Abstract
Background
Interspecific hybrids of frogs of the genus Xenopus result in sterile hybrid males and fertile hybrid females. Previous work has demonstrated a dramatic asymmetrical pattern of misexpression in hybrid males compared to the two parental species with relatively few genes misexpressed in comparisons of hybrids and the maternal species (X. laevis) and dramatically more genes misexpressed in hybrids compared to the paternal species (X. muelleri). In this work, we examine the gene expression pattern in hybrid females of X. laevis × X. muelleri to determine if this asymmetrical pattern of expression also occurs in hybrid females.
Results
We find a similar pattern of asymmetry in expression compared to males in that there were more genes differentially expressed between hybrids and X. muelleri compared to hybrids and X. laevis. We also found a dramatic increase in the number of misexpressed genes with hybrid females having about 20 times more genes misexpressed in ovaries compared to testes of hybrid males and therefore the match between phenotype and expression pattern is not supported.
Conclusion
We discuss these intriguing findings in the context of reproductive isolation and suggest that divergence in female expression may be involved in sterility of hybrid males due to the inherent sensitivity of spermatogenesis as defined by the faster male evolution hypothesis for Haldane's rule.
Background
Frogs of the genus Xenopus provide a striking exception to the most widespread generalization in evolutionary biology-Haldane's rule [1-6]. Haldane's rule states that the heterogametic sex (XY or ZW) typically suffers the most dysfunctional effects of interspecific hybridization [7] and the broad applicability of Haldane's rule across diverse groups of organisms suggests that common mechanisms may underlie postzygotic reproductive isolation [5]. Xenopus have ZW sex determination and Haldane's rule would predict that hybrid females should suffer the most dramatic effects of hybridization but contrary to expectation, F1 hybrid males are completely sterile and hybrid females are fertile [1,2].
Analyses of spermatogenesis in hybrid males of X. laevis × X. muelleri have shown that males have a dramatically lower abundance of motile sperm, increased numbers of undifferentiated sperm cells, and larger mature sperm cells compared to parental species [2]. The gene expression pattern for hybrid males shows a striking asymmetric pattern in that relatively few genes are differentially expressed between hybrids and the maternal species (X. laevis) whereas there are dramatically more genes differentially expressed between hybrid males and the paternal species, X. muelleri. These results suggest intriguing mechanisms operating on the transcriptome in hybrid males of Xenopus that may reflect strong maternal and/or species dominance effects [2].
Hybrid females are just as fertile as conspecific species [1] and given the phenotype of hybrid females, a reasonable prediction would be that gene expression should be similar compared to the two parental species. However, given the asymmetrical pattern of expression operating in hybrid males, it is of interest to investigate the pattern of gene expression in hybrid oogenesis, particularly since oogenesis in hybrids does not seem to be affected by the hybrid genetic background compared to hybrid males.
In this study, we analyzed the gene expression pattern of adult ovary in hybrid females of X. laevis × X. muelleri compared to the two parental species. Our analyses reveal a pattern of asymmetrical gene expression like that in testes of hybrid males but surprisingly there is a dramatic increase in the number of genes misexpressed in hybrid female ovaries compared to the two parental species relative to hybrid males. This increased level of gene misexpression suggests that oogenesis can tolerate dramatically more misexpression compared to spermatogenesis and points further evidence to the sensitive spermatogenesis component of the faster male evolution hypothesis for Haldane's rule.
Results
There was a substantial amount of differential expression in hybrid ovary compared to the ovaries of the two parental species. Using adjusted significance tests (P < 0.05), about 14% (1,616/11,485) of genes were differentially expressed in hybrid females compared to females of X. laevis and 63% (7,279/11,485) of genes were differentially expressed between hybrids and X. muelleri (Fig. 1). The number of genes upregulated in hybrids relative to X. laevis compared to the number of genes upregulated in X. laevis relative to hybrids was the same (839 vs. 777; G = 2.38; df = 1; P > 0.05) but there were significantly more genes upregulated in X. muelleri compared to hybrids (4,349 vs. 2,930; G = 139.2; df = 1; P < 0.0001). Many of the top 30 most differentially expressed genes for each class of gene expression behavior are expressed sequence tags (ESTs) with little functional information but our results imply that these sequences play a role in oogenesis in Xenopus. Of the top 30 candidate genes with known function many have a documented role in oogenesis in other organisms (Table 1, 2, 3, 4). Comparing the two lists of differentially expressed genes showed that about 68% (1105/1616) were common to both X. laevis vs. hybrids and X. muelleri vs. hybrids. This common set of differentially expressed genes suggests a set of genes that are uniquely expressed in hybrids relative to the two parental species.
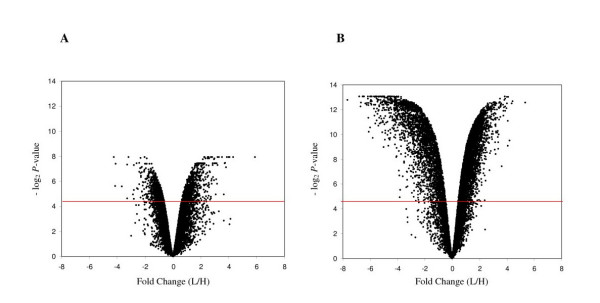
Figure 1.
Volcano plots of gene expression. Volcano plots from FDR corrected t-tests of statistical significance (vertical axis) against magnitude of expression change (horizontal axis), where each point corresponds to a gene/transcript. Expression change (fold-change) is defined as a log2-transformed ratio of mean nonhybrid to mean hybrid expression level. (A) Xenopus laevis (L) vs. Hybrids (H); (B) Xenpous muelleri (M) vs. Hybrids (H). The red horizontal line denotes FDR adjusted alpha 0.05. The horizontal deviation from 0 towards the right or left reflects hybrid underexpression or overexpression, respectively.
Table 1.
Candidate transcripts upregulated in X. laevis compared to hybrids.
| ProbeID | GenBankID | Target Gene | Gene Symbol | Description/Molecular Function | Mean Laevis | SD Laevis | Mean Hybrid | SD Hybrid | L-H | adj.P.Val |
|---|---|---|---|---|---|---|---|---|---|---|
| Xl.1473.1.A1_at | AW147858 | EST | Weakly similar to hypothetical protein MGC3731 (H. sapiens) | 10.18 | 0.56 | 4.28 | 0.43 | 5.90 | 0.0041 | |
| Xl.7484.1.A1_at | BJ081331 | EST | MGC84382 | Intracellular signaling cascade | 7.33 | 0.54 | 3.01 | 0.25 | 4.32 | 0.0041 |
| Xl.14874.1.A1_at | BM180490 | EST | Highly similar to T2D1_HUMAN TRANSCRIPTION INITIATION FACTOR TFIID 250 KD SUBUNIT | 7.35 | 0.35 | 3.29 | 0.54 | 4.06 | 0.0041 | |
| Xl.2557.1.S1_at | BG730898 | EST | Weakly similar to A39599 55 K erythrocyte membrane protein – human (H. sapiens) | 10.12 | 0.30 | 6.10 | 0.18 | 4.02 | 0.0041 | |
| Xl.4581.1.A1_at | CB562594 | EST | LOC398314 | Dihydrolipoamide acetyltransferase | 7.34 | 0.40 | 3.45 | 0.37 | 3.88 | 0.0041 |
| Xl.14122.1.A1_at | BJ079520 | EST | LOC494727 | 9.51 | 0.42 | 5.70 | 1.02 | 3.81 | 0.0072 | |
| Xl.12012.2.A1_at | BM191810 | EST | Weakly similar to FW1A_HUMAN F-boxWD-repeat protein | 7.39 | 0.34 | 3.63 | 0.77 | 3.76 | 0.0057 | |
| Xl.23885.1.S1_at | BQ388097 | EST | 7.76 | 0.50 | 4.05 | 0.59 | 3.71 | 0.0057 | ||
| Xl.3628.1.A1_at | BG023013 | EST | 7.18 | 1.34 | 3.52 | 0.77 | 3.66 | 0.0325 | ||
| Xl.24509.1.A1_at | BJ078115 | EST | MGC132176 | Heme oxygenase (decyclizing) activity | 9.04 | 0.30 | 5.50 | 0.56 | 3.53 | 0.0041 |
| Xl.14330.2.S1_at | BG021901 | EST | MGC85135 | Nucleic acid binding | 8.63 | 0.27 | 5.34 | 0.36 | 3.29 | 0.0041 |
| Xl.10705.1.A1_at | BJ051966 | EST | LOC495457 | Weakly similar to A36368 transcription factor CBF, CCAAT-binding – (H. sapiens) | 8.67 | 0.34 | 5.45 | 0.79 | 3.22 | 0.0064 |
| Xl.1973.1.S1_at | BQ383513 | EST | MGC78898 | Ubiquitin-protein ligase activity | 7.41 | 0.19 | 4.19 | 0.39 | 3.22 | 0.0041 |
| Xl.4825.1.A1_at | BG514303 | EST | 10.16 | 0.84 | 6.99 | 0.62 | 3.17 | 0.0182 | ||
| Xl.14885.1.A1_at | BM180520 | EST | MGC82215 | 8.79 | 0.46 | 5.64 | 0.23 | 3.15 | 0.0057 | |
| Xl.16512.1.A1_at | BJ050593 | EST | 7.03 | 0.48 | 3.91 | 1.11 | 3.12 | 0.0142 | ||
| Xl.1448.2.S1_a_at | BG408234 | EST | Moderately similar to pallidin; pallid (mouse) homolog, pallidin (H. sapiens) | 7.59 | 0.44 | 4.50 | 1.91 | 3.10 | 0.0325 | |
| Xl.16106.1.A1_at | BG161783 | EST | LOC495116 | 8.24 | 0.23 | 5.18 | 0.69 | 3.05 | 0.0057 | |
| Xl.25509.1.A1_at | BJ084136 | EST | LOC495259 | 6.09 | 0.34 | 3.09 | 0.23 | 3.00 | 0.0044 | |
| Xl.25610.1.A1_at | BF025269 | EST | Similar to thyroid hormone receptor associated protein 3 (predicted) [Rattus norvegicus] | 7.16 | 0.09 | 4.19 | 0.77 | 2.98 | 0.0057 | |
| Xl.10826.1.A1_at | CB560349 | EST | MGC82938 | Moderately similar to AAKG_HUMAN 5-AMP-activated protein kinase, (H. sapiens) | 8.37 | 0.31 | 5.42 | 0.37 | 2.95 | 0.0047 |
| Xl.8907.1.S1_at | AW632884 | proliferation-2G4 | Pa2g4 | Xenopus laevis, Similar to proliferation-associated 2G4 | 6.95 | 0.49 | 4.00 | 1.03 | 2.95 | 0.0148 |
| Xl.6727.1.A1_at | BG234472 | EST | LOC780754 | Regulation of transcription, DNA-dependent | 7.28 | 0.75 | 4.33 | 0.33 | 2.95 | 0.0149 |
| Xl.2780.1.S1_at | BQ400343 | TIA1 | tial1-a | Nucleic acid binding | 6.41 | 0.58 | 3.52 | 0.64 | 2.89 | 0.0124 |
| Xl.16426.1.A1_at | BJ045456 | EST | 6.58 | 0.40 | 3.71 | 0.46 | 2.87 | 0.0063 | ||
| Xl.6421.1.A1_at | AW633197 | EST | MGC68457 | 8.68 | 0.38 | 5.82 | 0.21 | 2.86 | 0.0057 | |
| Xl.22941.1.A1_at | BJ048949 | EST | MGC83726 | Moderately similar to A57088 nucleoporin-like protein Rab;regulation of GTPase activity | 6.79 | 0.15 | 3.98 | 0.43 | 2.81 | 0.0041 |
| Xl.25368.2.A1_at | BI444111 | EST | MGC69123 | Weakly similar to IFR1_HUMAN INTERFERON-RELATED DEVELOPMENTAL REGULATOR 1(H. sapiens) | 6.21 | 0.61 | 3.42 | 0.56 | 2.79 | 0.0139 |
| Xl.14796.1.A1_at | BM179493 | EST | MGC82526 | 7.10 | 0.69 | 4.32 | 0.68 | 2.78 | 0.0182 | |
| Xl.19146.3.A1_at | BJ057192 | EST | MGC81986 | 7.88 | 0.52 | 5.11 | 0.90 | 2.78 | 0.0151 |
Top 30 candidate transcripts upregulated in X. laevis and differentially expressed between females of X. laevis and hybrids. Expression values are in log2 scale; SD = standard deviation of expression values. P values are adjusted according to FDR moderated t-tests.
Table 2.
Candidate transcripts upregulated in hybrids compared to X. laevis.
| ProbeID | GenBankID | Target Gene | GeneSymbol | Description/Molecular Function | Mean Laevis | SD Laevis | Mean Hybrid | SD Hybrid | L-H | adj.P.Val |
|---|---|---|---|---|---|---|---|---|---|---|
| Xl.4605.1.A1_at | BG552470 | EST | MGC83384 | 3.41 | 0.39 | 7.63 | 0.44 | -4.22 | 0.0041 | |
| Xl.19075.1.A1_at | BI675584 | EST | Weakly similar to HES2 (Hairy and enhancer of split 2) (H. sapiens) | 5.53 | 1.29 | 9.67 | 0.27 | -4.14 | 0.0205 | |
| Xl.24502.1.S1_at | BJ098608 | EST | Weakly similar to forkhead box P2; (H. sapiens) | 4.56 | 0.70 | 8.65 | 0.00 | -4.09 | 0.0060 | |
| Xl.23573.1.S1_at | BC041496.1 | thymine-DNA glycosylase | TDG | Hydrolase activity, acting on glycosyl bonds | 5.55 | 1.16 | 9.22 | 0.15 | -3.67 | 0.0210 |
| Xl.16239.1.A1_at | BJ039322 | EST | 5.39 | 1.36 | 8.66 | 0.46 | -3.27 | 0.0405 | ||
| Xl.8630.1.S1_s_at | BC045272.1 | MGC53990 | MGC53990 | Similar to serum-inducible kinase; protein serine/threonine kinase act. | 4.74 | 0.36 | 8.01 | 0.75 | -3.26 | 0.0062 |
| Xl.21956.1.S1_at | BC042271.1 | MGC53461 | MGC53461 | 8.39 | 0.22 | 11.57 | 0.00 | -3.18 | 0.0041 | |
| Xl.24699.1.S1_at | CB984321 | EST | MGC79012 | Highly similar to alpha cardiac actin (H. sapiens) | 3.65 | 0.36 | 6.51 | 1.79 | -2.85 | 0.0327 |
| Xl.10600.1.S1_at | BE491023 | EST | 4.88 | 0.94 | 7.68 | 0.25 | -2.80 | 0.0256 | ||
| Xl.11324.1.A1_at | BG552091 | EST | 3.44 | 0.46 | 6.23 | 0.11 | -2.79 | 0.0064 | ||
| Xl.13010.1.A1_at | BJ099673 | EST | 5.43 | 0.46 | 8.22 | 1.89 | -2.79 | 0.0417 | ||
| Xl.14915.1.A1_at | BM180884 | EST | 5.11 | 0.30 | 7.72 | 0.35 | -2.61 | 0.0057 | ||
| Xl.13666.1.A1_x_at | BJ091634 | EST | 7.61 | 0.80 | 10.17 | 0.37 | -2.57 | 0.0230 | ||
| Xl.24516.1.S1_at | CB560511 | EST | LOC495356 | Weakly similar to Apolipoprotein E precursor (H. sapiens); lipid binding | 6.41 | 0.60 | 8.93 | 1.00 | -2.52 | 0.0244 |
| Xl.13831.3.S1_at | BJ075928 | EST | 5.64 | 0.45 | 8.05 | 0.17 | -2.41 | 0.0094 | ||
| Xl.24058.1.S1_at | BI940804 | EST | MGC82121 | Highly similar to histone H2A.FZ variant, isoform 1(H. sapiens) | 6.99 | 0.28 | 9.40 | 0.09 | -2.41 | 0.0057 |
| Xl.16509.1.A1_at | BJ084267 | EST | 4.04 | 0.35 | 6.44 | 1.00 | -2.40 | 0.0185 | ||
| Xl.433.2.S1_at | BC044959.1 | neurotrophin receptor B | trkb-b | Protein amino acid phosphorylation | 3.68 | 0.40 | 6.01 | 0.00 | -2.33 | 0.0076 |
| Xl.19610.1.A1_at | BJ084191 | EST | Similar to Angiopoietin-1 receptor precursor (mTIE2) | 4.48 | 0.05 | 6.81 | 0.36 | -2.33 | 0.0043 | |
| Xl.75.1.S1_at | D78003.1 | c4 | c4 | Endopeptidase inhibitor activity i fourth component of complement | 4.09 | 0.47 | 6.41 | 0.00 | -2.32 | 0.0101 |
| Xl.882.1.S1_at | U07179.1 | Ldehydrogenase A | ldha | Oxidoreductase activity | 5.29 | 0.94 | 7.60 | 0.06 | -2.31 | 0.0387 |
| Xl.17327.1.A1_at | BI448285 | EST | MGC68503 | 4.77 | 0.49 | 7.07 | 0.19 | -2.29 | 0.0117 | |
| Xl.747.1.S1_at | AF170341.1 | galectin-1 | MGC64502 | Sugar binding | 5.51 | 0.21 | 7.80 | 0.55 | -2.29 | 0.0075 |
| Xl.545.1.S1_at | AF170344.1 | metastasis associated 1 | mta2 | Transcription factor activity, regulation of transcription | 7.85 | 0.51 | 10.14 | 0.13 | -2.29 | 0.0124 |
| Xl.23647.1.S1_at | BC047974.1 | cell death 2 | pdcd2 | Apoptosis | 4.11 | 0.36 | 6.34 | 0.40 | -2.23 | 0.0096 |
| Xl.19047.1.A1_at | BI478140 | Coatomerprotein | copa | ER to Golgi vesicle-mediated transport i | 8.05 | 0.35 | 10.28 | 0.01 | -2.23 | 0.0071 |
| Xl.9113.1.A1_at | BG346438 | chimerin | chn1 | Signal transduction | 6.10 | 0.76 | 8.32 | 0.12 | -2.22 | 0.0276 |
| Xl.16847.1.A1_at | BJ052360 | EST | 4.22 | 0.74 | 6.44 | 0.36 | -2.22 | 0.0279 | ||
| Xl.13666.1.A1_at | BJ091634 | EST | 6.81 | 0.77 | 8.97 | 0.54 | -2.16 | 0.0350 | ||
| Xl.721.1.S1_at | L09728.1 | transcription factor DLL4 | Dlx2 | Regulation of transcription, DNA-dependent | 7.89 | 0.58 | 10.04 | 0.35 | -2.15 | 0.0199 |
Top 30 candidate transcripts upregulated in hybrids and differentially expressed between females of X. laevis and hybrids. Expression values are in log2 scale; SD = standard deviation of expression values. P values are adjusted according to FDR moderated t-tests.
Table 3.
Candidate transcripts upregulated in X. muelleri compared to hybrids.
| Probe ID | GenBank ID | Target Gene | Gene Symbol | Description/Molecular Function | Mean Muell. | SD Muell. | Mean Hybrid | SD Hybrid | M-H | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Xl.12012.2.A1_at | BM191810 | EST | Weakly similar to FW1A_HUMAN F-boxWD-repeat protein 1B (H. sapiens) | 8.50 | 0.22 | 3.16 | 0.55 | 5.35 | 0.0002 | |
| Xl.7034.1.S1_at | BC043865.1 | LOC398646 | LOC398646 | Similar to pantophysin, transporter activity | 10.03 | 0.23 | 5.60 | 0.29 | 4.44 | 0.0002 |
| Xl.5802.1.A1_x_at | AW764672 | EST | 9.84 | 0.32 | 5.46 | 0.27 | 4.38 | 0.0002 | ||
| Xl.5299.1.S1_at | BI445766 | SEB-4 | seb4-a | Nucleic acid binding | 8.67 | 0.44 | 4.47 | 1.24 | 4.19 | 0.0014 |
| Xl.17322.1.A1_a_at | BJ077543 | EST | Weakly similar to myeloidlymphoid or mixed-lineage leukemia 2; ALL1-related gene (H. sapiens) | 7.00 | 0.09 | 2.90 | 0.14 | 4.10 | 0.0001 | |
| Xl.3326.2.S1_a_at | X63427.1 | Bmp7 | MGC68434 | Bone morphogentic protein, ossification, growth factor activity | 8.03 | 0.53 | 3.94 | 0.43 | 4.09 | 0.0005 |
| Xl.24194.1.S1_at | CD362680 | EST | MGC68920 | Ribosome biogenesis and assembly | 8.36 | 0.60 | 4.27 | 1.21 | 4.09 | 0.0019 |
| XlAffx.1.12.S1_at | AF256087.1 | Xcat 2 | Xcat 2 | Xenopus borealis Xcat-2 | 10.81 | 0.09 | 6.79 | 0.43 | 4.02 | 0.0002 |
| Xl.23898.1.A1_x_at | BF428365 | EST | MGC82089 | Membrane alanyl aminopeptidase activity | 8.12 | 0.12 | 4.12 | 0.11 | 4.00 | 0.0001 |
| Xl.25735.1.S1_at | BE026658 | EST | Weakly similar to GRF1_HUMAN G-rich sequence factor-1 (GRSF-1) (H. sapiens) | 9.66 | 0.23 | 5.68 | 0.47 | 3.97 | 0.0002 | |
| Xl.14298.1.A1_at | BQ383420 | EST | Moderately similar to MOB-LAK (Homo sapiens) (H. sapiens) | 7.73 | 0.29 | 3.82 | 0.46 | 3.90 | 0.0003 | |
| Xl.4311.1.A1_at | BM261211 | EST | 6.75 | 0.24 | 2.86 | 0.37 | 3.89 | 0.0002 | ||
| Xl.25283.1.S1_s_at | BU904283 | EST | MGC85348 | Highly similar to RL2B_HUMAN 60S ribosomal protein L23a (H. sapiens); structural constituent of ribosome | 11.11 | 0.08 | 7.22 | 0.18 | 3.88 | 0.0001 |
| Xl.24302.1.A1_at | BG555239 | EST | 9.69 | 0.04 | 5.83 | 0.05 | 3.86 | 0.0001 | ||
| Xl.61.1.S1_s_at | Y17861.1 | LAP2 | LAP2 | Lamina associated polypeptide 2; nuclear envelope | 10.11 | 0.12 | 6.28 | 0.21 | 3.82 | 0.0001 |
| Xl.15150.1.A1_at | BJ097608 | EST | 7.54 | 0.32 | 3.75 | 0.22 | 3.79 | 0.0002 | ||
| Xl.6902.1.A1_at | BM261049 | EST | MGC68575 | Highly similar to B-cell CLLlymphoma 11A (zinc finger protein); (H. sapiens); nucleic acid binding | 7.37 | 0.47 | 3.60 | 0.04 | 3.76 | 0.0003 |
| Xl.8049.1.S1_a_at | BC041550.1 | Similar to VAMP | MGC53868 | Similar to VAMP (vesicle-associated membrane protein)-associated protein A, structural molecule activity | 8.22 | 0.28 | 4.47 | 0.43 | 3.75 | 0.0003 |
| Xl.6272.1.A1_at | AW782701 | MGC83120 | MGC83120 | Highly similar to Calcium-binding protein p22 (Calcium-binding protein CHP) (H. sapiens); calcium ion binding | 10.36 | 0.23 | 6.65 | 0.28 | 3.71 | 0.0002 |
| Xl.8805.1.S1_s_at | CB564916 | ribosomal protein L4 | rpl-4 | Ribosomal protein L1; structural constituent of ribosome | 11.01 | 0.19 | 7.37 | 1.79 | 3.64 | 0.0059 |
| Xl.2546.1.S1_at | CD324865 | Psma2 | Psma2 | Proteasome subunit XC3; ubiquitin-dependent protein catabolism | 8.19 | 0.25 | 4.58 | 0.10 | 3.61 | 0.0002 |
| Xl.17949.1.S1_at | BG022283 | EST | MGC68573 | Cytochrome-c oxidase activity | 7.41 | 0.23 | 3.83 | 0.21 | 3.58 | 0.0002 |
| Xl.25755.1.A1_at | CB756768 | EST | LOC734179 | Moderately similar to SYQ_HUMAN Glutaminyl-tRNA synthetase(H. sapiens), glutamate-tRNA ligase activity, protein biosynthesis | 7.67 | 0.18 | 4.09 | 0.30 | 3.58 | 0.0002 |
| Xl.7619.1.S1_a_at | BC045223.1 | zf-e326 | zf-e326 | Intracellular signaling cascade | 9.85 | 0.09 | 6.28 | 0.41 | 3.57 | 0.0002 |
| Xl.23754.1.S1_at | AW147985 | EST | LOC495016 | 8.87 | 0.30 | 5.32 | 0.01 | 3.55 | 0.0002 | |
| Xl.23241.1.S1_at | CA988460 | EST | 8.23 | 0.37 | 4.68 | 0.37 | 3.55 | 0.0004 | ||
| Xl.7661.1.S1_at | BJ097640 | EST | LOC495305 | Weakly similar to MCA3_HUMAN Multisynthetase complex auxiliary component p18 (H. sapiens) | 11.18 | 0.14 | 7.64 | 0.40 | 3.54 | 0.0002 |
| Xl.2200.1.A1_at | BM179326 | EST | Calcium ion binding | 6.64 | 0.02 | 3.13 | 0.11 | 3.51 | 0.0001 | |
| Xl.1140.1.S1_s_at | X63425.1 | Bmp2 | Bmp2 | Bone morphogenetic protein 2; growth factor activity; ossification | 9.29 | 0.18 | 5.79 | 0.07 | 3.50 | 0.0002 |
| Xl.15786.1.A1_at | BJ056161 | EST | MGC83224 | tRNA processing | 8.88 | 0.19 | 5.40 | 0.02 | 3.48 | 0.0002 |
Top 30 candidate transcripts upregulated in X. muelleri and differentially expressed between females of X. muelleri and hybrids. Expression values are in log2 scale; SD = standard deviation of expression values. P values are adjusted according to FDR moderated t-tests
Table 4.
Candidate transcripts upregulated in hybrids compared to X. muelleri.
| ProbeID | GenBank ID | Target Gene | GeneSymbol | Description/Molecular Function | Mean Muell. | SD Muell. | Mean Hybrid | SD Hybrid | M-H | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Xl.4276.1.S1_at | X53745.1 | Cyclin A1 | LOC397885 | Regulation of progression through cell cycle | 5.12 | 0.58 | 12.76 | 0.10 | -7.64 | 0.0001 |
| Xl.8319.1.S1_at | BJ098891 | Herz03 | Herz03 | 4.84 | 0.22 | 11.65 | 0.03 | -6.80 | 0.0001 | |
| Xl.21809.1.S1_at | BC041555.1 | MGC53900 | MGC53900 | Similar to calcium modulating ligand | 3.00 | 0.31 | 9.78 | 0.23 | -6.79 | 0.0001 |
| Xl.17345.1.A1_at | BJ053357 | EST | MGC115708 | Weakly similar to MIC2_HUMAN T-cell surface glycoprotein E2 precursor (H. sapiens) | 3.33 | 0.21 | 10.03 | 0.99 | -6.70 | 0.0002 |
| Xl.4744.1.S1_at | BE491637 | LOC495025 | Moderately similar to ubiquitin thiolesterase (H. sapiens); ubiquitin-dependent protein catabolism | 4.78 | 0.60 | 11.43 | 0.16 | -6.65 | 0.0002 | |
| Xl.6585.1.S1_at | BJ080015 | Similar to HIV-1 rev binding protein 2 | HRB2 | RNA binding | 3.22 | 0.20 | 9.75 | 0.14 | -6.53 | 0.0001 |
| Xl.21357.2.S1_at | BJ045324 | Claudin7L1 | MGC53400 | Xenopus laevis cldn7L1 mRNA for Claudin7L1, structural molecule activity | 3.90 | 0.27 | 10.42 | 0.03 | -6.51 | 0.0001 |
| Xl.14775.1.A1_at | BM179359 | EST | Weakly similar to POL2_MOUSE Retrovirus-related POL polyprotein (M. musculus) | 3.81 | 0.18 | 10.23 | 0.22 | -6.43 | 0.0001 | |
| Xl.3668.1.S1_at | AF450296.1 | XLCL2 | LOC397879 | Xenopus laevis F-box protein (PXP17), meiosis | 4.46 | 0.60 | 10.82 | 0.06 | -6.36 | 0.0002 |
| Xl.3401.2.A2_at | BG016692 | EST | LOC446970 | Similar to axotrophin; likely ortholog of mouse axotrophin (H. sapiens), protein binding | 3.72 | 0.63 | 9.95 | 0.14 | -6.24 | 0.0002 |
| Xl.1018.1.A1_at | U44950.1 | Vitelline envelope glycoprotein | lzpb-a | Xenopus laevis vitelline envelope 37 k glycoprotein xlZPB | 4.10 | 0.53 | 10.31 | 0.29 | -6.22 | 0.0002 |
| Xl.3862.2.S1_x_at | CD361360 | Translation factor sui1 | gc20 | Translation initiation factor activity | 3.29 | 0.41 | 9.45 | 0.17 | -6.16 | 0.0001 |
| Xl.7151.1.S1_at | BJ089477 | EST | MGC68561 | Moderately similar to hypothetical protein FLJ10738 (H. sapiens); 3'-5' exonuclease activity | 4.69 | 0.31 | 10.84 | 0.21 | -6.15 | 0.0001 |
| Xl.3536.2.S1_x_at | BF615663 | EST | LOC495200 | Highly similar to transcription factor BTF3a – (H. sapiens) | 4.19 | 0.26 | 10.32 | 1.21 | -6.13 | 0.0004 |
| Xl.23448.1.S1_at | BC041216.1 | SWI/SNF | smarce1 | Similar to SWISNF, actin dependent regulator of chromatin, regulation of transcription | 3.75 | 0.62 | 9.88 | 0.23 | -6.13 | 0.0002 |
| Xl.2060.1.A1_x_at | BJ055271 | EST | 3.61 | 0.40 | 9.69 | 1.64 | -6.08 | 0.0008 | ||
| Xl.576.1.S1_at | AF184090.1 | fatvg | fatvg | 4.94 | 0.56 | 11.02 | 0.03 | -6.08 | 0.0002 | |
| Xl.24785.1.S1_at | BM261081 | EST | MGC81067 | 4.41 | 0.07 | 10.41 | 0.03 | -5.99 | 0.0001 | |
| Xl.4504.1.A1_at | BJ076394 | EST | Weakly similar to ACRC protein; putative nuclear protein (H. sapiens) | 4.11 | 1.10 | 10.10 | 0.06 | -5.99 | 0.0007 | |
| Xl.7045.1.S1_a_at | BQ398421 | EST | Weakly similar to CTF1_HUMAN Cardiotrophin-1 (CT-1) (H. sapiens) | 2.67 | 0.16 | 8.64 | 0.24 | -5.97 | 0.0001 | |
| Xl.7252.1.S1_at | AY172320.1 | Germes | LOC398520 | 3.60 | 0.18 | 9.55 | 0.27 | -5.96 | 0.0001 | |
| Xl.2565.3.S1_x_at | CB561588 | Similar to alpha-Tubulin at 84B | MGC53359 | Xenopus laevis, Similar to alpha-Tubulin at 84B, microtubule-based movement | 4.44 | 0.41 | 10.39 | 0.27 | -5.95 | 0.0002 |
| Xl.7837.1.A1_at | BF232270 | EST | MGC132211 | Highly similar to hypothetical protein FLJ10900 (H. sapiens), electron transport | 2.94 | 0.43 | 8.88 | 0.02 | -5.94 | 0.0001 |
| Xl.25809.1.A1_at | BE026874 | EST | MGC80281 | Histidine catabolism | 4.26 | 0.49 | 10.18 | 0.36 | -5.92 | 0.0002 |
| Xl.6605.1.A1_at | AW632842 | EST | 3.59 | 0.26 | 9.50 | 0.02 | -5.91 | 0.0001 | ||
| Xl.4170.2.A1_at | BQ398301 | LOC494857 | LOC494857 | Cell differentiation | 4.21 | 0.59 | 10.11 | 0.11 | -5.90 | 0.0002 |
| Xl.1014.1.S1_at | U46131.1 | Cdc21 protein | cdc21 | Xenopus laevis DNA replication initiator protein, DNA replication initiation, regulation of transcription | 4.14 | 0.46 | 10.00 | 0.08 | -5.86 | 0.0002 |
| Xl.2839.1.S1_at | BC041270.1 | Protein translocation complex | sec61beta | Similar to protein translocation complex beta | 5.91 | 0.17 | 11.76 | 0.08 | -5.85 | 0.0001 |
| Xl.25536.1.A1_at | BE677987 | EST | Weakly similar to hypothetical protein MGC2577 (H. sapiens) | 4.36 | 1.14 | 10.15 | 0.93 | -5.79 | 0.0012 | |
| Xl.14065.1.A1_at | AW147826 | EST | 3.61 | 0.15 | 9.33 | 0.12 | -5.72 | 0.0001 |
Top 30 candidate transcripts upregulated in hybrids and differentially expressed between females of X. muelleri and hybrids. Expression values are in log2 scale; SD = standard deviation of expression values. P values are adjusted according to FDR moderated t-tests.
Gene expression between the two parental species was also dramatically different. More than 76% (8,741/11,485) of genes were differentially expressed between females of X. laevis and X. muelleri. Of these differentially expressed genes, about 60% (5,203/8,741) were upregulated in X. muelleri relative to X. laevis (5,203 vs. 3,538; G = 159.5; df = 1; P < 0.0001). Comparing the overlap in genes differentially expressed in the two hybrid contrasts to the three classes of expression behavior between X. laevis and X. muelleri (X. laevis > X. muelleri; X. laevis <X. muelleri; X. laevis = X. muelleri) shows a general pattern of semidominance in expression behavior (Table 5). For example, of the 839 genes upregulated in hybrids relative to X. laevis; 90% were upregulated in X. muelleri relative to X. laevis. Similarly, of the 2,930 genes that were upregulated in hybrids relative to X. muelleri, 91% were upregulated in X. laevis compared to X. muelleri. These results suggest a general pattern of intermediate expression in hybrids and are consistent with a semidominant model of expression difference even despite the asymmetrical pattern of misexpression in hybrids compared to the two parental species.
Table 5.
Overlap of transcripts from comparisons of hybrids and both species.
| L < H | L > H | M < H | M > H | |
|---|---|---|---|---|
| L > M | 24 | 626 | 2654 | 24 |
| L < M | 753 | 65 | 9 | 3997 |
| L = M | 62 | 86 | 267 | 328 |
| Total | 839 | 777 | 2930 | 4349 |
Comparison in the overlap of transcripts recovered as differentially expressed from the two contrasts with hybrids (Xenopus laevis (L) vs. hybrids (H) and X. muelleri (M) vs. hybrids) and the interspecies contrast (Xenopus laevis vs. X. muelleri). The congruence between patterns of expression behavior in hybrids compared to the interspecies comparison suggests a model of semidominance where hybrids have an intermediate level of expression compared to the two parental species
Discussion
Our analysis of hybrid females relative to the two parental species provides key insight into the process of oogenesis in hybrid females and the two parental species. There is an asymmetrical pattern of differential expression with about 4.5 times more genes differentially expressed between hybrids and Xenopus muelleri compared to X. laevis. This result implies that strong maternal and/or species dominance effects act in oogenesis and these are reflected in the hybrid transcriptome. Hybrid females have a general pattern of semidominance in gene expression with the majority of genes being expressed at intermediate levels compared to the two parental species. Finally, there is a dramatic divergence in gene expression in the ovary between the two parental species with more than 76% of genes differentially expressed between X. laevis and X. muelleri. This suggests that the process of oogenesis differs widely at the gene expression level between these two species of Xenopus.
It is important to consider the methodology used to gather the samples of RNA for this study. Samples of ovary (50 mg portions) were dissected and then homogenized in RNA extraction solution. Therefore, we gathered a sample of ovary rather than the entire ovary and this sample is a heterogeneous representation of oogenesis, rather than a direct assessment of specific stages of oocyte development. Given the heterogeneous nature of the tissue used to gather RNA, it is even more surprising that we found such strong effects. Increased heterogeneity among samples would decrease the ability to reject the null hypothesis that gene expression for a particular gene is the same between hybrids and conspecifics. Increased heterogeneity among samples would increase the standard error and thereby decrease power to reject the null hypothesis. In fact though, even despite the heterogeneous nature of the samples collected, we still reject a large portion of null hypotheses suggesting that our microarray results represent real biological effects, rather than statistical artifacts. Additionally, our results remain robust even when using different normalization techniques (scaling and Robust Multichip Averaging) providing further confidence that our results are not statistical artifacts (not shown).
The top 30 candidate genes for the contrasts between hybrids and the two parental species provide many genes with known roles in mitosis, meiosis, and oogenesis in general (Table 1, 2, 3, 4). One EST, MGC132176, is predicted to have heme oxygenase activity and this EST was upregulated 12 times in X. laevis relative to hybrids. Heme oxygenase plays a role in regulating ovarian steroidgenesis in rats and our results suggest this may be the case in Xenopus [8] as well. Of the genes with known function, many have been documented to play a role in oogenesis. For example, the proliferation associated protein PA2G4, which was upregulated in X. laevis about 8 times higher than in hybrids, has been previously isolated from Xenopus oocytes and is believed to play an important role in DNA replication and cell cycle progression [9]. One EST that is similar to the human transcription factor Hairy and enhancer of split 2, was upregulated 18 times in hybrids relative to X. laevis, and is known to be regulated by reproductive hormones in adult rat ovary [10]. Neurotrophin receptor B (Trkb-b) was upregulated 5 times in hybrids relative to X. laevis and plays a critical role in ovulation, steroid secretion, and follicular development in the ovary of rodents and humans [11-15].
Examining candidate gene lists for the Xenopus muelleri vs. hybrid comparison also reveal many genes involved in oogenesis. For example two bone morphogenetic proteins, Bmp7 and Bmp2, are 17 and 11 times respectively upregulated in X. muelleri relative to hybrid females. Bone morphogenetic proteins are part of a class of proteins involved in the development and patterning of the adult ovary [16,17]. Xcat-2 was upregulated 17 times more in X. muelleri relative to hybrids and is involved in the formation of germ plasm during stage I oocytes of Xenopus [18,19]. Another gene of interest is LAP2, upregulated 14 times higher in X. muelleri compared to hybrids and specific isoforms of LAP2 are expressed exclusively in the ovary of anurans and salamanders [20-22]. Cyclin A1, the most divergently expressed gene, was upregulated nearly 200 times higher in hybrids relative to X. muelleri and expressed the same in X. laevis and hybrids. Cyclin A1 plays a major role in mammalian gametogenesis and meiosis [23] and is a partner of Cdk2, a key gene involved in the cell cycle both in mitosis and meiosis. Female and male knockout Cdk2 mice are viable but both infertile [24]. Curiously, disruption of Cyclin A1 expression results in male infertility but not female infertility and specifically causes the developmental arrest of spermatogenesis during meiosis I [25,26]. A vitelline envelope glycoprotein, lzpb-a, was upregulated 75 times in hybrids relative to X. muelleri, and vitelline envelope proteins have an obvious role in the formation of oocytes during amphibian oogenesis [27]. Finally, Germes, a gene that localizes to the germ plasm during early oogenesis in Xenopus [28] was upregulated 62 times higher in hybrids compared to X. muelleri.
Perhaps the most surprising result of our analyses is that hybrid females are fertile yet have a dramatic increase in gene misexpression compared to hybrid males which are completely sterile [2]. These results would seem to contradict what we might intuitively predict; specifically that it seems reasonable to assume that normal phenotypes should have greater similarity in expression profiles and perturbed phenotypes should have greater divergence in expression. Hybrid males, which are completely sterile, have only 56 genes misexpressed in testes compared to both parental species whereas hybrid females, which are fertile, have nearly 20 times more genes misexpressed (1,105) in ovaries. However, these results are consistent with patterns of sex-biased gene expression in which female-biased genes were found to be more divergently expressed between species compared to male-biased genes [29] providing further evidence that this pattern represents real biological effects. Thus, we are left with a question, how can the process of oogenesis tolerate such dramatic differences in the level of gene expression, whereas the process of spermatogenesis in hybrid males has relatively few genes misexpressed yet results in complete sterility.
To date, there has been little exploration of this question because studies of gene expression and reproductive isolation have focused on the sterility phenotype which typically involves males. However hybrid females of Drosophila melanogaster and D. simulans, which are sterile, have been analyzed and hybrids had a majority of genes misexpressed compared to the two species [30]. Recent work has shown that critical genes involved in mammalian female reproduction undergo rapid diversification due to retrotransposed genes in Mus musculus [31] and these results may provide a clue to the divergent expression pattern occurring in females of Xenopus which could be a general pattern of female reproduction.
Xenopus do not conform to a fundamental generalization in evolutionary biology-Haldane's rule [1-7]. Patterns of sex-biased gene expression and comparisons between taxa in which the sex chromosome constitution is reversed suggest that the sensitive spermatogenesis component of the faster-male evolution hypothesis [3,32] is the best explanation for sterile males in Xenopus even though females are the heterogametic sex. Given the divergent pattern of expression in hybrids and females between species, we suggest the following scenario to explain hybrid male sterility in Xenopus.
First, oogenesis relies on a staggering amount of gene expression with up to 45% of all mouse genes and 55% of all Drosophila genes expressed in the mature oocyte [31,33] and additionally this abundant transcription results in maternally deposited RNAs and proteins which foster oocyte growth and early development [31,34]. In particular, much of this RNA deposition functions to localize coding and non-coding RNAs essential to germ cell development into a distinct subcellular domain that can be moved into the vegetal cortex of the oocyte. Interestingly, RNAs localized in the germ plasm may not be translated for years highlighting the importance of the germ plasm as a storage unit for RNA and furthermore many of these stored RNAs are involved in translational regulation of germ cell specific expression [35-38].
We find dramatic differences in gene expression between females of two species of Xenopus and many of the most dramatic differences have to do with Early/METRO pathway (e.g. Germes, Fatvg, Cyclin A1) of germ plasm specification [38]. These dramatic, sometimes 200 times different, RNA abundance levels indicate a major difference in the amount of key genes involved in germ plasm specification and maternally loaded RNAs between species. This result would suggest that each species has a divergent way of completing oogenesis with regard to gene expression.
During fertilization, sperm fertilize an egg and this starts the dramatic changes that turn the mature oocyte into a functioning zygote [39]. Each sperm delivers a haploid paternal genome along with mature RNA that initiates and directs subsequent development [40]. The interaction between the paternal genome and stored maternal RNA must coevolve in such a way to ensure successful development. Consider now sperm from a different species, adapted for fertilizing eggs of its conspecific species, that now successfully fertilizes an egg from a different species. As our data suggest, the paternal genome will now interact with a radically different embryo with drastically different amounts of stored maternal RNAs. We therefore suggest the possibility that disruption of spermatogenesis in adult hybrid males occurs because of radically divergent expression in females during oogenesis. Oocytes armed with pools of RNA adapted for one species, work in the sense that they can be fertilized and develop but, the initial differences in maternally stored RNAs generate subsequent dysfunctions in males because molecular interactions that generate the adult testis and subsequent spermatogenesis are misregulated due to the differences in maternally stored RNA populations. Spermatogenesis is special in the sense that during early development key factors fail to interact properly to generate a normally functioning testis.
Several genes from our microarray results suggest directions by which this hypothesis could be tested. One example is Cyclin A1 which was upregulated about 200 times more in hybrids and 170 times more in X. laevis compared to X. muelleri. Knocking out Cyclin A1 in mice causes completely sterility and the interaction between Cyclin A1 and cdk2 is crucial for normal development [24,25]. Cyclin A1 is also known to be maternally deposited in Xenopus and regulates the progression of the cell cycle and apoptosis [41]. Our hypothesis suggests that factors like Cyclin A1 which are loaded into embryos in drastically different amounts may play a role in misdirecting the development of the hybrid testis.
Conclusion
Our work provides an important first glimpse into the expression pattern of hybrid females and parental species. We find an asymmetrical expression pattern similar to the pattern of expression in hybrid male Xenopus and allotetraploid Arabidopsis [2,42]. However, hybrid females have a dramatic increase in the number of misexpressed genes compared to sterile males and we suggest that this gene expression divergence plays a role in hybrid male sterility. Our results call for attention as to how divergent expression in females plays a role in reproductive isolation between species.
Methods
Microarray Experiments
RNA was extracted from adult ovary in Xenopus laevis (n = 4), hybrids of X. laevis × X. muelleri (n = 2) and X. muelleri (n = 3). Hybrid individuals were produced by crossing maternal X. laevis with paternal X. muelleri. Origin of parents and methodology for creating hybrids has been described elsewhere [2,29]. Sufficient numbers of normal hybrid females from the reciprocal cross were unable to be produced because the reciprocal cross produces increased mortality and the offspring that survive have a high proportion of limb abnormalities [1]. Individual adults were euthanized with MS-222 and 50 mg of ovary was dissected and homogenized in RNA extraction solution using a hand held pestle. RNA was recovered using GeneHunter and Ambion RiboPure total RNA kits. Samples of RNA were checked for purity by examination of the 28S and 18S ribosomal RNA bands from denaturing gel electrophoresis, by 260/280 ratios from scans with a Nanodrop ND 1000 spectrophotometer, and by readouts of the Agilent Bioanalyzer. Total RNA samples were prepared and hybridized to Affymetrix Xenopus laevis GeneChip Genome Arrays at the University of Texas Southwestern Medical Center Microarray Array Core Facility following standard Affymetrix protocols. Affymetrix Microarray Analysis Suite (MAS) v.5.0 was used to scan and process each microarray chip. The signals of quality control and poly(A) transcripts revealed that hybridizations were of high quality in all chips. Quality control probe sets (i.e., spike in and housekeeping genes) were removed in subsequent statistical analyses. Hybridizing RNA from a heterospecific species to a microarray designed for a related species can have a dramatic impact on the signal recovered from microarrays [43-46]. To control for this effect, we used an electronic mask generated from hybridizing genomic DNA from X. laevis and X. muelleri onto the X. laevis microarray [2]. This mask which screens out probes that have significant sequence divergence in X. muelleri provides 11,485 probesets/genes for further analysis.
Data Analysis
We conducted three separate comparisons to uncover patterns of differential expression between Xenopus laevis and hybrids, X. muelleri compared to hybrids, and X. laevis compared to X. muelleri. First, the Xenopus laevis and hybrid chips were normalized using Robust Multichip Averaging (RMA) express software [47] using default parameters for background correction and quantile normalization. These RMA normalized data were then imported into the R statistical environment and tested for differences in expression between X. laevis and hybrids for each of the 11,485 genes using a moderated t-statistic based on an empirical Bayes method in the Limma package found in Bioconductor [48]. The TopTable function was then used to output the False Discovery Rate (FDR)-adjusted P-values and we considered genes with adjusted P-values less than 0.05 to be differentially expressed. Goodness of fit tests (G), based on the difference between the observed and the expected (under the null hypothesis of equal class probabilities) number of genes, were performed to test whether there was enrichment in the number of genes up-regulated in particular comparisons [49]. We normalized X. muelleri and hybrid chips together using RMA and repeated the analyses to uncover differential expression between X. muelleri and hybrids. Finally, we normalized X. laevis and X. muelleri chips together using RMA and repeated the analysis to uncover genes misexpressed between the two species. Separate normalizations were performed for each comparison in keeping with the assumptions of RMA normalization.
Authors' contributions
JHM conceived of the study, collected the data, performed the statistical analyses, and wrote the manuscript. PM participated in its design and coordination and edited the manuscript. All authors read and approved the final manuscript.
Contributor Information
John H Malone, Email: malonej@niddk.nih.gov.
Pawel Michalak, Email: michalak@uta.edu.
Acknowledgements
This work was conducted following the protocols of the University of Texas-Arlington Animal Care Committee (Protocol No. A05.001). We thank Professor R. C. Tinsley for donating specimens of Xenopus muelleri for use in this study, Funding was provided by a National Science Foundation Dissertation Improvement Grant (DEB-0508882) and Texas Academy of Science Student Research Grant to JHM.
References
- Malone JH. PhD thesis, Department of Biology. The University of Texas Arlington; 2007. Genetic determinants of reproductive isolation in Xenopus. [Google Scholar]
- Malone JH, Chrzanowski TH, Michalak P. Sterility and gene expression in hybrid males of Xenopus laevis and X. muelleri. PLoS ONE. 2007;22:e781. doi: 10.1371/journal.pone.0000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone JH, Michalak P. Physiological sex predicts hybrid sterility regardless of genotype. Science. 2008;319:59. doi: 10.1126/science.1148231. [DOI] [PubMed] [Google Scholar]
- Kobel HR. In: The Biology of Xenopus. Tinsley RC, Kobel HR, editor. Oxford, Clarendon Press; 1996. Allopolyploid speciation; pp. 391–401. [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland: Sinauer Associates, Inc; 2004. [Google Scholar]
- Orr HA, Presgraves DC. Speciation by postzygotic isolation: forces, genes, and molecules. BioEssays. 2000;22:1085–1094. doi: 10.1002/1521-1878(200012)22:12<1085::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Haldane JBS. Sex ratio and unisexual sterility in animal hybrids. J Genet. 1922;12:101–109. [Google Scholar]
- Alexandreanu IC, Lawson DM. Heme oxygenase in the rat ovary: immunohistochemical localization and possible role in steroidogenesis. Exp Biol Med. 2003;228:59–63. doi: 10.1177/153537020322800108. [DOI] [PubMed] [Google Scholar]
- Izuta S, Kitahara M, Hamaguchi T. Regulation of eukaryotic DNA replication by proliferation associated protein, PA2G4, in vitro. Nuc Acid Symp Ser. 2004;48:285–286. doi: 10.1093/nass/48.1.285. [DOI] [PubMed] [Google Scholar]
- Yamada K, Kawata H, Mizutani T, Arima T, Yazawa T, Matsuura K, Shou Z, Sekiguchi T, Yoshino M, Kajitani T, Miyamoto K. Gene expression of basic helix-loop-helix transcription factor, SHARP-2, is regulated by gonadotropins in the rat ovary and MA-10 cells. Biol Reprod. 2004;70:76–82. doi: 10.1095/biolreprod.103.020107. [DOI] [PubMed] [Google Scholar]
- Waraksa JA, Lindsay RM, Ip NY, Hutz RJ. Neurotrophin-3 augments steroid secretion by hamster ovarian follicles in vitro. Zool Sci. 1995;12:499–502. doi: 10.2108/zsj.12.499. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Hill DF, Costa ME, Dees WL, Lara HE, Ojeda SR. A role for Trk A nerve growth factor receptors in mammalian ovulation. Endocrinology. 1996;137:198–209. doi: 10.1210/endo.137.1.8536613. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Mayerhofer A, Ojeda SR. Participation of nerve growth factor in the regulation of ovarian function. Zygote. 1996;4:309–312. doi: 10.1017/s0967199400003300. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Dissen GA, Parrott JA, Hill DF, Mayerhofer D, Garfield RE, Costa ME, Skinner MK, Ojeda SR. Involvement of nerve growth factor in the ovulatory cascade: trk A receptor activation inhibits gap junctional communication between thecal cells. Endocrinology. 1996;137:5662–5670. doi: 10.1210/endo.137.12.8940397. [DOI] [PubMed] [Google Scholar]
- Seifer DB, Feng B, Sheldon RM. Immunocytochemical evidence for the presence and location of the neurotrophin-Trk receptor family in adult human preovulatory ovarian follicles. Am J Obs Gyn. 2006;194:1129–1136. doi: 10.1016/j.ajog.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-b superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- Von Schalburg KR, McCarthy SP, Rise ML, Hutson JC, Davidson WS, Koop BF. Expression of morphogenic genes in mature ovarian and testicular tissues: potential stem-cell niche markers and patterning factors. Mol Reprod Dev. 2006;73:142–152. doi: 10.1002/mrd.20359. [DOI] [PubMed] [Google Scholar]
- Kloc M, Etkin LD. Two distinct pathways for the localization of RNAs at the vegetal cortex in Xenopus oocytes. Development. 1995;121:287–297. doi: 10.1242/dev.121.2.287. [DOI] [PubMed] [Google Scholar]
- Zhou Y, King ML. Localization of Xcat-2 RNA, a putative germ plasm component, to the mitochondrial cloud in Xenopus stage I oocytes. Development. 1996;122:2947–2953. doi: 10.1242/dev.122.9.2947. [DOI] [PubMed] [Google Scholar]
- Lang C, Paulin-Levasseur M, Gajewski A, Alsheimer M, Benavente R, Krohne G. Molecular characterization and developmentally regulated expression of Xenopus lamina-associated polypeptide 2 (XLAP2) J Cell Sci. 1999;112:749–759. doi: 10.1242/jcs.112.5.749. [DOI] [PubMed] [Google Scholar]
- del Pino EM, Sáenz FE, Pérez OD, Brown FD, Avila ME, Barragán VA, Haddad N, Paulin-Levasseur M, Krohne G. The LAP2 (lamina-associated polypeptide 2) expression in fish and amphibians. Int J Dev Biol. 2002;46:327–334. [PubMed] [Google Scholar]
- Brown FD, del Pino EM, Krohne G. Bidder's organ in the toad Bufo marinus: effects of orchidectomy on the morphology and expression of lamina-associated polypeptide 2. Dev Growth Differ. 2002;44:527–535. doi: 10.1046/j.1440-169x.2002.00665.x. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum AL. Cell-cycle regulation and mammalian gametogenesis: a lesson from the unexpected. Mol Reprod Dev. 2006;73:939–942. doi: 10.1002/mrd.20536. [DOI] [PubMed] [Google Scholar]
- Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol. 2003;13:1775–1785. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Liu D, Matzuk MM, Sung WK, Guo Q, Wang P, Wolgemuth DJ. Cyclin A1 is required for meiosis in male mouse. Nat Genet. 1998;20:377–380. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- Persson JL, Zhang Q, Wang XY, Ravnik SE, Muhlrad S, Wolgemuth DJ. Distinct roles for the mammalian A-type cyclins during oogenesis. Reproduction. 2005;130:411–422. doi: 10.1530/rep.1.00719. [DOI] [PubMed] [Google Scholar]
- Kubo H, Kawano T, Tsubuki S, Kotani M, Kawasaki H, Kawashima S. Egg envelope glycoprotein gp37 as a Xenopus homolog of mammalian ZP1, based on cDNA cloning. Dev Growth Differ. 2000;42:419–27. doi: 10.1046/j.1440-169x.2000.00526.x. [DOI] [PubMed] [Google Scholar]
- Berekelya LA, Ponomarev MB, Luchinskaya NN, Belyavsky AV. Xenopus Germes encodes a novel germ plasm-associated transcript. Gene Exp Patterns. 2003;3:521–524. doi: 10.1016/s1567-133x(03)00055-3. [DOI] [PubMed] [Google Scholar]
- Malone JH, Hawkins DL Jr, Michalak P. Sex-biased gene expression in a ZW sex determination system. J Mol Evol. 2006;63:427–436. doi: 10.1007/s00239-005-0263-4. [DOI] [PubMed] [Google Scholar]
- Ranz JM, Namgyal K, Gibson G, Hartl DL. Anomalies in the expression profile of interspecific hybrids of Drosophila melanogaster and Drosophila simulans. Genome Res. 2004;14:373–379. doi: 10.1101/gr.2019804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evsikov AV, Graber JH, Brockman MJ, Hampl A, Holbrook AE, Singh P, Eppig JJ, Solter D, Knowles BB. Cracking the egg: molecular dynamics and evolutionary aspects of the transition from the fully grown oocyte to embryo. Genes Dev. 2006;20:2713–2727. doi: 10.1101/gad.1471006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-I, Davis AW. Evolution of postmating reproductive isolation: the composite nature of Haldane's rule and its genetic basis. Am Nat. 1993;142:187–212. doi: 10.1086/285534. [DOI] [PubMed] [Google Scholar]
- Tadros W, Goldman AL, Babak T, Menzies F, Vardy L, Orr-Weaver T, Hughes TR, Westwood JT, Smibert CA, Lipshitz HD. SMAUG is a major regulator of maternal mRNA destabilization in Drosophila and its translation is activated by the PAN GU kinase. Dev Cell. 2007;12:143–155. doi: 10.1016/j.devcel.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Seydoux G. Mechanisms of translational control in early development. Curr Opin Genet Dev. 1996;6:555–561. doi: 10.1016/s0959-437x(96)80083-9. [DOI] [PubMed] [Google Scholar]
- MacArthur H, Bubunenko M, Houston D, King ML. Xcat2 RNA is a translationally sequestered germ plasm component in Xenopus. Mech Dev. 1999;84:75–88. doi: 10.1016/s0925-4773(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Houston DW, King ML. Germ plasm and molecular determinants of germ cell fate. Curr Top Dev Biol. 2000;50:155–182. doi: 10.1016/s0070-2153(00)50008-8. [DOI] [PubMed] [Google Scholar]
- Houston DW, King ML. A critical role for Xdazl, a germ-plasm- localized RNA, in the differentiation of primordial germ cells in Xenopus. Development. 2000;127:447–456. doi: 10.1242/dev.127.3.447. [DOI] [PubMed] [Google Scholar]
- King ML, Messitt TJ, Mowry KL. Putting RNAs in the right place at the right time: RNA localization in the frog oocyte. Biol Cell. 2005;97:19–33. doi: 10.1042/BC20040067. [DOI] [PubMed] [Google Scholar]
- Stitzell ML, Seydoux G. Regulation of the oocyte-to-zygote transition. Science. 2007;316:407–408. doi: 10.1126/science.1138236. [DOI] [PubMed] [Google Scholar]
- Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA. Reproductive biology: delivering spermatozoan RNA to the oocyte. Nature. 2004;429:154. doi: 10.1038/429154a. [DOI] [PubMed] [Google Scholar]
- Carter AD, Wroble BN, Sible JC. Cyclin A1/Cdk2 is sufficient but not required for the induction of apoptosis in early Xenopus laevis embryos. Cell Cycle. 2006;5:2230–2036. doi: 10.4161/cc.5.19.3262. [DOI] [PubMed] [Google Scholar]
- Wang J, Tian L, Lee H-S, Wei NE, Jiang H, Watson B, Madlung A, Osborn TC, Doerge RW, Comai L, Chen ZJ. Genomewide nonadditive gene regulation in Arabidopsis allotetraploids. Genetics. 2006;172:507–517. doi: 10.1534/genetics.105.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzhdin SV, Wayne ML, Harmon KL, McIntyre LM. Common pattern of evolution of gene expression level and protein sequence in Drosophila. Mol Biol Evol. 2004;21:1308–1317. doi: 10.1093/molbev/msh128. [DOI] [PubMed] [Google Scholar]
- Gilad Y, Rifkin SA, Bertone P, Gerstein M, White KP. Multi-species microarrays reveal the effect of sequence divergence on gene expression profiles. Genome Res. 2005;15:674–680. doi: 10.1101/gr.3335705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad Y, Borevitz J. Using DNA microarrays to study natural variation. Curr Opin Genet Dev. 2006;16:553–558. doi: 10.1016/j.gde.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Oshlack A, Chabot AE, Smyth GK, Gilad Y. Using DNA microarrays to study gene expression in closely related species. Bioinformatics. 2007;23:1235–1242. doi: 10.1093/bioinformatics/btm111. [DOI] [PubMed] [Google Scholar]
- RMAExpress. http://rmaexpress.bmbolstad.com/
- Smyth G. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Statistical Applications in Genetics and Molecular Biology. 2004;3:Article 3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3. New York: W.H.Freeman; 2001. [Google Scholar]