phytoprostanes suggest the existence of more than one receptor and of differences in the corresponding signal transduction pathways.
This study has uncovered some of the downstream factors in the response to OPDA and PPA1. The high abundance of TGA motifs in the promoters of PPA1-induced genes prompted us to investigate whether TGA transcription factors are involved in mediating responses to OPDA and phytoprostanes. In the mutant tga2-5-6, which is defective in the expression of TGA2, TGA5, and TGA6, 60% of the genes that are induced by PPA1 in the wild type were not responsive to PPA1. In the case of OPDA, 30% of the OPDA-inducible genes did not respond to OPDA in tga2-5-6 (Figure 3). This suggests that TGA factors indeed contribute to PPA1 and OPDA signaling. Besides this similarity in OPDA and PPA1 signal transduction mechanisms, these data also point to differences between both oxylipins, since a higher proportion of PPA1 induction than of OPDA induction is dependent on TGA2, TGA5, and TGA6.
While the induction of several genes by phytoprostanes and OPDA is signaled through TGA factors, it remains unclear whether TGA factors are also involved in mediating some responses to JA. Analysis of the expression of two genes that are induced by PPA1, OPDA, and JA indicates that TGA factors might also be involved in JA responses concurrently with COI1 (see Supplemental Figure 12 online). This suggests that oxylipin signaling is not mediated by two strictly separated pathways dependent on either COI1 or TGAs but that the situation is more complex. Even though uncovering the function of TGA factors in mediating cyclopentenone effects is an important step in the elucidation of oxylipin signaling, this is only the beginning. Genetic screens as well as the analysis of mutants with defects in distinct signal transduction components are required to unravel the mechanisms involved in oxylipin responses.
TGA transcription factors belong to the family of basic Leu zipper transcription factors and bind to activating sequence 1–like elements that constitute salicylic acid (SA)–inducible cis elements. TGA factors are positive regulators of the expression of pathogenesis-related (PR) genes and GSTs in tobacco as well as in Arabidopsis (Chen and Singh, 1999; Johnson et al., 2001). The tga2-5-6 triple mutant is compromised in SA-mediated effects such as systemic acquired resistance and the induction of PR1 expression by SA analogs (Zhang et al., 2003). The mutant is also affected in the repression of PDF1.2 expression by SA (Ndamukong et al., 2007), indicating that these transcription factors are also negative regulators. Here, we show that this mutant is also impaired in some responses to OPDA and PPA1. This indicates that SA, OPDA, and PPA1 use a common family of transcription factors and corresponding promoter elements. There is some overlap in gene regulation by SA and OPDA/PPA1 (19% of PPA1-induced genes are also induced by SA; Table 3), but most SA- or PPA1-responsive genes are not regulated by both signals. This suggests that the signaling of oxylipin responses by TGA transcription factors is not mediated through SA. Notably, TGA transcription factors have also been implicated in the activation of xenobiotic-responsive promoters (Baerson et al., 2005). The activity of TGA factors is regulated by the interaction with other proteins. The proteins TGA2, TGA5, and TGA6 interact with NPR1, a central regulator of SA responses. TGA2 was recently reported to also interact with a glutaredoxin that inhibits the expression of the JA-responsive gene PDF1.2. However, expression of GST6 seems to be independent of NPR1 and glutaredoxin (Uquillas et al., 2004; Ndamukong et al., 2007), indicating the existence of additional factors regulating TGA activity. The identification of additional interaction partners of TGA factors may help to dissect the mechanisms that regulate the functions of TGA factors in response to different stimuli.
Phytoprostane and OPDA Metabolism
Why are nonenzymatically formed oxylipins perceived and how do they induce responses in the plant? The accumulation of an array of nonenzymatically produced oxylipins upon biotic and abiotic stress has been reported (Thoma et al., 2003; Montillet et al., 2004; Block et al., 2005; Grun et al., 2007). Chemically oxidized lipids are formed in complex membrane lipids in situ and can be liberated from membranes by unknown lipases that may initiate both membrane repair and signaling. Moreover, levels of esterified and free oxidized lipids are excellent markers of oxidative membrane damage in both plants (Imbusch and Mueller, 2000) and animals (Morrow, 2006). Therefore, oxidized lipids may be interpreted by cells as oxidative stress signals (Mueller, 2004). Moreover, since many of the released oxidized lipids, such as the cyclopentenone compounds, are chemically reactive electrophiles, rapid metabolism of endogenous electrophiles is required to cope with the consequences of oxidative stress.
Recently, it was proposed that a subgroup of oxylipins, RES, induce a common cluster of defense genes (Almeras et al., 2003; Weber et al., 2004; Farmer and Davoine, 2007). RES are characterized by their inherent chemical reactivity, which allows them to modify proteins. According to this concept, OPDA as well as A1- and dJ1-phytoprostanes would be classified as RES, because these compounds contain an α,β-unsaturated carbonyl structure and can bind to free thiol groups. By contrast, B1-phytoprostanes and JA are weak electrophiles that cannot modify proteins directly. However, RNA gel blot gene expression analysis of oxylipin-treated Arabidopsis leaves revealed a mixed result indicating that chemical reactivity and gene induction do not always correlate (Figure 2). From the 13 PPA1-responsive genes tested, only OPR1/2 was found to be strongly induced by JA in the cell culture system. All other genes were differentially induced by OPDA and phytoprostanes. Apparently, all tested genes were induced by more than one oxylipin species. Hence, the biological activities of OPDA, phytoprostanes, and other oxidized lipids partially overlap and do not strictly correlate with their electrophilicity. Notably, the three phytoprostanes tested in this experiment represent only a small fraction of a much larger array of oxidized lipids that are produced simultaneously during oxidative stress. These results suggest that the induction of many detoxification and stress genes (i.e., GSTs) can be triggered by several oxidized lipids. For example, the electrophilic compound malondialdehyde, which, like phytoprostanes and jasmonates, is mainly derived from linolenic acid, also induces OPR1/2 and several GSTs and heat shock proteins (Weber et al., 2004). Additionally, the tocopherol-deficient mutant vte2-1 contains dramatically increased levels of several nonenzymatically formed oxylipins, including phytoprostanes and malondialdehyde, and exhibits increased expression of OPR1 and several GSTs (Sattler et al., 2006; Menè-Saffrané et al., 2007). Therefore, an additive gene-inducing effect of oxidized lipids appears to be more likely the rule than the exception. On the one hand, detoxification of reactive lipids might be an important general mechanism to reduce oxidative stress, but on the other hand, metabolism/detoxification may also alter/terminate the signal represented by a number of oxidized lipids. Metabolites of oxidized lipids are often less reactive but, in addition, might possess biological activities different from those of cyclopentenone oxylipins.
Modification of proteins by oxylipins has been shown in animals to regulate protein activity and function. If this is also a regulatory mechanism in plants, strict control of the levels of these compounds would be important. The chemical reactivity and protein-modifying capacity of cyclopentenones can be eliminated (e.g., by reduction of the ring double bond by OPR enzymes or conjugation to GSH by GSTs). Here, we show that OPDA and PPA1 not only induce several OPR and GST enzymes but are also substrates of at least two strongly induced enzymes, OPR1 and GST6, in vitro. Both types of enzymes have been reported to display a broad substrate specificity (Costa et al., 2000; Wagner et al., 2002). Hence, it is expected that induction of the detoxification enzymes can be triggered by several oxidized lipids, which in turn can be metabolized by one or several of these enzymes along with a variety of other oxidized lipids. In vivo, exogenously added OPDA is rapidly metabolized by OPRs (Delker et al., 2007). In addition, the OPDA-GSH conjugate has been shown to accumulate in tobacco plants treated with cryptogein (Davoine et al., 2006). When PPA1 were infiltrated into Arabidopsis leaves, PPA1 levels decreased rapidly and <50% of the administered PPA1 could be recovered after 30 min (190.3 ± 19.3 μg/g dry weight immediately after infiltration, 38.6 ± 5.2 μg/g dry weight after 30 min). Metabolite analysis revealed a rapid accumulation of the PPA1-GSH adduct, while OPR products (cyclopentanone derivatives of PPA1) were barely detectable. Both GSH adducts of OPDA and PPA1 could be identified in Arabidopsis leaves at low levels and after treatment with P. syringae at elevated levels (Figure 7). Moreover, the isomer pattern of PPA1-GSH adducts clearly indicated that the conjugate was formed enzymatically by GST(s) and not through the relatively slow nonenzymatic Michael addition reaction. The following sequence of events can be hypothesized: (1) pathogen infection increases ROS and activates the jasmonate pathway, (2) leading to the formation of both chemically and enzymatically oxidized cyclopentenones and other RES; (3) these RES enhance the expression of detoxification enzymes, (4) resulting in the accumulation of GSH conjugates and other lipid metabolites. In animals, the conjugation with GSH is usually followed by further metabolism and excretion (Alary et al., 2003). In plants, the subsequent fate of the membrane-impermeable GSH adducts is unknown. Conjugation of RES with GSH might render them inactive or alter their biological activity. The high number of PPA1-induced ABC transporters indicates that these substances might be transported to and stored in the vacuole. Further investigations are needed to elucidate in detail the function and mechanism of cyclopentenone metabolism.
METHODS
Oxylipins
JA was prepared by alkaline hydrolysis of MeJA. OPDA was synthesized from linolenic acid using linseed acetone powder and purified by HPLC as described (Parchmann et al., 1997). The phytoprostanes A1, B1, and dJ1 were prepared and characterized for their purity as described previously (Thoma et al., 2003). All oxylipins were diluted to a final concentration of 75 μM in 0.5% methanol before administration. Controls were either treated with 0.5% methanol in water or not treated as indicated.
Cell Suspension Cultures
A mixotrophic cell suspension culture of Arabidopsis thaliana has been established from callus culture (Laboratoire de Cytologie Expérimental et Morphogenèse Végétale, Université Piérre et Marie Curie) and was cultivated in Gamborg's B5 medium supplemented with 2% sucrose on a rotary shaker at 26°C. The culture was subcultured weekly, and 3-d-old cell cultures were used for experiments. Cell cultures were divided into 10-mL aliquots at 1 d prior to treatments. Lipids were dissolved in methanol at a concentration of 15 mM and added to the cell culture to yield a final concentration of 75 μM. Controls were treated with the same volume of methanol.
Plant Material
For oxylipin treatments, Arabidopsis plants (ecotype Columbia [Col-0], tga2-5-6, and GST6:Luc) were grown in Murashige and Skoog liquid medium containing 2% (w/v) sucrose on an orbital shaker under 9 h of light (180 μmol·m−2·s−1 or μE·m−2·s−1) at 22°C. The mutant tga2-5-6 was kindly supplied by X. Li (University of British Columbia). The GST6:luciferase line was kindly provided by K. Singh (Centre for Environmental and Life Sciences, Australia). Seeds were surface-sterilized and sown on 24-well plates containing 1 mL of medium per well, 10 seeds per well. After 7 d, the medium was exchanged against 1 mL of fresh medium, and 3 d later, the plants were treated with 75 μM OPDA, phytoprostanes A1, B1, and dJ1, or JA.
Bacterial Cultivation and Treatment of Plants
The bacterial strain Pseudomonas syringae pv tomato DC3000 with a plasmid containing the avrRpm1 gene was used (provided by B. Staskawicz, University of Berkeley). The bacteria were cultured in King's B medium containing 50 mg/L rifampicin and 10 mg/L tetracycline at 28°C. For inoculation, bacteria were pelleted in a centrifuge, resuspended in 10 mM MgCl2, and adjusted to an OD600 of 0.2 (equivalent to 108 colony-forming units/mL). Arabidopsis plants (Col-0) were grown in soil at 22°C under a 9-h photoperiod (180 μmol·m−2·s−1 or μE·m−2·s−1). For treatment with Pseudomonas syringae pv tomato DC3000, 6-week-old plants showing a uniform appearance were infiltrated with the bacterial suspension using a needleless syringe. Control plants were infiltrated with 10 mM MgCl2. Plants were harvested at 10 h after infiltration and immediately frozen in liquid nitrogen.
RNA Gel Blot Analysis
Plant material (cell culture material or 10-d-old seedlings) was homogenized by a grinding mill, and total RNA was isolated with TriFast (Peqlab) according to the manufacturer's protocol. RNA was separated on 1% agarose gels and blotted on nitrocellulose membranes. Probes were randomly labeled with [α-32P]dATP as described in the manufacturer's protocol (Fermentas). Hybridization was performed in 50% formamide, 5× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate), 0.1% SDS, and 5× Denhardt's solution (1× Denhardt's solution is 0.02% Ficoll, 0.02% polyvinylpyrrolidone, and 0.02% BSA) at 42°C for 18 h. The membranes were washed with decreasing salt concentrations at 42°C. A final washing step was performed in 0.2× SSC and 0.1% SDS. Filters were exposed to a screen for 3 to 48 h, and screens were scanned with a phosphorimager (Fuji BAS2000).
DNA for probes was prepared using the following primers: for GST6 (At2g47730), forward, 5′-AGTCAAGAGCCATCACACAGTACC-3′, and reverse, 5′-CTACTGCTTCTGGAGGTCAATAACC-3′, 449 bp; for GSTU24 (At1g17170), forward, 5′-GTGAATGTTACGGCGAGAAGG-3′, and reverse, 5′-TACTCCAACCCAAGTTTCTTCCTAC-3′, 341 bp; for GSTU19 (At1g78380), forward, 5′-ACTAGGACAAGCCATTAAATCCAG-3′, and reverse, 5′-GACATTGCGTTGATTGGATTCTAC-3′, 371 bp; for CYP81D11 (At3g28740), forward, 5′-TATGAGCCGTCGGATCTCATC-3′, and reverse, 5′-GACATTGCGTTGATTGGATTCTAC-3′, 498 bp; for UGT73B2 (At4g34135), forward, 5′-CCGGAAGCTTCTAACCCTGC-3′, and reverse, 5′-AAGACTTGTGTTCCACGGCAC-3′, 542 bp; for MRP1 (At1g30400), forward, 5′-CGGAGAATCTTCTTTCAAATGGA3′, and reverse, 5′-AGAGATGTCACTTTGGTTTAGAGAGTC3′, 368 bp; for PDR12 (At1g15520), forward, 5′-GTTTCTTGAGTTTCCAGAGGAGTTTC3′, and reverse, 5′-CCAAGCGAGTCCTAGTATGAGAAGAAAC-3′, 302 bp; for HSP70 (At3g12580), forward, 5′-CGTGTAGAGTATTATGCCCAGTCG-3′, and reverse, 5′-CGATCAAGGACGAGAAGATCG-3′, 464 bp; for HSP17.6 (At2g29500), forward, 5′-CCAGAGATCTGAATAGACTTAACATCAG-3′, and reverse, 5′-TCTTCAACAACAGACGAAGC-3′, 439 bp; for OPR1/2 (At1g76680/90), forward, 5′-ATGGAGCTAATGGCTATCT-3′, and reverse, 5′-GGTAATCGGTGTAACCGAC-3′, 549 bp; for ELI3-2 (At4g37990), forward, 5′-GAGGAAGATGGTAATGGGAAGTATG-3′, and reverse, 5′-CTATTCATTTATTGGATTAAGCATACCA-3′, 328 bp; for AOX3 (At1g32350), forward, 5′-CAGAGTTTGAACGGTCCAATATG-3′, and reverse, 5′-ATGAACATAACCCAATCTGAAGATC-3′, 498 bp; and for TOLB (At4g01870), 5′-TCACGAGCAACCTGATCG-3′, and reverse, 5′-GTGGTAGCCGAGGAACTC-3′, 613 bp.
Quantitative Real-Time RT-PCR
Total RNA was extracted from mixotrophic cell cultures using the plant RNeasy extraction kit (Qiagen) according to the manufacturer's protocol. First-strand cDNA synthesis and quantitative real-time RT-PCR experiments were performed as described previously (Szyroki et al., 2001) using Lightcycler 3.1 (Roche). Primers used were as follows: for OPR1 (At1g76680), forward, 5′-GCACCGCTGAATAAGTACG-3′, and reverse, 5′-GTTAAGTTATGTTGGTCTC-3′, 256 bp; for OPR2 (At1g76690), forward, 5′-CTCCTATCTCTTGTACGGG-3′, and reverse, 5′-TGTGAGAACAGCTGCTAT-3′, 492 bp; and for OPR3 (At2g06050), forward, 5′-GCAGAGTATTATGCTCAAC-3′, and reverse, 5′-GAGGTTTCGGGTACTTCAC-3′, 322 bp. The number of transcripts was normalized to the constitutively expressed Actin2/8 mRNA (An et al., 1996).
Root Growth Analysis
Sterilized seeds were sown on vertically oriented square Petri dishes (120 mm × 120 mm) containing Murashige and Skoog medium supplemented with 2% (w/v) sucrose, 3% agar, and oxylipins in a final concentration of 25 μM. Freshly prepared plates were always used to avoid product breakdown or instability. Plates were stored overnight at 4°C and then placed in a 9-h light (180 μmol·m−2·s−1 or μE·m−2·s−1)/15-h dark regime at 22°C.
Bacterial Expression of GST6
A plasmid containing the GST6 (At2g47730) coding sequence (CDS) in the vector pUNI51, obtained from F. Theodoulou (Crop Performance and Improvement Division, Hertfordshire, UK), served as a template for amplification of the CDS lacking the predicted chloroplast localization sequence (the first 144 bp of GST6). Specific oligonucleotides (forward, 5′-CATCCCATGGCCAGTATCAAGGTTCACG-3′, and reverse, 5′-CTCGAGCTGCTTCTGGAGGTCAATAACC-3′) were used to amplify bases 145 to 789 of the CDS, introducing NcoI and a novel XhoI restriction site at position 790 of the coding sequence. This 645-bp CDS fragment was cloned into pGEM T vector (Promega) and subcloned into pET28 (Novagen) via NcoI and HindIII. For bacterial expression, Escherichia coli BL21 cells harboring the GST6 expression plasmid were cultured at 37°C on a rotary shaker (200 rpm) until the culture reached the log phase. Isopropylthio-β-d-galactoside was added to the culture (final concentration, 1 mM), and the bacteria were incubated at 28°C for 24 h. Cells of a 200-mL suspension were harvested and lysed by sonication. The crude extract was cleared by centrifugation (10,000g). For metal affinity chromatography, Ni-Tris-carboxymethyl ethylene diamine resin (Machery-Nagel) was utilized. The purification was monitored by SDS-PAGE (12.5%) under denaturing conditions (see Supplemental Figure 11 online) and determination of specific activity using 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. The assay was performed according to Mannervik and Guthenberg (1981) in a final volume of 1.0 mL containing 925 μL of 100 mM potassium phosphate buffer, pH 6.5, 25 μL of CDNB (40 mM in ethanol), 20 μL of GSH (50 mM), and 10 μg of purified enzyme. The reaction was followed with a spectrophotometer at 340 nm during 20 min. A molar extinction coefficient of 9.6 mM−1 cm−1 for CDNB was used for the calculations. The amount of soluble proteins was determined according to the method of Bradford (1976) using BSA as a standard. Specific activities of the enzyme preparations were between 53 and 64 nkat/mg protein
Bacterial Expression of OPR Isoenzymes
Plasmids for the expression of OPR1 and OPR3 isoenzymes containing the complete coding sequence of OPR1 and OPR3 in the protein expression vector pQE-30 (Schaller and Weiler, 1997; Schaller et al., 2000) were obtained from F. Schaller (University of Bochum). The (His)6-tagged proteins were purified with Ni-Tris-carboxymethyl ethylene diamine affinity columns as described above. The purification was monitored by SDS-PAGE (12.5%) under denaturing conditions (see Supplemental Figure 11 online).
Enzyme Assay for OPR Activity
The activity of OPR was determined as described by Costa et al. (2000). Assay reactions were performed at 25°C in 1 mL of potassium phosphate buffer, pH 7.5, containing 120 μM NADPH, 10 μg of purified protein, and different oxylipin substrates at a concentration of 0.1 mM. A molar extinction coefficient of 6.3 mM−1 cm−1 for NADPH was used for the calculations.
For the identification of products, the assay (total volume of 0.5 mL instead of 1 mL, 1 mM NADPH instead of 0.12 mM) was allowed to proceed for 30 min at 25°C. The reaction was stopped by setting the pH to 4 by the addition of 10 μL of HOAc and directly analyzed by LC-MS/MS. LC-MS/MS analyses were performed using a 1200 Agilent HPLC system coupled to a Micromass Quattro Premier triple-quadrupole mass spectrometer (Waters). The column (Purospher Star-RP 18ec, 2 × 125 mm i.d., 5-μm particle size; Merck) was eluted with a linear mobile phase gradient (0.2 mL/min flow rate) starting from 1 mM ammonium acetate:acetonitrile (55:45, v/v) at 0 min to ammonium acetate:acetonitrile (05:95, v/v) at 25 min. The mass spectrometer was operated in the negative electrospray ionization (ESI) mode. CID fragment spectra were recorded using argon (0.3 mL/min) as the collision gas (26 and 22 eV of collision energy for OPDA and PPA1, respectively).
Analysis of Cyclopentanone Effects on GST6:Luciferase Expression
GST6:luciferase plants were grown in liquid medium as described above. OPDA and PPA1 reduction products (cyclopentanones) were prepared by enzymatic reduction of OPDA and PPA1 using OPR3 and OPR1, respectively. The assays were essentially done as described above except that enzyme incubations were performed in the growth medium of the plants instead of phosphate buffer (1 mL of Murashige and Skoog liquid medium with 2% [w/v] sucrose containing 120 μM NADPH and 100 μM OPDA or PPA1 with or without 10 μg of purified OPR3 or OPR1 protein, respectively). This was important to ensure the health of the plants after addition of the incubation mixture. The reaction was monitored by UV light absorption. After 25 min at 25°C, the enzymatic reaction was stopped by heating the mixture for 10 min at 95°C. After cooling, the growth medium of the plants was replaced by the incubation mixture or plants were treated with 0.5% methanol in water containing 120 μM NADPH (controls). After 1 h of incubation, 50 μL of luciferin solution (1 mM Na-luciferin, 1 mM ATP, and 0.01% Triton X-100) was added to each well. Luminescence was measured at 10-min intervals for 12 h using a photon-counting video system (Visitron Systems). Quantitative evaluation of the images was performed using the program MetaMorph.
Analysis of Oxylipin GSH Adducts
For analysis of the oxylipin GSH adducts, frozen leaves were ground and extracted with 50 mM borate buffer, pH 4.0. The homogenate was centrifuged at 4000g at 4°C for 15 min. The supernatant was transferred into an HPLC vial, and 10 μL was analyzed by LC-MS/MS analysis. LC-MS/MS analyses were performed using a 1200 Agilent HPLC system coupled to a Micromass Quattro Premier triple-quadrupole mass spectrometer (Waters). The column (Purospher Star-RP 18ec, 2 × 125 × mm i.d., 5-μm particle size; Merck) was eluted with a linear mobile phase gradient (0.2 mL/min flow rate) starting from acetonitrile:water:formic acid (15:85:0.1, v/v) at 0 min to acetonitrile:water:formic acid (45:55:0.1, v/v) at 30 min. The mass spectrometer was operated in the positive ESI mode in the MRM mode. Argon (0.3 mL/min) was used as the collision gas (22 eV of collision energy).
Reference substances of PPA1 and OPDA adducts were generated by nonenzymatic formation as described by Davoine et al. (2005). The reaction mixture (1 mL), containing 1 mM GSH and 0.1 mM of the different oxylipins in 20 mM borate buffer, pH 10, was incubated at room temperature for 2 h. For enzymatic, GST6-catalyzed formation of adducts, incubations were performed in the presence of purified GST6 (200 μg of protein) at pH 7.5 for 15 min. The production of GSH adducts was monitored by LC-MS/MS in the positive ESI mode. CID fragment spectra were first recorded on a Micromass Quatro Premier mass spectrometer (Waters). Argon (0.3 mL/min) was used as the collision gas (22 eV of collision energy). Adducts were identified by four characteristic MRM transitions {PPA1-GSH: m/z 616→598 ([M+H]+ − H2O), 616→308 ([M+H]+ − PPA1), 616→273 ([M+H]+ − PPA1 − 2H2O), and 616→179 (Glu); OPDA-GSH: m/z 600→308 ([M+H]+ − OPDA), 600→293 ([M+H]+ − GSH), 600→275 ([M+H]+ − GSH − H2O), and 600→179 (Glu)}.
Microarray Analysis
For microarray analysis, total RNA was extracted using the RNeasy plant mini kit (Qiagen) according to the manufacturer's protocol. One microgram of total RNA was linearly amplified and biotinylated using the One-Cycle target-labeling kit (Affymetrix) according to the manufacturer's instructions. Labeling and hybridization were conducted by the microarray facility of the Universitaetsklinikum Tuebingen, Germany. Fifteen micrograms of labeled and fragmented copy RNA was hybridized to Arabidopsis ATH1 Gene Chip arrays (Affymetrix). After hybridization, the arrays were washed and stained in a Fluidics Station 450 (Affymetrix) with the recommended washing procedure. Biotinylated copy RNA bound to target molecules was detected with streptavidin-coupled phycoerithrin, biotinylated anti-streptavidin IgG antibodies, and again streptavidin-coupled phycoerithrin according to the protocol. Arrays were scanned using the GCS3000 Gene Chip scanner (Affymetrix) and GCOS 1.3 software. Scanned images were subjected to visual inspection to control for hybridization artifacts and proper grid alignment and analyzed with Microarray Suite 5.0 (Affymetrix) to generate report files for quality control.
Three independent replicates were done for each treatment. For statistical data analysis, the CEL files were imported into Genespring 7.1 (Agilent Technologies) using Genespring's implementation of GC-RMA for normalization and probe summarization (Wu et al., 2003). Additionally, genes were median-centered by dividing all signal values for a gene by the median of all signals for that gene. Transcripts with a high variance in replicate measurements were removed. From the remaining set, genes that showed an at least twofold increase or decrease in average expression were analyzed by Welsh's t test for significant differences and corrected for multiple testing according to Benjamini and Hochberg (1995). Functions of differentially expressed transcripts were annotated using the NetAFFX analysis center.
Cell Cycle Analysis
Cell suspension cultures of tobacco (Nicotiana tabacum) cv Bright Yellow 2 were cultivated in Murashige and Skoog medium, pH 5.8, supplemented with 3% sucrose, 0.1 g/L myo-inositol, 0.2 g/L KH2PO4, 1 mg/L thiamine, and 0.2 mg/L 2,4-D on a rotary shaker at 26°C. Cultures were subcultured weekly, and 7-d-old cell cultures were used for the experiments. Synchronization and determination of the mitotic index were essentially done as described by Sano et al. (2006). For synchronization, 10-mL culture aliquots were treated with aphidicolin (5 mg/L; Sigma-Aldrich). After 23 h, aphidicolin was removed by washing the cells with 10 volumes of washing solution (100 mM MES, pH 5.8, 3% sucrose, and 0.2 mg/mL 2,4-D) using cellulose filters in 5-mL empty glass columns. Cells were transferred back to 10 mL of fresh medium. OPDA or PPA1 was added at a final concentration of 15 μM to the synchronized culture. The oxylipin stock solutions were diluted 1:200, resulting in a final methanol concentration in the treated culture of 0.5%. An appropriate volume of methanol was added to control cell cultures. Nuclei were stained with 0.5 μg/mL 4′,6-diamidino-2-phenylindole dihydrochloride and 1% glutaraldehyde at 8, 8.5, and 9 h after oxylipin addition. The mitotic index was determined with a fluorescence microscope. At least 1000 nuclei were examined for each sample. Cell death was analyzed by determining the percentage of blue-stained cells after incubating the sample with 0.2% trypan blue for 5 min.
Websites
Gene annotation was performed using the NetAFFX analysis center (www.affymetrix.com/analysis/index.affx) and MapMan (http://gabi.rzpd.de/projects/MapMan). Gene regulation analyses were done using the website www.genevestigator.ethz.ch (Zimmermann et al., 2004). Analysis of gene homology was performed using NCBI/BLAST (www.ncbi.nlm.nih.gov).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: At3g28740 (CYP81D11), At4g34135 (UGT73B2), At2g47730 (GST6), At1g17170 (GSTU24), At1g78380 (GSTU19), At1g30400 (MRP1), At1g15520 (PDR12), At3g12580 (HSP70), At2g29500 (HSP17.6), At1g76680 (OPR1), At1g76690 (OPR2), At2g06050 (OPR3), At4g37990 (ELI3-2), At1g32350 (AOX3), and At4g01870 (TOLB). The microarray data were deposited in the Gene Expression Omnibus (accession number GSE10749; http://www.ncbi.nlm.nih.gov/projects/geo/query/acc.cgi?acc=GSE10749).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Loading Controls for RNA Gel Blot Analysis of Cell Culture Samples.
Supplemental Figure 2. Loading Controls for RNA Gel Blot Analysis of Plant Samples.
Supplemental Figure 3. RNA Gel Blot Analysis of Plants Treated with 75 μM PPA1 or 75 μM OPDA after 2, 4, and 6 h.
Supplemental Figure 4. RNA Gel Blot Analysis of the Oxylipin Response in the tga2-5-6 Mutant after 2, 4, and 6 h.
Supplemental Figure 5. Inhibition of Root Growth by OPDA and JA in opr3.
Supplemental Figure 6. Spectrophotometric Analysis of OPDA and PPA1 Reduction.
Supplemental Figure 7. Fragment Spectra of Substrates and Products of OPR Assays.
Supplemental Figure 8. Effects on Biological Activity of OPDA and PPA1 of Metabolism by OPR Enzymes.
Supplemental Figure 9. Fragment Spectra of the PPA1-GSH Adduct.
Supplemental Figure 10. Fragment Spectra of the OPDA-GSH Adduct.
Supplemental Figure 11. Purification of the Recombinant OPR1, OPR3, and GST6 Proteins.
Supplemental Figure 12. Regulation of Gene Expression by Oxylipins in the Mutants tga2-5-6 and coi1.
Supplemental Table 1. Differentially Expressed Genes Upregulated by PPA1 Treatment in Arabidopsis Cell Culture.
Supplemental Table 2. Differentially Expressed Genes Downregulated by PPA1 Treatment in Arabidopsis Cell Culture.
Supplemental Table 3. Four Data Sheets of Differentially Expressed Genes Regulated by PPA1 or OPDA Treatment in Wild-Type and tga2-5-6 Arabidopsis Plants.
Supplemental Table 4. Differentially Expressed Genes Upregulated by PPA1 as Well as OPDA Treatment in Arabidopsis Plants.
Supplemental Table 5. Differentially Expressed Genes in Wild-Type and tga2-5-6 Controls.
Supplementary Material
Acknowledgments
Special thanks go to the working group of P. Ache (Julius-von-Sachs-Institute, University of Wuerzburg) for technical support on real-time experiments and to H. Warzecha (Institute of Botany, University of Darmstadt) and C. Gatz (Albrecht-von-Haller-Institute, University of Goettingen) for fruitful discussion regarding TGA factor signaling. We are grateful to F. Theodoulou for supplying the GST6-CDS prior to publication. This work was supported by the German Research Foundation through SFB 567 and GK 1342.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Susanne Berger (berger@biozentrum.uni-wuerzburg.de).
Online version contains Web-only data.
References
- Alary, J., Gueraud, F., and Cravedi, J.P. (2003). Fate of 4-hydroxynonenal in vivo: Disposition and metabolic pathways. Mol. Aspects Med. 24 177–187. [DOI] [PubMed] [Google Scholar]
- Almeras, E., Stolz, S., Vollenweider, S., Reymond, P., Mene-Saffrane, L., and Farmer, E.E. (2003). Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 34 205–216. [DOI] [PubMed] [Google Scholar]
- An, Y.Q., McDowell, J.M., Huang, S., McKinney, E.C., Chambliss, S., and Meagher, R.B. (1996). Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 10 107–121. [DOI] [PubMed] [Google Scholar]
- Baerson, S.R., Sanchez-Moreiras, A., Pedrol-Bonjoch, N., Schulz, M., Kagan, I.A., Agarwal, A.K., Reigosa, M.J., and Duke, S.O. (2005). Detoxification and transcriptome response in Arabidopsis seedlings exposed to the allelochemical benzoxazolin-2(3H)-one. J. Biol. Chem. 280 21867–21881. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodological) 57 289–300. [Google Scholar]
- Block, A., Schmelz, E., Jones, J.B., and Klee, H.J. (2005). Coronatine and salicylic acid: The battle between Arabidopsis and Pseudomonas for phytohormone control. Mol. Plant Pathol. 6 79–83. [DOI] [PubMed] [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal. Biochem. 72 248–254. [DOI] [PubMed] [Google Scholar]
- Chen, W., and Singh, K.B. (1999). The auxin, hydrogen peroxide and salicylic acid induced expression of the Arabidopsis GST6 promoter is mediated in part by an ocs element. Plant J. 19 667–677. [DOI] [PubMed] [Google Scholar]
- Chini, A., Fonseca, S., Fernandez, G., Adie, B., Chico, J.M., Lorenzo, O., Garcia-Casado, G., Lopez-Vidriero, I., Lozano, F.M., Ponce, M.R., Micol, J.L., and Solano, R. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448 666–671. [DOI] [PubMed] [Google Scholar]
- Costa, C.L., Arruda, P., and Benedetti, C.E. (2000). An Arabidopsis gene induced by wounding functionally homologous to flavoprotein oxidoreductases. Plant Mol. Biol. 44 61–71. [DOI] [PubMed] [Google Scholar]
- Dathe, W., Rönsch, H., Preiss, A., Schade, W., Sembdner, G., and Schreiber, K. (1981). Endogenous plant hormones of the broad bean, Vicia faba L. (−)-jasmonic acid, a plant growth inhibitor in pericarp. Planta 153 530–535. [DOI] [PubMed] [Google Scholar]
- Davoine, C., Douki, T., Iacazio, G., Montillet, J.L., and Triantaphylides, C. (2005). Conjugation of keto fatty acids to glutathione in plant tissues. Characterization and quantification by HPLC-tandem mass spectrometry. Anal. Chem. 77 7366–7372. [DOI] [PubMed] [Google Scholar]
- Davoine, C., Falletti, O., Douki, T., Iacazio, G., Ennar, N., Montillet, J.L., and Triantaphylides, C. (2006). Adducts of oxylipin electrophiles to glutathione reflect a 13 specificity of the downstream lipoxygenase pathway in the tobacco hypersensitive response. Plant Physiol. 140 1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delker, C., Stenzel, I., Hause, B., Miersch, O., Feussner, I., and Wasternack, C. (2006). Jasmonate biosynthesis in Arabidopsis thaliana—Enzymes, products, regulation. Plant Biol. 8 297–306. [DOI] [PubMed] [Google Scholar]
- Delker, C., Zolman, B.K., Miersch, O., and Wasternack, C. (2007). Jasmonate biosynthesis in Arabidopsis thaliana requires peroxisomal beta-oxidation enzymes—Additional proof by properties of pex6 and aim1. Phytochemistry 68 1642–1650. [DOI] [PubMed] [Google Scholar]
- Devoto, A., Ellis, C., Magusin, A., Chang, H.S., Chilcott, C., Zhu, T., and Turner, J.G. (2005). Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol. Biol. 58 497–513. [DOI] [PubMed] [Google Scholar]
- Farmer, E.E., and Davoine, C. (2007). Reactive electrophile species. Curr. Opin. Plant Biol. 10 380–386. [DOI] [PubMed] [Google Scholar]
- Gobel, C., Feussner, I., Hamberg, M., and Rosahl, S. (2002). Oxylipin profiling in pathogen-infected potato leaves. Biochim. Biophys. Acta 1584 55–64. [DOI] [PubMed] [Google Scholar]
- Grun, G., Berger, S., Matthes, D., and Mueller, M.J. (2007). Early accumulation of non-enzymatically synthesized oxylipins in Arabidopsis thaliana after infection with Pseudomonas syringae. Funct. Plant Biol. 34 65–71. [DOI] [PubMed] [Google Scholar]
- Imbusch, R., and Mueller, M.J. (2000). Analysis of oxidative stress and wound-inducible dinor isoprostanes F(1) (phytoprostanes F(1)) in plants. Plant Physiol. 124 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal, M., Evans, P., Lledo, A., Verdaguer, X., Pericàs, M.A., Riera, A., Loeffler, C., Sinha, A.K., and Mueller, M.J. (2005). Total synthesis and biological activity of 13,14-dehydro-12-oxo-phytodienoic acids (deoxy-J1-phytoprostanes). ChemBioChem 6 276–280. [DOI] [PubMed] [Google Scholar]
- Johnson, C., Boden, E., Desai, M., Pascuzzi, P., and Arias, J. (2001). In vivo target promoter-binding activities of a xenobiotic stress-activated TGA factor. Plant J. 28 237–243. [DOI] [PubMed] [Google Scholar]
- Lam, E., Benfey, P.N., Gilmartin, P.M., Fang, R.X., and Chua, N.H. (1989). Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc. Natl. Acad. Sci. USA 86 7890–7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler, C., Berger, S., Guy, A., Durand, T., Bringmann, G., Dreyer, M., von Rad, U., Durner, J., and Mueller, M.J. (2005). B1-phytoprostanes trigger plant defense and detoxification responses. Plant Physiol. 137 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo, O., Chico, J.M., Sanchez-Serrano, J.J., and Solano, R. (2004). JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16 1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik, B., and Guthenberg, C. (1981). Glutathione transferase (human placenta). Methods Enzymol. 77 231–235. [DOI] [PubMed] [Google Scholar]
- Mariani, V., Gilles, S., Jakob, T., Thiel, M., Mueller, M.J., Ring, J., Behrendt, H., and Traidl-Hoffmann, C. (2007). Immunomodulatory mediators from pollen enhance the migratory capacity of dendritic cells and license them for Th2 attraction. J. Immunol. 178 7623–7631. [DOI] [PubMed] [Google Scholar]
- Menè-Saffrané, L., Davoine, C., Stolz, S., Majcherczyk, P., and Farmer, E.E. (2007). Genetic removal of tri-unsaturated fatty acids suppresses developmental and molecular phenotypes of an Arabidopsis tocopherol-deficient mutant: Whole-body mapping of malondialdehyde pools in a complex eukaryote. J. Biol. Chem. 282 35749–35756. [DOI] [PubMed] [Google Scholar]
- Montillet, J.L., Cacas, J.L., Garnier, L., Montane, M.H., Douki, T., Bessoule, J.J., Polkowska-Kowalczyk, L., Maciejewska, U., Agnel, J.P., Vial, A., and Triantaphylides, C. (2004). The upstream oxylipin profile of Arabidopsis thaliana: A tool to scan for oxidative stresses. Plant J. 40 439–451. [DOI] [PubMed] [Google Scholar]
- Montillet, J.L., Chamnongpol, S., Rusterucci, C., Dat, J., van de Cotte, B., Agnel, J.P., Battesti, C., Inze, D., Van Breusegem, F., and Triantaphylides, C. (2005). Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 138 1516–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow, J.D. (2006). The isoprostanes—Unique products of arachidonate peroxidation. Their role as mediators of oxidant stress. Curr. Pharm. Des. 12 895–902. [DOI] [PubMed] [Google Scholar]
- Mueller, M.J. (2004). Archetype signals in plants: The phytoprostanes. Curr. Opin. Plant Biol. 7 441–448. [DOI] [PubMed] [Google Scholar]
- Ndamukong, I., Abdallat, A.A., Thurow, C., Fode, B., Zander, M., Weigel, R., and Gatz, C. (2007). SA-inducible Arabidopsis glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1.2 transcription. Plant J. 50 128–139. [DOI] [PubMed] [Google Scholar]
- Parchmann, S., Gundlach, H., and Mueller, M.J. (1997). Induction of 12-oxo-phytodienoic acid in wounded plants and elicited plant cell cultures. Plant Physiol. 115 1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano, T., Higaki, T., Handa, K., Kadota, Y., Kuchitsu, K., Hasezawa, S., Hoffmann, A., Endter, J., Zimmermann, U., Hedrich, R., and Roitsch, T. (2006). Calcium ions are involved in the delay of plant cell cycle progression by abiotic stresses. FEBS Lett. 580 597–602. [DOI] [PubMed] [Google Scholar]
- Sasaki-Sekimoto, Y., et al. (2005). Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J. 44 653–668. [DOI] [PubMed] [Google Scholar]
- Sattler, S.E., Mene-Saffrane, L., Farmer, E.E., Krischke, M., Mueller, M.J., and DellaPenna, D. (2006). Nonenzymatic lipid peroxidation reprograms gene expression and activates defense markers in Arabidopsis tocopherol-deficient mutants. Plant Cell 18 3706–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, F., Biesgen, C., Mussig, C., Altmann, T., and Weiler, E. (2000). 12-Oxophytodienoate reductase 3 (OPR3) is the isoenzyme involved in jasmonate biosynthesis. Planta 210 979–984. [DOI] [PubMed] [Google Scholar]
- Schaller, F., and Weiler, E.W. (1997). Molecular cloning and characterization of 12-oxophytodienoate reductase, an enzyme of the octadecanoid signaling pathway from Arabidopsis thaliana. Structural and functional relationship to yeast old yellow enzyme. J. Biol. Chem. 272 28066–28072. [DOI] [PubMed] [Google Scholar]
- Stintzi, A., Weber, H., Reymond, P., Browse, J., and Farmer, E.E. (2001). Plant defense in the absence of jasmonic acid: The role of cyclopentenones. Proc. Natl. Acad. Sci. USA 98 12837–12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatek, A., Lenjou, M., Van Bockstaele, D., Inze, D., and Van Onckelen, H. (2002). Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiol. 128 201–211. [PMC free article] [PubMed] [Google Scholar]
- Szyroki, A., Ivashikina, N., Dietrich, P., Roelfsema, M.R., Ache, P., Reintanz, B., Deeken, R., Godde, M., Felle, H., Steinmeyer, R., Palme, K., and Hedrich, R. (2001). KAT1 is not essential for stomatal opening. Proc. Natl. Acad. Sci. USA 98 2917–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki, N., et al. (2005). 12-Oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 139 1268–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm, O., Blasing, O., Gibon, Y., Nagel, A., Meyer, S., Kruger, P., Selbig, J., Muller, L.A., Rhee, S.Y., and Stitt, M. (2004). MAPMAN: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 37 914–939. [DOI] [PubMed] [Google Scholar]
- Thines, B., Katsir, L., Melotto, M., Niu, Y., Mandaokar, A., Liu, G., Nomura, K., He, S.Y., Howe, G.A., and Browse, J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448 661–665. [DOI] [PubMed] [Google Scholar]
- Thoma, I., Loeffler, C., Sinha, A.K., Gupta, M., Krischke, M., Steffan, B., Roitsch, T., and Mueller, M.J. (2003). Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J. 34 363–375. [DOI] [PubMed] [Google Scholar]
- Traidl-Hoffmann, C., Mariani, V., Hochrein, H., Karg, K., Wagner, H., Ring, J., Mueller, M.J., Jakob, T., and Behrendt, H. (2005). Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J. Exp. Med. 201 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uquillas, C., Letelier, I., Blanco, F., Jordana, X., and Holuigue, L. (2004). NPR1-independent activation of immediate early salicylic acid-responsive genes in Arabidopsis. Mol. Plant Microbe Interact. 17 34–42. [DOI] [PubMed] [Google Scholar]
- Usadel, B., et al. (2005). Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes, and comparison with known responses. Plant Physiol. 138 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits, L., and Memelink, J. (2000). ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289 295–297. [DOI] [PubMed] [Google Scholar]
- Vellosillo, T., Martinez, M., Lopez, M.A., Vicente, J., Cascon, T., Dolan, L., Hamberg, M., and Castresana, C. (2007). Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell 19 831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, U., Edwards, R., Dixon, D.P., and Mauch, F. (2002). Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol. Biol. 49 515–532. [DOI] [PubMed] [Google Scholar]
- Wasternack, C. (2007). Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. (Lond.) 100 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, H., Chételat, A., Reymond, P., and Farmer, E.E. (2004). Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J. 37 877–888. [DOI] [PubMed] [Google Scholar]
- Wu, Z., Irizarry, R.A., Gentleman, R., Murillo, F.M., and Spencer, F. (2003). A Model Based Background Adjustment for Oligonucleotide Expression Arrays. (Baltimore, MD: Johns Hopkins University).
- Xie, D., Feys, B., James, S., Nieto-Rostro, M., and Turner, J. (1998). COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280 1091–1094. [DOI] [PubMed] [Google Scholar]
- Yamane, H., Takagi, H., Abe, H., Yokota, T., and Takahashi, N. (1981). Identification of jasmonic acid in three species of higher plants and its biological activities. Plant Cell Physiol. 22 689–697. [Google Scholar]
- Yan, Y., Stolz, S., Chételat, A., Reymond, P., Pagni, M., Dubugnon, L., and Farmer, E.E. (2007). A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19 2470–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Tessaro, M.J., Lassner, M., and Li, X. (2003). Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 15 2647–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, P., Hirsch-Hoffmann, M., Hennig, L., and Gruissem, W. (2004). GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136 2621–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.