Abstract
Reports of beneficial effects of exercise on psychological health in humans are increasingly supported by basic research studies. Exercise is hypothesized to regulate antidepressant-related mechanisms and we therefore characterized the effects of chronic exercise in mouse behavioral paradigms relevant to antidepressant actions. Mice given free access to running wheels showed antidepressant-like behavior in learned helplessness, forced-swim (FST) and tail suspension paradigms. These responses were similar to responses of antidepressant drug-treated animals. When tested under conditions where locomotor activity was not altered, exercising mice also showed reduced anxiety compared to sedentary control mice. In situ hybridization analysis showed that BDNF mRNA was increased in specific subfields of hippocampus after wheel running. We chose one paradigm, the FST, in which to investigate a functional role for brain-derived neurotrophic factor (BDNF) in the behavioral response to exercise. We tested mice heterozygous for a deletion of the BDNF gene in the FST after wheel-running. Exercising wild-type mice showed the expected antidepressant-like behavioral response in the FST but exercise was ineffective in improving FST performance in heterozygous BDNF knockout mice. A possible functional contribution of a BDNF signaling pathway to FST performance in exercising mice was investigated using the specific MEK inhibitor PD184161 to block the MAPK signaling pathway. Subchronic administration of PD184161 to exercising mice blocked the antidepressant-like behavioral response seen in vehicle-treated exercising mice in the FST. In summary, chronic wheel-running exercise in mice results in antidepressant-like behavioral changes that may involve a BDNF related mechanism similar to that hypothesized for antidepressant drug treatment.
Keywords: exercise, antidepressant, mouse, behavior, anxiety, BDNF
1. Introduction
Regular participation in physical exercise confers a variety of protective and beneficial effects on physical health including reduced risk for cardiovascular disease, diabetes and cancer (Tran et al., 1983; Bonen, 1995; O’Connor et al., 1995; Shepard and Shek, 1995; see Penedo and Dahn, 2005). A positive influence of exercise on psychological health has also been convincingly shown in cross-sectional studies and in randomized clinical trials that assessed the efficacy of exercise as a treatment intervention. In these studies, exercise was shown to improve cognitive function and reduce depressive symptomology and clinical depression, effects that are most clearly demonstrated in aged populations (see Churchill et al., 2002; Colcombe and Kramer, 2003; Farmer et al., 1988; Camacho et al., 1991; Babyak et al., 2000; Lampinen et al., 2000; Galper et al., 2006; Strawbridge et al., 2002; Dunn et al., 2005).
The clinical findings of beneficial effects of exercise on cognition and mood are supported by basic research studies. Memory and spatial learning are improved in exercising rodents compared to sedentary controls (Fordyce and Farrar, 1991, van Praag et al., 1999). Chronic exercise was shown to reduce depressive-like behavior in rats exposed to uncontrollable stress and in the Flinders Sensitive Line of “depressed” rats (Greenwood et al., 2003; Bjornebekk, et al., 2005). There is also evidence that exercise can contribute to recovery after injury. Exercise can facilitate recovery of function both in humans and in animal models that include brain and spinal cord injury (Grealy et al., 1999; Griesbach et al., 2004; Engesser-Cesar et al., 2005). Identifying mechanisms responsible for the ability of exercise to maintain and improve neural function is a current, extensive research focus (Cotman and Berchtold, 2002).
We are interested in processes regulated by exercise that might be relevant to antidepressant mechanisms. One candidate hypothesized to mediate neural plasticity in response to both antidepressant drugs and exercise is brain-derived neurotropic factor (BDNF) (Cotman and Berchtold, 2002; Duman and Monteggia, 2006). Up-regulation of hippocampal BDNF is a well-documented result of chronic antidepressant drug treatment that occurs with a time course suggesting relevance to time-dependent adaptive mechanisms (Nibuya et al., 1995; Duman et al., 1997; Duman and Monteggia, 2006). Exercise increases trophic factors in brain and spinal cord and upregulation of BDNF in the hippocampus has been one of the most robust, sustained and consistently demonstrated changes (Neeper et al., 1995, 1996; Cotman and Berchtold, 2002; Molteni et al., 2002; Gomez-Pinilla et al., 2002). These findings along with the well-known role of BDNF in neuronal plasticity have lead to the postulated role of BDNF as central to functional consequences of exercise (Russo-Neustadt et al., 2000; 2001; Cotman and Berchtold, 2002; Molteni et al., 2002). The functional relevance of BDNF to antidepressant-related behavior is supported by studies that have demonstrated antidepressant-like effects of intrahippocampal or intracranial BDNF administration in rodent behavioral models (Suciak et al., 1997; Shiriyama et al., 2002; Hoshaw et al., 2005).
In the present paper we characterized effects of chronic exercise in mice in behavioral paradigms that are sensitive to antidepressant effects and in anxiety tests. The results suggest a beneficial effect of chronic exercise on these behavioral features in mice and add to basic research supporting the clinical association between physical activity and mental health status. This characterization will be useful to the investigation of molecular pathways that underlie antidepressant actions and we demonstrate sensitivity of the effect of exercise in one behavioral paradigm to manipulation of a BDNF signaling pathway.
2. Results
The distance run by male C57/Bl6 mice over a 24 hr period increases during the first week of running wheel access. Mice initially run approximately 5 km over a 24 hr period and increase their 24 hr running distance throughout the first week of running wheel access. Following the first week, mice maintain relatively constant daily running distances between 12 and 14 kilometers per 24 hr (Fig. 1). The body weights of exercising mice were not different from those of sedentary control mice (data not shown).
Figure 1.
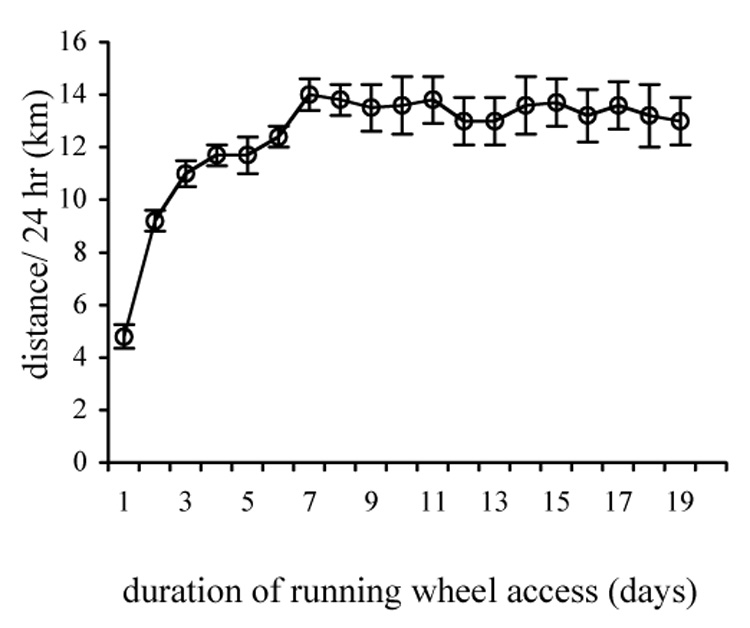
Average distance run per 24 hr over 3 weeks of running-wheel access for a typical group of C57 Bl/6 male mice. Average daily distance run increases over the first week of wheel access and then remains at 12–14 km per 24 h over the remainder of the wheel-access period. n = 8.
Effect of Wheel Running on Performance in Depression Tests
Preliminary studies indicated that LH performance was moderately improved in mice that ran for 7 days but not significantly in mice that ran for 5 days (data not shown). Mice given chronic homecage access to running wheels for 4 weeks showed robust improvements in learned helplessness (LH) performance and we chose 3 and 4 week running durations for further characterization of behavioral effects of chronic exercise. In the LH paradigm, exercising mice had decreased escape latencies and decreased number of escape failures compared to those of sedentary control mice (Fig. 2a). The effect of exercise in LH is similar in direction and magnitude to the effect of chronic administration of the antidepressant drug amitriptylline (Fig. 2a). Acute antidepressant drug effects can be difficult to demonstrate in mouse LH and we used amityiptylline administration in the drinking water which is an effective chronic treatment in the LH paradigm that does not require daily injections (Caldarone et al., 2003).
Figure 2.
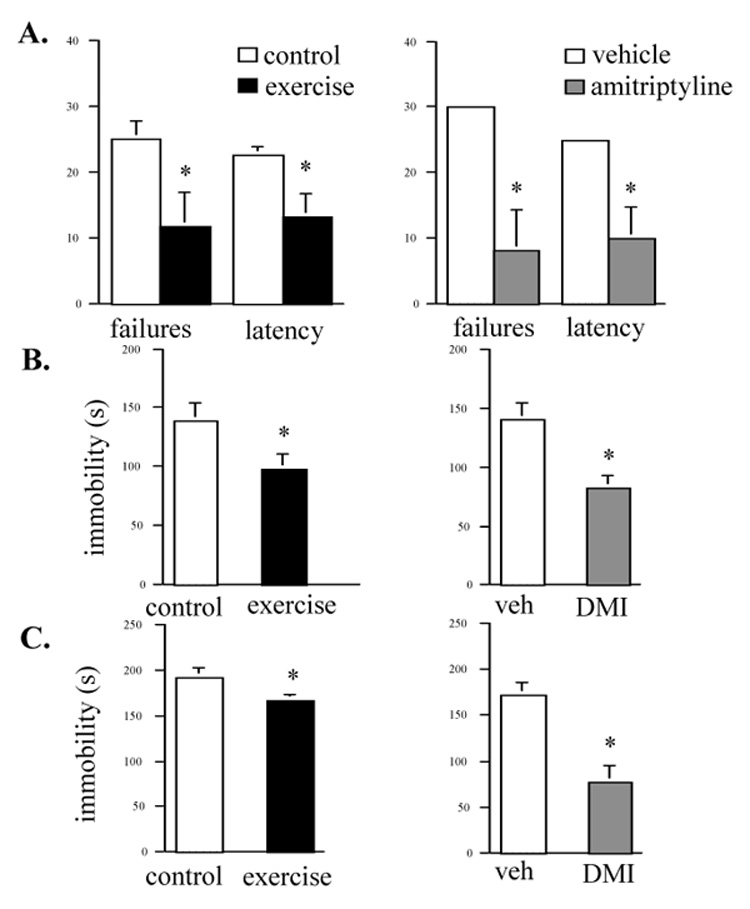
Exercising mice show antidepressant-like behavioral responses in 3 depression tests. A. Learned helplessness was quantified as number of escape failures or escape latencies in an active avoidance test. Escape failures and latencies were decreased in mice given chronic running-wheel access (left panel * p = 0.03, n = 8/group). Mice received inescapable shock training (180 footshocks, 0.3mA, 4 sec duration) 24 hr prior to a 30-trial active avoidance test. The magnitude and direction of this effect is similar to the effect of chronic amitriptylline (200 mg/L in drinking water) (right panel * p −0.03, n = 5/group). B. Forced-swim test immobility is decreased in chronic exercising mice compared with sedentary controls (left panel). * p = 0.05, n = 8/group. This effect is similar to the effect of antidepressant drug desipramine (DMI) (20 mg/kg, i.p.). * p = 0.02, n = 5/group. C. Immobility in the tail suspension test is also decreased in exercising mice (left panel, * p = 0.05, n = 7–8/group). The effect of desipramine (DMI) (10 mg/kg, i.p.) is shown in the right panel for comparison (* p = 0.02, n = 6/group).
Mice were also tested in the forced-swim (FST) and tail suspension (TST) tests, antidepressant drug-responsive behavioral paradigms. Mice given chronic access to running wheels showed antidepressant-like effects in the FST and in TST (Fig. 2b, 2c). The effects of exercise in these tests were also similar to antidepressant drug effects although the magnitude of the exercise effect was smaller in the TST (Fig. 2b, 2c). Desipramine is a tricyclic antidepressant drug which, like amityiptylline, blocks the reuptake of both serotonin and norepinephrine and desipramine. Desipramine is known to produce a reliable response in FST and TST as previously reported (Duman et al., 2007). One-week of running-wheel access did not alter immobility in the FST or TST tests (data not shown).
We assessed the likelihood of generally altered activity levels contributing to the improved performance of exercising mice in the depression tests. Surprisingly, we found that chronic exercising mice showed decreased levels of locomotor activity compared to the activity levels of control mice (Fig. 3a). Locomotor activity was tested during the first part of the light cycle, soon after the dark cycle running period, which is also when the depression-related behavioral testing was done. It is possible that fatigue following a running period could contribute to the reduced locomotor activity in exercising mice at this time. In support of this, we found that the locomotor activity of exercising mice was the same as that of sedentary control mice when the activity was tested 24 hr after the last wheel running (Fig. 3b). Importantly, at the time point when the depression-related behavioral testing was done, locomotor activity is decreased in exercising mice, indicating that the altered immobility of exercising mice in these models of depression is not an artifact of generally increased locomotor activity. Antidepressant-like behavioral responses are also observed when mice are tested 24 hr after last wheel access (data not shown), a time point when there are no locomotor activity differences between groups (Fig. 3b).
Figure 3.
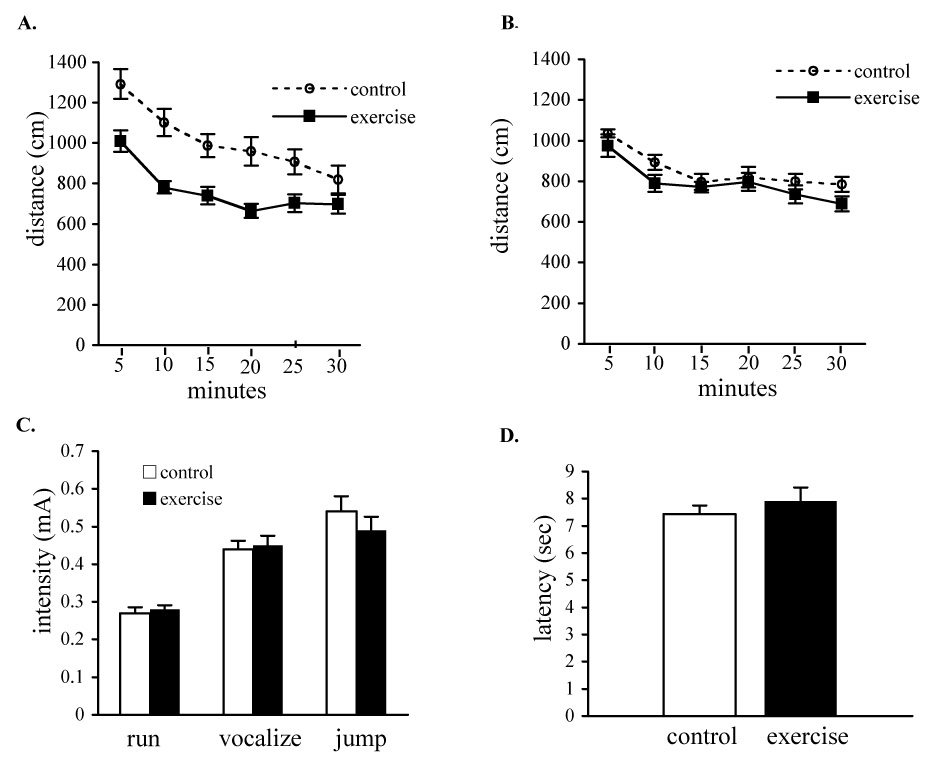
Locomotor activity is not up-regulated in exercising mice. A. Activity levels in a locomotor activity test are decreased in exercising mice (F 1,30= 17.02, p = 0.0003). Mice were placed in standard homecages and locomotor activity was quantified by video tracking for 30 minutes. B. Locomotor activity is not significantly changed in exercising mice relative to controls when the locomotor activity test is done 24 h after the last wheel running period (F1,13=1.96, p = 0.18). Panels A and B represent separate experiments on independent sets of mice. C. Footshock sensitivity was not significantly altered in exercising mice. Sensitivity to footshock was determined by measuring the lowest footshock that elicited incremental behavioral responses (flinch, run, vocalize). (F 1,34= 0.66, p = 0.42). D. Thermal pain sensitivity was not altered in exercising mice. Mice were individually placed on a 52°C hotplate and the latency to lift a paw in response to the heat was recorded for each animal.
We also assessed pain sensitivity in a footshock response test since altered pain sensitivity could contribute to altered responding in the learned helplessness paradigm. We therefore tested for behavioral responding to a series of footshocks and assessed pain sensitivity in a hotplate test. Exercising mice did not differ from controls in thresholds for behavioral responses to footshock or in thermal sensitivity (Fig. 3c,d) indicating that the differential responding of exercising mice in learned helplessness is not a consequence of altered stimulus sensitivity.
Effect of Wheel Running on Performance in Anxiety Tests
Exercising mice were tested for measures of anxiety in the elevated plus-maze (EPM) and the open field. In the EPM, chronic exercising mice spent greater amounts of time in the light arms and had a greater percentage of light arm entries indicating reduced anxiety compared with control mice (Fig. 4a). Total arm entries were decreased in the exercising mice consistent with the reduced activity of chronic exercising mice seen in the locomotor test. Exercising mice also showed increased time and entries in the light arms when they were tested in the EPM 24 hr after last wheel access, however these mice showed increased activity in the maze as indicated by increased total arm entries compared with control mice (Fig. 4b). Thus exercising mice showed decreased anxiety in the EPM regardless of the direction of locomotor activity changes. In the open field test, center time and center distance were decreased in exercising mice relative to controls (Fig. 4c), suggesting an increase in anxiety. However the total locomotor distance in the open field was significantly decreased in exercising mice making interpretation of the results of this test less clear (Fig. 4c). An open field test was also conducted 24 hr after last wheel access in separate groups of mice in order to assess anxiety parameters in groups of mice that do not differ in locomotor activity. In this experiment, the center time and center distance were significantly increased in exercising mice compared with controls and the overall locomotor distance traveled in the open field was not different between groups (Fig. 4d). Increased center time and center distance indicate decreased anxiety in the open field.
Figure 4.
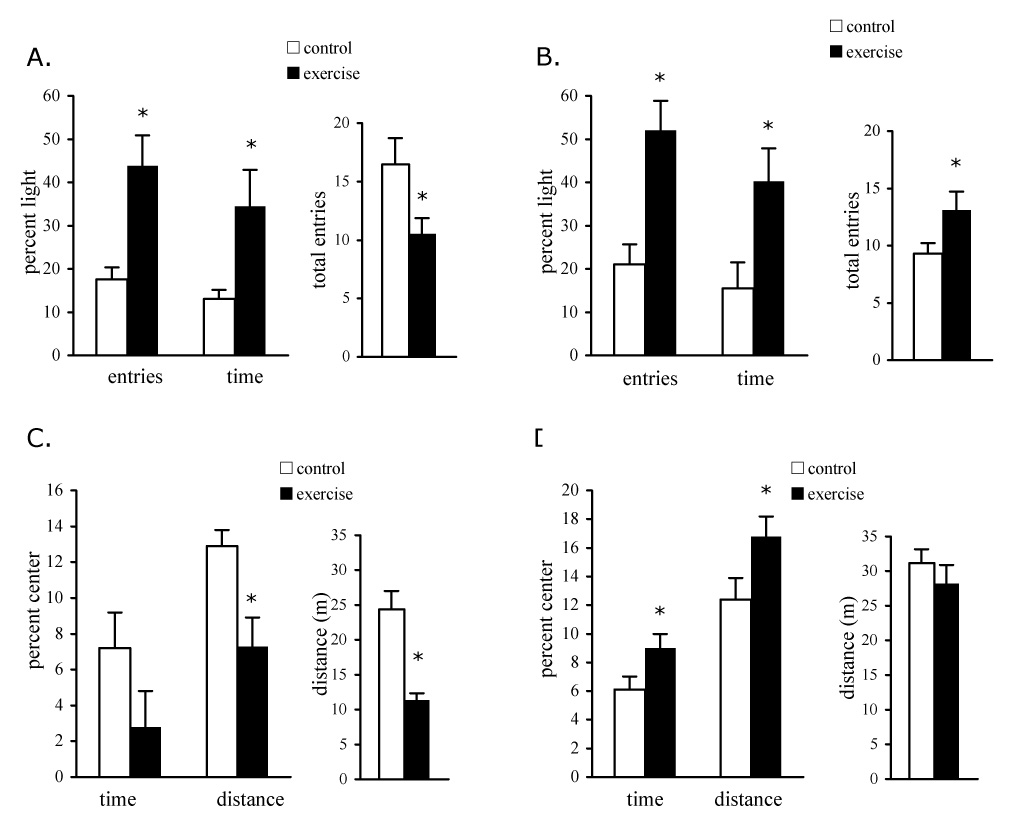
Exercising mice show decreased anxiety in the elevated plus-maze and in the open field, dependent on time of testing. A. Mice freely explored the elevated plus-maze for 5 minutes in dim lighting (40 lux). The percentage of open arm entries and the time spent in the open arms were greater in exercising mice compared to sedentary controls. Total arm entries in the maze were decreased. * p ≤ 0.03, n = 15/group. B. The percentage of open arm entries and the time spent in the open arms were greater in exercising mice compared to sedentary controls when testing occurred 24 hr after last wheel access. Total arm entries in the maze were increased. * p ≤ 0.05, n = 12/group. C. Mice were placed in the center of a brightly lit (500 lux) open field and allowed to freely explore for 5 minutes. The time spent in the center of the open field and the distance traveled in the center relative to the peripheral zones of the open field were decreased in exercising mice compared to sedentary control mice. * p ≤ 0.04, n = 8/group. D. The time spent in the center of the open field and the distance traveled in the center relative to the peripheral zones of the open field were increased in exercising mice compared to sedentary control mice when testing occurred 24 hr after last wheel access. * p ≤ 0.05, n = 8/group.
BDNF and MAPK Signaling in Wheel-Running Mice
Up-regulation of BDNF in hippocampus has been demonstrated in rats after exercise and induction of BDNF has been postulated to contribute to behavioral effects of exercise in rats (Neeper et al., 1995, 1996; Cotman and Berchtold, 2002; Russo-Neustadt et al., 2001). We investigated the regulation of BDNF mRNA by exercise within the hippocampus of mice. In situ hybridization analysis revealed that BDNF mRNA was increased in hippocampal area CA1 after 3 weeks of wheel-running exercise (p = 0.02) and was increased in the dentate gyrus after 1 or 3 weeks of exercise (p ≤ 0.01) (Fig. 5A). BDNF expression was not altered in area CA3 or in frontal cortex after exercise (Fig. 5A). Exercise has been shown to regulate the expression and phosphorylation of MAPK I and II in hippocampus (Shen et al., 2001; Molteni et al., 2002). We have observed that levels of phosphorylated MAPK kinase (pMEK), as well as levels of phosphorylated ERK I and II were increased in hippocampus of exercising mice (not shown).
Figure 5.
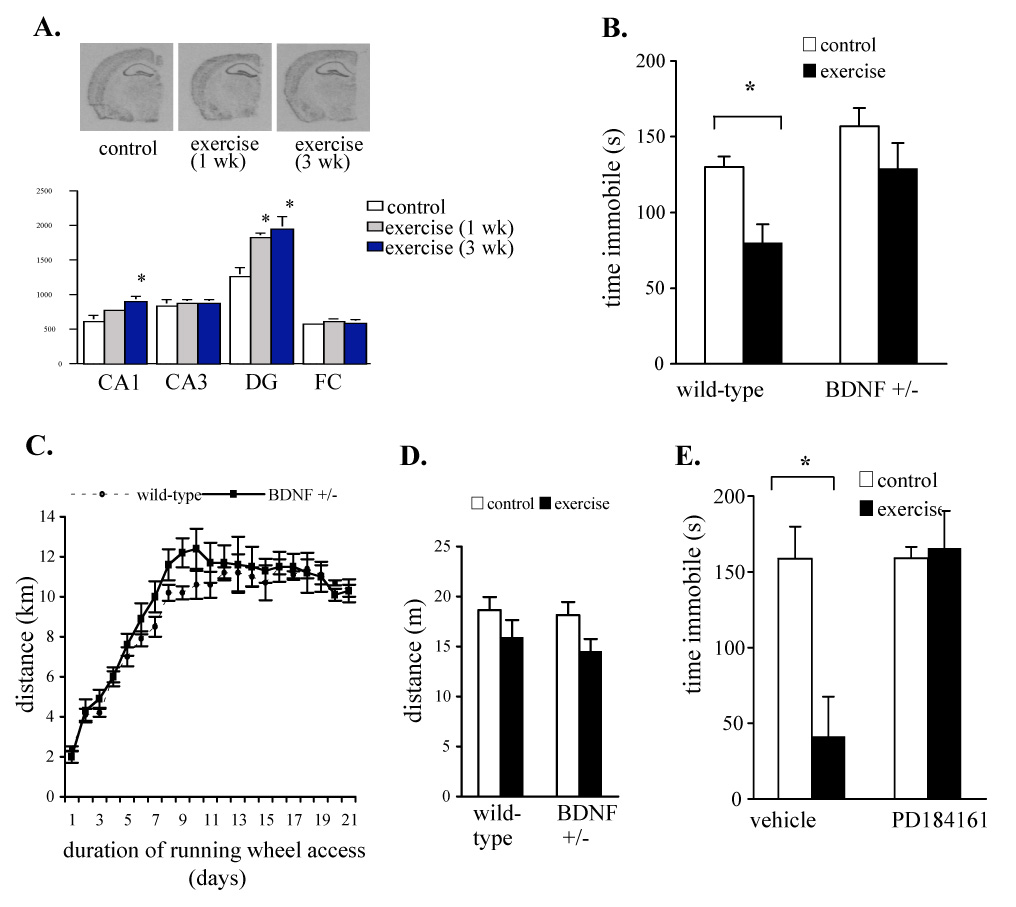
Chronic exercise upregulates BDNF in hippocampus and decreasing BDNF or blocking MAPK signaling blocks the antidepressant-like effect of exercise in the FST. A. Influence of wheel-running on BDNF mRNA expression in the hippocampus. Levels of BDNF mRNA in area CA1 after 3 wks of wheel running (* p = 0.02) and in dentate gyrus (dg) after 1 or 3 weeks of wheel running (* p < 0.01) were significantly increased as shown by in situ hybridization. BDNF mRNA was not altered in CA3 or in frontal cortex. n = 4–5 per group. B. Heterozygous BDNF deletion mice (BDNF +/−) do not show an antidepressant-like behavioral response to exercise in the FST. BDNF +/− mice and wild-type littermate controls (BDNF +/+) were given homecage running-wheel access for 3 weeks and were compared with sedentary mice of both genotypes in the FST. Immobility was decreased in exercising wild-type BDNF +/+ mice compared with sedentary wild-type counterparts indicating an antidepressant-like effect of exercise in these mice. Exercise did not significantly alter immobility in BDNF +/− mice compared to sedentary BDNF +/− mice. Two-way ANOVA: significant effect of genotype (F1,53 = 9.36, p = 0.004) and treatment (F1,53 = 9.33, p = 0.04), no significant genotype × treatment interaction (F1,53 = 0.72, p = 0.40). Post-hoc pairwise comparison: wild-type sedentary vs. wild-type exercise (* p = 0.008); BDNF +/− sedentary vs. BDNF +/− exercise (p = 0.12). The trend towards an increase in immobility in the sedentary BDNF +/− vs. wild-type mice was not significant (p = 0.12). n = 14–15 per group. C. Average distance run per 24 hr over the last 2 weeks of running-wheel access is not different for BDNF +/− and wild-type mice. Repeated measures ANOVA, no effect of group (F1,130 = 0.70 , p =0.42). BDNF +/− mice briefly had higher running distances than wild-type mice at the end of the acquisition period (days 8–10). Repeated measures ANOVA, no effect of group (F1,126=2.24, p = 0.15), distance × group interaction (F1,126 = 2.62, p=0.02), n = 8/group. D. Locomotor activity in control and exercising mice is not altered by genotype. Mice were placed in standard homecages and locomotor activity was quantified by video tracking and totaled over 10 minutes. Two-way ANOVA indicated a significant effect of exercise (F 1,28= 4.99, p = 0.03) and no significant effect of genotype (F 1,28= 0.45, p = 0.51) or exercise × genotype interaction (F 1,28= 0.11, p = 0.74). E. The antidepressant-like behavioral response of exercising mice in the forced-swim test is blocked by treatment with PD184161. PD184161 was administered subchronically (30 mg/kg, i.p) to exercising and sedentary control mice after a day-1 swim test at 23.5 and 5 hr before a day-2 FST. This administration did not alter the behavior of the control mice in the FST but it prevented the anti-immobility effect of exercise (main effect F3,12 = 8.08, p = 0.003). Post-hoc pair-wise comparisons indicated that the immobility of PD184161-treated exercising mice was significantly greater than that of vehicle-treated exercising mice (p = 0.001) and did not differ from that of control mice.
In order to investigate a possible role for BDNF in the behavioral effect of exercise in the FST, we used mice heterozygous for a deletion of the BDNF gene (BDNF +/−). BDNF +/− mice have half the normal complement of BDNF, and in contrast to the homozygous knockout mice have a normal lifespan and no overt deficits (Conover et al., 1995; Enfors et al., 1994). Behavioral features of the BDNF +/− mice include aggression and hyperphagia and they do not typically show a behavioral deficit in the FST in the absence of a challenge (Lyons et al., 1999; Duman et al., 2007). We assessed the behavior of BDNF +/− mice and wild-type controls in the FST after 3 weeks of homecage running wheel access. Chronic exercise decreased the immobility of wild-type mice in the FST indicating an antidepressant-like effect compared with sedentary wild-type mice (Fig. 5b). However, the immobility of exercising BDNF +/− mice did not differ significantly from their sedentary BDNF +/− counterparts (Fig. 5b). This suggests that exercise cannot effectively produce antidepressant-like behavior in the FST in BDNF-deficient mice. This cannot be explained by less wheel-running in BDNF +/− mice. Comparison of wheel-running distances indicated that BDNF +/− mice were not significantly different from wild-type control mice over most of the 3-week exercise period, with the exception of a brief period when BDNF +/− mice ran more than wild-type mice (Fig. 5c). We assessed general locomotor activity levels in exercising and control BDNF+/− and wild-type mice. Chronic exercising mice of both genotypes showed reduced locomotor activity compared to the sedentary counterparts and the activity of the two genotypes did not significantly differ for sedentary or for exercising groups (Fig. 5d). Thus there is no selective locomotor effect in BDNF +/− mice that can account for their lack of response to exercise compared with wild-type mice.
In order to investigate a possible contribution of a BDNF-regulated signaling pathway to the behavioral effect of exercise in the FST, we used an inhibitor of MAPK kinase (MEK) to block the phosphorylation and activation of MAP kinase. Pharmacologic inhibitors of MEK have been used to assess roles for MAPK signaling in synaptic plasticity and function in a variety of systems (Atkins et al., 1998; Sharma et al., 2003). In this study we used PD184161 which is a potent and specific MEK inhibitor that can influence behavior following systemic administration (Davies et al., 2000; Yung et al., 2004; Duman et al., 2007). We administered PD184161 subchronically (30 mg/kg, i.p) to exercising and sedentary control mice after a day-1 swim test at 23.5 and 5 hr before a second day swim test. This administration of PD184161 does not alter locomotor activity (Duman et al., 2007). PD184161 administration did not alter the behavior of the control mice in the FST but it prevented the anti-immobility effect that is normally seen in exercising mice in the FST (main effect F3,12 = 8.08, p = 0.003) (Fig. 5e). Post-hoc pair-wise comparisons indicated that the immobility of PD184161-treated exercising mice was significantly greater than that of vehicle-treated exercising mice (p = 0.001) and did not differ from that of sedentary control mice. This result indicates that blocking the phosphorylation of MAPK prevents the antidepressant-like effect of exercise from being expressed in the FST.
3. Discussion
We have shown that chronic exercise in mice results in antidepressant-like effects in 3 behavioral tests for antidepressant-responsiveness and can reduce anxiety-related behavior. These behavioral responses of exercising mice are similar to responses of antidepressant drug-treated animals, suggesting that exercise may promote mechanisms that overlap with those of antidepressant drugs. Upregulation of BDNF in response to exercise occurred in specific subfields of hippocampus in response to exercise. Our findings that exercise was ineffective in altering FST behavior in heterozygous BDNF knockout mice and that blocking MEK signaling blocked the effect of exercise in the FST suggest a functional role for BDNF and the MAPK signaling pathway in the behavioral consequences of exercise.
Exercising mice showed antidepressant-like behavior in the LH, FST and TST paradigms. The effects of exercise were similar to the effects of antidepressant drugs in these tests. Responding in each of these tests includes a motor activity component and it is conceivable that upregulation of basal/general activity levels in chronic exercising mice could have contributed to antidepressant-like profiles. We found that the antidepressant-like effects of exercise are not artifacts of generally upregulated locomotor activity. In fact, the antidepressant-like effect of exercise is apparent when the locomotor activity of mice is reduced relative to sedentary controls. Additionally, we ruled out a possible influence of altered pain sensitivity in contributing to the LH responses of exercising mice. This antidepressant-like effect of exercise in mice is consistent with studies where wheel-running was shown to prevent or reduce escape deficits in learned helplessness models in rats (Dishman et al., 1997; Greenwood et al., 2003; 2005; Bjornebekk et al., 2005) and with clinical studies showing that exercise is beneficial to depressed patients (Dunn et al., 2005; Galper et al., 2006).
Chronic exercise also altered anxiety-related measures in two behavioral paradigms in mice. Exercising mice showed behavioral features of decreased anxiety in the EPM. This effect occurred independent of activity changes related to testing time. We found that the direction of behavioral changes in exercising mice in the open field was related to overall activity and testing time. Decreases in center parameters and decreased overall activity occurred when testing was conducted after nightly wheel-running. By contrast, center parameters were significantly increased when testing occurred 24 hr after wheel access. There were no activity differences between exercising and control mice at this time. The literature reports of the effects of exercise in anxiety tests in rats are mixed, but are in agreement with our results suggesting that the direction of changes after chronic exercise, particularly in the open field tests are associated with overall activity and testing time. Increased anxiety parameters in the open field are reported in chronic wheel-running rats which also show decreased overall open field activity, while increased open field locomotion and decreased anxiety measures after wheel-running are reported in exercising animals tested 24hr after wheel use (Hoffmann et al., 1987; Dishman et al., 1996; Burghardt et al., 2004). Reduced locomotor activity may confound interpretation of behavior in anxiety tests. Data from animals without locomotor depression in the open field (related to testing time) demonstrate decreased anxiety in exercise groups and are consistent with clinical studies that indicate beneficial effects of exercise for anxiety (Steptoe et al., 1989; Broocks et al., 1998).
The neurotrophic hypothesis of depression postulates that up-regulation of neurotrophins is important to behavioral effects of antidepressant drugs. Neurotrophins have also been suggested as candidates for mediating plasticity related changes in response to chronic exercise (Cotman and Berchtold, 2002; Duman and Monteggia, 2006). Upregulation of hippocampal BDNF as a result of chronic exercise in rats has been hypothesized to be related to behavioral improvement, including antidepressant effects of exercise (Cotman and Berchtold, 2002; Russo-Neustadt et al., 2000; 2001), although this has not been directly tested. In the present study, we found that BDNF mRNA was increased in mouse hippocampus after 1 and 3 weeks of wheel running. The increase was most pronounced in the dentate gyrus granule cell layer of the hippocampus BDNF mRNA in hippocampus has been shown to increase as early as after 2 days of running in rats or after 6 hr running in rats that were previously trained to run (Neeper et al., 1995, 1996; Oliff et al., 1998; Garza et al., 2004). Behavioral improvements have not been demonstrated at these time points after exercise although to our knowledge this has not been rigorously examined.
Exogenous administration of BDNF into hippocampus or midbrain has been shown to improve behavioral outcome in rodent depression models (Suciak et al., 1997; Shiriyama et al., 2002; Hoshaw et al., 2005). This may not be the same for all paradigms as suggested by Greenwood et al., (2007) who showed that behavioral response was independent of hippocampal BDNF levels in their paradigm of learned helplessness where testing occurred in a novel environment. That study did however, show that wheel running protected against suppression of BDNF due to stress and suggested that this could counteract other behavioral consequences of reduced BDNF. We found the effects of wheel-running exercise on depression-related behavior, while observed as early as 1 week, are more robust after 3 or 4 weeks of exercise. Exercise (4 and 6 weeks) has been shown to reduce depressive-like behavior in rats (Greenwood et al., 2003; Bjornebekk, et al., 2005). Hippocampal BDNF is upregulated at these time points when behavioral improvements are observed for rats (Widenfalk et al., 1999; Adlard et al., 2004) and for mice (Adlard and Cotman, 2004; Adlard et al., 2005; present study). Our findings suggest that BDNF-related mechanisms could play a role in behavioral effects of exercise in mice.
In order to test the hypothesis that exercise-induced activation of BDNF and related signaling pathways influences depression-related behavior, we assessed performance of BDNF mutant mice in the FST. We found that exercise was not effective in significantly reducing FST immobility in BDNF-deficient mice. Different levels of exercise participation are not a factor in this behavioral difference, as the distances run by wild-type controls and BDNF +/− mice were similar over the chronic exercise period. Also, there were no differences in locomotor activity that could account for the behavioral effect. The activity of the sedentary mice did not differ by genotype and activity was similarly decreased in exercising groups for both genotypes. These results suggest that BDNF plays a role in determining FST performance after chronic exercise. It is possible that BDNF upregulation as a result of exercise leads to increased expression of the neurotrophic factor regulated gene, VGF (nonacronymic), which we have recently shown to be involved in the actions of BDNF and exercise (Hunsberger et al., 2007).
In order to investigate a possible functional contribution of a BDNF signaling pathway to antidepressant-related behavioral consequences of exercise, we used the specific MEK inhibitor PD184161 to block the MAPK signaling pathway (Davies et al., 2000; Yung et al., 2004; Duman et al., 2007). We selected the FST as the most suitable paradigm for this study based on dosing requirements. Long-term treatment with PD184161 is not recommended due to its relative insolubility/vehicle requirements and acute PD184161 treatment alone produces antidepressant-like behavioral effects (Duman et al., 2007). A two-day FST is a commonly used paradigm in which to test subchronic drug administration. Our prior study indicated that PD184161 is tolerated when given in a modified subchronic regimen and produces effects that are not expected to confound the interpretation of responses in exercising mice. In the present study, we found that subchronic administration of PD184161 in exercising mice blocked the antidepressant-like behavioral response seen in vehicle-treated exercising mice in the FST. This suggests that MAPK signaling mediates this behavioral response in exercising mice. Phosphorylation and expression of MAPK in hippocampus are increased in exercising animals (Shen et al., 2001; Molteni et al., 2002; Russell and Duman, unpublished observation) and MEK phosphorylation also appears to be increased (Russell and Duman, unpublished observation) suggesting upregulation of the MAPK signaling pathway by exercise. This is also consistent with our recent report that exercise increases the BDNF-MAPK signaling pathway, determined by analysis of gene expression profiling (Hunsberger et al., 2007). We have previously reported evidence for a similar requirement for intracellular signaling through MEK for antidepressant drug response (Duman et al., 2007). However, consistent regulation of components of the MAPK pathway by antidepressant drugs remains to be shown.
In summary, chronic wheel-running exercise in mice results in antidepressant-like behavioral changes that may involve a BDNF-related mechanism similar to that hypothesized for antidepressant drug treatment. Our behavioral findings will be useful to the investigation of molecular pathways that underlie antidepressant behavioral responses and further support the importance of neurotrophic mechanisms in treatments that induce neural and behavioral plasticity.
4. Experimental Procedure
Animals
Male C57Bl/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME) and were singly housed in standard mouse cages (Nalgene) with ad libitum access to food and water. Mice were obtained at 8–10 weeks of age and allowed a minimum of 1 week to acclimate before use in experiments. BDNF heterozygous knockout mice were obtained from Jackson Laboratories (Bar Harbor, ME) and bred with C57Bl/6 mice at the Yale facility in order to generate BDNF heterozygous knockout offspring and wild-type littermate controls. Mice were maintained on a 12-hour light-dark cycle with lights on at 7 am. The cages of exercising mice were equipped with running wheels (34.5 cm diameter) attached to mechanical counters. The counters were connected to a CLOCKLAB data collection system (Actimetrics, Evanston, IL) and wheel-running activity was recorded continuously throughout the experiments. For chronic exercise experiments, mice were given continual wheel access for 3–4 weeks up until the time of behavioral testing or tissue collection for biochemical analysis, except 24 hr wheel removal was used before selected anxiety or locomotor testing as indicated. Animal maintenance and use procedures were in accordance with the NIH Guide for Care and Use of Laboratory Animals and were approved by the Yale University Animal Care and Use Committee.
Drugs
Amitriptylline and desipramine were obtained from Sigma Chemical Co. (St. Louis, MO), and dissolved in saline. PD184161 (gift from Pfizer, Groton, CT) was dissolved in DMSO.
Behavioral tests
Learned Helplessness
Learned helplessness experiments were performed according to standard published procedures with minor modifications (Caldarone et al., 2003; Duman et al., 2007). Inescapable shock training and active avoidance testing were administered in shuttle boxes (Gemini II Avoidance System, San Deigo Instruments, San Deigo, CA). Scrambled shocks were delivered by an internal shock source to a grid floor of stainless steel bars.
Animals were given a single session of inescapable shock exposure (IES) (180 scrambled footshocks, 0.3 mA shock amplitude, 4 sec duration, 30 sec average interval) during which they were confined to one side of the shuttle box. Control animals were exposed to the box for the same period of time without receiving shocks. Shuttle escape performance was tested 24 hr after IES. Mice were given 30 shuttle escape trials (0.3 mA footshocks, 25 sec maximum duration, average interval of 30 sec). The shuttle door opened at the beginning of the shock and each trial was terminated when the mouse crossed into the adjacent ‘nonshock’ compartment, or when a 25 shock duration was reached. Latency to escape to the nonshock side of the shuttle box and escape failure number were recorded by the avoidance system software.
Forced Swim
The forced swim test was performed according to standard published procedures with minor modifications (Caldarone et al., 2003; Duman et al., 2007). Mice were placed in a glass cylinder (12 cm diameter) filled to a depth of 10 cm with water (23–25°C). A 6-min swim test session was videotaped and the time spent immobile during the last 4 min was recorded by an observer. Immobility is defined as a lack of movement but includes the presence of movements that are necessary to keep the head above water. A 2-day swim test procedure was used for the MEK blockade experiment and to test the BDNF mutant mice. An initial 10-min swim session was conducted on day 1 followed by a 6-min swim test 24 hrs later. Immobility was scored during the last 4 minutes of the test. PD184161 (30 mg/kg) or vehicle (DMSO) were administered (i.p.) at 23.5 and 5 hours before the second swim test (Duman et al., 2007).
Tail Suspension
The tail suspension procedure was performed according to the method of Steru et al., (1985). Mice were suspended by adhesive tape placed approximately 1 cm from the tip of the tail. The tail was taped to a piece of suspended tubing so that the mouse was suspended 50 cm from the benchtop. Immobility time (defined as lack of all movement except for whisker movement and respiration) was recorded for 6 min.
Elevated Plus Maze
The elevated plus-maze method follows published procedures and was used with minor modifications (Lister, 1987). Mice were placed in the center of the maze (30 cm above floor, four 30 × 5 cm arms two of which are enclosed by dark walls) facing an open arm. They were subsequently allowed to explore the maze for 5 min and were scored (number of arm entries and time spent on the arms) by an observer blind to genotype and treatment history.
Open Field
Animals were placed in the center of a brightly lit (650 lux) white Plexiglas box (50 × 50 cm) and videotaped with a Noldus EthovisionPro Video Tracking System in the absence of an observer for 5 min. Distance traveled and percentage of time spent in the central vs. peripheral zones of the field were calculated by Ethovision behavioral analysis software.
Pain sensitivity
Mice were tested for thermal sensitivity in a hotplate test. A hotplate (IITC, Inc.) (29 × 27 cm) within a clear Plexiglas enclosure was kept at 52°C and the latency to lift or lick a paw was recorded. For the assessment of footshock sensitivity thresholds, individual animals were exposed to scrambled footshocks of 1 sec duration and increasing intensities (0.1 mA increments). The shocks were spaced 30 sec apart and the occurrence of behavioral responses (flinch, run, vocalize and jump) at each shock intensity were recorded.
Locomotor activity
Locomotor activity was measured by video tracking (Ethovision Pro, Noldus Inc.) in standard mouse home cages. Data were analyzed using Ethovision behavioral analysis software.
In Situ hybridization
In situ hybridization for BDNF mRNA was carried out as described previously (Nibuya et al., 1995). Cryostat cut coronal sections (14 µm thickness) were fixed in 4% formaldehyde, acetylated and dried. Slides were incubated with 35S-labeled antisense riboprobes against the coding exon V (rat BDNF cDNA was obtained from Regeneron, Tarrytown, NY). After hybridization, sections were washed, dried and exposed to BioMax Film (Kodak, Rochester, NY). Levels of BDNF mRNA were analyzed using the Macintosh-based National Institutes of Health Image Analyzer program, version 1.57 (Bethesda, Maryland). Hippocampal subregions were analyzed by outlining the area of interest; an equivalent area was outlined for each sample. For each animal, the optical density measurements from both sides of four individual sections were analyzed, yielding eight measurements, from which the mean was calculated. To correct for nonlinearity, 14C step standards were used for calibration. The results were then subjected to statistical analysis, using between-subjects analysis of variance (ANOVA) followed by the post-hoc Newman-Keuls test.
Statistics
For analysis of two groups, data were subjected to Students t-test. For analysis of genotype and treatment effects in the FST data were analyzed by two-factor ANOVA. Fischer’s protected least significant difference was used for post hoc pairwise comparisons of group means. Locomotor activity data were analyzed by ANOVA with repeated measures. In all cases comparisons were considered significant for p < 0.05.
Acknowledgements
This project was supported by MH25642, MH45481, the Connecticut Mental Health Center, and the Veterans Affairs PTSD Program, West Haven, CT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124(4):9. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363(1):43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. 2005. Neurobiol Aging. 2005;26(4):511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1(7):602–607. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Bonen A. Benefits of exercise for type II diabetics: convergence of epidemiologic, physiologic, and molecular evidence. Can J Appl Physiol. 1995;20(3):261–279. doi: 10.1139/h95-020. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int J Neuropsychopharmacol. 2005;8(3):357–368. doi: 10.1017/S1461145705005122. [DOI] [PubMed] [Google Scholar]
- Broocks A, Bandelow B, Pekrun G, George A, Meyer T, Bartmann U, Hillmer-Vogel U, Rüther E. Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. Am J Psychiatry. 1998;155:603–609. doi: 10.1176/ajp.155.5.603. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019(1–2):84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Karthigeyan K, Harrist A, Hunsberger JG, Wittmack E, King SL, Jatlow P, Picciotto MR. Sex differences in response to oral amitriptyline in three animal models of depression in C57BL/6J mice. Psychopharmacology (Berl) 2003;170(1):94–101. doi: 10.1007/s00213-003-1518-7. [DOI] [PubMed] [Google Scholar]
- Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol. 1991;134(2):220–231. doi: 10.1093/oxfordjournals.aje.a116074. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenbough WT. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23:941–955. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P, et al. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375(6528):235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, Dunn AL, Youngstedt SD, Davis JM, Burgess ML, Wilson SP, Wilson MA. Increased open field locomotion and decreased striatal GABAA binding after activity wheel running. Physiol Behav. 1996;60(3):699–705. doi: 10.1016/0031-9384(96)00102-3. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN, Burke KA, Yoo HS, Mougey EH, Meyerhoff JL. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Res Bull. 1997;42(5):399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiat. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Enfors P, Lee K-F, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar c, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma. 2005;(1):157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- Farmer ME, Locke BZ, Moscicki EK, Dannenberg AL, Larson DB, Radloff LS. Physical activity and depressive symptoms: the NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1988;128(6):1340–1351. doi: 10.1093/oxfordjournals.aje.a115087. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Farrar RP. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav Brain Res. 1991;46(2):123–133. doi: 10.1016/s0166-4328(05)80105-6. [DOI] [PubMed] [Google Scholar]
- Galper DI, Trivedi MH, Barlow CE, Dunn AL, Kampert JB. Inverse association between physical inactivity and mental health in men and women. Med Sci Sports Exerc. 2006;38(1):173–178. doi: 10.1249/01.mss.0000180883.32116.28. [DOI] [PubMed] [Google Scholar]
- Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA. Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacol Biochem Behav. 2004;77(2):209–220. doi: 10.1016/j.pbb.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88(5):2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Grealy MA, Johnson DA, Rushton SK. Improving cognitive function after brain injury: the use of exercise and virtual reality. Arch Phys Med Rehabil. 1999;80(6):661–667. doi: 10.1016/s0003-9993(99)90169-7. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23(7):2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005;1033(2):164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Thompson RS, Fleshner M. Learned helplessness is independent of levels of brain-derived neurotrophic factor in the hippocampus. Neuroscience. 2007;144(4):1193–1208. doi: 10.1016/j.neuroscience.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125(1):129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Thorén P, Ely D. Effect of voluntary exercise on open-field behavior and on aggression in the spontaneously hypertensive rat (SHR) Behav Neural Biol. 1987;47(3):346–355. doi: 10.1016/s0163-1047(87)90461-4. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037(1–2):204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, Duman RS. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007. 2007;13(12):1476–1482. doi: 10.1038/nm1669. [DOI] [PubMed] [Google Scholar]
- Lampinen P, Heikkinen RL, Ruoppila I. Changes in intensity of physical exercise as predictors of depressive symptoms among older adults: an eight-year follow-up. Prev Med. 2000;30(5):371–380. doi: 10.1006/pmed.2000.0641. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92(2):180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96(26):15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16(6):1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor GT, Hennekens CH, Willett WC, Goldhaber SZ, Paffenbarger RS, Jr., Breslow JL, Lee IM, Buring JE. Physical exercise and reduced risk of nonfatal myocardial infarction. Am J Epidemiol. 1995;142(11):1147–1156. doi: 10.1093/oxfordjournals.aje.a117573. [DOI] [PubMed] [Google Scholar]
- Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61(1–2):147–153. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18(2):189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21(5):679–682. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101(2):305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res. 2001;120(1):87–95. doi: 10.1016/s0166-4328(00)00364-8. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Sherff CM, Shobe J, Bagnall MW, Sutton MA, Carew TJ. Differential role of mitogen-activated protein kinase in three distinct phases of memory for sensitization in Aplysia. J Neurosci. 2003;23(9):3899–3907. doi: 10.1523/JNEUROSCI.23-09-03899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Tong L, Balazs R, Cotman CW. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107(2):219–229. doi: 10.1016/s0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]
- Shephard RJ, Shek PN. Cancer, immune function, and physical activity. Can J Appl Physiol. 1995;20(1):1–25. doi: 10.1139/h95-001. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56(1):131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Strawbridge WJ, Deleger S, Roberts RE, Kaplan GA. Physical activity reduces the risk of subsequent depression for older adults. Am J Epidemiol. 2002;156(4):328–334. doi: 10.1093/aje/kwf047. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Edwards S, Moses J, Mathews A. The effects of exercise training on mood and perceived coping ability in anxious adults from the general population. J Psychosom Res. 1989;33(5):537–547. doi: 10.1016/0022-3999(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Tran ZV, Weltman A, Glass GV, Mood DP. The effects of exercise on blood lipids and lipoproteins: a meta-analysis of studies. Med Sci Sports Exerc. 1983;15(5):393–402. [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Olson L, Thorén P. Deprived of habitual running, rats downregulate BDNF and TrkB messages in the brain. Neurosci Res. 1999;34(3):125–132. doi: 10.1016/s0168-0102(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Yung HW, Wyttenbach A, Tolkovsky AM. Aggravation of necrotic death of glucose-deprived cells by the MEK1 inhibitors U0126 and PD184161 through depletion of ATP. Biochem Pharmacol. 2004;68(2):351–360. doi: 10.1016/j.bcp.2004.03.030. [DOI] [PubMed] [Google Scholar]


