Heat-shock protein Hsp33 from S. cerevisiae was crystallized and diffraction data were collected to 2.7 Å resolution.
Keywords: heat-shock proteins, Hsp33
Abstract
The heat-shock protein Hsp33 from the yeast Saccharomyces cerevisiae has been overexpressed, purified and crystallized. A crystal was obtained using the hanging-drop vapour-diffusion method and a data set was collected to 2.7 Å resolution. The crystal belongs to space group P43212, with unit-cell parameters a = b = 96.43, c = 132.22 Å, α = β = γ = 90°. The asymmetric unit is assumed to contain two subunits of Hsp33, with a V M value of 2.96 Å3 Da−1 and a solvent content of 58.41%.
1. Introduction
To survive in ever-changing environments, both prokaryotic and eukaryotic organisms need to respond swiftly and appropriately in order to adapt to drastic environmental changes by adopting a specific genomic expression strategy: some genes are apparently induced, while others are repressed. During this response, a set of inducible genes encoding heat-shock proteins (Hsps), which were initially found in the response to heat shock (Lindquist & Craig, 1988 ▶), play essential roles in the correct folding and degeneration of nascent and stress-induced misfolded proteins.
The expression of HSP genes is regulated by the binding of heat-shock factors to the upstream sequences, named heat-shock elements (HSEs; Mager & Kruijff, 1995 ▶). Hsps are preferentially expressed on heat shock, cold shock, oxidative stress, osmotic pressure, starvation etc. Hsp families, including Hsp110, Hsp90, Hsp70, Hsp27 and the small Hsps, are evolutionally conserved (Lindquist & Craig, 1988 ▶). They function in various processes such as DNA replication, cell division, transcription, translation, protein folding, membrane-protein functions and protein transport (Miura et al., 2006 ▶).
The YOR391C gene of the yeast Saccharomyces cerevisiae codes for a hypothetical 25.9 kDa protein of 237 amino-acid residues which belongs to the DJ-1/ThiJ/PfpI superfamily. The YOR391Cp protein has two close homologues: it has 99.5% homology (only one differing residue) to YPL280Wp and 99.0% homology (only two differing residues) to YMR322Cp. In addition, YOR391Cp has 69% sequence identity and 21% similarity with YDR533Cp. These four S. cerevisiae proteins, YDR533Cp, YPL280Wp, YOR391Cp and YMR322Cp, were renamed Hsp31, Hsp32, Hsp33 and Hsp34, respectively, based on the structural and putative functional similarity between YDR533Cp and Escherichia coli Hsp31 (Wilson et al., 2004 ▶) as well as the high homology among the latter three proteins. The crystal structure of E. coli Hsp31 revealed a putative catalytic triad that is common in the DJ-1/ThiJ/PfpI superfamily and led to the presumption that Hsp31 is a molecular chaperone and protease (Quigley et al., 2003 ▶). An aminopetidase activity with broad specificity has been identified, but no significant protease activity has yet been reported (Malki et al., 2005 ▶). Comparison of the structures of Hsp31 and Hsp33 will provide us with more hints relevant to the investigation of their biochemical functions.
2. Materials and methods
2.1. Cloning and expression
The coding region of the HSP33 gene was amplified by PCR with S. cerevisiae S288C genomic DNA as the template and inserted into a modified pET28a vector between the NdeI and NotI sites, with an additional six-His coding sequence at the 5′ end of the genes. The construct was transformed into E. coli Rosetta (DE3) (Novagen) and the transformant bacteria were grown in 2×YT medium at 310 K to an A 600nm of 0.6–0.8. Expression of Hsp33 was induced by addition of 0.25 mM IPTG and the cells were grown for a further 28 h. The cells were collected by centrifugation. The pellets were resuspended in 10 ml 300 mM NaCl, 20 mM Tris pH 6.1 and 20 mM β-mercaptoethanol. After being lysed by three freeze–thaw cycles followed by sonication, the soluble proteins in the supernatant were recovered by centrifugation at 20 000g for 30 min.
2.2. Purification
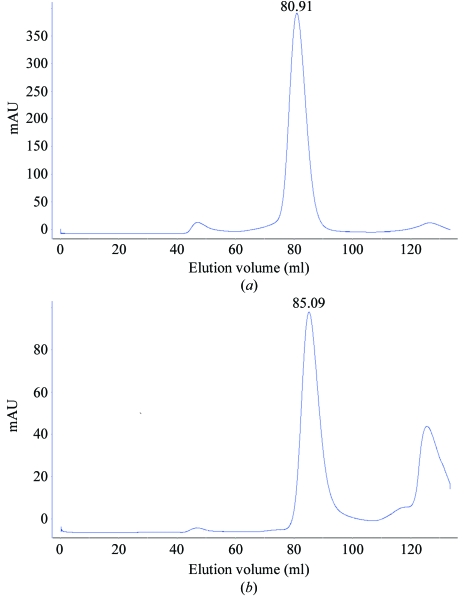
The supernatant was filtered and loaded onto a 2.5 ml Ni2+-chelating Sepharose Fast Flow column (Amersham Biosciences) equilibrated with lysis buffer. The column was washed with the same buffer containing 10 mM imidazole and the recombinant Hsp33 was then eluted with lysis buffer containing 250 mM imidazole. The eluted protein was further purified by gel filtration using a HiLoad 16/60 Superdex 75 column (Amersham Biosciences) equilibrated with buffer containing 20 mM Tris pH 6.1, 200 mM NaCl and 14 mM β-mercaptoethanol. The purity of the protein was checked by SDS–PAGE. Hsp33 eluted from the column at almost the same volume (85.09 ml) as Hsp31 (80.91 ml) (Fig. 1 ▶), which has been reported to form dimers (Graille et al., 2004 ▶); thus, Hsp33 also should be present as dimers in solution.
Figure 1.
Gel filtration of (a) Hsp31 and (b) Hsp33 using a HiLoad 16/60 Superdex 200 column.
2.3. Crystallization and data collection
Hsp33 was crystallized using the hanging-drop vapour-diffusion method. The crystals were grown from a mixture of 2 µl 10 mg ml−1 protein and 2 µl reservoir solution [15–18% PEG 3000, 200 mM (NH4)2SO4, 100 mM trisodium citrate pH 5.6, 5% glycerol]. Crystals of 0.2 × 0.2 × 2.0 mm in size appeared after 7–15 d incubation at a constant temperature of 288 K and were soaked in cryoprotectant buffer [30% glycerol, 18% PEG 3000, 200 mM (NH4)2SO4, 100 mM trisodium citrate dihytrate pH 5.6] prior to flash-freezing in liquid nitrogen.
Diffraction data were collected from a loop-mounted crystal using an RA-Mirco7 rotating copper-anode X-ray generator (Rigaku, Japan) with a MAR345 imaging-plate detector (MAR Research, Germany). The exposure time for each image was 30 min. X-ray crystallographic data were processed using AUTOMAR v.1.6 (MAR Research). The original data extended to 2.3 Å resolution, but the diffraction spots between 2.3 and 2.7 Å were too weak to be used in structure determination. A summary of the data-collection statistics used for structure determination is given in Table 1 ▶. The crystals belong to space group P43212, with unit-cell parameters a = b = 96.43, c = 132.22 Å, α = β = γ = 90°.
Table 1. Data-collection statistics for crystals of Hsp33.
Values in parentheses are for the last resolution shell.
| Resolution (Å) | 30.0–2.7 |
| Wavelength (Å) | 1.54179 |
| Space group | P43212 |
| Unit-cell parameters (Å, °) | a = b = 96.43, c = 132.22, α = β = γ = 90 |
| Total No. of reflections | 67420 |
| Total No. of unique reflections | 26349 |
| Rmerge† (%) | 10.57 (28.64) |
| Completeness (%) | 95.4 |
| I/σ(I) | 4.5 (2.2) |
| Resolution (Å) | 2.7 |
R
merge = 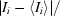
 , where I
i is the average intensity over symmetry-equivalent reflections.
, where I
i is the average intensity over symmetry-equivalent reflections.
3. Results and discussion
The open reading frame of HSP33/YOR391Cp is 714 bp in length and codes for 237 amino-acid residues. The molecular weight of Hsp33 is 27.0 kDa as determined by SDS–PAGE, which matches the theoretical molecular weight of 26 880.5 kDa. During gel-filtration chromatography, the main peak was observed at an elution volume of 85.09 ml and was very close to the elution volume of Hsp31 (80.91 ml). This implied that Hsp33 forms dimers in solution as reported for Hsp31 (Graille et al., 2004 ▶).
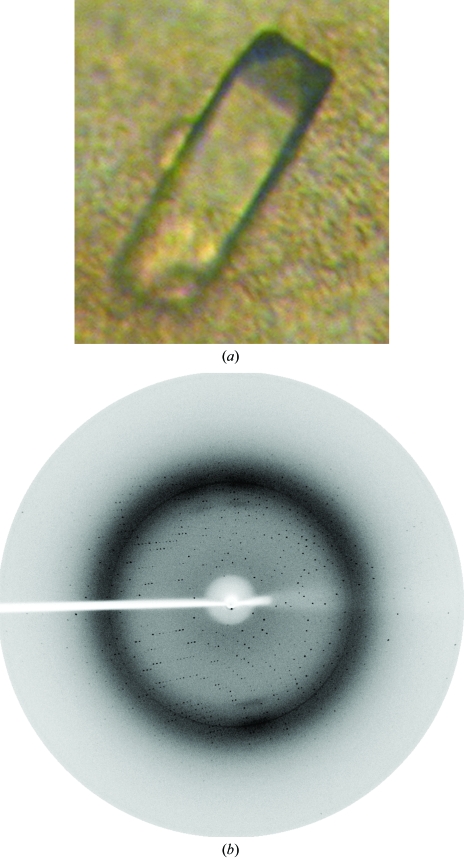
A Hsp33 crystal was obtained by the hanging-drop vapour-diffusion method in buffer consisting of 15–18% PEG 3000, 0.2 M ammonium sulfate, 0.1 M trisodium citrate pH 5.6 and 5% glycerol. After 7–15 d, a single crystal suitable for X-ray diffraction formed with dimensions of 0.2 × 0.2 × 2.0 mm (Fig. 2 ▶ a). The data revealed significant diffraction to 2.7 Å resolution (Fig. 2 ▶ b). Data processing showed that the crystal belongs to space group P43212 and data statistics are presented in Table 1 ▶. The Matthews coefficient suggests that there are two subunits in the asymmetric unit, giving a V M value of 2.96 Å3 Da−1 and a solvent content of 58.41% (Matthews, 1968 ▶). Molecular replacement was performed with CCP4 (Collaborative Computational Project, Number 4, 1994 ▶) using Hsp31 (PDB code 1qvz; 68% identity) as a search model and two solutions were found, one for each monomer in the asymmetric unit. The space group was confirmed as P43212. Structure refinement and analysis are in progress.
Figure 2.
(a) Crystal of S. cerevisiae Hsp33 obtained using the hanging-drop vapour-diffusion method. The average dimensions of these crystals were 0.2 × 0.2 × 2.0 mm. (b) X-ray diffraction pattern at a resolution of 2.7 Å.
Acknowledgments
We thank Mr Xiao-Xiao Ma for his help during gel-filtration chromatography. This work was supported by the Chinese National Natural Science Foundation (30470366 and 30121001), the 100 Talents Project of the Chinese Academy of Science and a start-up fund from USTC, the 973 Projects (2006CB806501 and 2006CB910200) and the Human Liver Structural Proteomics Project from the Ministry of Science and Technology of China.
References
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Graille, M., Quevillon-Cheruel, S., Leulliot, N., Zhou, C. Z., de la Sierra Gallay, I. L., Jacquamet, L., Ferrer, J.-L., Liger, D., Poupon, A., Janin, J. & van Tilbeurgh, H. (2004). Structure, 12, 839–847. [DOI] [PubMed] [Google Scholar]
- Lindquist, S. & Craig, E. A. (1988). Annu. Rev. Genet.22, 631–677. [DOI] [PubMed] [Google Scholar]
- Mager, W. H. & De Kruijff, A. J. (1995). Microbiol. Rev.59, 506–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malki, A., Caldas, T., Abdallah, J., Kern, R., Eckey, V., Kim, S. J., Cha, S. S., Mori, H. & Richarme, G. (2005). J. Biol. Chem.280, 14420–14426. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Miura, T., Minegishi, H., Usami, R. & Abe, F. (2006). Extremophiles, 10, 279–284. [DOI] [PubMed] [Google Scholar]
- Quigley, P. M., Korotkov, K., Baneyx, F. & Hol, W. G. (2003). Proc. Natl Acad. Sci. USA, 100, 3137–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, M. A., St Amour, C. V., Collins, J. L., Ringe, D. & Petsko, G. A. (2004). Proc. Natl Acad. Sci. USA, 101, 1531–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]