Abstract
Purpose
The Wnt pathway is an essential signaling cascade that regulates multiple processes in developing and adult tissues, including differentiation, cellular survival, and stem cell proliferation. The authors recently demonstrated altered expression of Wnt pathway genes during photoreceptor death in rd1 mice, suggesting an involvement for Wnt signaling in the disease process. In this study, the authors investigated the role of Wnt signaling in retinal degeneration.
Methods
The Wnt signaling reporter mouse line Tcf-LacZ was crossed with retinal degeneration rd1 mice, and β-galactosidase expression was used to localize Wnt signaling during photoreceptor death. To analyze the role of Wnt signaling activation, primary mixed retinal cultures were prepared, and XTT and TUNEL assays were used to quantify cell death. Luciferase reporter assays were used to measure Wnt signaling.
Results
The canonical Wnt signaling pathway was activated in Müller glia and the ganglion cell layer during rod photoreceptor degeneration in rd1/Tcf-LacZ mice. Wnt signaling was confirmed in cultured primary Müller glia. Furthermore, Wnt signaling activators protected photoreceptors in primary retinal cultures from H2O2-induced oxidative stress. The Wnt ligands Wnt5a, Wnt5b, Wnt10a, and Wnt13 were expressed in the degenerating retina and are candidate Wnt signaling activators in vivo.
Conclusions
This study is the first demonstration that Wnt signaling is activated in the degenerating retina and that it protects retinal cultures from oxidative stress. These data suggest that Wnt signaling is a component of the glial protective response during photoreceptor injury. Therefore, inducing Wnt activation, alone or in combination with growth factors, may increase the threshold for apoptosis and halt or delay further photoreceptor degeneration.
The Wnt signaling pathway is an essential intercellular communication pathway that controls many processes in embryonic and adult tissues, ranging from body axis determination and axonal outgrowth to cellular proliferation and differentiation.1,2 Altered Wnt signaling is a contributing factor in several ocular diseases and malignancies of the eye.3–5 Despite increasing recognition that the Wnt pathway regulates vertebrate eye development,3,6–14 the expression and function of Wnt signaling has not been systematically examined in the degenerating retina.
The canonical Wnt pathway is the best understood of the Wnt pathways in mammalian tissues.15 Secreted Wnt ligands bind to the coreceptors Frizzled and LRP5/6 at the plasma membrane, inducing activation of the protein Dishevelled (Dsh) and leading to stabilization of the central mediator β-catenin. Stabilized β-catenin translocates to the nucleus and binds to Tcf/Lef type transcription factors, leading to transcription of Wnt target genes. In the absence of Wnt ligands, β-catenin is phosphorylated and ubiquitinated by the cytoplasmic APC-GSK3β-axin destruction complex, which leads to β-catenin degradation by the proteosome.
We identified expression changes in Wnt signaling genes during rod and cone death in the rd1 retinal degeneration mouse in a recent microarray study.16 Several of these genes are expressed in Müller glia16 (Hackam A, unpublished observations, 2005), which is a retinal cell type that contributes to photoreceptor protection during retina damage. Previous studies also demonstrated that Wnt inhibitor proteins SFRP1, 2, 3, and 5 were upregulated in Müller glia and photoreceptors in retinitis pigmentosa tissue.17,18 Together, these findings raise questions about whether the Wnt pathway is active during retinal degeneration and, if so, what its role is.
A potential function for the Wnt pathway during retinal degeneration is suggested by reports that Wnt signaling is antiapoptotic in many tissues. In an Alzheimer disease cellular model, Wnt pathway activation by antisense knockdown of the inhibitor Dkk119 or addition of the activator Wnt3a ligand20 significantly decreased apoptosis in cultured neurons exposed to β-amyloid peptide. Additionally, reduced Wnt signaling led to increased neuronal death in mouse cranial neural crest cells21 and Drosophila neurons.22 In vivo studies supported these in vitro observations by demonstrating that Wnt activation significantly protected hippocampal CA1 neurons in a rat four-vessel occlusion global ischemia model.23 The antiapoptotic activity of the Wnt pathway in neurons in these examples provides a compelling rationale to explore whether Wnt signaling regulates cell survival in the retina. In this study, we characterized activated Wnt signaling in vivo during retinal degeneration and analyzed the effect of Wnt pathway activators on primary dissociated retinal cultures.
Materials and Methods
Reagents
Canonical Wnt signaling was induced by the chemical Wnt signaling activators SB216763 (40 μM, resuspended in dimethyl sulfoxide [DMSO]), LiCl (40 mM) (both from Sigma, St. Louis, MO), recombinant Wnt3a (50−150 ng/mL, resuspended in PBS; R&D Systems, Minneapolis, MN), or conditioned media containing Wnt3a (Wnt3a-CM) prepared from mouse L cells (ATCC, Manassas, VA) stably expressing Wnt3a, mixed in a 1:1 ratio with normal media. Control conditioned media were obtained from parental L cells (Ctrl-CM). To ensure that consistent amounts of Wnt3a were present in the media among experiments, we prepared the conditioned media in large batches and froze single-use aliquots.
Primary Retina Cultures and Primary Müller Glia Cultures
All procedures involving mice were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care and Use Committee at the University of Miami. Retinas from wild-type (wt) postnatal day (P) 8 mice were dissected free of choroidal vessels and dissociated by activated papain for 30 minutes at 37°C, using protocols modified from Barres et al.24 and Wahlin et al.25 Neurobasal medium containing 1 × lo ovomucoid (LoOvo) plus DNAse I (Invitrogen, Carlsbad, CA) was added, and the cells were centrifuged at 800 rpm for 8 minutes at room temperature. The cell pellet was resuspended in neurobasal-LoOvo medium without DNAse, recentrifuged, and resuspended in neurobasal medium containing l-glutamine, B27, and antibiotics. Cells were plated onto poly-d-lysine/laminin– coated 96-well dishes at 2.5 × 105 cells per well. Cultures with fibroblast or endothelial growth, which appeared as large, flat cells, were excluded. Immunostaining with cell type marker antibodies demonstrated that the retinal cultures were almost entirely composed of Müller glia and photoreceptors (see Results). Antibodies against the following cell-type marker proteins were used: calbindin (horizontal cells and a subset of amacrine cells), 1:500 dilution (Novus Biologicals, Littleton, CO), glutamine synthetase (Müller glia), 1:300 dilution (Sigma, St. Louis, MO), Pax6 (amacrine, ganglion), 1:100 dilution (Santa Cruz Biotechnology, Santa Cruz, CA), vimentin (astrocytes and Müller glia), 1:200 (Sigma), rhodopsin (rod photoreceptors), 1:300 (Chemicon, Temecula, CA), PKCα (rod bipolar), 1:200 (Novus Biologicals), and Thy1 (ganglion cells) 1:100 (Santa Cruz Biotechnology).
The cultures were plated onto glass chamber slides (Laboratory-Tek; Life Technologies, Gaithersburg, MD), fixed with 4% fresh paraformaldehyde at room temperature, blocked in 10% goat serum/0.3% Triton X-100 in PBS, and incubated for 1 hour at room temperature with the primary antibody diluted in 2% goat serum /0.3% Triton X-100 in PBS. After washing, the cells were incubated for 30 minutes with secondary antibodies (Molecular Probes, Eugene, OR), washed, counterstained with DAPI, and viewed under a fluorescence microscope (Axiovert 200; Carl Zeiss, Oberkochen, Germany), and images were captured with a digital camera (Axiocam; Carl Zeiss). Photographic and microscopic settings were kept constant for comparisons between samples and controls.
Primary Müller glia cultures were prepared from the primary retinal cultures on the first day after the initial seeding. Attached neurons were removed by mechanical disruption (gently agitating the culture dish and pipetting the culture media across the cells), according to the procedure of Hauck et al.26 The growth media were supplemented with 10% FCS to permit proliferation. The attached cells in the culture were identified as Müller glia based on morphology and immunostaining for glutamine synthetase, and the absence of rhodopsin-positive cells indicated the effectiveness of the disruption procedure.
Viability and TUNEL Assays
Wnt pathway regulators or control media were added to the primary retinal cultures in triplicate wells with or without H2O2 for 24 hours. XTT assays were used to measure viability using WST-1 reagent (Roche Diagnostics, Nutley, NJ) or another reagent (Cell Titer Blue; Promega, Madison, WI), which was added for 1 hour at 37°C and was quantified using an ELISA plate reader. Average absorbance measured for media plus treatment was subtracted from each test sample.4 Each experiment was performed at least three times on different days.
TUNEL assays were performed using a fluorescein in situ apoptosis detection kit (ApopTag; Chemicon) according to the manufacturer's directions. Retinal cultures were immunostained with anti– glutamine synthetase and anti–rhodopsin antibodies to identify TUNEL-positive cells. Cultures were counterstained with DAPI and viewed at 400×magnification. Total cells were counted using DAPI staining, and apoptotic cells were quantified by counting green fluorescence nuclear TUNEL staining that colocalized with glutamine synthetase or rhodopsin to determine the percentage of apoptotic cells per treatment. Cultures were counted in a masked fashion.
Wnt Activity Luciferase Assays
Primary retinal cultures and Müller glia cultures were cotransfected at a 4:1 ratio of the TOP-FLASH luciferase reporter plasmid (a generous gift from Randall Moon, HHMI, University of Washington) and a LacZ-containing plasmid using electroporation (AMAXA Biosystems, Gaithersburg, MD). Briefly, 5 × 106 cells were suspended in neuron nucleofector buffer (AMAXA Biosystems) with 5 μg total plasmid DNA. The mixtures were transferred into a single 2-mm nucleofector cuvette and electroporated with a preset program. Warm media were added immediately after nucleofector electroporation, and the cells were seeded from the cuvette into multiple wells in a tissue culture plate. Transfection efficiency was approximately 30% to 40%, as assessed by transfection of a green fluorescence protein– containing plasmid. Twenty-four hours after transfection, Wnt pathway activators were added for another 24 hours. Cell lysates were then collected in lysis buffer (Reporter; Promega),4 and luciferase activity was measured in a luminometer (Lumistar Galaxy; BMG Labtech, Offenburg, Germany) and normalized to β-galactosidase activity.4 Wnt signaling activity is expressed as luciferase units/β-galactosidase units.
In Vivo Analysis of Wnt Signaling
Tcf-LacZ mice27 were bred with rd1 mice for two generations to obtain mice that were heterozygous for Tcf-LacZ and homozygous for rd1. Genotyping was performed by PCR. Eyes were enucleated from age-matched Tcf-LacZ and rd1/Tcf-LacZ littermates, fixed in 4% paraformaldehyde, incubated in increasing sucrose concentrations (5%−20%), and embedded in OCT and flash frozen, as described.28 Sections were cut at 10-μm thickness and stained using X-gal after standard procedures and counterstained with the nuclear stain DAPI to localize the retinal layers. For immunohistochemistry, the slides were blocked in normal goat serum and incubated with anti–β-galactosidase antibody (Promega) overnight at 4°C, washed in PBS, and incubated with secondary antibody.28 Sections were counterstained with DAPI and viewed using a fluorescence microscope (Axiovert 200; Carl Zeiss), and images were captured with a digital camera (Axiocam; Carl Zeiss). Photographic and microscopic settings were kept constant for comparisons between samples and controls. X-gal stain and β-galactosidase expression was specific to mice containing the transgene, and no β-galactosidase was detected in the nontransgenic littermates.
β-Galactosidase Assays
Primary Müller glia cultures were prepared from Tcf-LacZ mice, as described, and incubated with Wnt3a-CM or Ctrl-CM for 24 hours. Cells were then lysed in lysis buffer (Reporter; Promega), and 20 μL lysate was mixed with β-galactosidase assay reagents (0.1 M MgCl2, 4.5 M β-mercaptoethanol, 0.1 M sodium phosphate [pH 7.5], 0.73 mg/mL o-nitrophenyl-β-D-galactopyranoside) and was incubated at 37°C. The β-galactosidase product was measured at 420 nm in an ELISA reader using three replicates of each sample and normalized to protein concentrations, which were measured using the Lowry reagent (Bio-Rad, Hercules, CA).
Quantitative PCR
Total RNA was isolated from a pooled sample of four animals using phenol-based extraction (Trizol; Invitrogen, Carlsbad, CA), as described.16 The rd1 mice were on a C57Bl/6 background. One microgram total RNA was reverse transcribed into cDNA (Thermoscript; Invitrogen), and quantitative real-time PCR (QPCR) was performed using a thermocycler (iCycler; Bio-Rad) according to the ΔΔCt method.29 Serial dilutions of template (four dilutions, each in triplicate) were used to estimate amplification efficiency for each primer pair. Primer specificity was verified by visualizing the products on agarose gels. Amplification was normalized to the housekeeping gene β-actin or ARP.16 QPCR was performed in triplicate on biological replicates. Student's t-test (for two variables) or ANOVA (for multiple variables) was used to determine statistical significance. Primer sequences are included in Supplementary Table S1, online at http://www.iovs.org/cgi/content/full/48/12/5733/DC1.
Statistical Analysis
Values are reported as mean ± SD. Unpaired t-test or one-way ANOVA and Tukey post-test were used for statistical analyses.
Results
Wnt Activators Protect Retinal Cultures from Oxidative Stress
Primary retinal cultures allow rapid initial screening of potential survival factors and have been used to delineate many aspects of photoreceptor survival when fewer cell types and lack of systemic factors are desirable.30,31 To identify a potential function of Wnt signaling in the retina, we used primary dissociated retina cultures from P8 mice.
Immunostaining using antibodies against retinal cell type marker antibodies was used to characterize the cell types in the retinal cultures. Figure 1 demonstrates that the cultures were highly enriched (approximately 99%) for rod photoreceptors and Müller glia. There was prominent staining with antibodies against rhodopsin, which identifies rod photoreceptors (Figs. 1A, 1B) and glutamine synthetase, which identifies Müller glia (Figs. 1A, 1C). The cultures also stained strongly for the glia marker protein vimentin (Fig. 1E). Every vimentin-positive cell was positive for glutamine synthetase, indicating that Müller glia, not astrocytes, were the prominent glial type. Furthermore, GFAP-positive cells, which primarily had low staining intensity, were present at numbers equivalent to those of glutamine synthetase-positive cells and represented Müller glia (Fig. 1G). Additional cell types were present at low levels or were not represented. An antibody against IBA-1, which labels microglia and macrophages, had minimal staining and detected fewer than 10 cells/well (out of 2 × 105 plated cells). Similarly, the cultures also contained low levels of horizontal, amacrine, and ganglion cells (Figs. 1H–1J).
Figure 1.
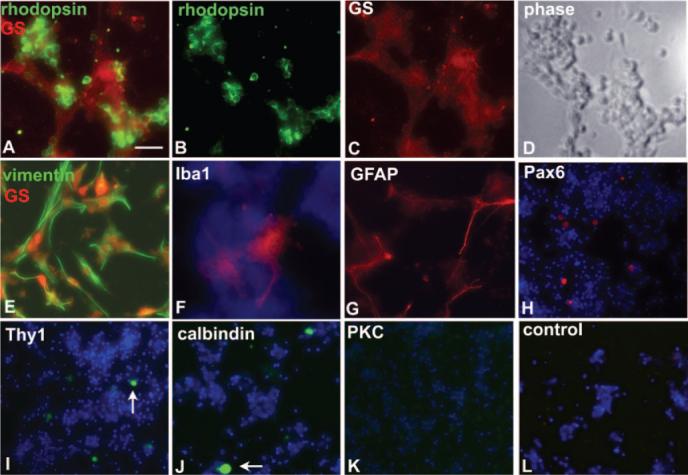
Characterization of the primary retinal cultures using immunostaining. (A–D) Prominent staining with antibodies against rhodopsin (A, B, green, rod photoreceptors) and glutamine synthetase (A, C, red, Müller glia). (D) Corresponding phase image for (A–C). (E) Every vimentin-positive cell was also positive for glutamine synthetase, indicating that Müller glia and not astrocytes were the prominent glial type. (F) Antibodies against IBA-1 (microglia, macrophages) had minimal staining and detected fewer than 10 cells/well (of 2.0 × 105 plated cells). DAPI-stained nuclei are blue. (G) GFAP-positive cells, which mostly had low staining intensity, were present at numbers equivalent to those of GS-positive cells and represented Müller glia. (H–J) Antibodies against Pax6 (H, amacrine and ganglion cells), Thy1 (I, ganglion cells), and calbindin (J, horizontal and amacrine cells) labeled fewer than a dozen cells per well (arrows). No cells were detected with antibodies against PKCα (K, rod bipolar cells), though the appropriate cell type was detected in retinal cross-sections. (L) Incubation with secondary antibodies only had no signal at the same or greater exposure time. Original magnification: (A–G) 40×; (H–L) 20×. Scale bars: (A–G) 20 μm; (H–L) 25 μm.
Careful comparison of immunostaining and DAPI-stained nuclei in low-density cultures indicated that a DAPI-stained cell that did not express rhodopsin or glutamine synthetase occurred rarely in the cultures. Of the 200,000 cells plated per well, only 30 to 40 were detected with nonglia and nonphotoreceptor cellular markers, allowing us to conclude that more than 99% of cells in the cultures were either Müller glia or rod photoreceptors. Immunostaining multiple culture preparations from different litters indicated no evident variability in cell composition. Although other populations of non-Müller glia and nonphotoreceptor cells might have been detected with additional antibodies, based on the DAPI-stained nuclei, we expected that other cell types were present at low numbers in the primary cultures.
Photoreceptor degeneration can be mimicked in culture using acute insult or genetic mutation. A key contributor to photoreceptor death in vivo is oxidative stress. During rod degeneration, high oxygen levels may result from decreased O2 uptake, which increases oxidative stress on photoreceptors, overwhelming protective mechanisms and leading to further cell death.32,33 Oxidative stress results in the production of toxic reactive oxygen species (ROS), including hydroxyl radical, superoxide anion radical, and hydrogen peroxide (H2O2). To mimic the cytotoxic effect of ROS, retinal cultures were incubated in H2O2, and cell death was measured by the XTT assay and TUNEL assay.
Incubating the cultures with 0.1 mM H2O2 to induce oxidative stress caused a 50% reduction in viability (Fig. 2A). Addition of the specific canonical Wnt signaling activator SB216763 to the H2O2–treated cultures significantly protected the retinal cultures, increasing the viability to the level of noninjured cultures (P < 0.001), whereas treatment with H2O2 plus the DMSO vehicle was equivalent to treatment with H2O2 alone. To measure which cells were dying and which were protected, we used a TUNEL assay to count apoptotic cells that coimmunostained with antibodies against rhodopsin or glutamine synthetase. Ninety-nine percent of the TUNEL-positive dead cells expressed rhodopsin, and 1% expressed glutamine synthetase, indicating that rod photoreceptors, not Müller glia, were sensitive to H2O2 (data not shown). Addition of the recombinant Wnt ligand activator Wnt3a to the H2O2-treated cultures significantly decreased the amount of photoreceptor death, confirming that Wnt signaling is neuroprotective (Fig. 2B). We also tested the effect of recombinant Wnt3a using the XTT assay. Wnt3a (50 ng/mL and 100 ng/mL) was added with the H2O2 and compared with cultures treated with H2O2 alone Fig. 2C, no Wnt). Wnt3a significantly increased the viability of the retinal cultures (P < 0.05; Fig. 2C). Phase-contrast images of retinal cultures treated with H2O2 and Wnt activators are shown in Supplementary Figure S1, http://www.iovs.org/cgi/content/full/48/12/5733/DC1.
Figure 2.
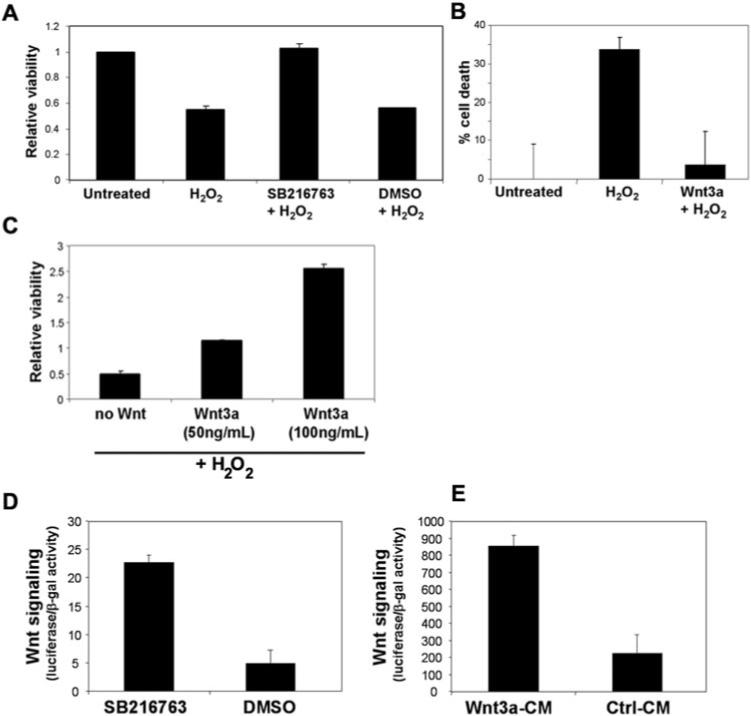
Wnt pathway activation protects primary retinal cultures from oxidative stress. (A) Viability of the retinal cultures was reduced by 50% by 0.1 mM H2O2. Wnt signaling activator SB216763 (40 μM) significantly increased the viability of the retinal cultures, whereas the vehicle control DMSO had no effect. Viability was measured by XTT assay and normalized to the untreated cultures. (B) TUNEL assay confirmed that photoreceptors in the cultures were rescued from H2O2 toxicity. Recombinant Wnt3a ligand (150 ng/mL) was used to activate the Wnt pathway, and rhodopsin+/TUNEL+ cells were counted. (C) Recombinant Wnt3a increased the viability of retinal cultures exposed to H2O2, measured by XTT assay. Two concentrations of Wnt3a were tested. The “no Wnt” cells were treated with H2O2 alone. (D, E) The Wnt signaling pathway was activated in the retinal cultures. Cultures were transfected with the Wnt reporter plasmid and a LacZ plasmid and then were incubated with the Wnt activators SB216763 and Wnt3a. Wnt signaling was measured by the luciferase reporter assay using β-galactosidase activity for normalization (values given in thousands).
Therefore, activating the Wnt pathway at its most upstream point using the Wnt3a ligand and at its most downstream point using the GSK3β inhibitor SB216763 protected the retinal cultures from H2O2-induced death. Although GSK3β inhibition may regulate other pathways that could potentially contribute to protection, the replication of our data with the recombinant Wnt3a ligand confirmed the prosurvival activity of the canonical Wnt pathway. These data are the first demonstration that canonical Wnt signaling activators protect retinal cultures from injury. Wnt activation alone, without H2O2, did not increase the viability of the cultures, indicating that Wnt activation did not increase the survival or proliferation of nonphotoreceptor cells (data not shown).
Activation of the Wnt signaling pathway was measured by transfecting the retinal cultures with plasmid (TOP-FLASH; Upstate Biotechnology), which contains a Wnt-responsive promoter, driving expression of luciferase.4 Incubation with SB216763 and Wnt3a ligand in conditioned media significantly increased reporter activity by up to 4.7-fold in the retinal cultures compared with the control treatments (Figs. 2D, 2E).
Wnt Activation in the Retina
To investigate whether Wnt signaling is an endogenous retinal neuroprotection mechanism, we determined whether the Wnt pathway is induced during degeneration. Differential expression of Wnt pathway genes have been identified in the degenerating retina by our group and others,16,18,34–36 and various Wnt inhibitor and activator genes were expressed in the retinas of mouse37,38 (Table 1), chick,39 and human.40 However, expression alone cannot predict which cells contain activated Wnt signaling. Therefore, to identify the temporal and spatial distribution patterns of functional canonical Wnt signaling during rod and cone death, we used the transgenic Wnt reporter mouse line Tcf-LacZ.27 In the Tcf-LacZ mice, expression of LacZ is induced by binding of endogenous β-catenin/Tcf complexes to the Tcf elements upstream of the LacZ transgene and β-galactosidase-positive cells correspond to active canonical Wnt signaling.
Table 1.
Differential Expression of Multiple Wnt Pathway Genes during Rod and Cone Photoreceptor Degeneration in rd1 Mice (on a C57B1/6 Background) Compared with wt C57B1/6 Mice
| Gene | P14 QPCR rd1/wt Ratio Rod Death | P50 QPCR rd1/wt Ratio Cone Death |
|---|---|---|
| Wnt5a | 1.4* | 4.7 |
| wnt5b | 1.9* | 2.9* |
| Wnt10a | ND in B16 | 1.1 |
| wnt13 | 1.11 | 0.82 |
| SFRP1 | 0.3* | 0.5* |
| SFRP2 | 2* | 1.8* |
| SFRP3 | 2.2* | 2.9* |
| SFRP4 | 0.94 | 2.3* |
| Frizzled3 | 1.2 | 2.5* |
| Frizzled4 | 0.18 | 0.11* |
| Frizzled6 | 2.45* | 1.6 |
QPCR ratios of rd1 to wt expression are shown. ND, not detected.
P < 0.05, Student's t-test.
We first demonstrated that nondegenerating Tcf-LacZ mice have active Wnt signaling in the ganglion cell layer (GCL) and in the inner portion of the inner nuclear layer (INL) in P19 mice (Fig. 3A, left). This pattern is consistent with in situ hybridization localization of genes in the Wnt pathway37 and indicates that the Wnt pathway has a normal role in postdevelopment RGCs and INL cells. Similar findings were recently reported by Liu et al.,41 who demonstrated that active Wnt signaling was present in subsets of amacrine cells in the INL cells and RGCs.
Figure 3.

Wnt signaling was activated during retinal degeneration. (A) The rd1/Tcf-LacZ mice (rd1, right) were compared with nondegenerating +/+-Tcf-LacZ littermate controls (wt, left). Wnt signaling (X-gal staining region (blue) is indicated by square brackets) in the INL layer was wider in the rd1 mice, indicating that an additional cell type(s) acquired Wnt signaling during degeneration. Retinas from P19 mice are shown. (B–E) β-galactosidase (β-gal, green, B) in the rd1/Tcf-LacZ retinas colocalized (arrows) with the Müller marker glutamine synthetase (GS; red, C). Choroidal vessels (B) are nonspecifically stained. (D, blue) DAPI-stained nuclei. (E) Merged image; original magnification, ×40. Retina from a P14 mouse is shown. (F) Higher magnification from an adjacent region of the tissue; original magnification, ×63. (G) Incubation with secondary antibodies only, at the same or greater exposure time, did not show any staining. (H, I) Region of the ONL of a P14 rd1/Tcf-LacZ retina showing colocalization of β-gal (green, H) and the microglia marker protein IBA-1 (red, I). Original magnification, ×40.
To localize Wnt signaling during retinal degeneration, we bred the Tcf-LacZ mice with rd1 retinal degeneration mice, which are homozygous for a mutation in the rod photoreceptor-specific cGMP phosphodiesterase β-subunit (PDEβ) and which exhibit rapid rod degeneration followed by prolonged cone degeneration. We generated an rd1/Tcf-LacZ line homozygous for rd1 and heterozygous for Tcf-LacZ and compared them with nondegenerating rd1/+-Tcf-LacZ and +/+-Tcf-LacZ littermate controls. As shown in Figure 3A, the INL staining in the rd1/Tcf-LacZ mice (Fig. 3A, right) widens compared with the nondegenerating littermate at P19 (Fig. 3A, left), suggesting that an additional cell type (or types) acquires Wnt signaling in response to rod degeneration. The new staining pattern is in the center and outer leaflets of the INL, in a pattern consistent with the localization of Müller glia cell bodies. The positions of the nuclei eliminated the possibility that the expanded LacZ staining was from “stretching” of the retina from a fixation artifact in the thinner retina. A similar expanded staining was seen during rod degeneration at P13 and P14.
Cells with newly activated Wnt signaling in rd1/Tcf-LacZ were identified as Müller glia because β-galactosidase (encoded by LacZ) colocalized with the cytoplasmic Müller marker glutamine synthetase (Figs. 3B–3F, P14 mice). These β-gal-positive cells did not colocalize with the cellular markers calretinin, calbindin, calretinin, Pax6, or rhodopsin (data not shown). However, a small number of β-gal-positive cells in the outer nuclear layer (ONL) colocalized with the microglia/macrophage marker IBA-1 (Figs. 3H, 3I), suggesting activation of Wnt signaling in microglia in addition to Müller glia.
LacZ staining was not detectable in photoreceptors (Fig. 3) or in RPE cells (rd1/Tcf-LacZ albino mouse; data not shown). This finding is reminiscent of the lack of growth factor signaling pathways and receptors in photoreceptors,42 though it is possible that transient Wnt signaling was not detectable. Therefore, Wnt pathway gene expression results in cell-specific summation of positive and negative regulators, leading to Wnt signaling in the INL and GCL and to lack of Wnt signaling in the RPE and photoreceptors.
Wnt Signaling Is Activated in Müller Glia
We next confirmed— using primary Müller glia cultures prepared from the mouse retina cultures—that Müller glia contain the required cellular pathways to respond to Wnt activators. To quantitate Wnt signaling, we transfected the primary Müller glia with the luciferase Wnt reporter plasmid and the LacZ plasmid and then incubated the cells with Wnt3a or control conditioned media. As shown in Figure 4A, Wnt signaling was dramatically induced in the primary Müller glia. Consistent with this finding is that β-galactosidase activity increased in response to Wnt3a in Müller glia derived from the Tcf-LacZ mice (Fig. 4B).
Figure 4.
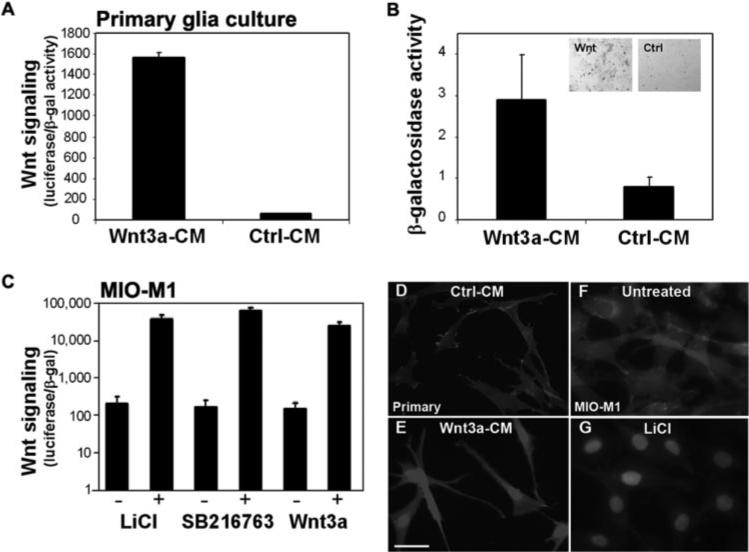
The Wnt signaling pathway is activated in Müller glia. (A) Addition of Wnt3a conditioned media (Wnt3a-CM) to primary Müller glia cultures increased activity of the Wnt luciferase reporter compared with Ctrl-CM. Luciferase activity is normalized to cotransfected β-galactosidase (values given in thousands). (B) Wnt3a also increased activity of β-galactosidase in Muller glia cultures from Tcf-LacZ retinas. B-gal activity is calculated per microgram of protein. Inset, blue: X-gal stain in Wnt3a-treated cultures (Wnt) and no stain in control treated cultures (Ctrl). (C) The MIO-M1 human Müller cell line activates Wnt signaling in response to Wnt3a ligand, LiCl, and SB216763 (+, treated with Wnt activator; –, control treatments (untreated for LiCl, DMSO for SB216763, control conditioned media for Wnt3a). A Wnt-responsive luciferase reporter was used to measure Wnt signaling in MIO-M1 cells as in (A). Log scale is shown. (A–C) Average ± SD. (D–G) Nuclear localization of β-catenin is used as an endogenous marker of Wnt signaling activation. Nuclear β-catenin is shown in primary Müller glia cultures (D, E) and the MIO-M1 Müller glia cell line (F, G) treated with the Wnt activators Wnt3a (E) and LiCl (G). The control for Wnt3a is Ctrl-CM (D) or untreated (F). Scale bar, 50 μm.
Upregulation and nuclear accumulation of endogenous β-catenin is an established marker of canonical Wnt signaling. Nuclear accumulation of β-catenin and increased cytoplasmic levels were observed in Wnt3a-treated primary Müller glia compared with control, indicating activation of the Wnt pathway (Figs. 4D, 4E).
To control for the potential presence of contaminating Wnt-responsive cells in the primary Müller glia cultures, we performed Wnt reporter luciferase assays on the MIO-M1 human Müller glia cell line.43 Wnt signaling was induced in response to the Wnt3a ligand and the chemical activators LiCl and SB216763 (Fig. 4C). Furthermore, incubation with these Wnt activators resulted in dramatic nuclear localization of β-catenin (shown for LiCl; Fig. 4G). The amount of β-catenin remaining in the cytoplasm on Wnt signaling activation was higher in the primary cultures than in the cell line (compare Figs. 4E and 4G). Although the response to Wnt activation by Wnt3a and LiCl was equivalent within each cell type, the cell line consistently responded better to all Wnt activators. This finding was consistent with the Wnt reporter luciferase assays that demonstrated higher Wnt signaling in MIO-M1 cells compared with the primary cultures (Figs. 4A, 4C). Despite the difference levels of Wnt signaling in the cell line and the primary cultures, the β-catenin and luciferase assay data demonstrated that Müller glia were Wnt-responsive cells.
Together, these data indicate that primary Müller glia and the Müller glia cell line contain the receptors and components of the Wnt pathway required for Wnt signaling. It has been established by several groups that Müller glia are the central mediators of growth factor protection in animal models of inherited and light-induced retinal degeneration.42,44 Therefore, the activation of Wnt signaling in Müller glia, together with the localization of Wnt regulators in the INL16,37 and the identification of Wnt signaling in Müller glia in the rd1/LacZ mice, indicate that Müller glia mediates Wnt activity during retinal degeneration.
Identification of Candidate Activators of Wnt Signaling during Retinal Degeneration
The increased distribution of Wnt signaling in Figure 3 predicts that Wnt pathway activators will be upregulated in the degenerating retina. To determine which Wnt ligands are potential activators in vivo, we identified Wnt signaling genes that are differentially expressed during photoreceptor death.
QPCR was performed on retinas from rd1 retinal degeneration mice. Gene expression was assessed at two time points, during the peak of rod degeneration at P14 and during cone degeneration at P50. We analyzed Wnt pathway genes that had previously been reported in mouse or human retina.8,16,17,37 Multiple Wnt pathway genes were expressed during rod and cone photoreceptor degeneration in rd1 mice. For example, Wnt10a and Wnt 13 are expressed (though not differentially) during cone degeneration based on the threshold amplification cycle below 32 and agarose gel confirmation of product size.
Differential expression of several Wnt pathway genes were also observed during rod and cone photoreceptor death, including the Wnt receptors Frizzled3 and Frizzled6 and the SFRP inhibitor genes (Table 1; QPCR ratios of age-matched rd1 to wt expression at P14 and P50; *P < 0.05, Student's t-test). The canonical ligand Wnt10a was expressed in the rd1 retina during rod degeneration at P14 but was undetectable in wt mice at this age. At P50 Wnt10a expression was equivalent in rd1 and wt retinas. Wnt5a and Wnt5b were expressed at higher levels in the rd1 retina at P50, whereas Wnt13 was expressed at similar levels in degenerating and wt retinas. Wnt5a activates the noncanonical or canonical/β-catenin Wnt pathway, depending on the receptor composition,45 and was previously identified in degenerating retina.35 Therefore, Wnt5a, Wnt5b, Wnt10a, and Wnt13 are potential mediators of Wnt signaling during degeneration.
Discussion
Photoreceptor death leads to many blinding conditions, including age-related macular degeneration and retinitis pigmentosa. A major focus of retinal research is the identification and characterization of novel photoreceptor protection factors. In the present study, we demonstrated that Wnt signaling activators protected retinal cultures from H2O2-induced oxidative stress. In the rd1/Tcf-LacZ retinal degeneration mouse, the distribution of Wnt signaling changed compared with age-matched wt animals. Wnt signaling became prominent in the INL in a pattern consistent with the induction of Wnt signaling in Müller glia. In contrast, Wnt signaling was not evident in photoreceptors. Wnt ligands activated the canonical Wnt pathway in cultured Müller glia, indicating that Müller glia contained the necessary receptors and intracellular components for Wnt signaling.
Several studies demonstrated altered expression of Wnt genes during retinal degeneration in rodent and human,16,18,34–36 suggesting that Wnt signaling may be a widespread retinal response to photoreceptor death and is not limited to the rd1 mutation. The genes identified in these studies are candidate mediators of Wnt signaling during retinal degeneration. In a previous study, we determined that the Wnt regulator gene Dkk3 was upregulated during photoreceptor death in rd1 mice and expressed in the INL.16 We recently demonstrated that Dkk3 potentiates Wnt signaling in cultured Müller glia5 and protects cell lines from apoptotic agents by inhibiting caspases (Nakamura REI, et al. IOVS 2006;47:ARVO E-Abstract 2575), suggesting that Dkk3 is a potential activator of Wnt signaling in Müller glia in vivo.
Our data suggest a model in which retinal injury stimulates Müller glia and leads to Wnt signaling activation. Wnt signaling may raise the threshold for apoptosis and reduce photoreceptor degeneration by directly affecting photoreceptor viability or by indirect effects, such as by regulating growth factor activity46,47 (Yi and Hackam, unpublished observations). In the presence of significant or continuing damage, these pathways are stimulated but are insufficient to prevent degeneration.5 An additional component of this model is that secretion of Wnt activators in Müller glia, such as the Wnt potentiator Dkk3,16 may amplify the protective response to injury by activating Wnt signaling in an autocrine manner and in a paracrine fashion in its neighboring cells. During retinal degeneration, Müller glia secrete prosurvival growth factors thought to protect remaining photoreceptors from apoptosis.48–51 Mimicking this intrinsic protective response by overexpressing growth factors reduces photoreceptor death in retinal culture, explants, and animal models.48,49,52 To determine whether our retinal culture results are translatable to photoreceptor protection in vivo, a compelling experiment will be to overexpress Wnt activators in an animal model to test whether elevated Wnt signaling delays or halts retinal degeneration.
Based on the retinal culture TUNEL assays, we consider that Wnt signaling induced neuroprotection in our study, not regeneration as reported in retinal explants.14 Understanding the conditions in which the Wnt pathway promotes neuroprotection over regeneration will be important for considering therapies involving the Wnt pathway. Although Wnt pathway activators are antiapoptotic in retinal culture and various normal and neoplastic tissues, Wnt signaling is thought to eliminate malformed photoreceptors in a wave of apoptosis in the developing Drosophila retina.53,54 Additionally, transgenic overexpression of β-catenin (Arm) in Drosophila leads to a neuronal progenitor differentiation defect and photoreceptor death.54 Identifying the underlying mechanisms of Wnt-regulated photoreceptor survival and death will address whether the differing response to Wnt activation in the retina is species specific or reflects opposite roles of Wnt signaling in the developing retina compared with the diseased retina.
Our study adds to an increasing number of reports on the role of the Wnt pathway in the retina (for reviews, see Refs. 3, 5, 55). The Wnt pathway is critical to retinal development8,56 and retinal stem cell proliferation,7,57,58 and mutations in Wnt pathway genes cause several retinal diseases. Progressive rod and cone death in the rd6 retinal degeneration mouse line is caused by a splice donor mutation in the membrane-type frizzled-related protein (Mfrp).36 The Mfrp gene contains domains found in the Wnt receptor Frizzled. Null mutations in MFRP cause extreme hyperopia in humans.59 Inactivating mutations in the Wnt coreceptors Frizzled4 (Fz4)10 and LRP511 cause familial exudative vitreoretinopathy (FEVR), a blinding disease characterized by disrupted peripheral retinal angiogenesis. Norrin, the protein mutated in Norrie disease, is a novel binding partner for Fz4.12 The retinal vascularization phenotype in Norrie disease is similar to FEVR and is caused by disrupted Wnt signaling as a result of decreased Norrin-Fz4 interactions.12
Interestingly, several of the Wnt ligands expressed in the retina (Table 1) activate noncanonical β-catenin–independent pathways. Noncanonical signaling is not mediated by β-catenin stabilization but instead is transmitted by calcium signaling, JNK activation, or protein kinase C pathways. The role of the noncanonical pathway in the retina is unknown, though in other tissues it attenuates the activity of canonical signaling.60 Future work will explore the role of β-catenin–independent Wnt signaling in the retina to facilitate understanding of how multiple Wnt signaling pathways mediate photoreceptor survival and regulate photoreceptor– Müller glia communication.
Acknowledgments
The authors thank Astrid Limb for the MIO-M1 cell line.
Supported by a Pediatric Ophthalmology Grant from the Knights Templar Eye Foundation, a Research for the Prevention of Blindness Career Development Award, the Karl Kirchgessner Foundation, and National Eye Institute Grant EY017837 (all ASH). Additional funding was provided by the Canadian Institute of Health Research (DD). Institutional support was provided by an unrestricted grant to Bascom Palmer Eye Institute from the Research to Prevent Blindness and National Eye Institute Core Grant P30 EY014801.
Footnotes
Disclosure: H. Yi, None; R.E.I. Nakamura, None; O. Mohamed, None; D. Dufort, None; A.S. Hackam, None
Supplementary Material
References
- 1.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 2.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 3.de Iongh RU, Abud HE, Hime GR. WNT/Frizzled signaling in eye development and disease. Front Biosci. 2006;11:2442–2464. doi: 10.2741/1982. [DOI] [PubMed] [Google Scholar]
- 4.Tell S, Yi H, Jockovich ME, Murray TG, Hackam AS. The Wnt signaling pathway has tumor suppressor properties in retinoblastoma. Biochem Biophys Res Commun. 2006;349:261–269. doi: 10.1016/j.bbrc.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Hackam A. The Wnt signaling pathway in retinal degenerations. IUBMB Life. 2005;57:381–388. doi: 10.1080/15216540500137586. [DOI] [PubMed] [Google Scholar]
- 6.Van Raay TJ, Moore KB, Iordanova I, et al. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46:23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- 8.Hunter DD, Zhang M, Ferguson JW, Koch M, Brunken WJ. The extracellular matrix component WIF-1 is expressed during, and can modulate, retinal development. Mol Cell Neurosci. 2004;27:477–488. doi: 10.1016/j.mcn.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masai I, Yamaguchi M, Tonou-Fujimori N, Komori A, Okamoto H. The hedgehog-PKA pathway regulates two distinct steps of the differentiation of retinal ganglion cells: the cell-cycle exit of retinoblasts and their neuronal maturation. Development. 2005;132:1539–1553. doi: 10.1242/dev.01714. [DOI] [PubMed] [Google Scholar]
- 10.Robitaille J, MacDonald ML, Kaykas A, et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet. 2002;32:326–330. doi: 10.1038/ng957. [DOI] [PubMed] [Google Scholar]
- 11.Toomes C, Bottomley HM, Jackson RM, et al. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am J Hum Genet. 2004;74:721–730. doi: 10.1086/383202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q, Wang Y, Dabdoub A, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 13.Das AV, Mallya KB, Zhao X, et al. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Osakada F, Ooto S, Akaqi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 16.Hackam AS, Strom R, Liu D, et al. Identification of gene expression changes associated with the progression of retina degeneration in the rd1 mouse. Invest Ophthalmol Vis Sci. 2004;45:2929–2942. doi: 10.1167/iovs.03-1184. [DOI] [PubMed] [Google Scholar]
- 17.Jones SE, Jomary C, Grist J, Stewart HJ, Neal MJ. Modulated expression of secreted frizzled-related proteins in human retinal degeneration. Neuroreport. 2000;11:3963–3967. doi: 10.1097/00001756-200012180-00012. [DOI] [PubMed] [Google Scholar]
- 18.Jones SE, Jomary C, Grist J, Stewart HJ, Neal MJ. Altered expression of secreted frizzled-related protein-2 in retinitis pigmentosa retinas. Invest Ophthalmol Vis Sci. 2000;41:1297–1301. [PubMed] [Google Scholar]
- 19.Caricasole A, Copani A, Caraci F, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer's brain. J Neurosci. 2004;24:6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez AR, Godov JA, Mullendorff K, Olivares GH, Bronfman M, Inestrosa NC. Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Exp Cell Res. 2004;297:186–196. doi: 10.1016/j.yexcr.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Brault V, Moore R, Kutsch S, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 22.Mirkovic I, Charish K, Gorski SM, McKnight K, Verheyen EM. Drosophila nemo is an essential gene involved in the regulation of programmed cell death. Mech Dev. 2002;119:9–20. doi: 10.1016/s0925-4773(02)00289-7. [DOI] [PubMed] [Google Scholar]
- 23.Cappuccio I, Calderone A, Busceti CL, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J Neurosci. 2005;25:2647–2657. doi: 10.1523/JNEUROSCI.5230-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 25.Wahlin KJ, Lim L, Grice EA, Campochiaro PA, Zack DJ, Adler R. A method for analysis of gene expression in isolated mouse photoreceptor and Muller cells. Mol Vis. 2004;10:366–375. [PubMed] [Google Scholar]
- 26.Hauck SM, Suppmann S, Ueffing M. Proteomic profiling of primary retinal Muller glia cells reveals a shift in expression patterns upon adaptation to in vitro conditions. Glia. 2003;44:251–263. doi: 10.1002/glia.10292. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed OA, Dufort D, Clarke HJ. Expression and estradiol regulation of Wnt genes in the mouse blastocyst identify a candidate pathway for embryo-maternal signaling at implantation. Biol Reprod. 2004;71:417–424. doi: 10.1095/biolreprod.103.025692. [DOI] [PubMed] [Google Scholar]
- 28.Hackam AS, Qian J, Liu D, et al. Comparative gene expression analysis of murine retina and brain. Mol Vis. 2004;10:637–649. [PubMed] [Google Scholar]
- 29.Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- 30.Xin Y, Fong YT, Wolf G, Wolf D, Cao W. Protective effect of XY99–5038 on hydrogen peroxide induced cell death in cultured retinal neurons. Life Sci. 2001;69:289–299. doi: 10.1016/s0024-3205(01)01122-5. [DOI] [PubMed] [Google Scholar]
- 31.Seigel GM, Chiu L, Paxhia A. Inhibition of neuroretinal cell death by insulin-like growth factor-1 and its analogs. Mol Vis. 2000;6:157–163. [PubMed] [Google Scholar]
- 32.Yu DY, Cringle SJ, Su EN, Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest Ophthalmol Vis Sci. 2000;41:3999–4006. [PubMed] [Google Scholar]
- 33.Yu DY, Cringle S, Valter K, Walsh N, Lee D, Stone J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest Ophthalmol Vis Sci. 2004;45:2013–2019. doi: 10.1167/iovs.03-0845. [DOI] [PubMed] [Google Scholar]
- 34.Jones SE, Jomary C, Grist J, Stewart HJ, Neal MJ. Identification by array screening of altered nm23–M2/PuF mRNA expression in mouse retinal degeneration. Mol Cell Biol Res Commun. 2000;4:20–25. doi: 10.1006/mcbr.2000.0250. [DOI] [PubMed] [Google Scholar]
- 35.Yu J, He S, Friedman JS, et al. Altered expression of genes of the Bmp/Smad and Wnt/calcium signaling pathways in the cone-only Nrl(super−/−) mouse retina, revealed by gene profiling using custom cDNA microarrays. J Biol Chem. 2004;279:42211–42220. doi: 10.1074/jbc.M408223200. [DOI] [PubMed] [Google Scholar]
- 36.Kameya S, Hawes NL, Chang B, Heckenlively JR, Naggert JK, Nishina PM. Mfrp, a gene encoding a frizzled related protein, is mutated in the mouse retinal degeneration 6. Hum Mol Genet. 2002;11:1879–1886. doi: 10.1093/hmg/11.16.1879. [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- 38.Monaghan AP, Kioschis P, Wu W, et al. Dickkopf genes are coordinately expressed in mesodermal lineages. Mech Dev. 1999;87:45–56. doi: 10.1016/s0925-4773(99)00138-0. [DOI] [PubMed] [Google Scholar]
- 39.Jin EJ, Burrus LW, Erickson CA. The expression patterns of Wnts and their antagonists during avian eye development. Mech Dev. 2002;116:173–176. doi: 10.1016/s0925-4773(02)00128-4. [DOI] [PubMed] [Google Scholar]
- 40.Chang JT, Esumi N, Moore K, et al. Cloning and characterization of a secreted frizzled-related protein that is expressed by the retinal pigment epithelium. Hum Mol Genet. 1999;8:575–583. doi: 10.1093/hmg/8.4.575. [DOI] [PubMed] [Google Scholar]
- 41.Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA. Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol Vis Sci. 2006;47:5088–5097. doi: 10.1167/iovs.06-0403. [DOI] [PubMed] [Google Scholar]
- 42.Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:927–936. [PubMed] [Google Scholar]
- 43.Limb GA, Salt TE, Munro PM, Moss SE, Khaw PT. In vitro characterization of a spontaneously immortalized human Muller cell line (MIO-M1). Invest Ophthalmol Vis Sci. 2002;43:864–869. [PubMed] [Google Scholar]
- 44.Harada T, Harada C, Nakayama N, et al. Modification of glialneuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron. 2000;26:533–541. doi: 10.1016/s0896-6273(00)81185-x. [DOI] [PubMed] [Google Scholar]
- 45.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patapoutian A, Backus C, Kispert A, Reichardt LF. Regulation of neurotrophin-3 expression by epithelial-mesenchymal interactions: the role of Wnt factors. Science. 1999;283:1180–1183. doi: 10.1126/science.283.5405.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuli R, Tuli S, Nandi S, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278:41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 48.Chaum E. Retinal neuroprotection by growth factors: a mechanistic perspective. J Cell Biochem. 2003;88:57–75. doi: 10.1002/jcb.10354. [DOI] [PubMed] [Google Scholar]
- 49.Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Liu C, Peng M, Laties AM, Wen R. Preconditioning with bright light evokes a protective response against light damage in the rat retina. J Neurosci. 1998;18:1337–1344. doi: 10.1523/JNEUROSCI.18-04-01337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bush RA, Williams TP. The effect of unilateral optic nerve section on retinal light damage in rats. Exp Eye Res. 1991;52:139–153. doi: 10.1016/0014-4835(91)90254-c. [DOI] [PubMed] [Google Scholar]
- 52.Adler R. Trophic interactions in retina development and in retinal degenerations: in vivo and in vitro studies. In: Adler R, Farber D, editors. The Retina: A Model for Cell Biology. Academic Press, Inc.; Orlando, FL: 1986. pp. 112–150. [Google Scholar]
- 53.Lin HV, Rogulja A, Cadigan KM. Wingless eliminates ommatidia from the edge of the developing eye through activation of apoptosis. Development. 2004;131:2409–2418. doi: 10.1242/dev.01104. [DOI] [PubMed] [Google Scholar]
- 54.Ahmed Y, Hayashi S, Levine A, Wieschaus E. Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell. 1998;93:1171–1182. doi: 10.1016/s0092-8674(00)81461-0. [DOI] [PubMed] [Google Scholar]
- 55.Yang XJ. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin Cell Dev Biol. 2004;15:91–103. doi: 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Raay TJ, Vetter ML. Wnt/frizzled signaling during vertebrate retinal development. Dev Neurosci. 2004;26:352–358. doi: 10.1159/000082277. [DOI] [PubMed] [Google Scholar]
- 57.Liu H, Xu S, Wang Y, et al. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev Biol. 2007;308:54–67. doi: 10.1016/j.ydbio.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 58.Cho SH, Cepko CL. Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006;133:3167–3177. doi: 10.1242/dev.02474. [DOI] [PubMed] [Google Scholar]
- 59.Sundin OH, Leppert GS, Silva ED, et al. Extreme hyperopia is the result of null mutations in MFRP, which encodes a Frizzled-related protein. Proc Natl Acad Sci USA. 2005;102:9553–9558. doi: 10.1073/pnas.0501451102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weidinger G, Moon RT. When Wnts antagonize Wnts. J Cell Biol. 2003;162:753–755. doi: 10.1083/jcb.200307181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


