An ω-amino acid:pyruvate transaminase from C. violaceum has been purified and crystallized in two crystal forms. The structure has been solved using molecular replacement.
Keywords: ω-transaminase, pyridoxal 5′-phosphate
Abstract
The enzyme ω-transaminase catalyses the conversion of chiral ω-amines to ketones. The recombinant enzyme from Chromobacterium violaceum has been purified to homogeneity. The enzyme was crystallized from PEG 4000 using the microbatch method. Data were collected to 1.7 Å resolution from a crystal belonging to the triclinic space group P1, with unit-cell parameters a = 58.9, b = 61.9, c = 63.9 Å, α = 71.9, β = 87.0, γ = 74.6°. Data were also collected to 1.95 Å from a second triclinic crystal form. The structure has been solved using the molecular-replacement method.
1. Introduction
The transaminases (aminotransferases; EC 2.6.1) are a large group of enzymes that catalyse the reversible transfer of an amino group from amino acids to oxo acids (Mehta et al., 1993 ▶). All transaminases use the cofactor pyridoxal 5′-phosphate (PLP; vitamin B6) for catalysis. They are ubiquitously present in microorganisms and in higher organisms and play an important role in amino-acid metabolism (Christen & Metzler, 1985 ▶).
The mechanism by which transaminase enzymes act consists of two half-reactions in which the cofactor alternates between two forms: the aldehyde (PLP) and amino (PMP) forms. An amino acid (amino donor) donates its amine group to the PLP form of the enzyme (E-PLP) to produce enzyme-pyridoxamine-5′-phosphate (E-PMP) and the corresponding keto acid. In the second half-reaction, the amino acceptor accepts the amino group from E-PMP to produce the corresponding amino acid and regenerates E-PLP (Shin & Kim, 2002 ▶; Eliot & Kirsch, 2004 ▶).
Transaminases can be divided into four subgroups based on their amino-acid sequence relationship (Mehta et al., 1993 ▶). Subgroup II contains ornithine (EC 2.6.1.13), 4-aminobutyrate (EC 2.6.1.19), acetylornithine (EC.2.6.1.11), diaminopelargonate (EC.2.6.1.62) and ω-amino acid:pyruvate transaminases (EC 2.6.1.18). Within this subgroup, the distal amino group of the substrate undergoes transamination. These transaminases can therefore be regarded collectively as ω-transaminases. Among these ω-transaminases, only ω-amino acid:pyruvate transaminase shows catalytic activity towards primary amines and aliphatic amines without a carboxyl group (Yonaha et al., 1987 ▶; Shin & Kim, 1999 ▶; Shin et al., 2003 ▶).
The major interest in bacterial ω-transaminases is their industrial importance, as they can be used as biocatalysts for the asymmetric synthesis of homochiral amines of high enantioselective purity (Shin & Kim, 1999 ▶; Cho et al., 2003 ▶; Yun et al., 2003 ▶). The ω-transaminases from Vibrio fluvialis JS17, Klebsiella pneumoniae JS2F and Bacillus thuringiensis JS64 show high enantioselectivity for certain enantiomers of chiral amines such as (S)-α-methylbenzylamine (Shin & Kim, 1998 ▶; Shin & Kim, 2001 ▶). The ω-amino acid:pyruvate transaminase from Chromobacterium violaceum has a molecular weight of 51 213 Da. It shows 38% sequence similarity to the ω-transaminase from V. fluvialis JS17. During the ω-transaminase reaction, the amino donor, for example methylbenzylamine, is converted to the ketone acetophenone and the amino acceptor pyruvate, which is converted to the α-amino acid alanine (Hwang & Kim, 2004 ▶). Their relaxed substrate specificity, rapid reaction rates and no requirement for external cofactor regeneration makes transaminase enzymes attractive biocatalysts compared with chemical methods for the production of chiral amines (Taylor et al., 1998 ▶).
Several structures of subgroup II transaminases have been determined to date. The most closely related structure to C. violaceum ω-transaminase is that of diaminopelargonic acid synthase (PDB code 1qj3; Kack et al., 1999 ▶), which shares 26% amino-acid sequence identity. The transaminases of known three-dimensional structure that have higher than 20% sequence identity to C. violaceum ω-transaminase are all dimeric molecules.
2. Materials and methods
2.1. Protein purification
The gene coding for the ω-amino acid:pyruvate transaminase was cloned into the expression vector pET29a (Novagen) incorporating a N-terminal six-His tag and was overexpressed in Escherichia coli BL21 Star (DE3) pLysS (Kaulmann et al., 2007 ▶). E. coli BL21 Star (pLysS) cells harbouring the pET29a vector containing the transaminase gene were grown on Luria–Bertani medium at 310 K to an optical density at 600 nm of 0.8 and were induced with 1 mM isopropyl β-d-1-thiogalactopyranoside for 5 h.
The harvested E. coli cells containing the expressed protein were resuspended in 50 mM Tris–HCl pH 7.5 (buffer A) and sonicated for ten 15 s cycles. The insoluble cell debris was removed by centrifugation at 12 000g for 20 min at 277 K.
The soluble fraction was applied onto a 30 ml nickel-affinity chromatography column (Nickel Sepharose Hi-Performance, GE Healthcare) pre-equilibrated with buffer A. A linear gradient was used over five column volumes leading to 100% buffer B (50 mM Tris–HCl pH 7.5, 1 M imidazole) at a flow rate of 3 ml min−1 and was collected in 5 ml fractions. Active fractions were pooled and the protein was precipitated with 80% ammonium sulfate, centrifuged at 12 000g for 20 min and redissolved in buffer C (50 mM Tris–HCl pH 7.5, 100 mM NaCl). The protein was applied onto a 120 ml gel-filtration column (Superdex 200 Hiload 16/60, GE Healthcare) pre-equilibrated with buffer C at a flow rate of 0.5 ml min−1 and collected in 1 ml fractions. All purification steps were carried out at 277 K. The purity of the protein was analysed by SDS–PAGE (Laemmli, 1970 ▶) and the concentration of the protein was estimated using the absorbance at A 280; the extinction coefficient was calculated to be 80 245 M −1 cm−1 from the protein sequence using the ExPASY database (Bairoch & Apweiler, 2000 ▶).
2.2. Crystallization
Crystallization was carried out by the microbatch method using an Oryx 8 automated robot (Douglas Instruments) at 290 K. Prior to concentration, 100 µM PLP was added to the protein solution. The protein was concentrated using a 30 kDa molecular-weight cutoff Vivaspin centrifugal concentrator (Sartorius, UK) to 10 mg ml−1 in 50 mM Tris–HCl pH 7.5, 100 mM NaCl. Initial crystallization screens were performed using Molecular Dimensions Structure Screens 1 and 2. 1 µl protein solution was added to 1 µl crystallization buffer, giving a final protein concentration of 5 mg ml−1. The droplets were covered with a 50:50(v:v) mix of silicon and paraffin oil.
Two potential crystallization conditions were found: 0.05 M HEPES pH 7.5, 5%(v/v) 2-propanol, 10%(w/v) PEG 4000 (condition 1) and 0.1 M lithium sulfate monohydrate, 0.05 M Tris–HCl pH 8.5, 15%(w/v) PEG 4000 (condition 2). However, for both conditions both the droplet and crystals appeared to be colourless. Crystallization was optimized for the second of these conditions over a range of concentrations of protein, lithium sulfate, Tris–HCl and PEG 4000. Further optimizations were carried out with the addition of PLP to the crystallization droplet to achieve a final concentration of 100 µM. Crystals grown under these conditions were of a yellow colour. Crystallization condition 1 was not pursued at this stage because of the quality of the crystals obtained using condition 2, which were of better quality and size.
2.3. Crystallographic data collection
Data were collected in-house using a rotating-anode generator (Bruker AXS) operated at 100 mA and 35 kV (3.5 kW) producing Cu Kα radiation. No cryogenic liquor was used and the crystals were transferred directly from the crystal drop into liquid nitrogen. Data were collected under cryocooling conditions (in a 100 K nitrogen-gas stream) using a MAR Research 345 image plate. The rotation increment for data collection was 0.5° per frame. Data were collected from two crystals. Data from the first crystal (colourless in appearance, grown without addition of PLP to the crystal droplet) were collected over 620°. The blind region was minimized by bending the loop after 360° of data collection. Data from a second crystal (yellow in colour, grown with PLP added to the crystal droplet) were collected over 245°. The data were processed using the programs DENZO and SCALEPACK (Otwinowski & Minor, 1997 ▶). The data sets were processed without specifying any resolution ranges corresponding to ice rings to be rejected; a significant number of reflections in these resolution ranges were rejected as having poor background.
3. Results and discussion
The purified recombinant ω-transaminase migrated on SDS–PAGE as a single band of approximately 50 kDa. However, gel-filtration analysis carried out in 50 mM Tris–HCl pH 7.5, 0.1 M NaCl estimated the protein to have a weight of approximately 100 kDa, suggesting that the enzyme exists as a homodimer.
Crystals of form A were grown in 0.1 M lithium sulfate, 0.05 M Tris–HCl pH 8.5, 15%(w/v) PEG 4000 in the absence of the addition of any PLP to the crystal droplet and grew to dimensions of 0.5 × 0.4 × 0.2 mm (Fig. 1 ▶). A crystal of this form diffracted to 1.67 Å in-house and belonged to the triclinic space group P1, with unit-cell parameters a = 58.9, b = 61.9, c = 63.9 Å, α = 71.9, β = 86.9, γ = 74.6°. The self-rotation function suggests that the unit cell of the triclinic crystal contains a transaminase dimer, which gives a solvent content of 40.4% and a V M = 2.08 Å3 Da−1 (Matthews, 1968 ▶).
Figure 1.
The triclinic form A crystal of C. violaceum ω-amino acid:pyruvate transaminase.
Crystal form B was grown in 0.15 M lithium sulfate, 0.05 M Tris–HCl pH 8.5, 12%(w/v) PEG 4000 with 100 µM PLP added to the crystal droplet. These crystals grew to dimensions of 0.4 × 0.3 × 0.15 mm and were slightly yellow in colour, suggesting the presence of PLP. These crystals had the same habit as crystal form A. The crystals diffracted to 1.95 Å in-house and belonged to the triclinic space group P1, with unit-cell parameters a = 61.9, b = 62.4, c = 119.4 Å, α = 74.7, β = 82.3, γ = 75.4°. This crystal form contains two transaminase dimers in the asymmetric unit that are related by pseudotranslation. The native Patterson synthesis calculated between 15 and 4 Å resolution shows a significant peak at (0.0, 0.5, 0.5) which is 14.1σ or 14% of the origin peak in height. The solvent content of the crystals has been estimated as 40.8%; V M = 2.09 Å3 Da−1. This triclinic unit cell appears to be comprised of two unit cells of crystal form A. It would appear that this doubling of the unit-cell volume is caused by the presence of PLP in the crystallization solution as the crystal of form B was yellow while the crystals of form A were colourless.
A range of mother liquors and cryoprotectants were tested using various mixtures of PEG 400, PEG 4000 and glycerol. A significant deterioration in crystal diffraction, with both reduced resolution and increased mosaicity, was observed for all cryoprotectants tested. As no suitable cryogenic liquor was found, the data sets were collected and were processed with ice rings. This resulted in some reflections in the corresponding resolution shells being lost during data processing. Freezing the crystal under silicon oil is being attempted in order to improve the ice-ring problem. A summary of the X-ray data statistics is shown in Table 1 ▶ for both crystal forms.
Table 1. Data-collection statistics.
Values in parentheses are for the outer resolution shell.
| Crystal form A | Crystal form B | |
|---|---|---|
| Resolution range (Å) | 15–1.67 (1.70–1.67) | 15–1.95 (1.98–1.95) |
| No. of measured reflections | 504954 | 231882 |
| No. of unique reflections | 86906 | 96563 |
| Completeness | 90.7 (81.1) | 79.7 (81.5) |
| I > 3σ(I) (%) | 73.6 (34.2) | 61.9 (40.8) |
| I/σ(I) | 25.1 (3.4) | 16.6 (2.2) |
| Rsym† (%) | 5.6 (25.1) | 4.6 (37.2) |
R
sym = 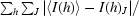
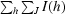 , where I(h) is the intensity of reflection h,
, where I(h) is the intensity of reflection h,  is the sum over all reflections and
is the sum over all reflections and  is the sum over J measurements of the reflection.
is the sum over J measurements of the reflection.
Molecular-replacement studies were carried out with the program MOLREP (Vagin & Teplyakov, 1997 ▶) using chain A of 7,8-diaminopelargonic acid synthase as a model (PDB code 1qj3; Kack et al., 1999 ▶), which has 26% amino-acid sequence identity to the ω-aminotransferase. The resulting model was refined in REFMAC (Murshudov et al., 1997 ▶). Manual model rebuilding was carried out using Coot (Emsley & Cowtan, 2004 ▶). Crystal form B was solved by molecular replacement (MOLREP) using the model partially refined in crystal form A.
No electron density corresponding to PLP was observed at the cofactor-binding site in crystal form A (colourless), suggesting that this crystal form is that of the apoenzyme. Crystal form B (yellow-coloured) contains PLP, but disappointingly with only partial occupancy, which differs between the subunits. We attribute this low occupancy to a low affinity of the protein for PLP and to the competition of sulfate ions for cofactor binding under the crystallization conditions. Crystallization condition 1 is currently being reinvestigated for the ability to obtain full PLP occupancy, since these conditions do not contain lithium sulfate.
The binding of PLP appears to cause significant conformational changes in the protein structure. These changes differ between the two dimers, which are crystallographically equivalent in crystal form A. This non-equivalence results in a doubling of the volume of the triclinic unit cell in crystal form B in relation to crystal form A. The structures of ω-transaminase obtained from both crystal forms are currently being refined.
Acknowledgments
The School of Biosciences is acknowledged for PhD studentship funding to CS. Professor John Ward and Dr Ursula Kaulmann are thanked for providing the E. coli overexpression system for C. violaceum ω-amino acid:pyruvate transaminase and for useful discussions.
References
- Bairoch, A. & Apweiler, R. (2000). Nucleic Acids Res.28, 45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, B., Cho, H., Park, S., Yun, H. & Kim, B. (2003). Biotechnol. Bioeng.81, 783–789. [DOI] [PubMed] [Google Scholar]
- Christen, P. & Metzler, D. E. (1985). Transaminases. New York: Wiley.
- Eliot, A. C. & Kirsch, J. F. (2004). Annu. Rev. Biochem.73, 383–415. [DOI] [PubMed] [Google Scholar]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- Hwang, B. Y. & Kim, B. G. (2004). Enzyme Microb. Technol.34, 429–436.
- Kack, H., Sandmark, J., Gibson, K., Schneider, G. & Lindqvist, Y. (1999). J. Mol. Biol.291, 857–876. [DOI] [PubMed] [Google Scholar]
- Kaulmann, U., Smithies, K., Smith, M., Hailes, H. & Ward, J. (2007). Submitted.
- Laemmli, U. K. (1970). Nature (London), 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Mehta, P. K., Hale, T. I. & Christen, P. (1993). Eur. J. Biochem.214, 259–261. [DOI] [PubMed] [Google Scholar]
- Murshudov, G. N., Vagin, A. A. & Dodson, E. J. (1997). Acta Cryst. D53, 240–255. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.267, 21839–21843. [DOI] [PubMed]
- Shin, J. S. & Kim, B. G. (1998). Biotechnol. Bioeng.60, 534–540. [DOI] [PubMed] [Google Scholar]
- Shin, J. S. & Kim, B. G. (1999). Biotechnol. Bioeng.65, 206–211. [PubMed] [Google Scholar]
- Shin, J. S. & Kim, B. G. (2001). Biosci. Biotechnol. Biochem.65, 1782–1788. [DOI] [PubMed] [Google Scholar]
- Shin, J. S. & Kim, B. G. (2002). Biotechnol. Bioeng.77, 832–837. [DOI] [PubMed] [Google Scholar]
- Shin, J. S., Yun, H., Jang, J. W., Park, I. & Kim, B. G. (2003). Appl. Microbiol. Biotechnol.61, 463–471. [DOI] [PubMed] [Google Scholar]
- Taylor, P. P., Pantaleone, D. P., Senkpeil, R. F. & Fotheringham, I. G. (1998). Trends Biotechnol.16, 412–418. [DOI] [PubMed] [Google Scholar]
- Vagin, A. A. & Teplyakov, A. (1997). J. Appl. Cryst.30, 1022–1025. [Google Scholar]
- Yonaha, K., Toyama, S. & Soda, K. (1987). Methods Enzymol.143, 500–504. [DOI] [PubMed] [Google Scholar]
- Yun, H., Yang, Y. H., Cho, B. K., Hwang, B. Y. & Kim, B. G. (2003). Biotechnol. Lett.25, 809–814. [DOI] [PubMed] [Google Scholar]



