Abstract
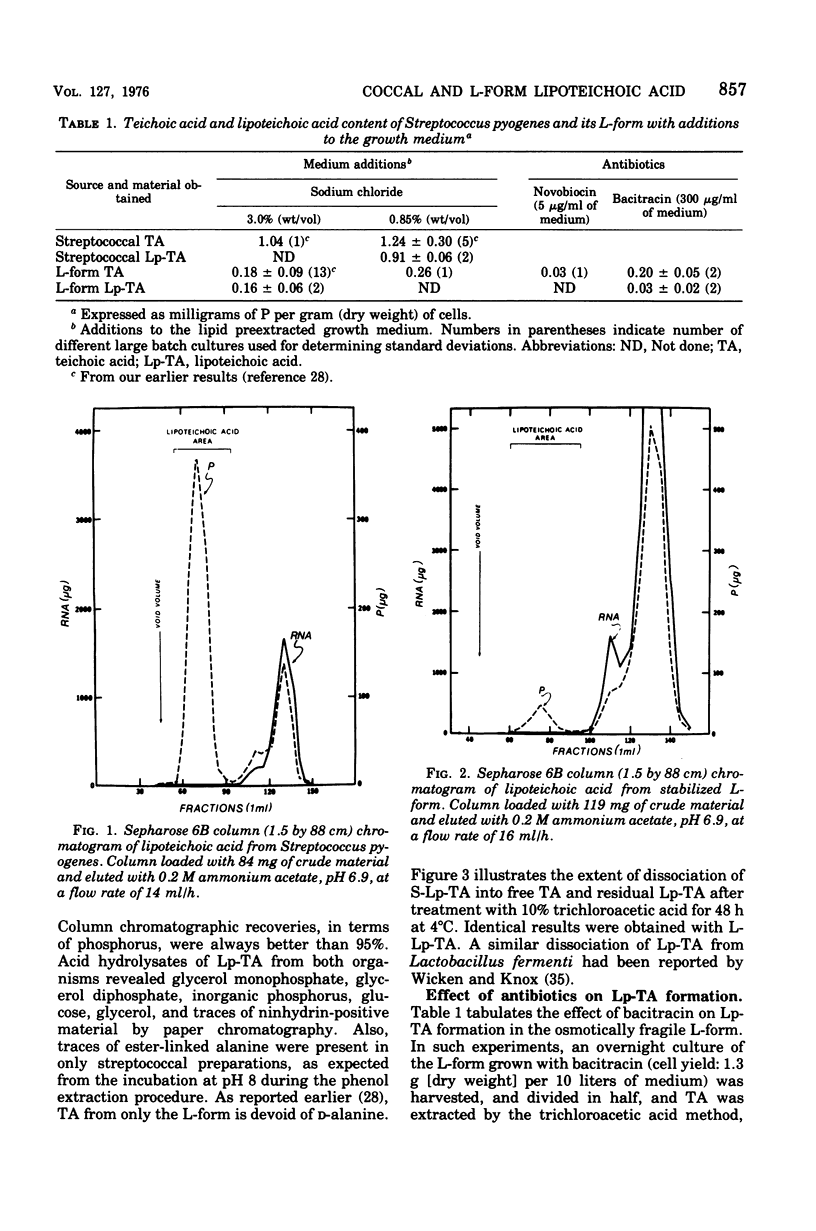
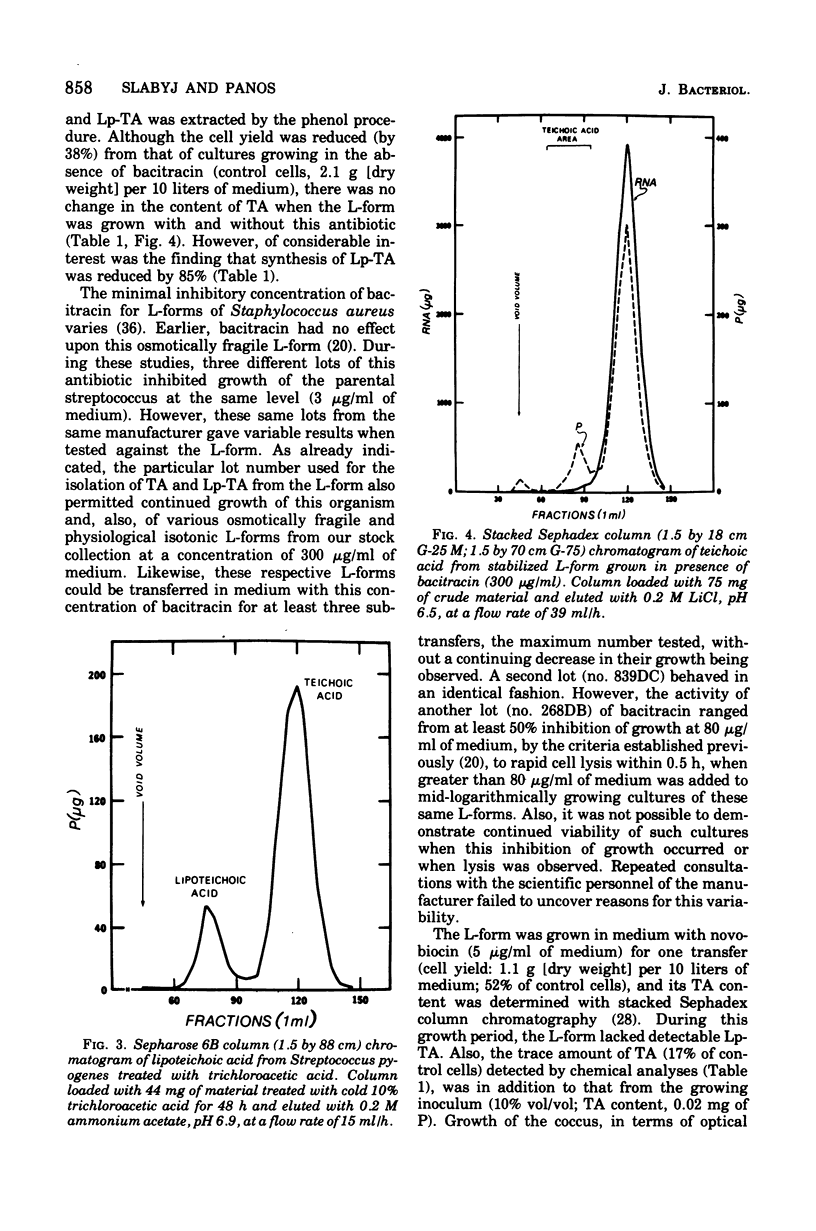
Membrane lipoteichoic acid continues to be synthesized by an osmotically fragile, stabilized L-form of Streptococcus pyogenes. Chromatographic and electrophoretic comparisons indicate that the lipid componenent of lipoteichoic acid in this L-form and its parental streptococcus is glycerophosphoryldiglucosyl diglyceride and not phosphatidylkojibiosyl diglyceride. Based upon dry weight determinations, the yield of lipoteichoic acid from the L-form is 0.19%, as compared with 0.97% from the streptococcus. When grown with bacitracin the L-form contains the same amount of teichoic acid as when grown without this antibiotic; however, its lipoteichoic acid content is reduced by 85%. Similarly, the L-form grown with novobiocin for 10 h contains only 17% of the teichoic acid found in control cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambron R. T., Pieringer R. A. The metabolism of glyceride glycolipids. V. Identification of the membrane lipid formed from diglucosyl diglyceride in Streptococcus faecalis ATCC 9790 as an acylated derivative of glyceryl phosphoryl diglucosyl glycerol. J Biol Chem. 1971 Jul 10;246(13):4216–4225. [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J., Shaukat G. A. The glycerol teichoic acid from walls of Staphylococcus epidermidis I2. Biochem J. 1968 Dec;110(3):583–588. doi: 10.1042/bj1100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevion M., Panos C., Linzer R., Neuhaus F. C. Incorporation of D-alanine into the membrane of Streptococcus pyogenes and its stabilized L-form. J Bacteriol. 1974 Dec;120(3):1026–1032. doi: 10.1128/jb.120.3.1026-1032.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevion M., Panos C., Paxton J. Membrane studies of Streptococcus pyogenes and its L-form growing in hypertonic and physiologically isotonic media. An electron spin resonance spectroscopy approach. Biochim Biophys Acta. 1976 Mar 5;426(2):288–301. doi: 10.1016/0005-2736(76)90338-2. [DOI] [PubMed] [Google Scholar]
- Cohen M., Panos C. Membrane lipid composition of Streptococcus pyogenes and derived L form. Biochemistry. 1966 Jul;5(7):2385–2392. doi: 10.1021/bi00871a031. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Fischer W., Ishizuka I., Landgraf H. R., Herrmann J. Glycerophosphoryl diglucosyl diglyceride, a new phosphoglycolipid from Streptococci. Biochim Biophys Acta. 1973 Mar 8;296(3):527–545. doi: 10.1016/0005-2760(73)90113-6. [DOI] [PubMed] [Google Scholar]
- Ganfield M. C., Pieringer R. A. Phosphatidylkojibiosyl diglyceride. The covalently linked lipid constituent of the membrane lipoteichoic acid from Streptococcus faecalis (faecium) ATCC 9790. J Biol Chem. 1975 Jan 25;250(2):702–709. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Hancock R., Fitz-James P. C. Some differences in the action of penicillin, bacitracin, and vancomycin on Bacillus megaterium. J Bacteriol. 1964 May;87(5):1044–1050. doi: 10.1128/jb.87.5.1044-1050.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrikson C. V., Panos C. Fatty acid composition, distribution, and requirements of two nonsterol-requiring mycoplasmas from complex but defatted growth media. Biochemistry. 1969 Feb;8(2):646–651. doi: 10.1021/bi00830a028. [DOI] [PubMed] [Google Scholar]
- Hughes A. H., Stow M., Hancock I. C., Baddiley J. Function of teichoic acids and effect of novobiocin on control of Mg2+ at the bacterial membrane. Nat New Biol. 1971 Jan 13;229(2):53–55. doi: 10.1038/newbio229053a0. [DOI] [PubMed] [Google Scholar]
- Jackson R. W., Moskowitz M. Nature of a red cell sensitizing substance from streptococci. J Bacteriol. 1966 Jun;91(6):2205–2209. doi: 10.1128/jb.91.6.2205-2209.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon O., Panos C. Adaptation of an osmotically fragile L-form of Streptococcus pyogenes to physiological osmotic conditions and its ability to destroy human heart cells in tissue culture. Infect Immun. 1976 Jan;13(1):252–262. doi: 10.1128/iai.13.1.252-262.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Moskowitz M. Separation and properties of a red cell sensitizing substance from streptococci. J Bacteriol. 1966 Jun;91(6):2200–2204. doi: 10.1128/jb.91.6.2200-2204.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panos C., Cohen M., Fagan G. Antibiotic inhibition and binding studies with a group A streptococcal L-form. J Gen Microbiol. 1967 Feb;46(2):299–304. doi: 10.1099/00221287-46-2-299. [DOI] [PubMed] [Google Scholar]
- Panos C., Cohen M., Fagan G. Lipid alterations after cell wall inhibition. Fatty acid content of Streptococcus pyogenes and derived L-form. Biochemistry. 1966 May;5(5):1461–1468. doi: 10.1021/bi00869a003. [DOI] [PubMed] [Google Scholar]
- Panos C., Leon O. Replacement of the octadecenoic acid growth-requirement for Acholeplasma laidlawii A by cis-9,10-methylenehexadecanoic acid, a cyclopropane fatty acid. J Gen Microbiol. 1974 Jan;80(1):93–100. doi: 10.1099/00221287-80-1-93. [DOI] [PubMed] [Google Scholar]
- Pieringer R. A., Ambron R. T. A method for the specific labeling of the glycerol in glyceride-containing lipids of Streptococcus faecalis ATCC 9790. J Lipid Res. 1973 May;14(3):370–372. [PubMed] [Google Scholar]
- Pieringer R. A. Biosynthesis of the phosphatidyl diglucosyl diglyceride of Streptococcus faecalis (ATCC 9730) from diglyucosyl diglyceride and phosphatidyl glycerol or diphosphatidyl glycerol. Biochem Biophys Res Commun. 1972 Oct 17;49(2):502–507. doi: 10.1016/0006-291x(72)90439-1. [DOI] [PubMed] [Google Scholar]
- Pieringer R. A. Phosphatidylkojibiosyl Diglyceride: metabolism and function as an anchor in bacterial cell membrane. Lipids. 1975 Jul;10(7):421–426. doi: 10.1007/BF02532448. [DOI] [PubMed] [Google Scholar]
- Reusch V. M., Panos C. Defective synthesis of lipid intermediates for peptidoglycan formation in a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1976 Apr;126(1):300–311. doi: 10.1128/jb.126.1.300-311.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH J. L., WEINBERG E. D. Mechanisms of antibacterial action of bacitracin. J Gen Microbiol. 1962 Jul;28:559–569. doi: 10.1099/00221287-28-3-559. [DOI] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci U S A. 1967 Mar;57(3):767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Teichoic acid of a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1973 Jun;114(3):934–942. doi: 10.1128/jb.114.3.934-942.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Toon P., Brown P. E., Baddiley J. The lipid-teichoic acid complex in the cytoplasmic membrane of Streptococcus faecalis N.C.I.B. 8191. Biochem J. 1972 Apr;127(2):399–409. doi: 10.1042/bj1270399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Driel D., Wicken A. J., Dickson M. R., Knox K. W. Cellular location of the lipoteichoic acids of Lactobacillus fermenti NCTC 6991 and Lactobacillus casei NCTC 6375. J Ultrastruct Res. 1973 Jun;43(5):483–497. doi: 10.1016/s0022-5320(73)90025-7. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. E. L FORMS OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Nov;33:325–334. doi: 10.1099/00221287-33-2-325. [DOI] [PubMed] [Google Scholar]
- Watkinson R. J., Hussey H., Baddiley J. Shared lipid phosphate carrier in the biosynthesis of teichoic acid and peptidoglycan. Nat New Biol. 1971 Jan 13;229(2):57–59. doi: 10.1038/newbio229057a0. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]


