SUMMARY
The trp RNA-binding attenuation protein (TRAP) regulates expression of the tryptophan biosynthetic (trp) genes in response to changes in intracellular levels of free L-tryptophan in many gram positive bacteria. When activated by binding tryptophan, TRAP binds to the mRNAs of several genes involved in tryptophan metabolism, and down-regulates transcription or translation of these genes. Anti-TRAP (AT) is an antagonist of TRAP that binds to tryptophan-activated TRAP and prevents it from binding to its RNA targets, and thereby up-regulates trp gene expression. The crystal structure shows that AT is a cone-shaped trimer (AT3) with the N-terminal residues of the three subunits assembled at the apex of the cone and that these trimers can further assemble into a dodecameric (AT12) structure. Using alanine-scanning mutagenesis we found four residues, all located on the “top” region of AT3, which are essential for binding to TRAP. Fluorescent labeling experiments further suggest that the top region of AT is in close juxtaposition to TRAP in the AT-TRAP complex. In vivo studies confirmed the importance of these residues on the top of AT in regulating TRAP mediated gene regulation.
Keywords: protein-protein interactions, RNA-binding protein, gene regulation, transcription attenuation, protein oligomerization, tryptophan genes
INTRODUCTION
In many gram positive bacteria, an RNA binding protein called TRAP (trp RNA-binding attenuation protein) regulates expression of genes involved in tryptophan metabolism (trp) in response to changes in intracellular levels of free L-tryptophan 1;2; 3. TRAP down-regulates both transcription and translation of the trp genes by binding to its RNA targets in response to elevated levels of tryptophan 3. An antagonist of TRAP, termed anti-TRAP (AT), has been identified in only a subset of the TRAP-containing bacilli including B. subtilis, B. licheniformis 4; 5, B. mojavensis and B. spizizenii (P. Gollnick unpublished observations). AT binds to tryptophan-activated TRAP and prevents it from binding to its RNA targets, and thus up-regulates expression of the trp genes 4; 6. Synthesis of AT is induced by accumulation of uncharged tRNATrp, however, neither tryptophan nor uncharged tRNATrp appear to bind to AT directly 7.
Tryptophan-activated TRAP binds to mRNA targets that contain multiple (9 to 11) NAG triplet repeats (N = G ≈ U > A > C) usually separated by two or three nonconserved “spacer” nucleotides 2; 3. TRAP regulates transcription of the trpEDCFBA operon by binding to the 5’ leader region of the nascent trp transcript 8. This binding promotes formation of an intrinsic transcription terminator that halts transcription within the leader region prior to the coding sequence of trpE9. There is also a stem-loop structure (5’SL) at the 5’ end of the leader region of trp operons regulated by TRAP 24 (J. Simon and P. Gollnick unpublished observations). Moreover, studies by Babitzke and coworkers have shown that the 5’SL is important for proper TRAP mediated control of transcription of the trp operon 24. Translation of trpE 10; 11, trpG12; 13, trpP 14; 15, and ycbK 16 is also regulated by TRAP. TRAP binding to trp operon transcripts that have escaped transcriptional control promotes formation of a stem-loop structure that sequesters the Shine-Dalgarno (SD) sequence of trpE, thus inhibiting translation initiation of this gene 3; 10. TRAP down regulates translation of trpG, trpP and ycbK by directly competing with ribosomes for binding to the translation start region of these genes 3.
TRAP consists of 11 identical 75-residue subunits arranged in a symmetrical ring 17; 18; 19. The secondary structure of TRAP is composed of 11 β-sheets, each containing 7 antiparallel β-strands; three strands from one subunit and four from the adjacent subunit 18. 11 tryptophan-binding pockets are formed at the interfaces between adjacent TRAP subunits 18. Structures of tryptophan-activated TRAP in complex with RNAs containing either 11 GAG or 11 UAG repeats show that the single-stranded RNA wraps around the outer perimeter of the protein ring and interacts primarily with residues Glu36, Lys37, Lys56 and Arg58 17; 20; 21; 22. The residues that are involved in binding tryptophan and those that bind to the RNA are conserved in all currently available TRAP sequences 23.
AT is a 53-residue polypeptide encoded by the rtpA gene, which is the first gene of the two-gene at operon 4 The other gene in this operon, ycbK, is located downstream of rtpA and encodes a protein that may be involved in tryptophan transport 16. Both transcription and translation of rtpA are regulated in response to accumulation of uncharged tRNATrp. Transcription of the at operon is regulated by the T-box antitermination mechanism, in which accumulation of uncharged tRNATrp enhances transcription of rtpA 16; 25 and ultimately increases expression of the trp genes 26. Translational control of rtpA expression involves a 10-codon open reading frame, rtpLP, located in the 5’ leader region upstream of rtpA 7; 27. Efficient translation through three consecutive Trp codons in rtpLP allows the ribosome to reach the stop codon, which is 6 nucleotides upstream of the SD sequence of rtpA 3. This positioning of the rtpLP-translating ribosome interferes with translation initiation of rtpA 27. In contrast, if the translating ribosome stalls on one of the three Trp codons in rtpLP due to insufficient levels of charged tRNATrp, the rtpA SD sequence remains exposed and rtpA is translated efficiently 3; 27.
The amino acid sequence of AT contains two CXXCXGXG (X is any amino acid) Zn-binding motifs similar to those found in the type I family of Hsp40 molecular chaperones 4. The cysteine residues in these motifs, and the presence of bound zinc are required for AT stability and function 28. Crystal structures of B. subtilis AT 26;29 and B. licheniformis AT (M. Shevetsov and A. Antson unpublished data) reveal that AT assembles into cone-shaped trimers (AT3) (Figure 1B). The N-terminal residues of the three subunits assemble at the apex, or “top” of the cone, and three zinc-binding domains extend down toward the base, or “bottom” of the cone. Four AT trimers further associate into dodecamers (AT12) via weak trimer-trimer interactions 26. Analytical ultracentrifugation studies showed that AT exists in a reversible equilibrium between trimers and dodecamers in solution, and provided evidence for an AT-TRAP complex composed of 12 AT subunits and 11 TRAP subunits 30. No complexes with mass corresponding to AT3-TRAP11, AT6-TRAP11, or AT9-TRAP11 were detected suggesting that AT binds to TRAP as a preformed dodecamer or that 4 AT trimers bind cooperatively 30. However, physiological concentrations of AT are much lower than those required for AT12 formation in vitro 31, suggesting that AT may function as a trimer in vivo.
Figure 1.
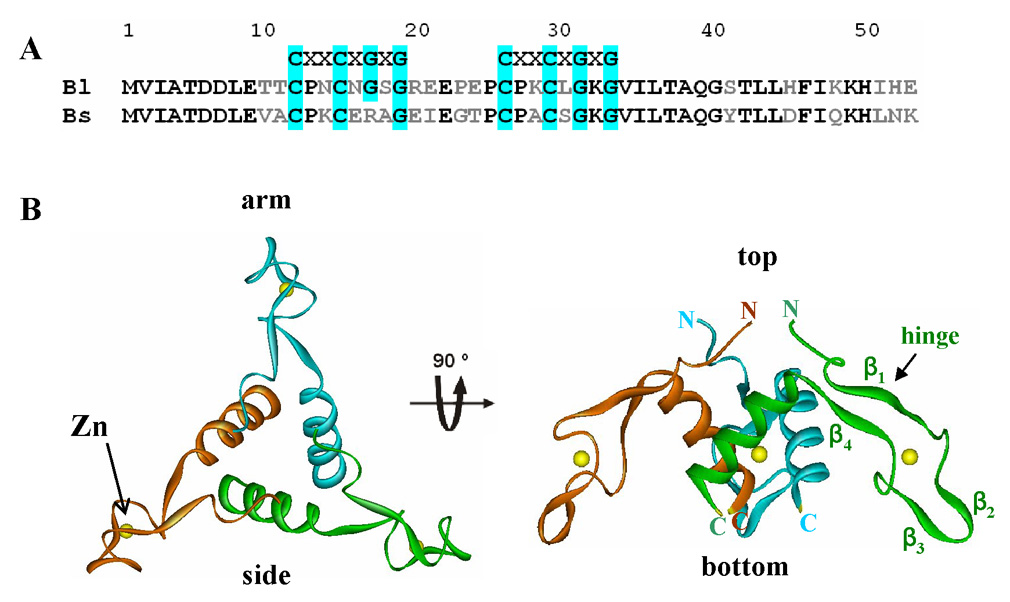
Amino Acid Sequence alignment and trimer structure of AT. (A). Alignment of amino acid sequences of B. licheniformis AT (Bl) and B. subtilis AT (Bs)50. The cysteine and glycine residues of the Zn-binding CXXCXGXG motif are highlighted in cyan. The identical and non-identical residues between the two sequences are marked in black and gray, respectively. (B). Ribbon diagram of the top view (left) and side view (right) of the B. subtilis AT3 structure. The three subunits are shown in red, green and blue ribbons. Zn atoms are shown as yellow spheres. The regions of the protein defined as top, bottom, and the Zn-binding arm are labeled. Individual β-strands (β1–β4) and the putative hinge region are labeled on the green subunit.
Studies of AT binding to mutant TRAP proteins indicate that TRAP residues involved in binding RNA are also recognized by AT 6 However, the residues of AT that are involved in binding to TRAP have not been identified. To identify these residues, we individually substituted 26 (including 21 surface residues) of the 53 amino acid residues of AT with alanine, and examined binding of the resulting mutant proteins to TRAP. Our results showed that four residues located on the “top” of the AT3 cone structure are important for binding to TRAP. The presence of tryptophan-activated TRAP interfered with the labeling of three different residues located near the top of AT3 with the fluorescent dye 5- Iodoacetamidofluorescein (5-IAF) indicating that these residues are less solvent exposed in the AT-TRAP complex than in free AT. In addition, in vivo studies of the activity of mutant AT proteins in B. subtilis confirmed the importance of residues near the top of AT3 in AT function. Together, these studies indicate that residues located on the top of the AT3 structure are directly involved in AT binding to TRAP. Based on this conclusion and on results from prior studies of the AT-TRAP interaction 4; 26; 30 we propose a model for the AT-TRAP complex in which 4 AT trimers bind to a TRAP 11mer. In this model each AT trimer binds at or near the RNA binding region of TRAP and thus AT binding is predicted to simply sterically interfere with RNA binding to TRAP.
RESULTS
Alanine-scanning of the surface residues of AT
In this study we utilized AT from two Bacillus species, B. subtilis and B. licheniformis. These two proteins show 66% sequence identity over their 53 amino acid length (Figure 1) 26 and both proteins assemble into very similar trimer structures (A. Antson and M. Shevetsov personal communication). Preliminary studies showed that AT from B. licheniformis was expressed to higher levels in E. coli than the B. subtilis protein. This difference was found to be due to a large number of rare codons in the B. subtilis gene (C. McElroy and M. Foster, Ohio State University). We performed our initial alanine scanning studies with B. licheniformis AT, which allowed easier production and purification of the mutant proteins. The sequence and structural similarities between the proteins from both bacilli suggest that information obtained from studying B. licheniformis AT will apply to both systems. This hypothesis was confirmed by making and testing several key Ala substitutions in B. subtilis AT.
To probe which amino acid residues of AT are critical for binding to TRAP, we performed alanine scanning mutagenesis of residues with solvent exposed side chains on the surface of the AT3. These residues are the best candidates for being involved in direct interactions with TRAP and substitution of these residues is unlikely to disrupt the structure of AT3. Residues with buried side chains or those involved in monomer-monomer interactions in AT3 were not substituted so as to avoid disrupting the protein’s structure. We also avoided changing the cysteines or glycines in the two CXXCXGXG Zn-binding domains (Figure 1A) because previous studies have shown that the bound Zn is required for AT structure and function 28. Consistent with this strategy, size exclusion chromatography and CD studies indicate that neither the subunit assembly nor the overall folding was significantly affected in any of the Ala-substituted AT proteins that we studied (See Below).
Residues on top of AT trimer are important for binding TRAP
WT AT and 21 alanine-substituted AT proteins from B. licheniformis were tested for binding to tryptophan-activated TRAP. WT AT binds to TRAP with an observed Kd of 3.1 µM (Table 1). This value appears to be high in view of the maximal in vivo levels AT having been measured to be approximately 100 nM 31. The reason for this discrepancy is unclear; nevertheless it represents a useful value to compare to the Ala-substituted AT proteins. Four of the alanine-substituted AT proteins, V02A, I03A, D06A, and D07A showed greater than 20-fold lower affinity for TRAP than WT AT (Table 1), suggesting that Val02, Ile03, Asp06 and Asp07 play important roles in binding to TRAP. The remaining alanine substituted proteins bound TRAP with no more than 3-fold lower affinity than WT AT (Table 1). The four residues implicated in TRAP binding are all located on the top of the AT trimer structure (Figure 2A), suggesting that this region of AT is critical for binding to TRAP.
Table 1.
Substitution of surface residues on B. licheniformis AT3
| AT protein | Residue location on AT3¶ | Kd (µM)‡ |
|---|---|---|
| W.T. | wild type | 3.1 ± 0.3 |
| V02A | top | n.b.* |
| I03A | top | n.b.* |
| T05A | close to the top | 3.8 ± 0.8 |
| D06A | top | 101.7 ± 8.5 |
| D07A | top | 64.3 ± 6.8 |
| T10A | arm | 1.6 ± 0.2 |
| N14A | arm | 3.7 ± 0.3 |
| N16A | arm | 4.1 ± 0.5 |
| R20A | end of the arm | 6.2 ± 0.7 |
| E21A | end of the arm | 1.9 ± 0.1 |
| E22A | end of the arm | 3.0 ± 0.3 |
| P23A | end of the arm | 1.4 ± 0.1 |
| E24A | end of the arm | 3.5 ± 0.3 |
| P25A | end of the arm | 1.0 ± 0.1 |
| I35A | arm | 6.7 ± 0.8 |
| T37A | near the top | 2.3 ± 0.3 |
| S41A | side | 6.9 ± 0.8 |
| H45A | side | 9.6 ± 0.8 |
| I51A | bottom | 4.0 ± 0.7 |
| H52A | bottom | 1.7 ± 0.1 |
| E53A | bottom | 2.8 ± 0.2 |
| V02L | top | n.b.* |
| D06S | top | n.b.* |
| D07T | top | n.b.* |
Location of AT residues examined by alanine substitution (See Figure 2A&B).
Each value is the average ± the standard deviation of at least two independent binding measurements, each done in triplicate.
n.b., no binding detected with AT3 up to 60 µM.
Figure 2.
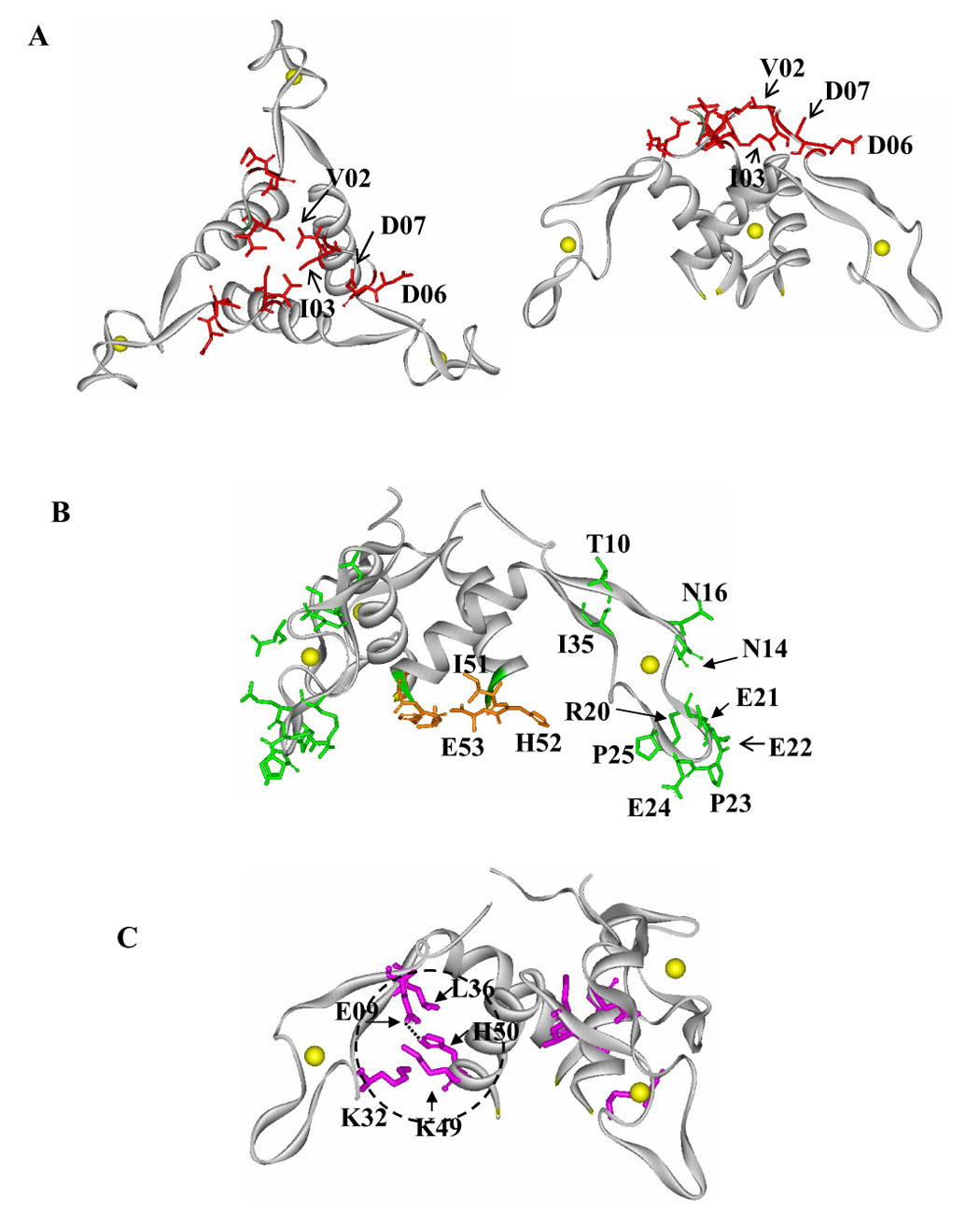
Locations of the AT residues examined by alanine substitution. The backbone of each subunit is shown as a solid gray ribbon and Zn atoms are shown as yellow spheres. (A) Side chains of AT residues for which alanine substitution significantly reduced the affinity for TRAP (Val02, Ile03, Asp06 and Asp07) are shown as red sticks on the top (left side) and side (right side) views of AT3. (B) The side chains of AT residues for which alanine substitution did not significantly alter interaction with TRAP are shown as green sticks on the Zn-binding region and orange on the bottom region of AT3. (C) The location of AT residues potentially involved in anchoring the Zn-binding arms to the AT3 core is circled with black dashed line. The side chains Glu09, Lys32, Leu36, Lys49 and His50 are shown in magenta sticks and a dotted line indicates the hydrogen bone between His50 and Glu09.
We made several additional substitutions of these critical residues on the top of AT3 to further probe the requirements of the side chains at these positions for interacting with TRAP. Changing Val02 to alanine eliminated detectable binding to TRAP (Table 1). This substitution shortens the side-chain and removes its β-branch suggesting that the side chain at position 2 needs to be hydrophobic and either larger than alanine or β-branched. To examine the importance of these features of residue 2 for binding to TRAP, we substituted Val02 with leucine, which is one methyl group larger than valine and maintains branching of the side chain. The V02L mutant protein also showed no detectable binding to TRAP (Table 1) suggesting a strict requirement for Val at residue 2. These observations suggest a tight fit of the two proteins at this location and a need for specific hydrophobic interaction involving the Val side chain.
Replacing Asp06 or Asp07 with alanine reduced the affinity of AT for TRAP by 20–30 fold (Table 1). These substitutions remove the charged polar side chains of the residues at position 6 or 7. We have found that the stability of the AT-TRAP complex is not sensitive to changes in ionic strength, suggesting that ionic interactions are not crucial for stabilizing this interaction (Y. Chen and P. Gollnick, unpublished observations). Together these observations suggest that a polar side chain may be required at residues 6 and 7. To test this possibility we replaced Asp06 and Asp07 with Serine and Threonine respectively, both of which have uncharged polar side chains. In both cases these substitutions were more deleterious than alanine replacement, and eliminated measurable binding to TRAP (Table 1). These data again suggest that a strict requirement of side chains of specific residues (in this case Asp06 and Asp07) on the top region of AT3 is needed for binding to TRAP.
The Zn-binding arms and the bottom region of AT3
AT contains two cysteine-rich CXXCXGXG motifs with sequence 4 and structural 26 similarity to the Zn-binding domain of the type I family of Hsp40 molecular chaperones (Figure 1A) 26; 32. The Zn-binding domains of members of the Hsp40 family, including E. coli DnaJ 26; 32 and Ydj1 of yeast 33, have been implicated in interacting with other proteins. Hence a model has been proposed for the interaction of AT with TRAP that involves a direct role of the Zn-binding domains of AT 26. However, alanine substitution for any residues in this part of AT including 20–25 of the β-hairpin (β2–β3) at the end of the Zn-binding arm; in the small loop adjacent to the Zn-binding domain (Asn14 or Asn16); or in the β-sheet (β1–β4) between the Zn-binding domain and the α-helical core of the protein (Thr10 or Ile35), had little or no effect on AT binding to TRAP (Table 1 and Figure 2B). These observations suggest that the side chains of residues located on the Zn-binding arms of AT do not directly interact with TRAP. However, these data do not rule out the possibility that the Zn-binding domains of AT interact with TRAP via their main-chain atoms.
Side chains of several residues on the bottom of AT3, including Ile51, His52 and Glu53 are solvent exposed (Figure 2B) and are thus potential candidates for interacting with TRAP. However, alanine substitution of any of these three residues had no significant affect on the affinity for TRAP (Table 1).
Alanine substitution of non-surface residues of AT
The crystal structure of AT indicates that each monomer has a hinge formed by the small antiparallel β-sheet composed of residues 9–11 (β1) and 34–36 (β4) (Figure 1B), which suggests that the Zn-binding arm may be flexible relative to the coil-coil core of the trimer 26. The side chains of a number of residues, including Glu09, Lys32, Leu36, Lys49, and His50, are located between the arm and the trimer core (Figure 2C) suggesting that these residues might be involved in stabilizing the local structure of AT3, which could be important for TRAP recognition. To test this hypothesis, we made alanine substitutions for these residues and examined the binding of resulting mutant proteins to TRAP. Two of these mutant proteins, E09A and H50A, showed significantly reduced binding to TRAP (Table 2). The side chains of Glu09 from the Zn-binding arm of one subunit and His50 from the helical core of the adjacent subunit form a hydrogen bond (Figure 2C) 26. Hence substituting either of these residues with alanine might destabilize local structure of AT by eliminating this hydrogen bond, and this could consequently alter the surface structure of AT recognized by TRAP. Consistent with this hypothesis, alanine substitution of Glu09 or His50 in AT resulted in either 11-fold reduced binding affinity or in no detectable binding to TRAP respectively (Table 2). However, the observed differences in the TRAP binding activity of these two mutant proteins suggests that the Ala substitutions affect more than just formation of the hydrogen bond between these two residues.
Table 2.
Alanine substitution of residues between the arm and the core of AT3
| AT protein | Kd (µM)‡ |
|---|---|
| W.T. | 3.1 ± 0.3 |
| E09A | 35.9 ± 3.4 |
| K32A | 6.6 ± 1.0 |
| L36A | 6.5 ± 0.7 |
| K49A | 12.6 ± 1.8 |
| H50A | n.b.* |
Each value is the average ± the standard deviation of at least two independent binding measurements, each done in triplicate.
n.b., no binding detected using AT3 up to 60 µM.
Alanine substitution of Lys32, Leu36 or Lys49 in this region of AT did not significantly alter binding to TRAP (Table 2). The side chains of these residues are not completely buried between the arm and the AT3 core, and they appear to be less critical than His50 and Glu09 in stabilizing the local structure necessary for TRAP recognition.
Analysis of the secondary and quaternary structure of alanine substituted AT proteins
One potential explanation for the reduced TRAP binding activity seen for the four alanine substituted AT proteins we identified is that these substitutions could disrupt the overall secondary structure of AT rather than directly affect residues that interact with TRAP. To examine this possibility we compared the CD spectra of WT AT and several of the mutant proteins that do not bind TRAP (Figure 3). The spectrum of WT AT shows negative peaks at 208 nm and 222 nm, as well as a positive peak at 195 nm, which are typical for proteins that are largely alpha helical, such as AT (Figure 1) 34. The spectra of four mutant AT proteins with alanine substitutions on the top of AT3 (V02A, I03A, D06A, and D07A) are all very similar to that of WT AT (Figure 3), suggesting that these substitutions did not significantly alter the overall secondary structure of AT. Other mutant AT proteins, including N16A, R20A, E22A, E24A, P25A, S41A and I51A, also had very similar spectra as WT AT (Data not shown). Only two mutant AT proteins, E09A (Figure 3) and H50A (data not shown), showed slightly diminished signal between 195 and 215 nm, which suggests that these mutant proteins contains more random coil than WT AT. As described in the previous section, there is a hydrogen bond between the side chains of Glu09 and His50 in the C-terminal α-helix of AT (Figure 2C). Our CD data are consistent with the proposal that this hydrogen bond is involved in maintaining the local structure of AT, which appears to be important for TRAP binding.
Figure 3.
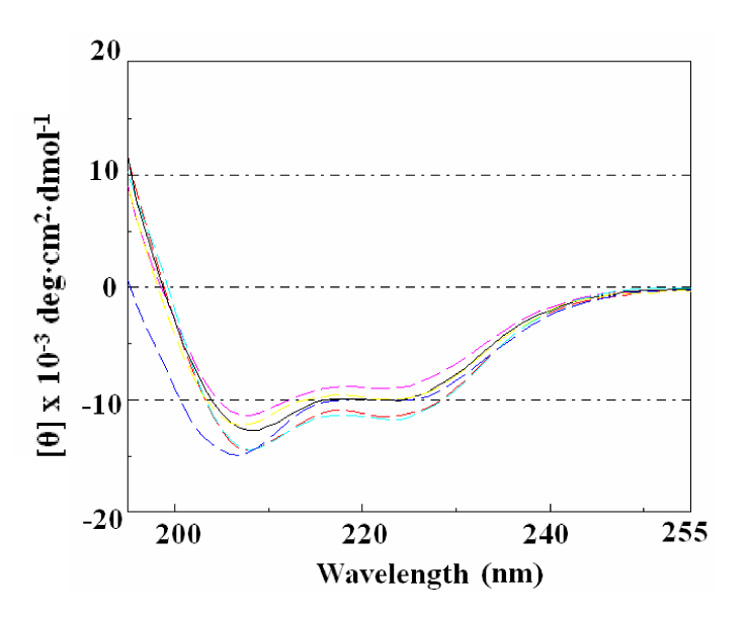
Circular dichroisim spectra of AT proteins. CD spectra of WT and several Ala-substituted AT proteins are shown in WT (black), V02A (red), I03A (yellow), D06A (magenta), D07A (cyan), and E09A (blue) lines. Each AT protein was 200 µg/ml in 50 mM sodium phosphate buffer (pH8.0) and the spectra were recorded at 37 °C.
Proper oligomerization of AT is likely critical for binding to TRAP. AT has been shown to exist as both trimers (AT3) and as dodecamers (AT12) in solution 30. Thus alanine substitutions that diminish the activity of AT could do so by altering oligomerization. We therefore examined the properties of WT and mutant AT proteins on size exclusion chromatography. WT AT (118 µM AT3) showed a predominant peak at 8.3 minutes after injection and a smaller peak at 9.5 minutes (data not shown). Based on protein MW standards, these times correspond to MWs of 74.6 kDa and 17.9 kDa respectively, which are close to the predicted MWs of AT12(67.2 kDa) and AT3 (16.8 kDa). All mutant AT proteins that we examined, including V02A, I03A, D07A and D07A, which showed diminished affinity for TRAP, as well as E09A, N16A, R20A, E22A, E24A, P25A, S41A, H50A and I51A showed very similar elution patterns as WT AT (data not shown). These results suggest that alanine substitution did not dramatically alter the quaternary structure of these proteins as compared to WT AT under the conditions of these experiments.
Fluorescent labeling AT
The results of alanine substitution studies identified four residues on the top region of AT3 that are critical for binding to TRAP (Table 1 and Figure 2). A simple interpretation of these observations is that the top region of AT3 interacts directly with TRAP. However, it is also possible that the deleterious effects of alanine substitution of residues Val02, Ile03, Asp06 and Asp07 are indirect. To further investigate which region of AT directly interacts with TRAP, we examined the solvent accessibility of several surface residues of AT when the protein was free as compared to when it was in complex with TRAP. To do so we used the fluorescent dye 5-Iodoacetamidofluorescein (5-IAF), which reacts with free sulfhydryl groups 35. WT AT contains four Cys residues but showed very low reactivity with 5-IAF (Figure 4C), presumably because the side chains of these 4 cysteine residues coordinate Zn and thus do not react with 5-IAF. We made cysteine substitutions for residues located on various region of the AT3 structure (Figure 4A). These included His52 on the bottom of AT3, Glu22 on the end of the Zn-binding arm, Thr10 and Asn16 on the arm between the bound Zn and the top of the trimer, and Thr05 near the top of the trimer. These residues were chosen because when they were substituted with alanine AT remained fully functional for binding TRAP (Table 1). Unfortunately it was not possible to place a cysteine at the very top of the AT3 structure because substituting residues in this region significantly reduced the affinity of AT for TRAP (Table 1). The rate of labeling these Cys-substituted proteins with 5-IAF in the absence and presence of tryptophan-activated TRAP was examined. Figure 4B shows an example of 5-IAF labeling T05C AT. In this case the rate of labeling was slower in the presence of tryptophan-activated TRAP than in its absence suggesting that residue 5 is less solvent accessible in the AT-TRAP complex than in free AT. The rate of labeling T05C AT was not affected if the reaction was performed with TRAP in the absence of tryptophan (data not shown). A simple interpretation of these observations is that residue 5 is near TRAP in the AT-TRAP complex. Similar studies showed 25–35% reduced labeling for T05C, T10C, and N16C in the presence of tryptophan-activated TRAP (Figure 4C); the Cys residues in these substituted proteins are near the top of AT3 (Thr05 and Thr10) or on the upper part of the arm (Asn16) of AT3 (Figure 4A). Two other mutant AT proteins that contain cysteine residues on the end of the Zn-binding arm (E22C) or on the bottom (H52C) of AT3 showed little or no difference in reactivity with 5-IAF in the absence or presence of TRAP (Figure 4C), indicating that these residues are equally solvent accessible in free AT and in the AT-TRAP complex. Together, these results are consistent with the alanine substitution results described above and further suggest that AT interacts with TRAP through the region on the top of the trimer.
Figure 4.
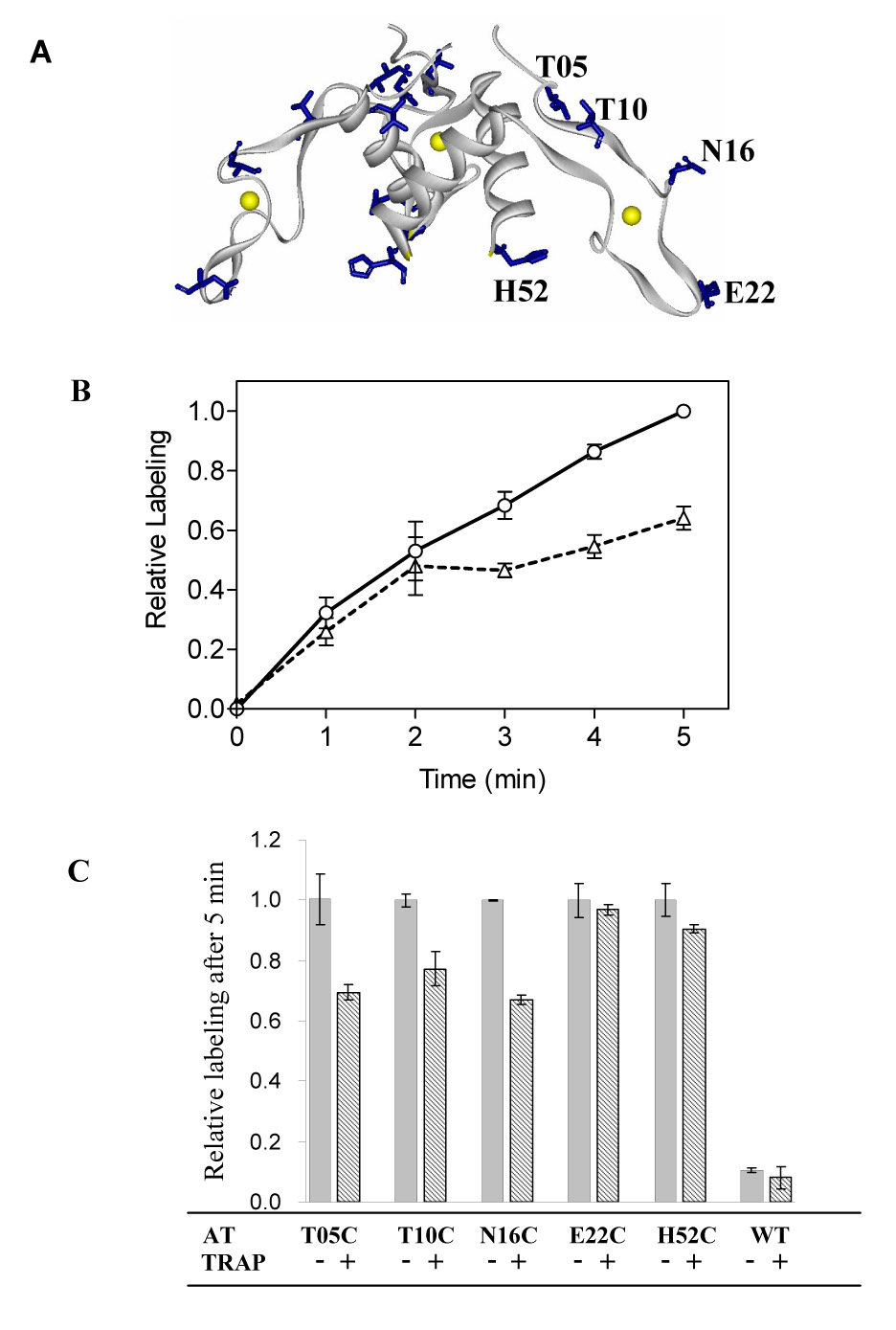
Labeling AT with 5-Iodoacetamidofluorescein (5-IAF). (A) The location of the side chains of AT residues Thr05, Thr10, Asn16, Glu22 and His52, which were substituted with cysteine for 5-IAF labeling studies are shown as blue sticks on a gray ribbon diagram of the backbone of AT3 with the Zn atoms shown as yellow spheres. (B) Example of the time course of 5-IAF labeling T05C AT in the absence (circles/ solid line) and presence (triangles/dashed line) of tryptophan-activated TRAP. The fluorescence intensity measured after labeling 5-minutes in the absence of TRAP was set to be 1.0 and all other measurements were normalized to this value. Each point represents the average of at least two independent experiments. (C). Comparison of the relative fluorescence intensity of WT and Cys-substituted AT proteins labeled with 5-IAF in the absence (solid column) and presence (striped column) of tryptophan-activated TRAP for 5 minutes. The average fluorescence intensity for the cysteine mutant AT proteins labeled in the absence of TRAP was set to be 1.0 and other measurements were normalized accordingly. Each column represents the average of at least three independent experiments.
Alanine substitution of residues in B. subtilis AT
The amino acid sequences and the trimer structures of AT from B. licheniformis and B. subtilis are very similar (Figure 1). Moreover, all four residues that were identified as being important for B. licheniformis AT binding to TRAP (Val02, Ile03, Asp06, Asp07) are conserved in both proteins (Figure 1A). These observations suggest that both proteins likely bind to TRAP by a similar mechanism. To test this hypothesis, we made four alanine substitutions in B. subtilis AT and examined their effects on binding to TRAP (Table 3). As seen with B. licheniformis AT, alanine substitutions of residues on the top region of B. subtilis AT3 (V02A and D06A) eliminated binding to TRAP, whereas substitutions of residues at the end of the Zn-binding arm (E22A) or on the bottom (N52A) of AT3 had little or no effect on binding (Table 3).
Table 3.
B. subtilis alanine substituted AT proteins binding to TRAP
| B.subtilis AT | Residue location on AT3 | Kd (µM)‡ |
|---|---|---|
| W.T. | wild type | 3.1 ± 0.1 |
| V02A | top | n.b.* |
| D06A | top | n.b.* |
| E22A | end of the arm | 5.9 ± 1.0 |
| N52A | bottom | 2.2 ± 0.5 |
Location of AT residues tested for alanine substitution (See Figure 5A).
Each value is the average ± the standard deviation of at least two independent binding measurements, each done in triplicate.
n.b., no binding detected with AT3 up to 60 µM.
In vivo studies of AT binding to TRAP using mutant AT proteins
We also examined the importance of residues on the top region of the AT trimer for AT function in vivo. To do so, we expressed WT and three mutant B. subtilis AT proteins (D06A and D07A on the top of AT3, and E16A on the Zn-binding are of AT3) in B. subtilis CYBS410pJS673, which carries a chromosomal deletion of the rtpA-ycbK region (rtpA encodes AT) and has a trpE’-’lacZ gene fusion under control of the trp promoter and regulatory leader region 16; 36. The AT proteins to be tested for their ability to modulate TRAP-mediated regulation of the trpE’-’lacZ fusion were expressed from the IPTG-inducible spac promoter on pDG148 4. TRAP regulated β-galactosidase expression 281-fold in response to tryptophan in this strain carrying the empty vector pDG148, and this regulation was not affected by IPTG (Table 4). As seen previously 4, IPTG induced expression of WT AT from pDG148 eliminated TRAP-mediated control of the trpE’-’lacZ fusion, resulting in elevated β-galactosidase expression in the absence or presence of tryptophan (Table 4). The presence of this plasmid also resulted in slightly elevated β-galactosidase expression in cells grown with tryptophan in the absence of IPTG, presumably due to a small amount of leaky expression of AT from the spac promoter. Expressing either D06A or D07A AT, which contain alanine substitutions for residues on the top of AT3 resulted in dramatically less inhibition of TRAP-mediated regulation of the trpE’-’lacZ fusion than seen with WT AT (Table 4). In contrast expressing E16A AT (alanine substitution in the Zn-binding arm) resulted in similar effects as expressing WT AT. These results together with those in the previous section are summarized in Figure 5, and are again consistent with the conclusion that the residues on top portion of AT3 are critical for binding to TRAP.
Table 4.
Effects of expressing WT and Alanine substituted AT on trp’E-’lacZ expression in vivo.
| β-galactosidase activity (Miller units) | ||||||
|---|---|---|---|---|---|---|
| −IPTG | +IPTG | |||||
| AT expressed | −Trp | +Trp | Fold Regulation* | −Trp | +Trp | Fold Regulation* |
| None (plasmid only) | 281 | 1 | 281 | 275 | 1 | 275 |
| WT | 219 | 6 | 36 | 375 | 382 | 1 |
| D06A | 253 | 3 | 84 | 252 | 7 | 36 |
| D07A | 246 | 1 | 246 | 352 | 3 | 117 |
| E16A | 258 | 6 | 43 | 644 | 575 | 1 |
Fold regulation is defined as the ratio of β-galactosidase units of −Trp/+Trp.
Figure 5.
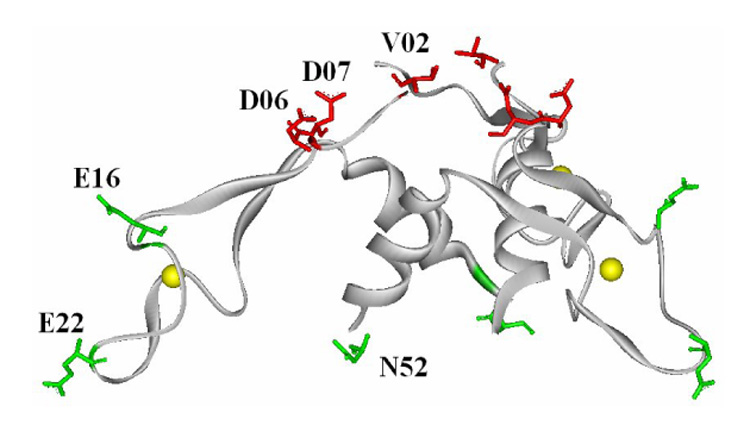
Summary of the effects of Ala substitution of residues in B. subtilis AT. The effects of alanine substitution of residues V02, D06, E22 and N52 were tested in vitro for binding to TRAP, and the effects of alanine substitution of D06, D07 and E16 were tested in vivo for the ability to affect TRAP mediated control of a trpE’-‘lacZ gene fusion. The backbone of each AT subunit is shown as a solid gray ribbon and Zn atoms are shown as yellow spheres. Residues for which alanine substitution significantly affected interaction with TRAP are marked in red stick diagrams, whereas those for which alanine substitution did not significantly alter interaction with TRAP are shown as green sticks.
DISCUSSION
Several studies have demonstrated that AT specifically binds to tryptophan-activated TRAP 4; 6; 30. Moreover, Valbuzzi et al. provided evidence that AT recognizes residues in the RNA binding region of TRAP, including Lys37, Lys56, and Arg58, which are located on the outer perimeter of the TRAP ring (Figure 6A)6. Less is known about how AT functions in the AT-TRAP complex. For example, the oligomeric state of AT in this complex is not clear. Ultracentrifugation studies have shown that AT exists in solution as both trimers (AT3) and dodecamers (AT12) 30. These studies also provided evidence for an AT:TRAP complex containing 12 AT subunits/TRAP 11mer. Since no complexes were detected with 3, 6 or 9 AT subunits/TRAP 11mer, it was suggested that AT binds to TRAP as a preformed 12mer 30. However, the concentrations of AT3 used in these studies, 12–48 µM (with equal fractions of AT3 and AT12 seen at 20 µM), are three orders of magnitude higher than have been measured for AT in vivo (~30 nM AT3) 31, suggesting that AT may function as a trimer in vivo In crystals of AT, four timers further assemble into a dodecamer (Figure 6B) 26, although there is no evidence that this is a biologically relevant assembly of AT.
Figure 6.
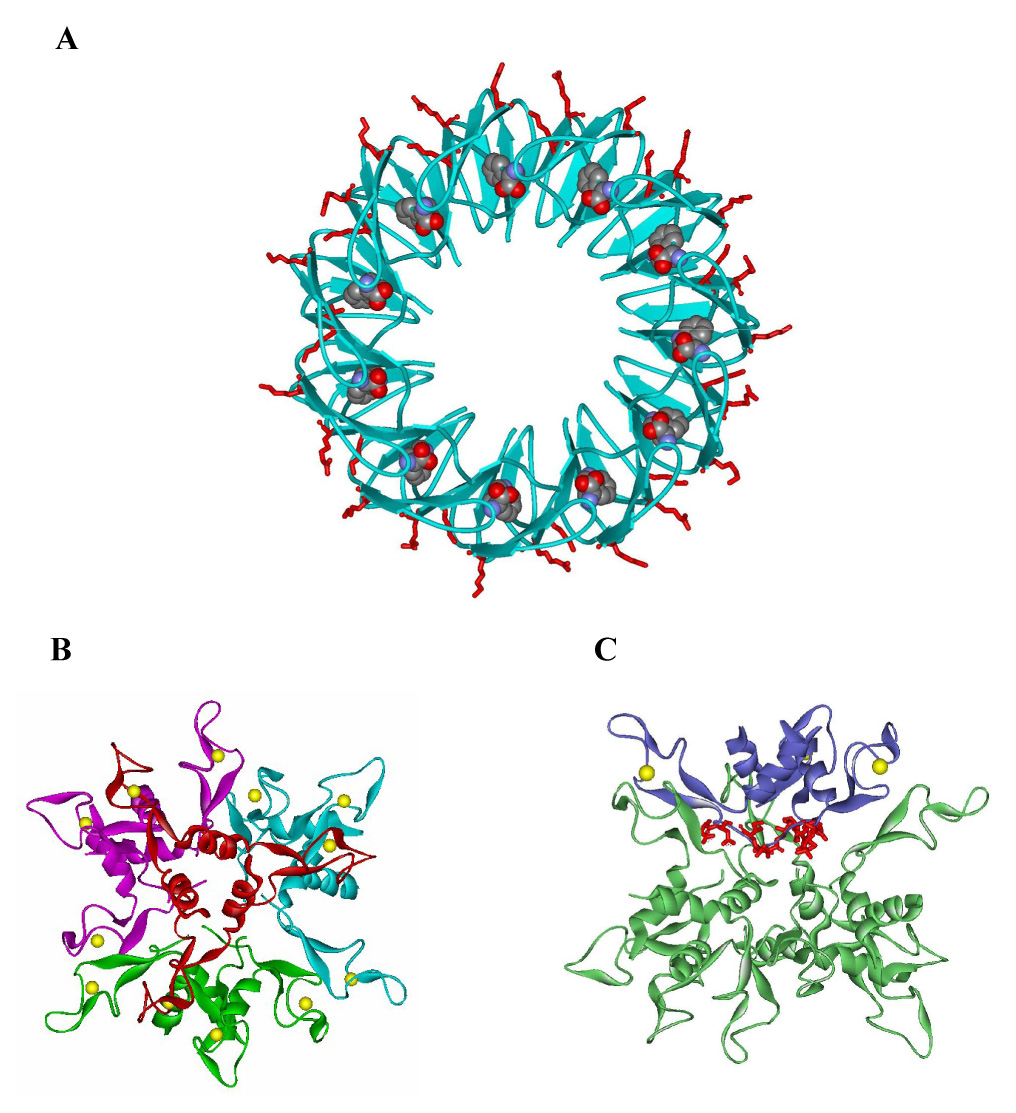
Structures of TRAP and AT dodecamer. (A) The backbone of the TRAP 11mer is shown in blue cartoons with the bound tryptophan molecules shown in Van der Waals spheres. The side chains of residues Lys37, Lys56, and Arg58, which interact with bound RNA are show as red sticks. (B and C) Ribbon diagrams of the dodecamer structure of B. subtilis AT. In panel B four AT trimers are shown in red, green, blue and magenta with the Zn atoms shown as yellow spheres. In panel C three of the AT trimers are shown in green ribbons and one trimer is highlighted in blue on which residues Val02, Ile03, Asp06, and Asp07 on the top region are shown as red sticks.
The alanine substitution studies described here identified four residues (Val02, Ile03, Asp06 and Asp07) in AT that are important for interaction with TRAP (Table 1 and Table 3). These residues are located on the top region of the AT trimer structure (Figure 2). This region of the each trimer is pointed inward toward the center of the tetrahedral dodecamer structure such that Val02, Ile03, Asp06 and Asp07 are not exposed to interact with TRAP (Figure 6C). If AT interacts with TRAP as the dodecamer seen in the crystal structure, then it is possible that alanine substitutions of these residues interfere with TRAP binding indirectly by interfering with formation of the dodecamer. However, several observations suggest that this is not the case. First, alanine substitution for residues in all other parts of the protein, including the Zn-binding arms and the bottom region, had little or no effect on TRAP binding (Table 1 and Figure 2). Thus if the deleterious effects of substituting residues on the top of AT3 are indirect, we were unable to identify any other region of the protein in which the amino acid side chains directly interact with TRAP. Secondly, none of the alanine substitutions that affected TRAP binding altered the behavior of the mutant proteins on size exclusion chromatography as compared to WT AT suggesting they do not alter the oligomerization properties of AT. Finally fluorescent labeling studies suggest that Thr05, Thr10 and Asn16, which are near the top region of AT3, are less solvent exposed in the presence of tryptophan-activated TRAP than in free AT. These residues, particularly residue 10, are buried in the B. subtilis AT12 structure and hence it is unlikely they would be even less exposed in the complex with TRAP if this dodecamer binds to TRAP.
Taken together the available data support the proposal that AT binds to TRAP as trimers via residues on the “top” of the of the trimer structure (Figure 1B & 2A). Based on this conclusion, we propose the model for the AT-TRAP complex shown in Figure 7. In this model 4 AT trimers bind to the outer perimeter of the TRAP ring at or near the residues that bind RNA. This model is consistent with the results presented here, as well as with data from previous studies. These include analytical ultracentrifugation studies that provided evidence for an AT-TRAP complex containing 12 AT subunits per TRAP 11mer 30, as well as studies of AT binding to mutant TRAP proteins, which suggest that AT binds to the same region of TRAP that binds to RNA 6. A similar, although less detailed, model has been previously proposed by Valbuzzi and Yanofsky 4.
Figure 7.
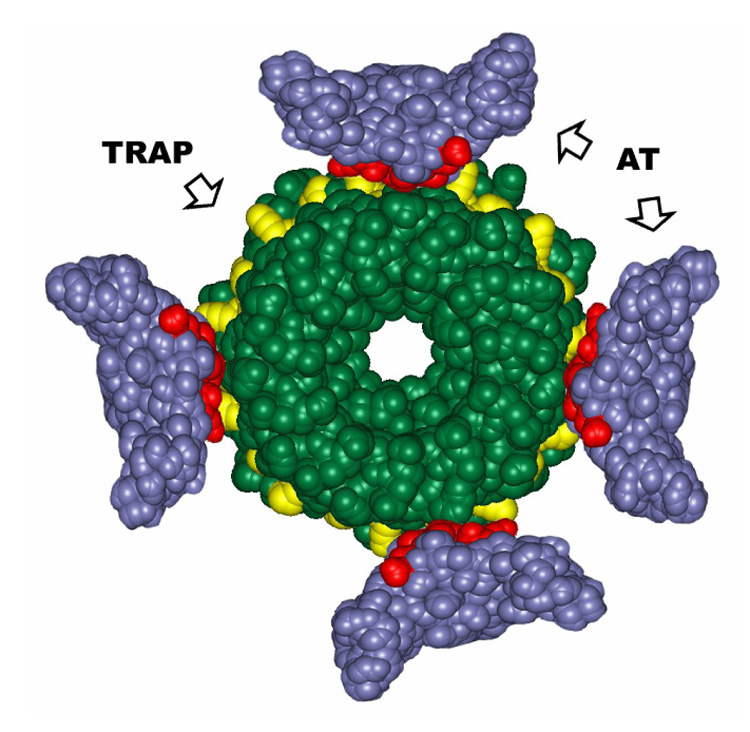
Model of AT-TRAP interaction. A TRAP 11mer and four AT trimers are shown as green and blue spacefilling models respectively and are drawn to scale. On TRAP, residues (Glu36, Lys37, Lys56, and Arg58) which interact with boud RNA are highlighted in yellow. On the four AT trimers, residues (Val2, Ile3, Asp6, Asp7), which are critical for binding to TRAP are highlighted in red. Four AT trimers are positioned around the outer perimeter of TRAP such that the AT residues (red) on top region of the trimers interact with the TRAP residues (yellow) that interact with the bound RNA.
An important feature of the model shown in Figure 7 is that AT binding would simply sterically interfere with the binding of RNA to TRAP. This feature differs from a previously proposed model in which AT binds to TRAP as dodecamer via the Zn-binding arms with the top region of each AT trimer buried within the dodecamer 26. In that model AT does not directly interact with the RNA binding region of TRAP and would not directly interfere with RNA binding to TRAP. To reconcile this model with prior studies showing that substitutions in the RNA binding region of TRAP interfere with AT binding 6, it was proposed that these substitutions in the RNA binding residues alter the conformation of TRAP in distant regions that AT recognizes 26. Similarly it was proposed that AT binding alters the conformation of the RNA binding region of TRAP indirectly through conformational changes such that it doesn’t bind RNA 26 Consistent with these proposals, recent studies have shown that tryptophan binding to TRAP 37 as well as substitution for an amino acid residue in the tryptophan binding pocket of TRAP 23 alter the conformation of distant regions of the protein. Nevertheless it is simpler to suggest that the residues in both AT (Val02, Ile03, Asp06, and Asp07) and in TRAP (Lys37, Lys56, and Arg58) that have been identified as being crucial for complex formation interact directly as we propose in Figure 7.
The suggestion that multiple AT trimers bind to the TRAP 11mer (Figure 7) raises interesting questions about the mechanism of AT’s action to prevent TRAP from binding RNA in order to regulate its target genes. For example it is not clear how many AT trimers must bind in order to inactivate TRAP. TRAP binds to 11 (G/U)AG repeats in the leader region of the trp operon to regulate transcription attenuation 3; 38. The bound RNA wraps around the outside of the TRAP ring with each triplet repeat interacting with two adjacent TRAP subunits 17; 19; 22. This binding prevents formation of an antiterminator RNA structure and allows an overlapping intrinsic terminator to form. TRAP may have to move along the nascent RNA transcript to wrap the RNA around the protein as part of its regulatory mechanism. Hence it is conceivable that as few as one or two bound AT trimers would interfere with this mechanism and prevent TRAP function. Consistent with this proposal, Yang and Yanofsky have shown that in vivo TRAP mediated regulation of the trp operon is completely alleviated under conditions when there are approximately 2 AT trimers per TRAP 11mer present 31.
It is also unclear at what point during transcription of the trp operon that AT can function to control TRAP. Prior studies have shown that AT cannot bind to TRAP after it has bound an RNA containing 11 (G/U)AG repeats 6 (Y. Chen and P. Gollnick unpublished observations. However, it is not known whether AT can bind to TRAP when it has bound less than 11 RNA triplets, such as would be the case if TRAP initiates binding to the RNA during transcription of the trp operon leader region when less than 11 triplet repeats are available. Moreover, studies by Babitzke and coworkers have shown that an RNA stem-loop that forms at the 5’ end of the trp mRNA just upstream of the first UAG repeat plays a role in attenuation control of transcription 24. Their current model proposes that during transcription of the trp leader region, TRAP first associates with the 5’ Stem-loop prior to binding to the (G/U)AG triplet repeats 24. It is possible that AT could bind to TRAP when it is associated with the 5’ Stem-loop prior to it’s interaction with the triplet repeats.
One awkward aspect of this new model is the symmetry mismatch for 4 AT3 binding to a TRAP 11-mer (Figure 7). However examples of such symmetry mismatch have been observed previously in other protein-protein interactions 39; 40; 41. Moreover, preliminary NMR studies of B. subtilis AT binding to TRAP are consistent with AT being symmetrical in the absence of TRAP and losing this symmetry upon forming a complex with TRAP (C. McElroy, M. Foster Ohio State University and P. Gollnick, unpublished observations). To reconcile this new model with the ultracentrifugation data 30, we suggest that under the conditions of those experiments (4–12 µM AT3) four AT trimers bind cooperatively to TRAP and hence complexes containing 3, 6 or 9 AT subunits per TRAP 11-mer were not observed. Further studies are clearly needed to further the models for the AT-TRAP interaction as well as its mechanism of action and we are currently pursuing such studies.
MATERIALS AND METHODS
Plasmids
Wild-type and mutant Bacillus licheniformis AT coding sequences were inserted between the NdeI and EcoRI sites of the expression plasmid pET17b (Novagen), to generate pETBlAT. B. subtilis AT proteins were expressed from pETBsAT, which contains the AT coding sequence, with codons optimized for E. coli, inserted between the NdeI and EcoRI sites of pET24a (Novagen). This plasmid was kindly provided by C. McElroy and M. Foster at Ohio State University. The native B. subtilis AT coding sequence was also inserted between the HindIII and SalI sites of the E. coli/B. subtilis shuttle plasmid pDG148 42; 43 to create pDG148rtpA, which was transformed into CYBS410pJS673 (see below) to express AT in B. subtilis. Expression of AT from this plasmid is controlled by the IPTG-inducible spac promoter 36.
Bacteria strains
E. coli strain K802 was used to propagate all plasmids. E. coli Rosetta(DE3) (Novagen) was used for expression of wild-type and mutant AT proteins. The B. subtilis strain used in this study, CYBS410pJS673-amyE∷[Ptrp(trpE’-’lacZ)]CmR · SpR · ΔrtpA-ycbK, was generously provided by Dr. Charles Yanofsky at Stanford University. The rtpA gene, which encodes AT, is deleted in this strain, which also carries a trpE’-’lacZ reporter fusion under control of the trp promoter and regulatory leader region.
Site-directed mutagenesis PCR
Mutant rtpA genes with amino acid substitutions in AT were created with the QuikChange II Site-Directed Mutagenesis Kit (Stratagene) using pETBlAT, pETBsAT or pDG148rtpA as templates. Each mutation was introduced using a pair of complementary mutagenic oligonucleotides (Integrated DNA Technologies) with the desired substitutions. PCR products were treated with DpnI (New England BioLabs) at 37°C for 1 hour to digest the original methylated plasmid template. The resulting PCR generated plasmids were transformed into E. coli K802, and individual clones were sequenced to confirm the presence of the desired substitution.
Purification of AT and TRAP proteins
Wild-type and mutant B. licheniformis and B. subtilis AT proteins were purified by slight modifications of a previously described procedure 4. E. coli Rosetta™ (DE3) (Novagen) transformed with the appropriate expression plasmid was grown in LB medium at 37°C until OD600 reached 0.4. AT expression was induced by adding isopropyl-1-thio-β-D-galactopyranoside (IPTG) to 1mM. After growth was continued for an additional 3 hours, cells were collected by centrifugation, resuspended in buffer A (100 mM Tris-Cl, pH 7.8, 1 mM dithiothreitol (DTT)). Cells were disrupted by two passages through a French Press cell at 10,000 psi (AMINCO). The lysate was cleared by centrifugation at 30,000 × g for 20 min. Streptomycin sulfate powder was added to the supernatant with stirring on ice until the final concentration was 3%, followed by stirring for an additional 20 min. After centrifugation at 30,000 × g for 20 min to remove the precipitate, the supernatant was heated to 90 °C for 8 min. The heat-treated extract was centrifuged at 30,000 × g for 20 min and the supernatant was dialyzed overnight at 5 °C versus buffer B (50 mM Tris-Cl, pH 7.8, 1 mM DTT). The sample was applied to a DEAE A-25 column (Pharmacia Fine Chemicals) equilibrated with Buffer B and eluted with a gradient from 0 to 0.5 M NaCl in Buffer B. Fractions containing AT were identified by electrophoresis on 15% SDS polyacrylamide gels, pooled and concentrated using Amicon Ultra-15 centrifugal concentrators (Millipore). After dialysis against buffer A, the sample was further purified over Sephadex G-75. AT-containing fractions were pooled, concentrated as described above, then dialyzed into buffer A containing 50% glycerol and stored at −20 °C. The purity of the protein was monitored by 15% SDS polyacrylamide gel electrophoresis and the concentration of AT was determined by BCA protein assay using BSA as standard (Pierce).
Wild-type B. stearothermophilus TRAP was expressed from pTZstmtrB in E. coli BL21(DE3) and purified as described previously 19. Wild-type B. subtilis TRAP was expressed in E. coli BL21(DE3) and purified by chromatography on phenyl agarose as described previously 44; 45.
RNA synthesis
(GAGUU)11 RNA was transcribed from HindIII-linearized pTZ18U-GAGTT11 template using T7 RNA polymerase and labeled with [α-32P] UTP (PerkinElmer Life Sciences) 46. The RNA was purified on a 6% polyacrylamide gel containing 8 M urea and extracted by a crush and soak protocol 47.
Filter binding assay of AT binding to TRAP
To measure AT binding to TRAP a previously developed filter binding assay for RNA binding to TRAP 47 was modified to incorporate competition between AT and RNA. In the presence of 1 mM tryptophan, RNA binding to TRAP reaches ~90% saturation at 20 nM TRAP. For the AT binding assay, 0 to 60 µM AT trimer was mixed with 20 nM TRAP in 95 µl Filter Binding Buffer (FBB) consisting of 16 mM HEPES pH8.9, 250 mM potassium glutamate, and 1 mM L-tryptophan. The binding reaction was incubated for 10 minutes at room temperature, which was determined to be sufficient to reach equilibrium. 1 fmol (10 pM) of 32 P-labeled (GAGUU)11 RNA (≈ 5000 dpm) and 10 µg of yeast tRNA were then added in 5 µl FBB and the reaction mixtures were incubated for an additional 10 min at room temperature. 50 µl aliquots of each binding reaction were filtered through a two membrane layer using a 96-well dot blot mini fold apparatus (Schleicher and Shuell) 48. The membranes consisted of 0.45 µm nitrocellulose (Schleicher and Shuell) on top, which retains the bound RNA, and Hybond-N+ (Amersham) on the bottom, which retains the unbound RNA. Each well was washed with 100 µl of FBB, both membranes were dried and exposed to a phosphorstorage screen (Molecular Dynamics), which was developed with a Storm phosphorimager (Molecular Dynamics). AT binding to TRAP was measured by its ability to inhibit RNA binding to TRAP. We defined % inhibition as RNAfree / (RNAbound + RNAfree), where RNAfree is the unbound RNA, and RNAbound is the RNA in complex with TRAP. The background of RNAfree in the absence of AT (20 nM TRAP) was subtracted from the RNAfree value for each sample. The data were normalized by setting the % inhibition at saturation (≥60 µM AT trimer) to 100%. The normalized % inhibition data were analyzed by fitting to a nonlinear single binding-site regression algorithm (Prism, GraphPad Software Inc.) to determine apparent dissociation constant (Kd) values.
Circular dichroism spectra
CD spectra (195–255 nm) of 200 µg/ml wild type and mutant AT proteins were recorded in 50mM sodium phosphate buffer (pH 8.0) at 37°C using a JASCO model J715 spectrapolarimeter.
Fluorescent labeling assay
5- Iodoacetamidofluorescein (5-IAF, Molecular Probes-Invitrogen) labels solvent-accessible sulfhydryl groups such as the side chain of cysteine. To test the solvent accessibility of various AT residues, 30 µM WT or Cys-substituted AT3 were incubated for 10 minutes in the absence and presence of 8.8 µM TRAP in 10 µl of 50 mM sodium phosphate buffer (pH 8.0) containing 1 mM tryptophan. 2.5 µM 5-IAF was then added and labeling reactions were incubated at room temperature. Aliquots of the labeling reactions were removed at various times and the reactions were stopped by adding 10 µl SDS-PAGE loading buffer containing 50 mM DTT, which reacts with the unincorporated 5-IAF. The samples were run on 15% SDS gels to separate labeled AT from unincorporated 5-IAF. Images of the gels were obtained with a 312-nm transilluminator and a Kodak DC290 digital camera. Intensities of the labeled AT bands were quantified using ImageQuant software (Molecular Dynamics).
β-galactosidase assay
B. subtilis CYBS410pJS673 transformed with pDG148rtpA containing the WT or mutant rtpA genes was grown at 37°C in medium consisting of VB minimal salts 49, 10mM Glucose, 0.2% acid-hydrolyzed casein, 1.32mM K2HPO4, 10 µM FeCl3, 5 µg/ml phleomycin (Sigma), in the absence or presence of 50 µg/ml L-tryptophan. Cells were harvested when the OD600 reached 0.4–0.6 and β-galactosidase activity was measured as described previously 8; 22.
Size exclusion chromatography
To compare the oligomerization states of WT and mutant AT proteins, size exclusion chromatography of AT was performed using a Biosep SEC-S2000 size exclusion column (300 × 7.8 mm; Phenomenex) and a Millipore Waters 501 HPLC system. Samples of 2 mg/ml WT or alanine substituted AT in 50 mM sodium phosphate (pH 8.0) were run on the column at a flow rate of 1 ml/min. Proteins were detected using a Millipore Waters 484 absorbance detector at wavelength of 215 nm.
ACKNOWLEDGEMENTS
The authors thank Alfred Antson for critical reading of the manuscript. We thank Gerald Koudelka and Todd Hennessey for help with CD and HPLC respectively. This work was supported by grant GM62750 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gollnick P. Regulation of the Bacillus subtilis trp operon by an RNA-binding protein. Mol Microbiol. 1994;11:991–997. doi: 10.1111/j.1365-2958.1994.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 2.Babitzke P. Regulation of transcription attenuation and translation initiation by allosteric control of an RNA-binding protein: the Bacillus subtilis TRAP protein. Curr Opin Microbiol. 2004;7:132–139. doi: 10.1016/j.mib.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Gollnick P, Babitzke P, Antson A, Yanofsky C. Complexity in regulation of tryptophan biosynthesis in Bacillus subtilis. Annu Rev Genet. 2005;39:47–68. doi: 10.1146/annurev.genet.39.073003.093745. [DOI] [PubMed] [Google Scholar]
- 4.Valbuzzi A, Yanofsky C. Inhibition of the B. subtilis regulatory protein TRAP by the TRAP-inhibitory protein, AT. Science. 2001;293:2057–2059. doi: 10.1126/science.1062187. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez-Preciado A, Yanofsky C, Merino E. Comparison of tryptophan biosynthetic operon regulation in different Gram-positive bacterial species. Trends Genet. 2007;23:422–426. doi: 10.1016/j.tig.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Valbuzzi A, Gollnick P, Babitzke P, Yanofsky C. The anti-trp RNA-binding attenuation protein (Anti-TRAP), AT, recognizes the tryptophan-activated RNA binding domain of the TRAP regulatory protein. J Biol Chem. 2002;277:10608–10613. doi: 10.1074/jbc.M111813200. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Yanofsky C. Tandem transcription and translation regulatory sensing of uncharged tryptophan tRNA. Science. 2003;301:211–213. doi: 10.1126/science.1084902. [DOI] [PubMed] [Google Scholar]
- 8.Kuroda MI, Henner D, Yanofsky C. cis-acting sites in the transcript of the Bacillus subtilis trp operon regulate expression of the operon. J Bacteriol. 1988;170:3080–3088. doi: 10.1128/jb.170.7.3080-3088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babitzke P, Yanofsky C. Reconstitution of Bacillus subtilis trp attenuation in vitro with TRAP, the trp RNA-binding attenuation protein. Proc Natl Acad Sci U S A. 1993;90:133–137. doi: 10.1073/pnas.90.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du H, Babitzke P. trp RNA-binding attenuation protein-mediated long distance RNA refolding regulates translation of trpE in Bacillus subtilis. J Biol Chem. 1998;273:20494–20503. doi: 10.1074/jbc.273.32.20494. [DOI] [PubMed] [Google Scholar]
- 11.Merino E, Babitzke P, Yanofsky C. trp RNA-binding attenuation protein (TRAP)-trp leader RNA interactions mediate translational as well as transcriptional regulation of the Bacillus subtilis trp operon. J Bacteriol. 1995;177:6362–6370. doi: 10.1128/jb.177.22.6362-6370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang M, de Saizieu A, van Loon AP, Gollnick P. Translation of trpG in Bacillus subtilis is regulated by the trp RNA-binding attenuation protein (TRAP) J Bacteriol. 1995;177:4272–4278. doi: 10.1128/jb.177.15.4272-4278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du H, Tarpey R, Babitzke P. The trp RNA-binding attenuation protein regulates TrpG synthesis by binding to the trpG ribosome binding site of Bacillus subtilis. J Bacteriol. 1997;179:2582–2586. doi: 10.1128/jb.179.8.2582-2586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarsero JP, Merino E, Yanofsky C. A Bacillus subtilis gene of previously unknown function, yhaG, is translationally regulated by tryptophan-activated TRAP and appears to be involved in tryptophan transport. J Bacteriol. 2000;182:2329–2331. doi: 10.1128/jb.182.8.2329-2331.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakhnin H, Zhang H, Yakhnin AV, Babitzke P. The trp RNA-binding attenuation protein of Bacillus subtilis regulates translation of the tryptophan transport gene trpP (yhaG) by blocking ribosome binding. J Bacteriol. 2004;186:278–286. doi: 10.1128/JB.186.2.278-286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarsero JP, Merino E, Yanofsky C. A Bacillus subtilis operon containing genes of unknown function senses tRNA Trp charging and regulates expression of the genes of tryptophan biosynthesis. Proc Natl Acad Sci U S A. 2000;97:2656–2661. doi: 10.1073/pnas.050578997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antson AA, Dodson EJ, Dodson G, Greaves RB, Chen X, Gollnick P. Structure of the trp RNA-binding attenuation protein, TRAP, bound to RNA. Nature. 1999;401:235–242. doi: 10.1038/45730. [DOI] [PubMed] [Google Scholar]
- 18.Antson AA, Otridge J, Brzozowski AM, Dodson EJ, Dodson GG, Wilson KS, Smith TM, Yang M, Kurecki T, Gollnick P. The structure of trp RNA-binding attenuation protein. Nature. 1995;374:693–700. doi: 10.1038/374693a0. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Antson AA, Yang M, Li P, Baumann C, Dodson EJ, Dodson GG, Gollnick P. Regulatory features of the trp operon and the crystal structure of the trp RNA-binding attenuation protein from Bacillus stearothermophilus. J Mol Biol. 1999;289:1003–1016. doi: 10.1006/jmbi.1999.2834. [DOI] [PubMed] [Google Scholar]
- 20.Hopcroft NH, Manfredo A, Wendt AL, Brzozowski AM, Gollnick P, Antson AA. The interaction of RNA with TRAP: the role of triplet repeats and separating spacer nucleotides. J Mol Biol. 2004;338:43–53. doi: 10.1016/j.jmb.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 21.Hopcroft NH, Wendt AL, Gollnick P, Antson AA. Specificity of TRAP-RNA interactions: crystal structures of two complexes with different RNA sequences. Acta Crystallogr D Biol Crystallogr. 2002;58:615–621. doi: 10.1107/s0907444902003189. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Chen X, Militello K, Hoffman R, Fernandez B, Baumann C, Gollnick P. Alanine-scanning mutagenesis of Bacillus subtilis trp RNA-binding attenuation protein (TRAP) reveals residues involved in tryptophan binding and RNA binding. J Mol Biol. 1997;270:696–710. doi: 10.1006/jmbi.1997.1149. [DOI] [PubMed] [Google Scholar]
- 23.Payal V, Gollnick P. Substitutions of Thr30 provide mechanistic insight into tryptophan-mediated activation of TRAP binding to RNA. Nucleic Acids Res. 2006;34:2933–2942. doi: 10.1093/nar/gkl383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGraw AP, Bevilacqua PC, Babitzke P. TRAP-5' stem loop interaction increases the efficiency of transcription termination in the Bacillus subtilis trpEDCFBA operon leader region. RNA. 2007;13:2020–2033. doi: 10.1261/rna.719507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putzer H, Condon C, Brechemier-Baey D, Brito R, Grunberg-Manago M. Transfer RNA-mediated antitermination in vitro. Nucleic Acids Res. 2002;30:3026–3033. doi: 10.1093/nar/gkf415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shevtsov MB, Chen Y, Gollnick P, Antson AA. Crystal structure of Bacillus subtilis anti-TRAP protein, an antagonist of TRAP/RNA interaction. Proc Natl Acad Sci U S A. 2005;102:17600–17605. doi: 10.1073/pnas.0508728102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen G, Yanofsky C. Features of a leader peptide coding region that regulate translation initiation for the anti-TRAP protein of B. subtilis. Mol Cell. 2004;13:703–711. doi: 10.1016/s1097-2765(04)00085-1. [DOI] [PubMed] [Google Scholar]
- 28.Valbuzzi A, Yanofsky C. Zinc is required for assembly and function of the anti-trp RNA-binding attenuation protein, AT. J Biol Chem. 2002;277:48574–48578. doi: 10.1074/jbc.M208980200. [DOI] [PubMed] [Google Scholar]
- 29.Shevtsov MB, Chen Y, Gollnick P, Antson AA. Anti-TRAP protein from Bacillus subtilis: crystallization and internal symmetry. Acta Crystallogr D Biol Crystallogr. 2004;60:1311–1314. doi: 10.1107/S0907444904011199. [DOI] [PubMed] [Google Scholar]
- 30.Snyder D, Lary J, Chen Y, Gollnick P, Cole JL. Interaction of the trp RNA-binding attenuation protein (TRAP) with anti-TRAP. J Mol Biol. 2004;338:669–682. doi: 10.1016/j.jmb.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 31.Yang WJ, Yanofsky C. Effects of tryptophan starvation on levels of the trp RNA-binding attenuation protein (TRAP) and anti-TRAP regulatory protein and their influence on trp operon expression in Bacillus subtilis. J Bacteriol. 2005;187:1884–1891. doi: 10.1128/JB.187.6.1884-1891.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabo A, Korszun R, Hartl FU, Flanagan J. A zinc finger-like domain of the molecular chaperone DnaJ is involved in binding to denatured protein substrates. Embo J. 1996;15:408–417. [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Z, Cyr DM. The conserved carboxyl terminus and zinc finger-like domain of the co-chaperone Ydj1 assist Hsp70 in protein folding. J Biol Chem. 1998;273:5970–5978. doi: 10.1074/jbc.273.10.5970. [DOI] [PubMed] [Google Scholar]
- 34.Visser L, Minnaar S, Webb GL. An automated procedure for circular dichroism and optical rotary dispersion spectroscopy. Anal Biochem. 1974;60:59–77. doi: 10.1016/0003-2697(74)90131-6. [DOI] [PubMed] [Google Scholar]
- 35.Cabiscol E, Levine RL. The phosphatase activity of carbonic anhydrase III is reversibly regulated by glutathiolation. Proc Natl Acad Sci U S A. 1996;93:4170–4174. doi: 10.1073/pnas.93.9.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yansura DG, Henner DJ. Use of the Escherichia coli lac repressor and operator to control gene expression in Bacillus subtilis. Proc Natl Acad Sci U S A. 1984;81:439–443. doi: 10.1073/pnas.81.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McElroy CA, Manfredo A, Gollnick P, Foster MP. Thermodynamics of tryptophan-mediated activation of the trp RNA-binding attenuation protein. Biochemistry. 2006;45:7844–7853. doi: 10.1021/bi0526074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gollnick P, Babitzke P. Transcription attenuation. Biochim Biophys Acta. 2002;1577:240–250. doi: 10.1016/s0167-4781(02)00455-4. [DOI] [PubMed] [Google Scholar]
- 39.Abrahams JP, Leslie AG, Lutter R, Walker JE. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 40.Hendrix RW. Symmetry mismatch and DNA packaging in large bacteriophages. Proc Natl Acad Sci U S A. 1978;75:4779–4783. doi: 10.1073/pnas.75.10.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortega J, Lee HS, Maurizi MR, Steven AC. ClpA and ClpX ATPases bind simultaneously to opposite ends of ClpP peptidase to form active hybrid complexes. J Struct Biol. 2004;146:217–226. doi: 10.1016/j.jsb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 42.Stragier P, Bonamy C, Karmazyn-Campelli C. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell. 1988;52:697–704. doi: 10.1016/0092-8674(88)90407-2. [DOI] [PubMed] [Google Scholar]
- 43.Yakhnin H, Babiarz JE, Yakhnin AV, Babitzke P. Expression of the Bacillus subtilis trpEDCFBA operon is influenced by translational coupling and Rho termination factor. J Bacteriol. 2001;183:5918–5926. doi: 10.1128/JB.183.20.5918-5926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li PT, Gollnick P. Using hetero-11-mers composed of wild type and mutant subunits to study tryptophan binding to TRAP and its role in activating RNA binding. J Biol Chem. 2002;277:35567–35573. doi: 10.1074/jbc.M205910200. [DOI] [PubMed] [Google Scholar]
- 45.Antson AA, Brzozowski AM, Dodson EJ, Dauter Z, Wilson KS, Kurecki T, Otridge J, Gollnick P. 11-fold symmetry of the trp RNA-binding attenuation protein (TRAP) from Bacillus subtilis determined by X-ray analysis. J Mol Biol. 1994;244:1–5. doi: 10.1006/jmbi.1994.1698. [DOI] [PubMed] [Google Scholar]
- 46.Otridge J, Gollnick P. MtrB from Bacillus subtilis binds specifically to trp leader RNA in a tryptophan-dependent manner. Proc Natl Acad Sci U S A. 1993;90:128–132. doi: 10.1073/pnas.90.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baumann C, Otridge J, Gollnick P. Kinetic and thermodynamic analysis of the interaction between TRAP (trp RNA-binding attenuation protein) of Bacillus subtilis and trp leader RNA. J Biol Chem. 1996;271:12269–12274. doi: 10.1074/jbc.271.21.12269. [DOI] [PubMed] [Google Scholar]
- 48.Wong I, Lohman TM. A double-filter method for nitrocellulose-filter binding: application to protein-nucleic acid interactions. Proc Natl Acad Sci U S A. 1993;90:5428–5432. doi: 10.1073/pnas.90.12.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogel HJ, Bonner DM. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- 50.Willcox BE, Gao GF, Wyer JR, Ladbury JE, Bell JI, Jakobsen BK, van der Merwe PA. TCR binding to peptide-MHC stabilizes a flexible recognition interface. Immunity. 1999;10:357–365. doi: 10.1016/s1074-7613(00)80035-7. [DOI] [PubMed] [Google Scholar]


