Abstract
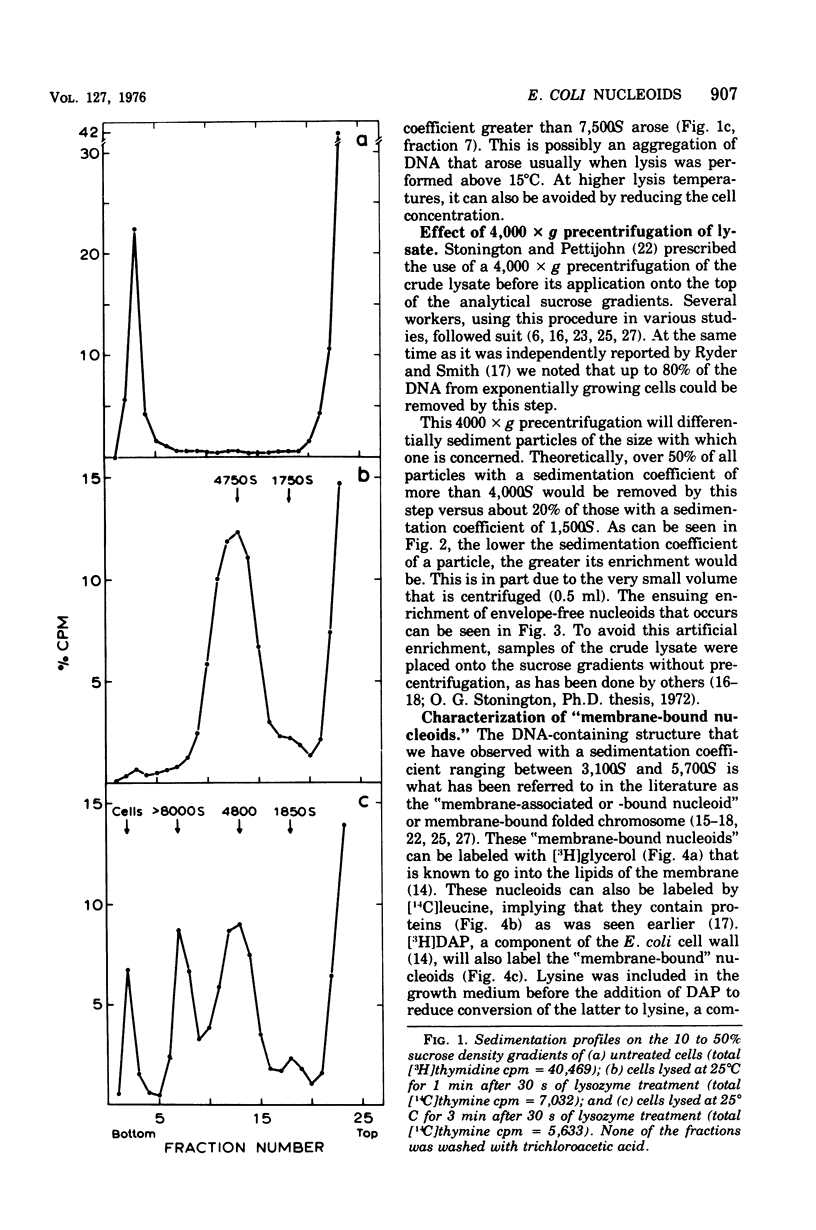
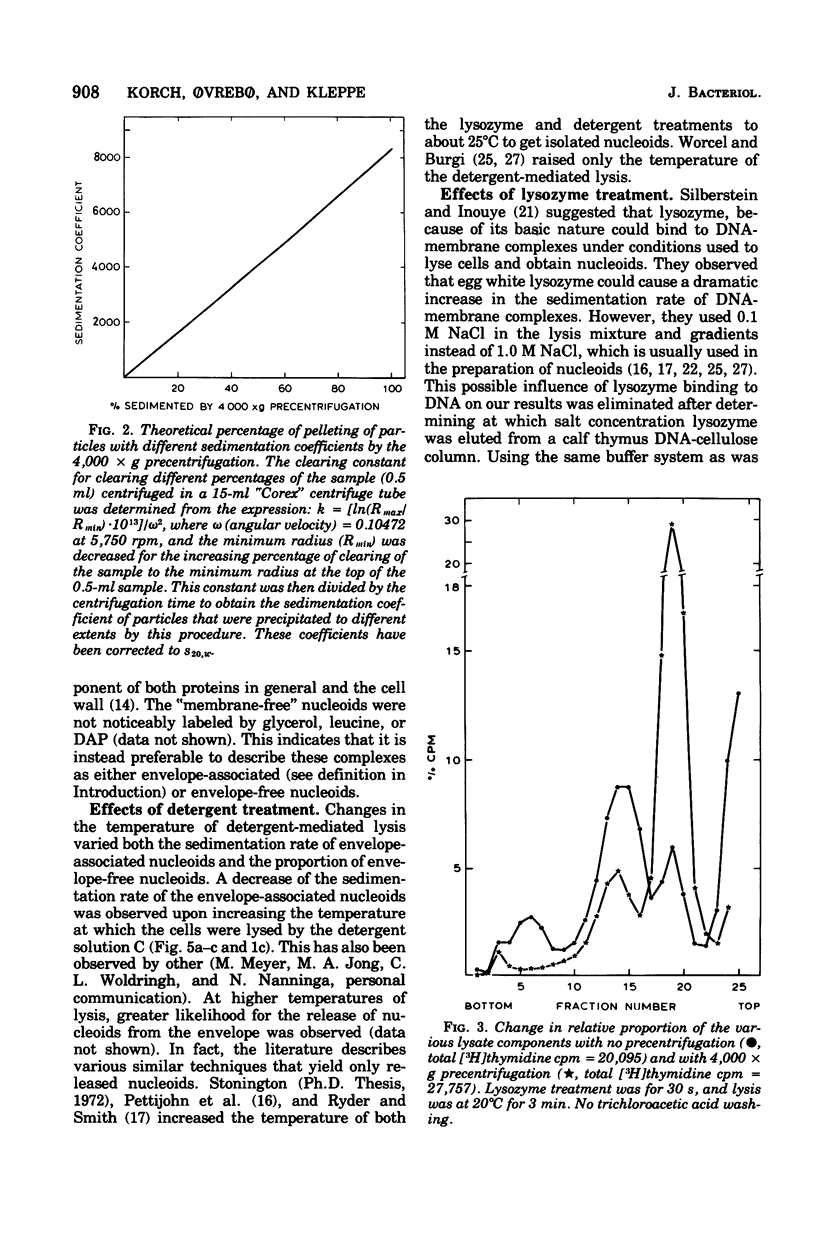
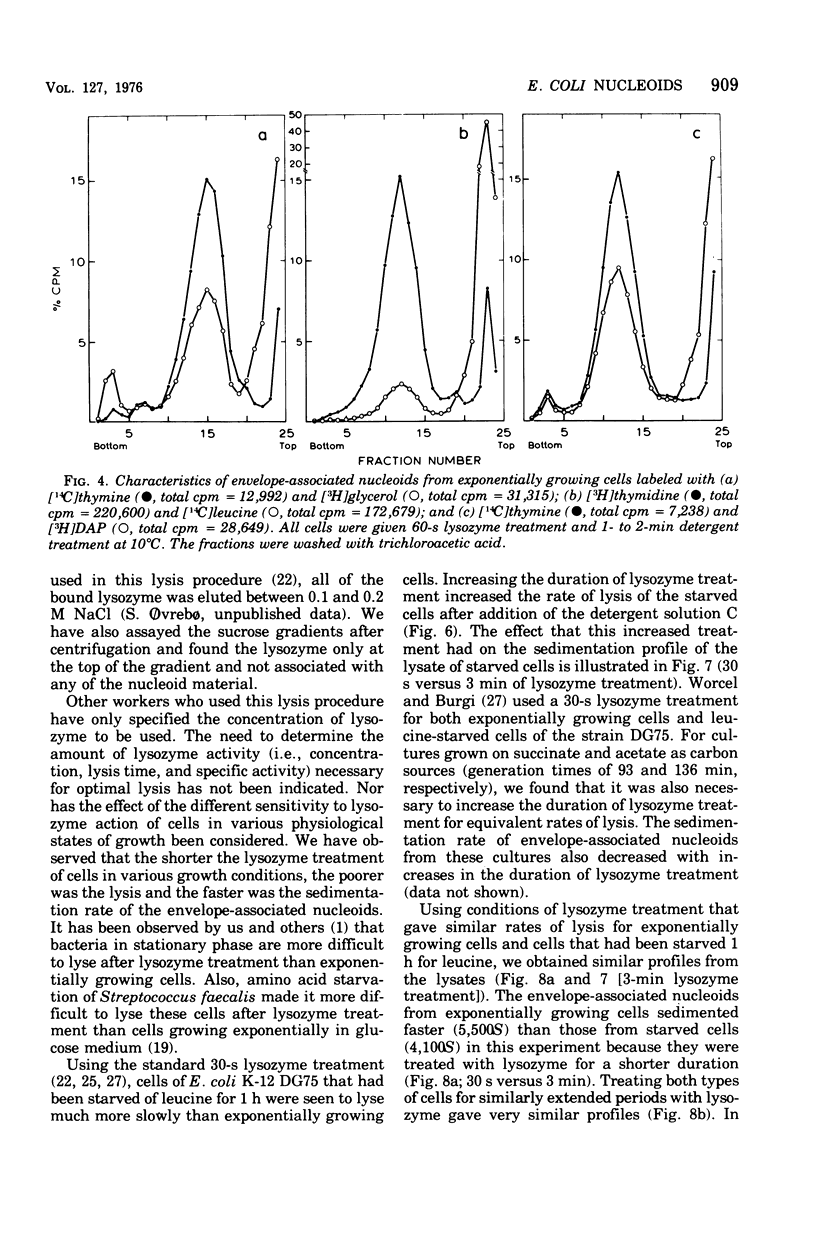
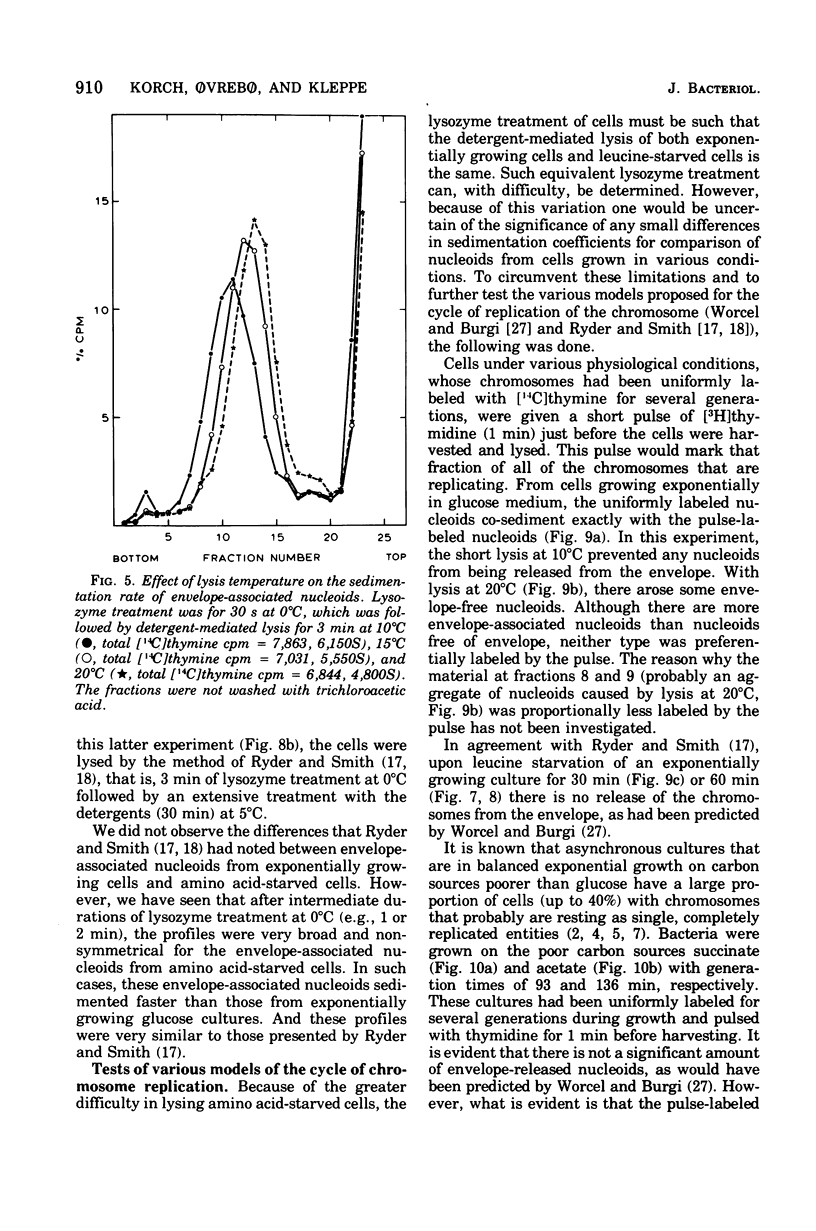
The folded chromosome of Escherichia coli has been investigated under various lysis and physiological conditions. A new gradient system was devised that allows excellent separation between unlysed cells and envelope-associated and envelope-free chromosomes. Isotope incorporation experiments showed that the fraction often called "membrane-bound nucleoids" contains cell wall in addition to nucleic acids, membranes, and proteins. The amount of lysozyme added and the lysozyme digestion time were found to be important when comparing the rate of sedimentation of envelope-associated chromosomes obtained under various physiological conditions. Amino acid-starved cells were found to be much harder to lyse with lysozyme than exponentially grown cells, The difference in sedimentation coefficient of envelope-associated chromosomes described earlier (Ryder and Smith, 1974) was not detected when the latter two types of cells had been given equivalent, but not identical, lysozyme treatment such that detergent-mediated lysis proceeded at the same rate. Analysis of pulse- and uniformly labeled chromosomes from amino acid-starved cultures revealed no preferential labeling of either envelope-associated or -released nucleoids. Nor was there a difference in sedimentation coefficient between uniform and pulse-labeled envelope-associated nucleoids. These results are in disagreement with the models for chromosome replication of Worcel and Burgi (1974) and Ryder and Smith (1974), respectively. Growing cells on carbon sources poorer than glucose demonstrated that the replicating chromosomes sediment faster than the bulk of envelope-associated nucleoids. The slower the growth rate, the greater this difference became. An alternative hypothesis regarding chromosome replication and its association with the cell envelope is presented.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgi A. W., Robinton J., Carlson C. L. Studies on the folded chromosome of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1974;38:43–51. doi: 10.1101/sqb.1974.038.01.007. [DOI] [PubMed] [Google Scholar]
- CHALOUPKA J., KRECKOVA P., RIHOVA L. Changes in the character of the cell wall in growth of Bacillus megaterium cultures. Folia Microbiol (Praha) 1962;7:269–274. doi: 10.1007/BF02928656. [DOI] [PubMed] [Google Scholar]
- CUMMINGS D. J. SEDIMENTATION AND BIOLOGICAL PROPERTIES OF T-PHAGES OF ESCHERICHIA COLI. Virology. 1964 Jul;23:408–418. doi: 10.1016/0042-6822(64)90264-8. [DOI] [PubMed] [Google Scholar]
- Chandler M., Bird R. E., Caro L. The replication time of the Escherichia coli K12 chromosome as a function of cell doubling time. J Mol Biol. 1975 May 5;94(1):127–132. doi: 10.1016/0022-2836(75)90410-6. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E., Bendigkeit H. E., Loken M. R. Onset of DNA synthesis during the cell cycle in chemostat cultures. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1611–1617. doi: 10.1073/pnas.57.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lane H. E., Denhardt D. T. The rep mutation. III. Altered structure of the replicating Escherichia coli chromosome. J Bacteriol. 1974 Nov;120(2):805–814. doi: 10.1128/jb.120.2.805-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark C. Regulation of deoxyribonucleic acid synthesis in Escherichia coli: dependence on growth rates. Biochim Biophys Acta. 1966 Jun 22;119(3):517–525. doi: 10.1016/0005-2787(66)90128-6. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Marunouchi T., Messer W. Replication of a specific terminal chromosome segment in Escherichia coli which is required for cell division. J Mol Biol. 1973 Jun 25;78(1):211–228. doi: 10.1016/0022-2836(73)90439-7. [DOI] [PubMed] [Google Scholar]
- Messer W. Initiation of deoxyribonucleic acid replication in Escherichia coli B-r: chronology of events and transcriptional control of initiation. J Bacteriol. 1972 Oct;112(1):7–12. doi: 10.1128/jb.112.1.7-12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Ryder O. A., Smith D. W. Isolation of membrane-associated folded chromosomes from Escherichia coli: effect of protein synthesis inhibition. J Bacteriol. 1974 Dec;120(3):1356–1363. doi: 10.1128/jb.120.3.1356-1363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder O. A., Smith D. W. Properties of membrane-associated folded chromosomes of E. coli related to initiation and termination of DNA replication. Cell. 1975 Apr;4(4):337–345. doi: 10.1016/0092-8674(75)90154-3. [DOI] [PubMed] [Google Scholar]
- SHUGAR D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952 Mar;8(3):302–309. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Phillips P. M., Riley L. S., Toennies G. LYSIS OF STREPTOCOCCUS FAECALIS. J Bacteriol. 1961 Jan;81(1):36–43. doi: 10.1128/jb.81.1.36-43.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein S., Inouye M. The effects of lysozyme on DNA--membrane association in Escherichia coli. Biochim Biophys Acta. 1974 Oct 11;366(2):149–158. [PubMed] [Google Scholar]
- Stonington O. G., Pettijohn D. E. The folded genome of Escherichia coli isolated in a protein-DNA-RNA complex. Proc Natl Acad Sci U S A. 1971 Jan;68(1):6–9. doi: 10.1073/pnas.68.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutas D. J., Wehner J. M., Koerner J. F. Unfolding of the host genome after infection of Escherichia coli with bacteriophage T4. J Virol. 1974 Feb;13(2):548–550. doi: 10.1128/jvi.13.2.548-550.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B., Pato M. L., Ward C. B., Glaser D. A. On the origin and direction of replication of the E. coli chromosome. Cold Spring Harb Symp Quant Biol. 1968;33:575–584. doi: 10.1101/sqb.1968.033.01.064. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. doi: 10.1016/0022-2836(74)90576-2. [DOI] [PubMed] [Google Scholar]



