Abstract
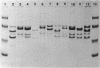
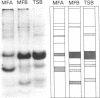
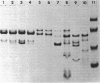
Variation in the protein and lipopolysaccharide composition of the meningococcal outer membrane may be due to either serotype differences or to changes in cultural conditions. There are 12 antigenically distinct serotypes of group B meningococci, and these are associated with distinct major outer membrane protein patterns on sodium dodecyl sulfate-polyacrylamide gels. In most strains the predominant outer membrane protein carries the serotype-specific determinant. Certain strains, when grown under similar conditions in different media showed an altered membrane composition. The type 2 strain, M986, grown in modified Frantz medium-A, had a reduced amount of the major 41,000-dalton protein while a 28,000-dalton protein predominated. The altered protein composition may be related to changes in cell metabolism as reflected by the pH of the medium after growth. Growth of the organism in Frantz medium-B caused a negligible drop in pH and the 41,000-dalton protein remained predominant. There was also variation associated with changes in the growth rate. Increasing the aeration caused a concomitant increase in growth rate and cell yield. We observed two quantitative changes in outer membrane proteins in four of seven strains examined: (i) where only a single major protein changed (three strains), and (ii) where an increase in one protein component was associated with a decrease in another protein (one strain). When the strains were grown in tryptic soy broth (Difco Laboratories, Detroit, Mich.) with either high or low aeration, the total protein in the outer membrane remained constant. In contrast, with high aeration there was a significant increase in lipopolysaccharide. These studies suggest that the cell surface proteins may be altered by the organism to meet a variety of environmental conditions.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Organization of proteins in the native and reformed outer membrane of Escherichia coli. Biochim Biophys Acta. 1972 Aug 9;274(2):478–488. doi: 10.1016/0005-2736(72)90193-9. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Frantz I. D. Growth Requirements of the Meningococcus. J Bacteriol. 1942 Jun;43(6):757–761. doi: 10.1128/jb.43.6.757-761.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Chapman S. S. Classification of Neisseria meningitidis group B into distinct serotypes. I. Serological typing by a microbactericidal method. Infect Immun. 1972 Jan;5(1):98–102. doi: 10.1128/iai.5.1.98-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Chapman S. S. Classification of Neisseria meningitidis group B into distinct serotypes. II. Extraction of type-specific antigens for serotyping by precipitin techniques. Infect Immun. 1972 Aug;6(2):127–133. doi: 10.1128/iai.6.2.127-133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Gotschlich E. C. An outer membrane protein of Neisseria meningitidis group B responsible for serotype specificity. J Exp Med. 1974 Jul 1;140(1):87–104. doi: 10.1084/jem.140.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller I., Henning U. Cell envelope and shape of Escherichia coli K12. Crosslinking with dimethyl imidoesters of the whole cell wall. Proc Natl Acad Sci U S A. 1974 May;71(5):2018–2021. doi: 10.1073/pnas.71.5.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller I., Hoehn B., Henning U. Apparent high degree of asymmetry of protein arrangement in the Escherichia coli outer cell envelope membrane. Biochemistry. 1975 Feb 11;14(3):478–484. doi: 10.1021/bi00674a003. [DOI] [PubMed] [Google Scholar]
- Henning U., Haller I. Mutants of Escherichia coli K12 lacking all 'major' proteins of the outer cell envelope membrane. FEBS Lett. 1975 Jul 15;55(1):161–164. doi: 10.1016/0014-5793(75)80983-5. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Seiler M. W. Immunochemical characterization of the outer membrane complex of Bacteroides fragilis subspecies fragilis. J Infect Dis. 1975 Oct;132(4):440–450. doi: 10.1093/infdis/132.4.440. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McDonald I. J., Adams G. A. Influence of cultural conditions on the lipopolysaccharide composition of Neisseria sicca. J Gen Microbiol. 1971 Feb;65(2):201–207. doi: 10.1099/00221287-65-2-201. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C. EFFECTS OF ENVIRONMENT ON THE COMPOSITION OF BACTERIAL CELLS. Annu Rev Microbiol. 1963;17:61–86. doi: 10.1146/annurev.mi.17.100163.000425. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A., Tempest D. W. Phenotypic variability of the envelope proteins of Klebsiella aerogenes. J Gen Microbiol. 1973 Oct;78(2):361–370. doi: 10.1099/00221287-78-2-361. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Johnson K. G., McDonald I. J. Envelope proteins in Neisseria. Can J Microbiol. 1975 Oct;21(10):1519–1534. doi: 10.1139/m75-224. [DOI] [PubMed] [Google Scholar]
- Sadoff J. C., Artenstein M. S. The outer cell-wall membrane of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S81–S93. doi: 10.1093/infdis/130.supplement.s81. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Kasper D. L., Veltri B. J., Artenstein M. S. Isolation and characterization of a native cell wall complex from Neisseria meningitidis. Infect Immun. 1972 Nov;6(5):835–851. doi: 10.1128/iai.6.5.835-851.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]