Abstract
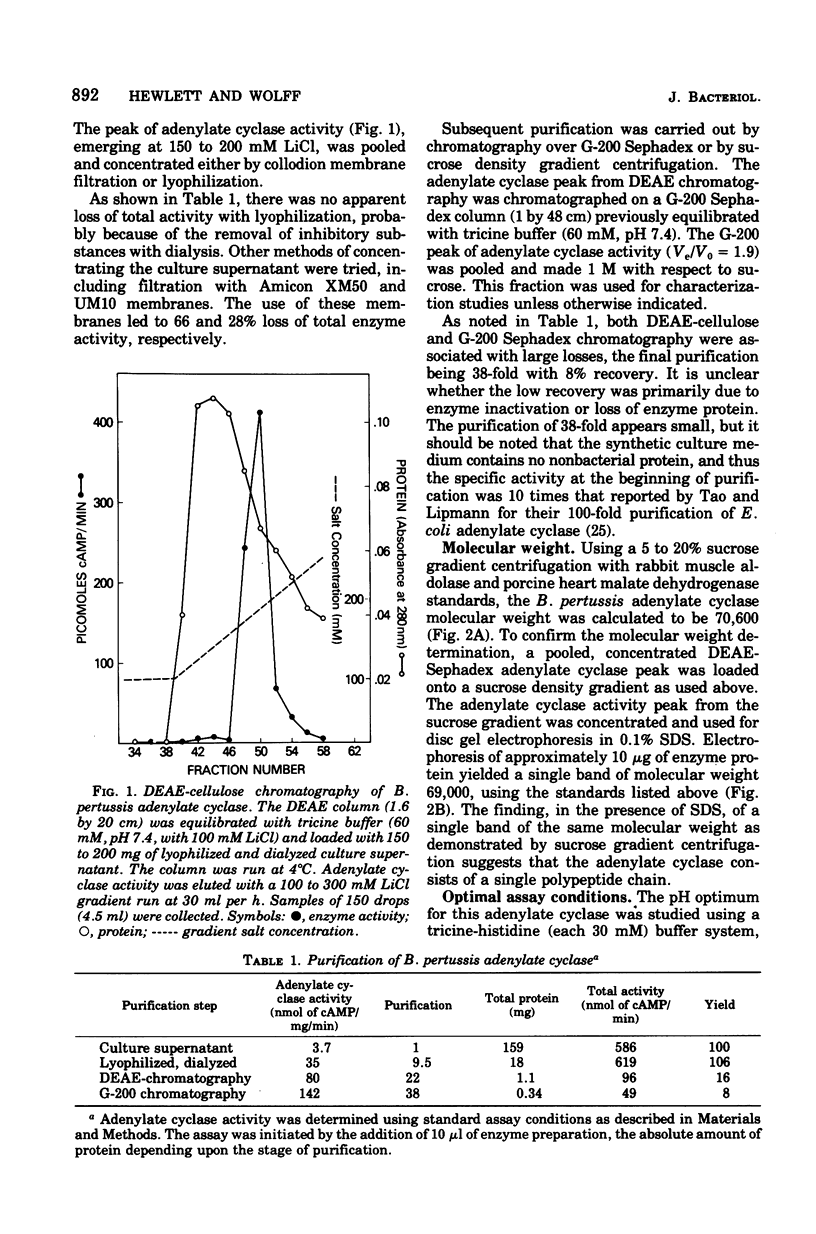
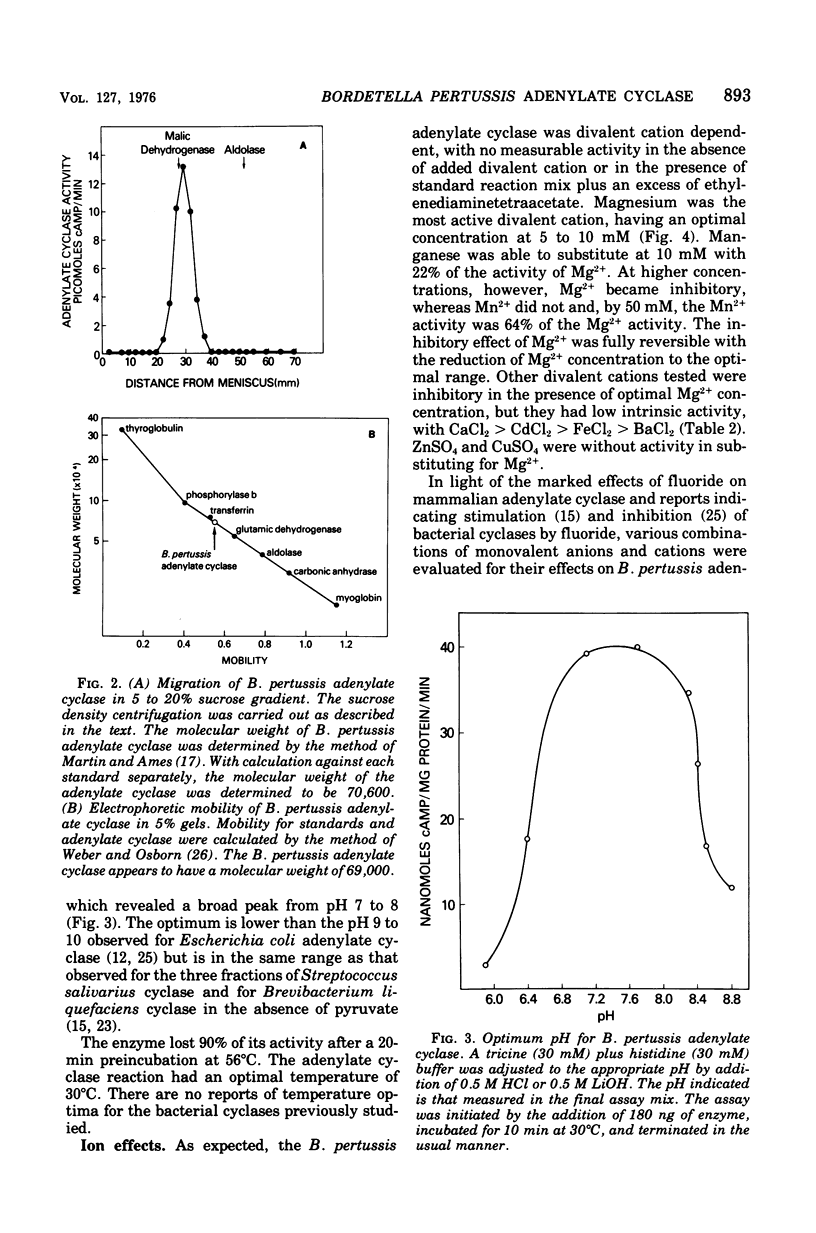
Culture medium of exponentially growing Bordetella pertussis (strain 114) contains significant quantities of soluble (100,000 X g for 1 h) adenylate cyclase. The enzyme was purified by chromatography on diethylaminoethyl-cellulose and Sephadex G-200. The purest material yielded a single band on sodium dodecyl sulfate-disc gel electrophoresis. It is heat labile, has a temperature optimum of 30 degrees C, a pH optimum of pH 7 to 8, and a Km for adenosine 5'-triphosphate of 0.4 mM, and requires Mg2+ for maximum activity. The molecular weight, by sodium dodecyl sulfate-disc gel electrophoresis and sucrose density gradient, is approximately 70,000. The enzyme is markedly inhibited by fluoride and weakly inhibited by monovalent salts, but its activity is not altered by alpha-keto acids of nonsubstrate nucleoside triphosphates. Thus, but its presence in the culture supernatant, its smaller molecular weight, and its insensitivity to alpha-keto acids and nucleotides, this enzyme differs from the bacterial adenylate cyclases previously described.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman R. K., Munoz J. Effects of epinephrine, norepinephrine and isoproterenol against challenge in Bordetella pertussis-treated and beta-adrenergic blocked mice. Life Sci II. 1971 May 22;10(10):561–568. doi: 10.1016/0024-3205(71)90192-5. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Gorman R. E., Thomas L. Selective stimulation of epinephrine-responsive adenyl cyclase in mice by endotoxin. Proc Soc Exp Biol Med. 1971 Dec;138(3):773–775. doi: 10.3181/00379727-138-35986. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Chen L. C., Curlin G. T., Evans D. G. Stimulation of adenyl cyclase by Escherichia coli enterotoxin. Nat New Biol. 1972 Apr 5;236(66):137–138. doi: 10.1038/newbio236137a0. [DOI] [PubMed] [Google Scholar]
- FISHEL C. W., SZENTIVANYI A., TALMAGE D. W. Sensitization and desensitization of mice to histamine and serotonin by neurohumors. J Immunol. 1962 Jul;89:8–18. [PubMed] [Google Scholar]
- FISHEL C. W., SZENTIVANYI A. THE ABSENCE OF ADRENALINE-INDUCED HYPERGLYCEMIA IN PERTUSSIS- SENSITIZED MICE AND ITS RELATION TO HISTAMINE AND SEROTONIN HYPERSENSITIVITY. J Allergy. 1963 Sep-Oct;34:439–454. doi: 10.1016/0021-8707(63)90009-1. [DOI] [PubMed] [Google Scholar]
- Fishel C. W., O'Bryan B. S., Smith D. L., Jewell G. W. Conversion of ATP by mouse lung preparations and by an extract of Bordetella pertussis. Int Arch Allergy Appl Immunol. 1970;38(5):457–465. doi: 10.1159/000230298. [DOI] [PubMed] [Google Scholar]
- Flawiá M. M., Torres H. N. Adenylate cyclase activity in Neurospora crassa. I. General properties. J Biol Chem. 1972 Nov 10;247(21):6873–6879. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Isaac P., Heal D. J., Bond R. P. Inhibition of adenyl cyclase by an exotoxin of Bacillus thuringiensis. Nature. 1975 Jan 3;253(5486):58–60. doi: 10.1038/253058a0. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Guerrant R. L., Evans D. J., Jr, Greenough W. B., 3rd Toxins of Vibrio cholerae and Escherichia coli stimulate adenyl cyclase in rat fat cells. Nature. 1974 May 24;249(455):371–373. doi: 10.1038/249371a0. [DOI] [PubMed] [Google Scholar]
- Hirata M., Hayaishi O. Adenyl cyclase of Brevibacterium liquefaciens. Biochim Biophys Acta. 1967 Nov 21;149(1):1–11. doi: 10.1016/0005-2787(67)90685-5. [DOI] [PubMed] [Google Scholar]
- Ide M. Adenyl cyclase of Escherichia coli. Biochem Biophys Res Commun. 1969 Jul 7;36(1):42–46. doi: 10.1016/0006-291x(69)90646-9. [DOI] [PubMed] [Google Scholar]
- Keller K. F., Fishel C. W. In vivo and in vitro manifestations of adrenergic blockade in Bordetella pertussis-vaccinated mice. J Bacteriol. 1967 Oct;94(4):804–811. doi: 10.1128/jb.94.4.804-811.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal R. L., Hamilton I. R. Purification and properties of adenyl cyclase from Streptococcus salivarius. J Biol Chem. 1971 May 25;246(10):3297–3304. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Parker C. W., Morse S. I. The effect of Bordetella pertussis on lymphocyte cyclic AMP metabolism. J Exp Med. 1973 Apr 1;137(4):1078–1090. doi: 10.1084/jem.137.4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M., Rosen S. M. A bacterial activator of frog erythrocyte adenyl cyclase. Arch Biochem Biophys. 1970 Nov;141(1):346–352. doi: 10.1016/0003-9861(70)90142-6. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Takai K., Kurashina Y., Suzuki-Hori C., Okamoto H., Hayaishi O. Adenylate cyclase from Brevibacterium liquefaciens. I. Purification, crystallization, and some properties. J Biol Chem. 1974 Mar 25;249(6):1965–1972. [PubMed] [Google Scholar]
- Tao M., Huberman A. Some properties of Escherichia coli adenyl cyclase. Arch Biochem Biophys. 1970 Nov;141(1):236–240. doi: 10.1016/0003-9861(70)90127-x. [DOI] [PubMed] [Google Scholar]
- Tao M., Lipmann F. Isolation of adenyl cyclase from Escherichia coli. Proc Natl Acad Sci U S A. 1969 May;63(1):86–92. doi: 10.1073/pnas.63.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wolff J., Cook G. H. Activation of thyroid membrane adenylate cyclase by purine nucleotides. J Biol Chem. 1973 Jan 10;248(1):350–355. [PubMed] [Google Scholar]
- Wolff J., Cook G. H. Charge effects in the activation of adenylate cyclase. J Biol Chem. 1975 Sep 10;250(17):6897–6903. [PubMed] [Google Scholar]
- Wolff J., Cook G. H. Choleragen stimulates steroidogenesis and adenylate cyclase in cells lacking functional hormone receptors. Biochim Biophys Acta. 1975 Dec 1;413(2):283–290. doi: 10.1016/0005-2736(75)90113-3. [DOI] [PubMed] [Google Scholar]


