Abstract
Background:
Increased spatial and temporal dispersion of repolarization contributes to ventricular arrhythmogenesis. Beat-to-beat fluctuations in T-wave timing are thought to represent such dispersion, and may predict clinical events.
Objectives:
We assessed whether a novel noninvasive measure of beat-to-beat instability in T-wave timing would provide additive prognostic information in post-myocardial infarction patients.
Methods:
We studied 678 patients from 12 hospitals with 32 lead 5-minute ECG recordings 6-8 weeks after myocardial infarction (MI). Custom software identified R wave to T wave intervals (RTI) and diastolic intervals (DI). Repolarization scatter (RTI:DIStdErr) was then calculated as the standard error about the RTI:DI regression line. In addition, left ventricular ejection fraction (LVEF), short-term HRV parameters and QT Variability Index were measured. Patients were followed for the composite endpoint of death or life threatening ventricular arrhythmia.
Results:
After a mean follow-up of 63 months, 134 patients met the composite endpoint. An RTI:DIStdErr over 5.50ms was associated with a 210% increase in arrhythmias or deaths (p<0.001). After adjusting for left venricular ejection fraction (LVEF), RTI:DIStdErr remained an independent predictor (p<0.001). RTI:DIStdErr was also independent of short-term HRV parameters and the QT Variability Index.
Conclusions:
Increased repolarization scatter, a measure of high frequency, cycle-length-dependent repolarization instability, predicts poor outcomes in patients after myocardial infarction.
Keywords: QT variability, repolarization, myocardial infarction, risk stratification
Background
Ventricular arrhythmias remain the most common cause of death in the United States1, 2. Our ability to identify patients at risk continues to be limited, in part due to the poor positive predictive value of current invasive and non-invasive testing3. Nevertheless, the financial and medical incentives for advancement in risk stratification remain very strong.
It is well established that myocardium with heterogeneous repolarization is prone to reentrant arrhythmias4-6. In normal hearts, repolarization is highly coordinated, both temporally and spatially, to avoid such heterogeneity. It has been well demonstrated in experimental and computer models that when small, beat-to-beat changes in diastolic intervals induce a large change in action potential duration (i.e. when the restitution7-10 slope exceeds one) the activation wavefront destabilizes after a short sequence of rapid beats11, 12. This may result in repolarization alternans that degenerates into fibrillation13. According to this paradigm, future improvements in arrhythmia risk stratification are likely to be obtained by the direct assessment of repolarization dynamicity rather than indirect measures of autonomic function.
The utility of assessing beat-to-beat repolarization stability to predict arrhythmias has indeed been established. Microvolt alternations in T-wave amplitude (MTWA) have been shown to predict clinical events in various clinical populations14-18. There is evidence in animal models that torsades de pointes may be better predicted by beat-to-beat variability of repolarization timing than by QT prolongation19-21. Furthermore, initial studies of the QT Variability Index (QTVI)22, which expresses a relation between QT interval variance and short-term heart rate variability, have shown its correlation with heart failure22, 23 and its value in predicting clinical events24-26.
In this paper, we have employed a novel analytic method to assess beat-to-beat variability of repolarization that quantifies instability in the repolarization-diastolic interval feedback system. We hypothesized that increased instability (scatter) in the timing of the T-wave peak would predict clinical events in patients with a recent myocardial infarction.
Methods
Patient Population
The study protocol was approved by the respective ethics committees and regulatory bodies at all involved centers. Patients were considered for enrollment if they were 18 years or older and if their treating physicians had diagnosed an acute myocardial infarction during their index hospitalization. Exclusion criteria were: inability to give informed consent, pacemaker dependence, preexisting severe contractile dysfunction (ejection fraction ≤ 25%) or cardiogenic shock.
Study Design
We studied 678 post-myocardial infarction patients from 12 hospitals in Utah and Pennsylvania. Recruitment was initiated by point-of-care clinicians who sought patients' permission to be contacted by study coordinators. Enrollment spanned from 1996 to 2003. After informed consent was obtained, we catalogued demographic data, left ventricular ejection fraction, myocardial injury serum markers, and infarct location by electrocardiogram. Patient care was dictated by the enrolling physician and was not altered by this protocol. Patients were then visited at their homes 6-8 weeks after hospital discharge where a study nurse updated the medical history, performed a limited transthoracic echocardiogram to measure left ventricular ejection fraction (LVEF), and performed a continuous 5 minute, 32-lead body surface mapping with the patient resting and supine. Follow-up was then continued by telephone and medical record review at six-month intervals to ascertain whether and when any clinical endpoints were met. At the completion of follow-up, the predictive performance of the described electrocardiographic measures were analyzed retrospectively.
The primary endpoint was a composite endpoint of death or documented ventricular arrhythmia. Using available medical records, obituaries, and interviews with family members when possible, we tried to categorize patient deaths as: arrhythmia, pump failure, infarct-related, or noncardiac. As noncardiac deaths comprised a high proportion of total events, we also assessed a secondary composite endpoint of cardiac deaths or documented ventricular arrhythmia.
Measurements
Ejection fraction
LVEF was estimated by single plane Simpson's method from apical four-chamber views.
Repolarization slope and scatter
Body surface mapping was performed using a custom-made 32-lead system as previously reported27. Briefly, 32 pre-specified thoracic lead positions were located using anatomical landmarks and silver-chloride electrodes embedded in nylon strips were affixed using conductive gel and adhesive. After confirming signal quality, all leads were sampled at 1kHz, filtered, and recorded for offline analysis with custom software. For beat detection, a lead was chosen with a clear, solitary, intrinsic negative QRS deflection. The recording from this lead was further processed with smoothing and first derivative filters, providing reliable high amplitude deflections for identifying distinct ventricular complexes. Premature ventricular complexes were manually identified and omitted. Maps obtained during a paced rhythm were excluded.
After QRS detection, the root mean square (RMS) signal was calculated for each patient using raw recordings from all leads. Baseline correction was performed on all beats by subtraction, zeroing the TP or PR segments. QRS and T-wave peaks were identified using a parabolic, least-mean-squared error estimate of the RMS ECG, which reduced the sensitivity of peak identification to high frequency noise. Cycle lengths (CL) were defined as the time between subsequent QRS peaks. The time of the RMS R peak has been shown to correlate with the mean depolarization time of the ventricles28, 29. Similarly, the time of the RMS T peak has been shown to correlate with mean ventricular repolarization time. RT Intervals (RTIs) were defined as the time between the local QRS peak and the T-wave peak in the RMS signal. This interval has been shown to correlate well with mean cellular action potential duration30, 31. The mean diastolic interval (DI) was estimated as the time from RMS T-wave peak of the preceding beat to the peak of the RMS R wave (both determined from local maxima in the 2nd derivative function), a measure that has been shown to correlate with the mean cellular diastolic recovery time28. For every patient, all RTIs were plotted as a function of the preceding DI. Simple linear regression was applied to the resulting scatterplot to ascertain the slope (RTI:DISlope) and y-intercept (RTI:DIY-intercept). Regression diagnostics then provided the standard error about the regression line (RTI:DIStdErr), a measure of ‘scatter’ in the repolarization / diastolic interval relationship. This term quantifies instability in repolarization timing that is not attributable to beat-to-beat changes in diastolic interval.
QT Variability Index
This time-series calculation has been previously described, and represents the log ratio between the QT interval and heart rate variabilities. Briefly, the above-described detection of basic ECG intervals was implemented and applied to the equation QTVIestimate = log10[(RTIVariance/(RTIMean)2)/(HRVariance/(HRMean)2)]. Of note, since we used 2nd derivative filter settings optimized for detecting the T-wave peak, we use RTI in this equation rather than the QT interval. Therefore, this metric varies slightly from QTVI as originally described since the Tpeak-Tend segment is not included. Also, as originally described, this measure was calculated twice for every patient (over two 256 beat epochs) and then averaged, but we forewent this averaging as only single measures of RTI:DIStdErr were performed.
Heart rate variability
To assess the relationship between repolarization scatter and high frequency heart rate variability (HRV), several HRV indices were estimated from the 5 minute recording, including rMMSD, pNN50, and ICC32-36. rMMSD is the root mean square of successive differences of normal RR intervals. pNN50 is the percent of successive normal RR interval differences that exceed 50ms. ICC is the interbeat correlation (the correlation coefficient of a Poincare plot, where every normal RR interval is plotted as a function of the preceding RR interval).
Statistical Analysis
Data distributions were first assessed for artifactual anomalies and normality. As the normality assumption was rejected for all prediction variables, estimates of means and confidence intervals (C.I.) about the mean for these variables were assessed using the bootstrap method. Based on whether the composite endpoint was met, each patient was categorized into either the Event Group or the Non-Event Group. Comparisons of non-normally distributed continuous variables between these sample groups were performed using the Wilcoxon signed rank test on unmatched data.
We used survival-time-data with lost-to-follow-up censoring to analyze the predictive performance of these measures. The optimal operating points (cutpoint where the sensitivity / specificity balance is optimized) for linear prediction variables were determined by maximizing the post-estimation c-statistic of a single variable Cox survival model. After identifying the optimal operating point, the accuracy for predicting events was assessed using survival analysis, with the statistical significance of differences between survival curves reported using log rank tests of equality.
Candidate predictors were assessed for collinearity before inclusion in multivariable analyses. Correlation involving non-normal distributions are reported using Spearman's rank test (ρ). Cox proportional hazards models were used to assess for interaction between binary predictor variables, with model comparisons using likelihood ratio tests. Associations with predictor variables and clinical variables, such as peak CK serum level, LVEF, and age were also assessed using a Cox proportional hazards model. P-values less than 0.05 were considered significant. All data are shown as mean±SD unless otherwise specified. All analyses were performed using Stata v9.0 for Macintosh.
Results
Patient characteristics
The mean age of the study group was 64±12.5 years and 76% were male. The distribution of race / ethnicity was Caucasian (94%), Hispanic (3.4%), black (1.3%), Asian (including Pacific Islander) (1.1%), and Native American (0.6%). The mean body weight was 188±40 pounds. Thrombolytic therapy was administered in 35% of patients. The mean peak serum creatine kinase enzyme level was 1684±2134ug/L, and the median peak creatine kinase-MB was 186±267ug/L. There were 198 anterior, 8 anterolateral, 27 lateral, 21 inferolateral, and 234 inferior infarcts. LVEF data were available at enrollment in 412 patients, with a mean of 53±13%. At post-discharge follow-up, the mean LVEF was 47±9%.
At the time ECG recordings were made, we were able to obtain medication lists for 615 (91%) of patients. Of these patients, 549 (89%) were taking aspirin, 73 (12%) digoxin, 395 (64%) beta-blockers, 253(41%) ace-inhibitors and 19 (3%) ARBs, and 297 (48%) statins. These rates must be interpreted in the context of the era during which most patients were enrolled.
After a mean follow-up of 63 months (3635 patient-years), 134 patients met the composite endpoint (123 deaths, and 11 documented ventricular arrhythmias). Causes of deaths were categorized as arrhythmia in 15, pump failure in 23, infarct-related in 10, and noncardiac causes in 79.
Using nonparametric tests of equality to compare clinical variables between groups, the Event Group had a lower LVEF, lower body weight, and more advanced age than the Non-Event Group (see Table 1). Using the chi-squared test to compare prevalences between groups, patients in the Event Group were less likely to have received thrombolytics and less likely to have been smokers. There were no significant differences between groups in peak CK or peak CK-MB.
Table 1.
Comparison of clinical characteristics between Event Group and Non-Event Group.
| Clinical Variable | Event Group | ←p-value→ | Non-Event Group |
|---|---|---|---|
| LVEF | 43% | p < 0.001 | 48% |
| Age | 73 years | p < 0.001 | 62 years |
| Thrombolytics | 24% | p < 0.01 | 37% |
| Male gender | 69% | p=0.02 | 80% |
| Weight | 182 pounds | p = 0.02 | 189 pounds |
| Smoker | 12% | p = 0.04 | 20% |
| Peak CK | 1685 ug/L | p = 0.1 | 1693 ug/L |
| Peak CK-MB | 192 ug/L | p = 0.17 | 186 ug/L |
Repolarization scatter
Example RTI:DI scatterplots from two representative patients are shown in Figure 1. Compared to the 560 patients in the Non-Event Group, patients in the Event Group had lower CLMean and higher RTI:DIStdErr, but other RTI:DI scatterplot characteristics were not different between groups (See Table 2.) The optimal operating points for these variables are also shown in Table 2, with the strongest predictor being RTI:DIStdErr at an operating point of 5.5ms, where the c-statistic was 0.6. The median RTI:DIStdErr was 5.1ms. The Kaplan-Meier clinical event curves of patients with positive versus negative RTI:DIStdErr were well separated (p<0.001) (See Figure 2). The hazard ratio for events comparing patients with positive versus negative RTI:DIStdErr tests was 2.1 (95% C.I. = 1.43 3.10). Event curves were less well separated using CLMean (p=0.006) as a predictor.
Figure 1.
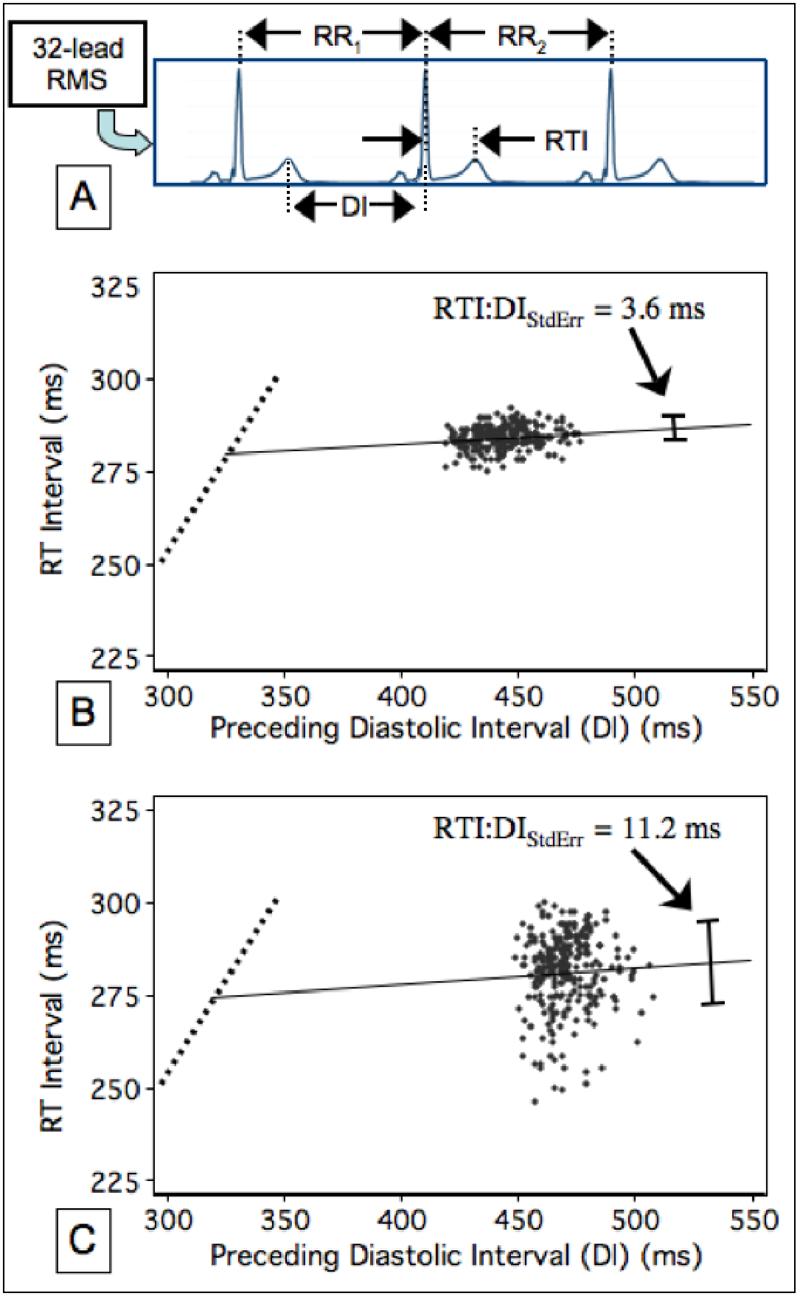
Assessment of the restitution curves. A) RT intervals (RTIs) are plotted as a function of preceding diastolic intervals (DI) as measured from the root mean square (RMS) of 32-lead resting ECG recordings. B) Example data from a patient in the Non-Event group. The steep portion of the restitution curve (dotted line) cannot be assessed with resting recordings because high heart rates are necessary to observe this restitution behavior. The linear regression estimate of this data is superimposed (solid line), along with RTI:DIStdErr (the standard error of the estimate (whisker bar)). C) Example data from a patient in the Event group, showing a significantly higher RTI:DIStdErr. The heart rate variability, as assessed with CLSD, was actually lower in panel C (5.1 vs. 12ms).
Table 2.
Relative predictive performance of candidate predictor variables. Means (95% C.I.) are compared between event-free patients and those with events. Optimal operating points (OOP) are used to predict survival curves, which are compared by log rank test.
| Predictor Variable | Patients with Events | ←p-Value→ | Event-Free Patients | OOP | Log Rank Test |
|---|---|---|---|---|---|
| RTI:DIStdErr* (ms) | 7.1 (5.1 9.2) | <0.001 | 5.46 (4.90 6.01) | ≥ 5.50 | p=0.001 (χ2=8.6) |
| QTc (ms) | 421 (415 426) | <0.001 | 408 (405 410) | >440 | p<0.001 (χ2=14) |
| CLMean(ms) | 871 (842 900) | <0.001 | 933 (919 946) | ≤ 930 | p=0.006 (χ2=7.5) |
| QTVI* | −0.93 (−1.08 −0.77) | NS | −1.05 (−1.10 −1.00) | ≥−1.19 | p=0.03 (χ2=4.65) |
| ICC | 0.554 (0.50 0.61) | 0.03 | 0.639 (0.62 0.66) | ≤ 0.81 | p=NS (χ2=2.00) |
| CLSD* (ms) | 40.1 (31 49) | NS | 32.6 (29.4 35.7) | ≤ 43.3 | p= NS (χ2=2.80) |
| RTIMean(ms) | 281 (271 291) | NS | 292 (287 298) | ≤ 284 | p= NS (χ2=0.18) |
| RTI:DISlope* | 0.023 (0.01 0.04) | NS | 0.030 (0.024 0.037) | ≥ 0.019 | p= NS (χ2=0.02) |
| RTI:DIY-intercept(ms) | 264 (255 274) | NS | 276 (270 283) | ≤ 271 | p= NS (χ2=0.15) |
| pNN50 (%) | 9.6% (6.1 13.2) | NS | 7.6% (6.3 9.0) | ≥ 0.5% | p= NS (χ2=0.01) |
| rMMSD (ms) | 36.9 (28 46) | NS | 28.8 (25 33) | ≤ 20.3 | p= NS (χ2=0.00) |
RT:DI = Linear regression estimate of preceding Diastolic Interval predicting RT interval
QTVI = QT Variabiliy Index
SD = Standard Deviation
StdErr = Standard Error about the estimate
Figure 2.
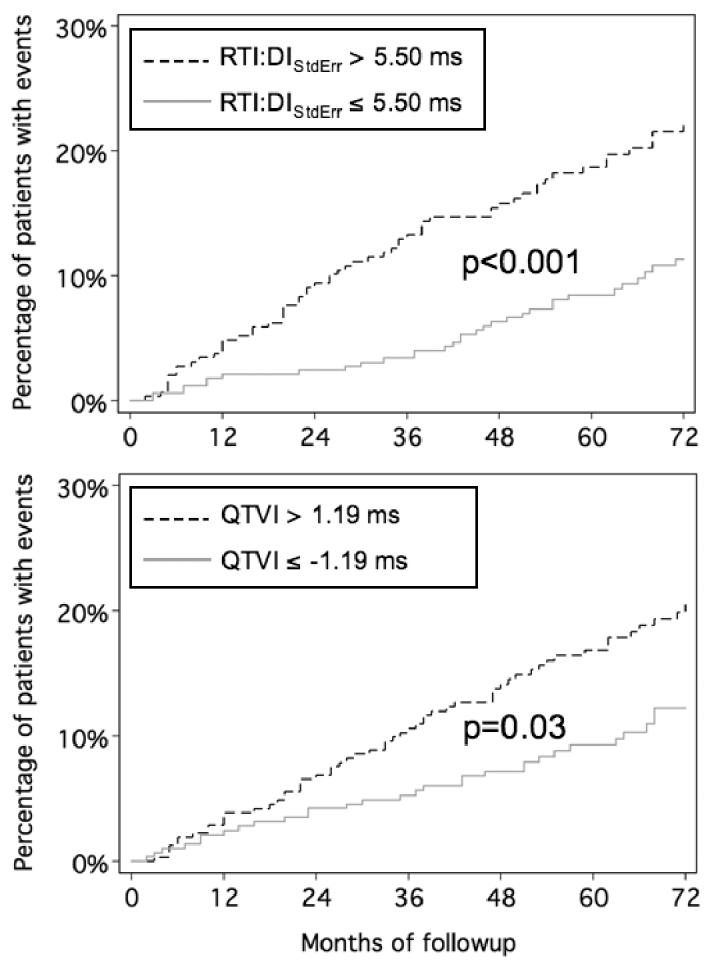
Kaplan-Meier clinical event curves of patients as stratified by RTI:DIStdErr or QTVI (QT Variability Index) predictions. Patients with a RTI:DIStdErr over 5.5ms sustain a 210% increase in the rate of clinical events compared to patients with RTI:DIStdErr below 5.5ms. QTVI was a weaker predictor, but itself identified a group with a 54% increase in rate of events.
When the predictive value of RTI:DIStdErr was re-analyzed using the secondary endpoint of cardiac deaths or documented ventricular arrhythmias (thus excluding noncardiac events), the optimal operating point dropped to 5.1ms, where the c-statistic improved to 0.62. The hazard ratio increased to 2.7 (1.4 4.7) and event curves remained well separated (p<0.01)(See Figure 3).
Figure 3.
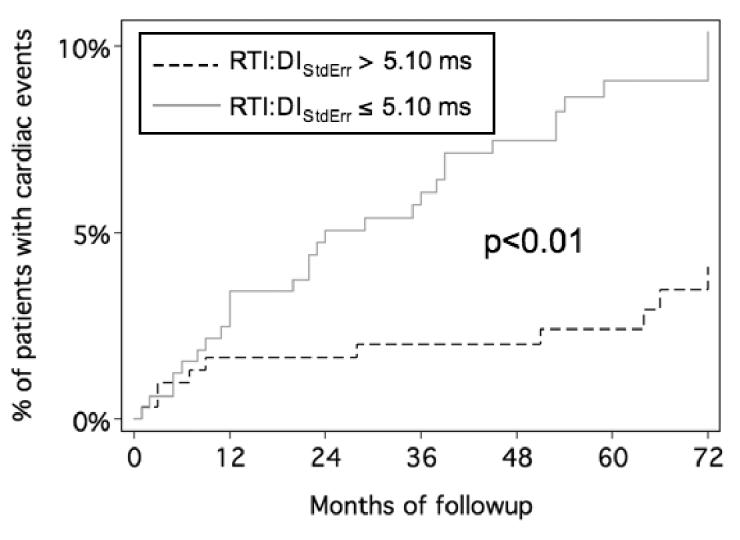
Kaplan-Meier curves for cardiac events as stratified by RTI:DIStdErr. Patients with a RTI:DIStdErr over 5.1ms sustain a 270% increase in the rate of cardiac events compared to patients with RTI:DIStdErr below 5.1ms.
There was no correlation between RTI:DIStdErr and mean heart rate, ρ = 0.03 (p=0.49). RTI:DIStdErr increased slightly with age, ρ = 0.10 (p=0.008) but did not vary with gender. Neither smoking nor the use of thrombolytics effected RTI:DIStdErr. There was a weak correlation between RTI:DIStdErr and LVEF, ρ = −0.09 (p=0.03), with slightly higher RTI:DIStdErr at lower ejection fractions. The median RTI:DIStdErr was 5.1 in patients on beta-blockers and 5.2 patients not taking beta-blockers (p=0.29). The only medications to influence RTI:DIStdErr in univariate analyses were digoxin (5.8 versus 5.0, p=0.02) and statins (5.3 vs. 4.9, p=0.02).
QT variability index
The bootstrap estimate of the population mean (95% C.I.) for QTVI was −1.03 (−1.09 −0.97). The optimal operating point was −1.19, which gave a c-statistic of 0.57. As stratified by this test, survival curves were separable (p=0.03) with a hazard ratio of 1.53 in patients with QTVI > 1.19 (see Figure 2). As shown in Figure 3, QTVI correlated only weakly with RTI:DIStdErr. In a multivariable Cox prediction model, RTI:DIStdErr remained a significant predictor of clinical events (p=0.001) even after adjusting for QTVI.
Heart rate variability indices
As shown in Table 1, ICC (interbeat correlation coefficient) demonstrated the strongest association with clinical events among HRV indices. The Non-Event Group had a mean ICC of 0.639, where the Event Group had a mean of 0.544 (p=0.03). However, survival curves were not separated using ICC alone as a predictor (p=0.16).
None of the HRV indices (CLSD, ICC, pNN50, and rMMSD) showed any correlation with RTI:DIStdErr (ρ = 0.04, 0.00, −0.04, and −0.04, respectively), demonstrating that repolarization scatter is independent of high frequency HRV. There was weak correlation between RTI:DIStdErr and CLMean, ρ = −0.15 (p=0.0001), with greater repolarization scatter at lower cycle lengths. Similarly, there was weak correlation between RTI:DIStdErr and RTIMean, QTc, and RTI:DIY-intercept, ρ = −0.13 (p=0.001), ρ = −0.13 (p=0.002), and ρ = −0.10 (p=0.01), respectively. There was no correlation between RTI:DIStdErr and RTI:DISlope, ρ = 0.00 (p=0.98). As expected, all measures of high frequency HRV were well correlated amongst each other. The weakest correlation amongst HRV variables was between ICC and CLSD, ρ = −0.24 (p<0.0001) and the strongest between pNN50 and rMMSD, ρ = 0.87 (p<0.0001).
Sixteen patients (3%) demonstrated atrial fibrillation during their 5 minute recordings. This pattern was more prevalent in the Event Group (6.1%) than in the Non-Event Group (1.2%), but 7 of the 8 events in subjects with atrial fibrillation were noncardiac. RTI:DIStdErr was higher in recordings with AF (median 7.4 versus 5.1ms, p=0.002). In this small group of patients with AF, RTI:DIStdErr did not appear to offer substantial risk stratification – and thus the inclusion of these patients should only weaken the overall predictive accuracy of RTI:DIStdErr. Indeed, if these patients are excluded from the analysis, the hazard ratio for the primary endpoint increases to 2.5, and remains significant (p=0.001). This small cohort of course demonstrated significant elevations in all measures of high frequency HRV.
Multivariable analyses
Several multivariable models were fitted to assess interactions between RTI:DIStdErr and other predictor variables. In a two-variable model including LVEF and RTI:DIStdErr, the independent predictive value of RTI:DIStdErr remained highly significant (p<0.001). Adding QTc and CLMean sequentially improved the overall model, with RTI:DIStdErr remaining significant (p=0.02). Adding ICC to the model with RTI:DIStdErr alone offered slight improvement and strengthened the independent effect of RTI:DIStdErr (p<0.001). The atrial fibrillation pattern was collinear with HRV measures, but when this variable was added to RTI:DIStdErr in predicting events, both atrial fibrillation (p=0.04) and RTI:DIStdErr (p<0.001) exhibited independent effects. None of the other predictor variables had significant influences on the multivariable model, as expected based on their weak predictive performances. This confirms the independence of RTI:DIStdErr from HRV that was suggested in the univariate analysis.
The hazard ratio imposed by an abnormal RTI:DIStdErr improved to 2.5 (95% C.I. 1.4 – 4.0) after adjusting for all medication classes. In this model, beta-blockers imposed a protective effect (HR 0.56, p<0.01) and ace-inhibitors imposed an increased risk (HR 1.4 p=0.05). However, these influences are confounded by contractile function as neither of these influences remain significant if you further adjust for ejection fraction.
Discussion
The main findings from this study are: 1) increased scatter about the flat portion of the repolarization / diastolic interval curve (RTI:DIStdErr) portends a significantly increased risk of ventricular arrhythmias or death in post-myocardial infarction patients, and 2) the predictive value of this measurement was independent from LVEF, QTc, short-term HRV parameters and the QT Variability Index. Our findings suggest that this noninvasive measurement of repolarization lability could provide a relatively simple and inexpensive new tool in risk stratifying patients with a history of prior MI.
Uniqueness from other predictive measures
Both spectral and time-domain MTWA techniques are highly sensitive to beat-to-beat changes in T-wave amplitude37, 38, but potentially insensitive to changes in T-wave timing. However, as there is increasing recognition of mechanistic ties between MTWA and the calcium handling alterations that manifest abrupt changes in repolarization timing in response to cycle length changes39-42, improved methods for assessing temporal perturbations in repolarization are needed. As our methods are solely intended for assessing the timing of T-waves (as an estimate of the beat-to-beat changes in mean cellular repolarization duration), they are likely to complement the use of MTWA in predicting events, though further work is required to explore this relationship.
Heart rate variability measures have developed rapidly in recent years, and have proven value in predicting outcomes, but the mechanistic links between HRV and arrhythmogenesis remain very indirect. Indeed, HRV measures provide valuable information about the function of the autonomic nervous system, a key component of the arrhythmogenic milieu. The effect of autonomic function on the actual onset and propagation of arrhythmias may occur via modulation of repolarization and its dispersion43-48. Temporal and spectral analyses of repolarization therefore appear to offer more direct assessment of the arrhythmogenic substrate. Our finding that high frequency HRV is independent of repolarization scatter strongly supports the notion that HRV and repolarization scatter will provide complementary prognostic information, and will allow independent assessments of both the systemic milieu and tissue substrate that underlie arrhythmogenesis.
At first glance, this measure of repolarization scatter is similar in concept to QTVI, which seeks to describe repolarization variability after adjusting for heart rate variability. The critical difference lies in the pair-wise nature of this proposed method. For any given levels of QT variance and RR variance, it is possible to have zero correlation or perfect correlation between the two intervals. If there is instability or time delay in the adaptation of repolarization timing in response to changes in diastolic interval, departure from perfect correlation occurs. The superior predictive performance of the scatter method in this study suggests that this instability is more important than the absolute variability of repolarization or heart rate.
Limitations
The main limitation of this study is that the numerical cutoffs for predictor variables were retrospectively determined. Although patients were longitudinally followed and the prediction variables were predetermined, the predictive performance was assessed in the same population used for predictor derivation, which increases the chance of type I statistical error. Prospective validation in a separate population is therefore needed. It should also be emphasized that HRV parameters were calculated from only 5-minute ECG recordings and therefore lack the reproducibility of parameters derived from longer recording periods. Also, the clinical endpoint we used is an imperfect marker for the occurrence of arrhythmias. Many patient deaths appeared to be unrelated to ventricular arrhythmias. In addition, there were likely clinically relevant ventricular arrhythmias that were not detected. However, short of implanting recording devices to detect all arrhythmias, we believe that all-cause mortality is the least ambiguous endpoint for this type of study. Misclassification of the cause of death is more likely to dilute the predictive accuracy of repolarization scatter than to inflate it. Indeed, the hazard ratio for events imposed by abnormally high repolarization scatter was higher when noncardiac events were excluded. Finally, our methods utilized nonstandard 32-lead mapping systems (to facilitate parallel efforts with methods requiring high spatial resolution). The feasibility of repolarization scatter would be improved if 12-lead recording systems could be used, but future study will be needed to validate this approach.
Conclusion
The findings from the present study suggest that repolarization scatter, quantified by the standard error about the regression estimate for RTI versus diastolic interval (RTI:DIStdErr), is predictive of deaths and arrhythmic events. A RTI:DIStdErr over 5.50 is associated with a 210% increase in the event rate. Repolarization scatter, which quantifies instability in the repolarization – diastolic interval feedback system, is distinct from the QT variability index, and independent of QTc and high frequency heart rate variability measures, therefore providing additive prognostic information in post-MI patients.
Figure 4.
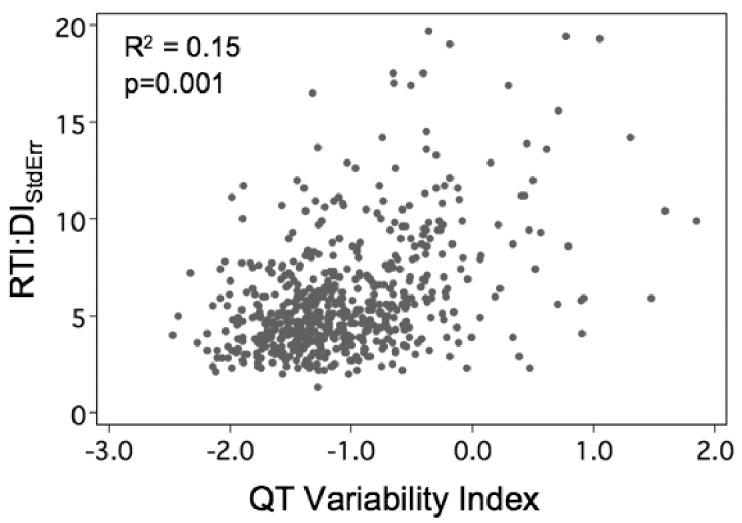
Scatterplot showing weak correlation between RTI:DIStdErr and QT Variability Index. The absence of a strong correlation between these two measures suggests they may be of complementary prognostic value.
Acknowledgments
This work was supported by grants from the NIH (HL52338 (RLL), 5T32HL007576-20 (NMS)) and a grant from the Department of Veterans Affairs (SEL).
Financial Support: This work was supported by grants from the NIH (HL52338 (RLL), 5T32HL007576-20 (NMS)) and a grant from the Department of Veterans Affairs (SEL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None.
References
- 1.Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980-2000. Jama. 2002 Dec 18;288(23):3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 2.Rea TD, Eisenberg MS, Sinibaldi G, White RD. Incidence of EMS-treated out-of-hospital cardiac arrest in the United States. Resuscitation. 2004 Oct;63(1):17–24. doi: 10.1016/j.resuscitation.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Wellens HJ. Cardiac arrhythmias: the quest for a cure: a historical perspective. J Am Coll Cardiol. 2004 Sep 15;44(6):1155–1163. doi: 10.1016/j.jacc.2004.05.080. [DOI] [PubMed] [Google Scholar]
- 4.Baker LC, London B, Choi BR, Koren G, Salama G. Enhanced dispersion of repolarization and refractoriness in transgenic mouse hearts promotes reentrant ventricular tachycardia. Circ Res. 2000 Mar 3;86(4):396–407. doi: 10.1161/01.res.86.4.396. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan VS, Downar E, Nanthakumar K, Parker JD, Ross HJ, Chan W, Picton P. Increased ventricular repolarization heterogeneity in patients with ventricular arrhythmia vulnerability and cardiomyopathy: a human in vivo study. Am J Physiol Heart Circ Physiol. 2006 Jan;290(1):H79–86. doi: 10.1152/ajpheart.00648.2005. [DOI] [PubMed] [Google Scholar]
- 6.Yuan S, Wohlfart B, Olsson SB, Blomstrom-Lundqvist C. The dispersion of repolarization in patients with ventricular tachycardia. A study using simultaneous monophasic action potential recordings from two sites in the right ventricle. Eur Heart J. 1995 Jan;16(1):68–76. doi: 10.1093/eurheartj/16.1.68. [DOI] [PubMed] [Google Scholar]
- 7.Bass BG. Restitution of the action potential in cat papillary muscle. Am J Physiol. 1975 Jun;228(6):1717–1724. doi: 10.1152/ajplegacy.1975.228.6.1717. [DOI] [PubMed] [Google Scholar]
- 8.Boyett MR, Jewell BR. A study of the factors responsible for rate-dependent shortening of the action potential in mammalian ventricular muscle. J Physiol. 1978 Dec;285:359–380. doi: 10.1113/jphysiol.1978.sp012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyett MR, Jewell BR. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol. 1980;36(1):1–52. doi: 10.1016/0079-6107(81)90003-1. [DOI] [PubMed] [Google Scholar]
- 10.Sosnowski M, Czyz Z, Tendera M. Scatterplots of RR and RT interval variability bring evidence for diverse non-linear dynamics of heart rate and ventricular repolarization duration in coronary heart disease. Europace. 2001 Jan;3(1):39–45. doi: 10.1053/eupc.2000.0144. [DOI] [PubMed] [Google Scholar]
- 11.Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000 Oct 3;102(14):1664–1670. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- 12.Pak HN, Hong SJ, Hwang GS, Lee HS, Park SW, Ahn JC, Moo Ro Y, Kim YH. Spatial dispersion of action potential duration restitution kinetics is associated with induction of ventricular tachycardia/fibrillation in humans. J Cardiovasc Electrophysiol. 2004 Dec;15(12):1357–1363. doi: 10.1046/j.1540-8167.2004.03569.x. [DOI] [PubMed] [Google Scholar]
- 13.Karma A. Electrical alternans and spiral wave breakup in cardiac tissue. Chaos. 1994 Sep;4(3):461–472. doi: 10.1063/1.166024. [DOI] [PubMed] [Google Scholar]
- 14.Chow T, Kereiakes DJ, Bartone C, Booth T, Schloss EJ, Waller T, Chung ES, Menon S, Nallamothu BK, Chan PS. Prognostic utility of microvolt T-wave alternans in risk stratification of patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2006 May 2;47(9):1820–1827. doi: 10.1016/j.jacc.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 15.Bloomfield DM, Steinman RC, Namerow PB, Parides M, Davidenko J, Kaufman ES, Shinn T, Curtis A, Fontaine J, Holmes D, Russo A, Tang C, Bigger JT., Jr. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004 Oct 5;110(14):1885–1889. doi: 10.1161/01.CIR.0000143160.14610.53. [DOI] [PubMed] [Google Scholar]
- 16.Bloomfield DM, Bigger JT, Steinman RC, Namerow PB, Parides MK, Curtis AB, Kaufman ES, Davidenko JM, Shinn TS, Fontaine JM. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006 Jan 17;47(2):456–463. doi: 10.1016/j.jacc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Chow T, Kereiakes DJ, Bartone C, Booth T, Schloss EJ, Waller T, Chung E, Menon S, Nallamothu BK, Chan PS. Microvolt T-wave alternans identifies patients with ischemic cardiomyopathy who benefit from implantable cardioverter-defibrillator therapy. J Am Coll Cardiol. 2007 Jan 2;49(1):50–58. doi: 10.1016/j.jacc.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 18.Chan PS, Bartone C, Booth T, Kereiakes D, Chow T. Prognostic implication of redefining indeterminate microvolt T-wave alternans studies as abnormal or normal. Am Heart J. 2007 Apr;153(4):523–529. doi: 10.1016/j.ahj.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Thomsen MB, Oros A, Schoenmakers M, van Opstal JM, Maas JN, Beekman JD, Vos MA. Proarrhythmic electrical remodelling is associated with increased beat-to-beat variability of repolarisation. Cardiovascular research. 2007 Feb 1;73(3):521–530. doi: 10.1016/j.cardiores.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen MB, Verduyn SC, Stengl M, Beekman JD, de Pater G, van Opstal J, Volders PG, Vos MA. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation. 2004 Oct 19;110(16):2453–2459. doi: 10.1161/01.CIR.0000145162.64183.C8. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen MB, Volders PG, Beekman JD, Matz J, Vos MA. Beat-to-Beat variability of repolarization determines proarrhythmic outcome in dogs susceptible to drug-induced torsades de pointes. J Am Coll Cardiol. 2006 Sep 19;48(6):1268–1276. doi: 10.1016/j.jacc.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 22.Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997 Sep 2;96(5):1557–1565. doi: 10.1161/01.cir.96.5.1557. [DOI] [PubMed] [Google Scholar]
- 23.Piccirillo G, Quaglione R, Nocco M, Naso C, Moise A, Lionetti M, Di Carlo S, Marigliano V. Effects of long-term beta-blocker (metoprolol or carvedilol) therapy on QT variability in subjects with chronic heart failure secondary to ischemic cardiomyopathy. Am J Cardiol. 2002 Nov 15;90(10):1113–1117. doi: 10.1016/s0002-9149(02)02778-9. [DOI] [PubMed] [Google Scholar]
- 24.Atiga WL, Calkins H, Lawrence JH, Tomaselli GF, Smith JM, Berger RD. Beat-to-beat repolarization lability identifies patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol. 1998 Sep;9(9):899–908. doi: 10.1111/j.1540-8167.1998.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 25.Atiga WL, Fananapazir L, McAreavey D, Calkins H, Berger RD. Temporal repolarization lability in hypertrophic cardiomyopathy caused by beta-myosin heavy-chain gene mutations. Circulation. 2000 Mar 21;101(11):1237–1242. doi: 10.1161/01.cir.101.11.1237. [DOI] [PubMed] [Google Scholar]
- 26.Piccirillo G, Magri D, Matera S, Magnanti M, Torrini A, Pasquazzi E, Schifano E, Velitti S, Marigliano V, Quaglione R, Barilla F. QT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: a prospective study. Eur Heart J. 2006 Nov 13; doi: 10.1093/eurheartj/ehl367. [DOI] [PubMed] [Google Scholar]
- 27.Lux RL, Smith CR, Wyatt RF, Abildskov JA. Limited lead selection for estimation of body surface potential maps in electrocardiography. IEEE Trans Biomed Eng. 1978 May;25(3):270–276. doi: 10.1109/TBME.1978.326332. [DOI] [PubMed] [Google Scholar]
- 28.Fuller MS, Sandor G, Punske B, Taccardi B, MacLeod RS, Ershler PR, Green LS, Lux RL. Estimates of repolarization dispersion from electrocardiographic measurements. Circulation. 2000 Aug 8;102(6):685–691. doi: 10.1161/01.cir.102.6.685. [DOI] [PubMed] [Google Scholar]
- 29.Fuller MS, Sandor G, Punske B, Taccardi B, MacLeod RS, Ershler PR, Green LS, Lux RL. Estimates of repolarization and its dispersion from electrocardiographic measurements: direct epicardial assessment in the canine heart. J Electrocardiol. 2000 Apr;33(2):171–180. doi: 10.1016/s0022-0736(00)80073-4. [DOI] [PubMed] [Google Scholar]
- 30.Lux RL, Fuller MS, MacLeod RS, Ershler PR, Punske BB, Taccardi B. Noninvasive indices of repolarization and its dispersion. J Electrocardiol. 1999;32(Suppl):153–157. doi: 10.1016/s0022-0736(99)90073-0. [DOI] [PubMed] [Google Scholar]
- 31.Haws CW, Lux RL. Correlation between in vivo transmembrane action potential durations and activation-recovery intervals from electrograms. Effects of interventions that alter repolarization time. Circulation. 1990 Jan;81(1):281–288. doi: 10.1161/01.cir.81.1.281. [DOI] [PubMed] [Google Scholar]
- 32.Raetz SL, Richard CA, Garfinkel A, Harper RM. Dynamic characteristics of cardiac R-R intervals during sleep and waking states. Sleep. 1991 Dec;14(6):526–533. doi: 10.1093/sleep/14.6.526. [DOI] [PubMed] [Google Scholar]
- 33.Schechtman VL, Harper RK, Harper RM. Analysis of beat-to-beat heart rate changes during sleep-waking states in normal infants. J Dev Physiol. 1993 Jun;19(6):263–271. [PubMed] [Google Scholar]
- 34.Schechtman VL, Raetz SL, Harper RK, Garfinkel A, Wilson AJ, Southall DP, Harper RM. Dynamic analysis of cardiac R-R intervals in normal infants and in infants who subsequently succumbed to the sudden infant death syndrome. Pediatr Res. 1992 Jun;31(6):606–612. doi: 10.1203/00006450-199206000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Woo MA, Stevenson WG, Moser DK, Trelease RB, Harper RM. Patterns of beat-to-beat heart rate variability in advanced heart failure. Am Heart J. 1992 Mar;123(3):704–710. doi: 10.1016/0002-8703(92)90510-3. [DOI] [PubMed] [Google Scholar]
- 36.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996 Mar;17(3):354–381. [PubMed] [Google Scholar]
- 37.Adam DR, Smith JM, Akselrod S, Nyberg S, Powell AO, Cohen RJ. Fluctuations in T-wave morphology and susceptibility to ventricular fibrillation. J Electrocardiol. 1984 Jul;17(3):209–218. doi: 10.1016/s0022-0736(84)80057-6. [DOI] [PubMed] [Google Scholar]
- 38.Estes NA, 3rd, Michaud G, Zipes DP, El-Sherif N, Venditti FJ, Rosenbaum DS, Albrecht P, Wang PJ, Cohen RJ. Electrical alternans during rest and exercise as predictors of vulnerability to ventricular arrhythmias. Am J Cardiol. 1997 Nov 15;80(10):1314–1318. doi: 10.1016/s0002-9149(97)00694-2. [DOI] [PubMed] [Google Scholar]
- 39.Bao M, Zhang J, Huang C, Jiang H, Liu J, Zhao D. Abnormal Intracellular Calcium Handling Underlying T-Wave Alternans and Its Hysteresis. Cardiology. 2006 Nov 3;108(3):147–156. doi: 10.1159/000096566. [DOI] [PubMed] [Google Scholar]
- 40.Goldhaber JI, Xie LH, Duong T, Motter C, Khuu K, Weiss JN. Action potential duration restitution and alternans in rabbit ventricular myocytes: the key role of intracellular calcium cycling. Circ Res. 2005 Mar 4;96(4):459–466. doi: 10.1161/01.RES.0000156891.66893.83. [DOI] [PubMed] [Google Scholar]
- 41.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004 Apr 30;94(8):1083–1090. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 42.Walker ML, Rosenbaum DS. Repolarization alternans: implications for the mechanism and prevention of sudden cardiac death. Cardiovascular research. 2003 Mar;57(3):599–614. doi: 10.1016/s0008-6363(02)00737-x. [DOI] [PubMed] [Google Scholar]
- 43.Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circ Res. 2000 Apr 14;86(7):816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 44.Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovascular research. 2001 May;50(2):409–416. doi: 10.1016/s0008-6363(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 45.Mantravadi R, Gabris B, Liu T, Choi BR, de Groat WC, Ng GA, Salama G. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circ Res. 2007 Apr 13;100(7):e72–80. doi: 10.1161/01.RES.0000264101.06417.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Opthof T, Coronel R, Vermeulen JT, Verberne HJ, van Capelle FJ, Janse MJ. Dispersion of refractoriness in normal and ischaemic canine ventricle: effects of sympathetic stimulation. Cardiovascular research. 1993 Nov;27(11):1954–1960. doi: 10.1093/cvr/27.11.1954. [DOI] [PubMed] [Google Scholar]
- 47.Opthof T, Misier AR, Coronel R, Vermeulen JT, Verberne HJ, Frank RG, Moulijn AC, van Capelle FJ, Janse MJ. Dispersion of refractoriness in canine ventricular myocardium. Effects of sympathetic stimulation. Circ Res. 1991 May;68(5):1204–1215. doi: 10.1161/01.res.68.5.1204. [DOI] [PubMed] [Google Scholar]
- 48.Takei M, Sasaki Y, Yonezawa T, Lakhe M, Aruga M, Kiyosawa K. The autonomic control of the transmural dispersion of ventricular repolarization in anesthetized dogs. J Cardiovasc Electrophysiol. 1999 Jul;10(7):981–989. doi: 10.1111/j.1540-8167.1999.tb01269.x. [DOI] [PubMed] [Google Scholar]


