Abstract
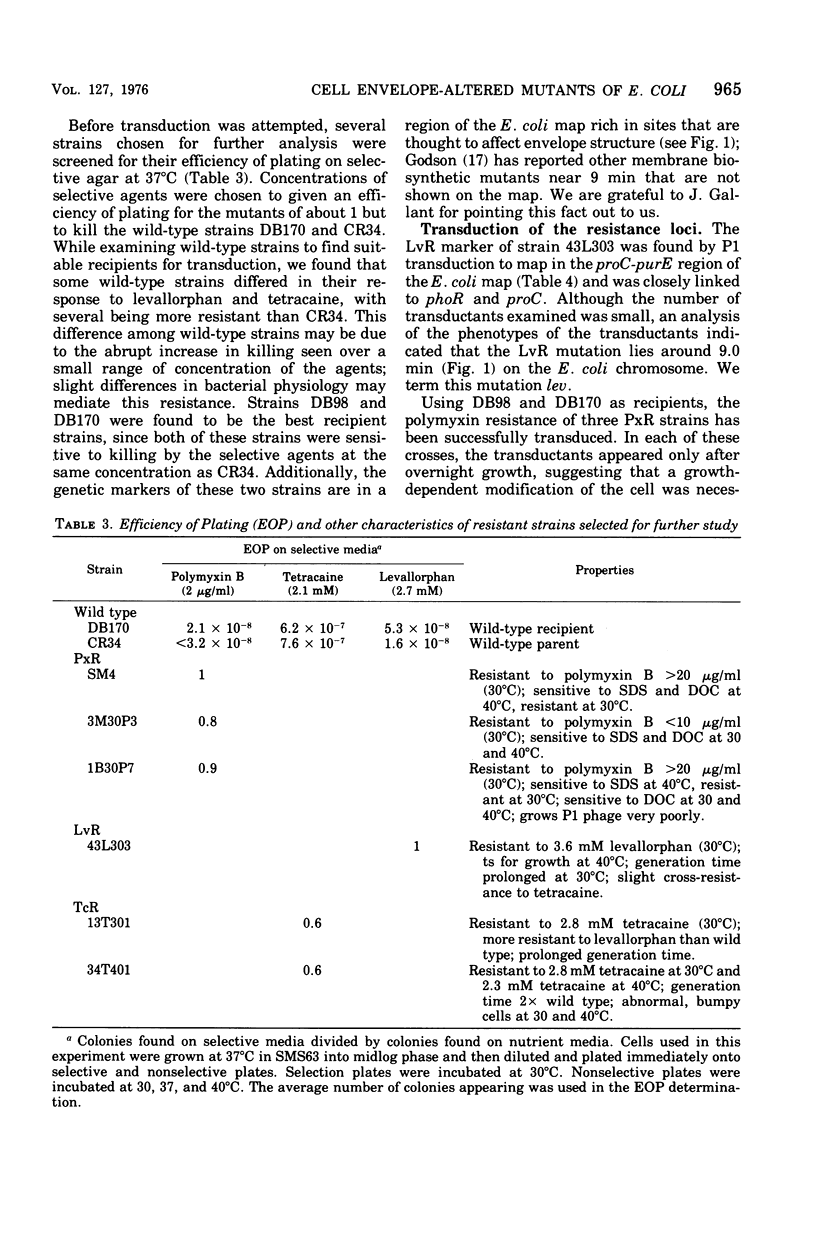
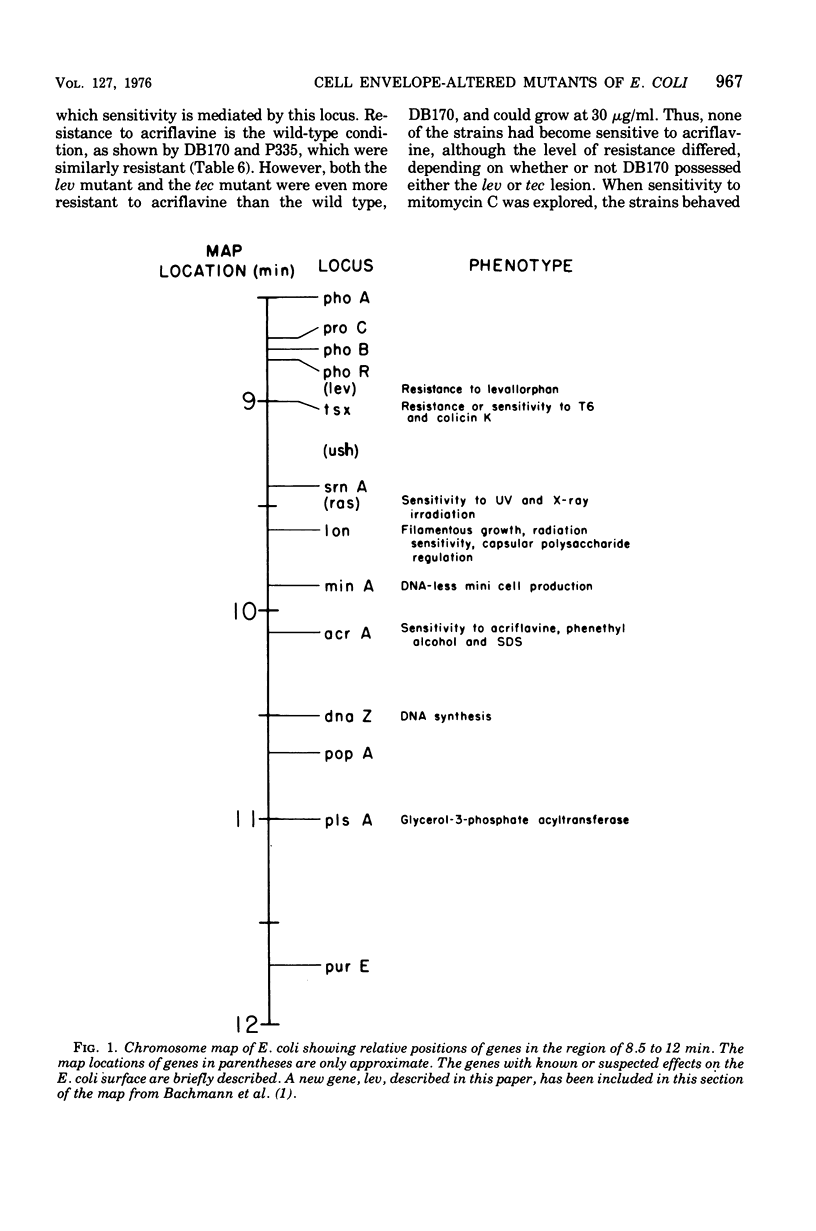
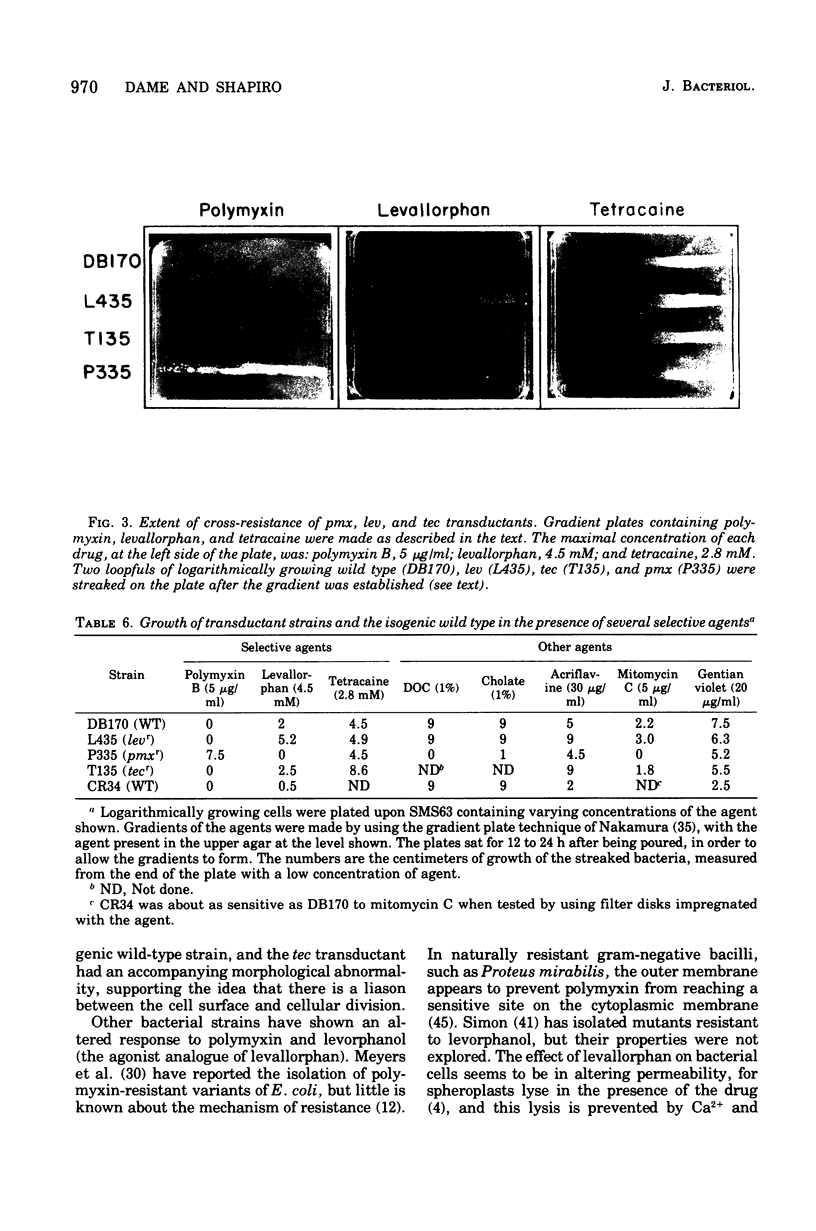
Mutants of Escherichia coli were isolated by their resistance to the bacteriocidal effects of the membrane-active drugs polymyxin B, levallorphan, and tetracaine. The mutants were examined for additional changes in cellular physiology evoked by the lesions; many polymyxin-resistant strains had a concomitant increased sensitivity to anionic detergents, and several strains of each type had concomitant alterations in generation time and morphology. Mutants of each class (polymyxin resistant, tetracaine resistant, and levallorphan resistant) were transduced into recipient strains. The levallorphan resistance site (lev) was located at approximately 9 min on the E. coli chromosome. Polymyxin (pmx) and tetracaine (tec) resistance loci were also transduced. The lev and tec strains had a slight prolongation of generation time, in contrast with their isogenic wild-type strains. The tec transductant produced long filaments in the absence of tetracaine and had an altered colonial morphology, it reverted at high frequency, with the morphological abnormalities reverting along with the tetracaine resistance. The pmx transductant had an increased sensitivity to levallorphan and to anionic detergents. In contrast, both lev and tec mutants were more resistant to acriflavine than was the wild type or the pmx transductant. The pmx, lev, and tec loci differed in sensitivity to mitomycin C; the lev strain was more resistant, the tec strain was more sensitive, and the pmx strain was much more sensitive than the wild type. There was no difference in sensitivity to several other dyes and detergents, colicins, or T bacteriophage between the transductant and isogenic wild-type strains. Thus, lev, tec, and pmx loci confer more subtle alterations in the permeability barrier than do lipopolysaccharide-deficient mutants previously studied.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsky A. Z., Armstrong J. B. Osmotic reversal of temperature sensitivity in Escherichia coli. J Bacteriol. 1973 Jan;113(1):76–81. doi: 10.1128/jb.113.1.76-81.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W. Bacterial transport. Annu Rev Biochem. 1974;43(0):123–146. doi: 10.1146/annurev.bi.43.070174.001011. [DOI] [PubMed] [Google Scholar]
- Boquet P. L., Devynck M. A., Monnier C., Fromageot P. Inhibition of stable RNA synthesis by levallorphan in Escherichia coli. Implication of compounds MS I and MS II. Eur J Biochem. 1973 Dec 3;40(1):31–42. doi: 10.1111/j.1432-1033.1973.tb03166.x. [DOI] [PubMed] [Google Scholar]
- Boquet P., Devynck M., Aurelle H., Fromageot P. On the bactericidal action of levallorphan. Irreversible alterations of the plasmic membrane. Eur J Biochem. 1971 Aug 25;21(4):536–541. doi: 10.1111/j.1432-1033.1971.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Hantke K. Biochemistry of bacterial cell envelopes. Annu Rev Biochem. 1974;43(0):89–121. doi: 10.1146/annurev.bi.43.070174.000513. [DOI] [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis H. L., Bloomstein M. I. Discussion paper: antibiotic-sensitive mutants of Escherichia coli possess altered outer membranes. Ann N Y Acad Sci. 1974 May 10;235(0):593–600. doi: 10.1111/j.1749-6632.1974.tb43293.x. [DOI] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. R., Nordström K., Englund P. Resistance of Escherichia coli to penicillins. IX. Genetics and physiology of class II ampicillin-resistant mutants that are galactose negative or sensitive to bacteriophage C21, or both. J Bacteriol. 1971 Dec;108(3):1210–1223. doi: 10.1128/jb.108.3.1210-1223.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEW A. V., SCHULMAN J. H. The absorption of polymyxin E by bacteria and bacterial cell walls and its bactericidal action. J Gen Microbiol. 1953 Dec;9(3):454–466. doi: 10.1099/00221287-9-3-454. [DOI] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins and colicinogenic factors. Symp Soc Exp Biol. 1958;12:104–122. [PubMed] [Google Scholar]
- Feingold D. S., HsuChen C. C., Sud I. J. Basis for the selectivity of action of the polymyxin antibiotics on cell membranes. Ann N Y Acad Sci. 1974 May 10;235(0):480–492. doi: 10.1111/j.1749-6632.1974.tb43285.x. [DOI] [PubMed] [Google Scholar]
- Fried V. A., Novick A. Organic solvents as probes for the structure and function of the bacterial membrane: effects of ethanol on the wild type and an ethanol-resistant mutant of Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):239–248. doi: 10.1128/jb.114.1.239-248.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N. Isolation and partial characterization of temperature-sensitive mutants in ten loci that affect cell membrane synthesis in Escherichia coli: isolation and genetic sorting. J Bacteriol. 1973 Feb;113(2):813–824. doi: 10.1128/jb.113.2.813-824.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U. L. Determination of cell shape in bacteria. Annu Rev Microbiol. 1975;29:45–60. doi: 10.1146/annurev.mi.29.100175.000401. [DOI] [PubMed] [Google Scholar]
- Johnson C. L., Goldstein M. A., Schwartz A. On the molecular action of local anesthetics. 1. The mitochondrian as a model membrane system for studying local anesthetic action. Mol Pharmacol. 1973 May;9(3):360–371. [PubMed] [Google Scholar]
- Koike M., Iida K. Effect of polymyxin on the bacteriophage receptors of the cell walls of gram-negative bacteria. J Bacteriol. 1971 Dec;108(3):1402–1411. doi: 10.1128/jb.108.3.1402-1411.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M., Iida K., Matsuo T. Electron microscopic studies on mode of action of polymyxin. J Bacteriol. 1969 Jan;97(1):448–452. doi: 10.1128/jb.97.1.448-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Shaprio B. M. Relationship between permeability, cell division, and murein metabolism in a mutant of Escherichia coli. J Bacteriol. 1972 Aug;111(2):499–509. doi: 10.1128/jb.111.2.499-509.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz P. J., Schaechter M. The attachment of the bacterial chromosome to the cell membrane. Int Rev Cytol. 1975;41:1–28. doi: 10.1016/s0074-7696(08)60964-x. [DOI] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Lopes J., Gottfried S., Rothfield L. Leakage of periplasmic enzymes by mutants of Escherichia coli and Salmonella typhimurium: isolation of "periplasmic leaky" mutants. J Bacteriol. 1972 Feb;109(2):520–525. doi: 10.1128/jb.109.2.520-525.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J., Inniss W. E. Electron microscopy of effect of polymyxin on Escherichia coli lipopolysaccharide. J Bacteriol. 1969 Nov;100(2):1128–1129. doi: 10.1128/jb.100.2.1128-1130.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu L. G., Legault-Hetu D. Decreased sensitivity to polymyxin B in colicin K tolerant cells of Escherichia coli K-12 in the presence of colicin K. Can J Microbiol. 1973 Mar;19(3):345–351. doi: 10.1139/m73-057. [DOI] [PubMed] [Google Scholar]
- Matsuzawa H., Matsuhashi M., Oka A., Sugino Y. Genetic and biochemical studies on cell wall peptidoglycan synthesis in Escherichia coli K-12. Biochem Biophys Res Commun. 1969 Aug 15;36(4):682–689. doi: 10.1016/0006-291x(69)90360-x. [DOI] [PubMed] [Google Scholar]
- Meyers E., Parker W. L., Brown W. E., Linnett P., Strominger J. L. EM49: a new polypeptide antibiotic active against cell membranes. Ann N Y Acad Sci. 1974 May 10;235(0):493–501. doi: 10.1111/j.1749-6632.1974.tb43286.x. [DOI] [PubMed] [Google Scholar]
- Monner D. A., Jonsson S., Boman H. G. Ampicillin-resistant mutants of Escherichia coli K-12 with lipopolysaccharide alterations affecting mating ability and susceptibility to sex-specific bacteriophages. J Bacteriol. 1971 Aug;107(2):420–432. doi: 10.1128/jb.107.2.420-432.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Nakamura H. Genetic determination of resistance to acriflavine, phenethyl alcohol, and sodium dodecyl sulfate in Escherichia coli. J Bacteriol. 1968 Oct;96(4):987–996. doi: 10.1128/jb.96.4.987-996.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Suganuma A. Membrane mutation associated with sensitivity to acriflavine in Escherichia coli. J Bacteriol. 1972 Apr;110(1):329–335. doi: 10.1128/jb.110.1.329-335.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Person S., Phillips S., Funk F. A determination of mutagen specificity in bacteria using nonsense mutants of bacteriophage T4. J Mol Biol. 1967 Jun 28;26(3):437–447. doi: 10.1016/0022-2836(67)90314-2. [DOI] [PubMed] [Google Scholar]
- SIMON E. J. INHIBITION OF BACTERIAL GROWTH BY DRUGS OF THE MORPHINE SERIES. Science. 1964 May 1;144(3618):543–544. doi: 10.1126/science.144.3618.543. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. Local anesthetics. I. The blocking potencies of some local anesthetics and of butyl alcohol determined on peripheral nerves. Acta Pharmacol Toxicol (Copenh) 1954;10(3):281–291. doi: 10.1111/j.1600-0773.1954.tb01344.x. [DOI] [PubMed] [Google Scholar]
- Siccardi A. G., Shapiro B. M. On the process of cellular division in Escherichia coli. IV. Altered protein composition and turnover of the membranes of thermosensitive mutants defective in chromosomal replication. J Mol Biol. 1971 Mar 28;56(3):475–490. doi: 10.1016/0022-2836(71)90395-0. [DOI] [PubMed] [Google Scholar]
- Simon E. J., Schapira L., Wurster N. Effect of levorphanol on putrescine transport in Escherichia coli. Mol Pharmacol. 1970 Nov;6(6):577–587. [PubMed] [Google Scholar]
- Singh A. P., Cheng K. J., Costerton J. W., Idziak E. S., Ingram J. M. Sensitivity of normal and mutant strains of Escherichia coli to actinomycin-D. Can J Microbiol. 1972 Jun;18(6):909–915. doi: 10.1139/m72-139. [DOI] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Mechanism of polymyxin B resistance in Proteus mirabilis. J Bacteriol. 1970 Oct;104(1):289–294. doi: 10.1128/jb.104.1.289-294.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Matsuhashi M. Increase in sensitivity to antibiotics and lysozyme on deletion of lipopolysaccharides in Escherichia coli strains. J Bacteriol. 1973 Apr;114(1):453–454. doi: 10.1128/jb.114.1.453-454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling-Häggström B., Normark S. Genetic and physiological analysis of an envB spherelike mutant of Escherichia coli K-12 and characterization of its transductants. J Bacteriol. 1975 Jul;123(1):75–82. doi: 10.1128/jb.123.1.75-82.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster N., Elsbach P., Rand J., Simon E. J. Effects of levorphanol on phospholipid metabolism and composition in Escherichia coli. Biochim Biophys Acta. 1971 Nov 5;248(2):282–292. doi: 10.1016/0005-2760(71)90016-6. [DOI] [PubMed] [Google Scholar]




