Abstract
Background
In people with diabetes, blood glucose levels should be monitored regularly to prevent serious complications associated with diabetes. This involves the invasive method of withdrawing blood, which causes inconvenience to patients. The objective of this study was to investigate the efficiency of the noninvasive electroporation and transcutaneous sampling (ETS) technique for predicting blood glucose levels.
Methods
In vitro studies were carried out in Franz diffusion cells using porcine epidermis to assess the feasibility of transcutaneous sampling of glucose. In vivo, the ETS technique was assessed in the diabetes-induced Sprague—Dawley rat model. Glucose was sampled following the application of 30 electrical pulses of 1 ms duration at 120 V/cm2, 1 Hz. Clarke error grid analysis was carried out for the venous blood glucose levels that were determined by the ETS with reference to those measured by a glucose meter.
Results
The amount of glucose sampled by the ETS method both in vitro and in vivo was proportional to the dermal glucose concentration. All data points from in vivo studies were in A and B zones of Clarke error grid analysis, and the mean absolute relative error was 12.8%.
Conclusion
Results of the present study demonstrate that ETS technique could be developed as a noninvasive method of predicting venous blood glucose levels in people with diabetes.
Keywords: electroporation and transcutaneous sampling, glucose, in vitro, in vivo
Introduction
Diabetes mellitus is a major health concern. Diabetes can lead to chronic complications such as heart disease, blindness, renal failure, peripheral vascular disease, and limb amputation.1,2 For proper diabetes management, it is very important that blood glucose levels are checked regularly. This involves the invasive method of collecting blood through a finger stick system, which has been a major inconvenience for patients.3 Some patients avoid glucose measurements due to needle phobia. To overcome this problem, different noninvasive blood glucose monitoring methods have been developed, including near-infrared spectroscopy, far-infrared spectroscopy, radio wave impedance, reverse iontophoresis, ultrasound, blister technique, and microneedles.4–8 However, none of the methods are in clinical use. Electroporation and transcutaneous sampling (ETS) is a noninvasive method of sampling drugs and analytes from the dermal extracellular fluid by reversible electrical permeabilization of the skin.9 The ETS technique involves application of a few short electrical pulses on the surface of the skin, which permeabilize the stratum corneum by creating transient aqueous pathways. The dermal glucose diffuses into the sampling fluid, which is in contact with the permeabilized region of the skin. The objective of this study was to investigate the feasibility of utilizing the ETS technique to sample glucose in the dermal extracellular fluid that correlates with blood glucose levels.
Materials and Methods
Chemicals
Glucose, alloxan monohydrate, and the glucose assay kit (GAHK-20) were purchased from Sigma-Aldrich Inc. (St. Louis, MO); 10X phosphate-buffered saline (PBS) premixed powder was obtained from EMD Chemicals (Gibbstown, NJ).
Skin
Porcine belly skin was obtained from a local abattoir. Pieces of skin wrapped in aluminum foil were heated to 60°C for 2 min, and the epidermis was gently peeled off the skin. The fresh epidermis was used for in vitro glucose sampling by electroporation.
In Vitro Experimental Arrangement
In vitro diffusion studies were carried out in Franz diffusion cells (Logan Instruments Ltd., Somerset, NJ) using porcine epidermis collected from three animals. The stratum corneum side of the skin was in contact with the upper sampling compartment and the ventral side with the reservoir compartment. The active diffusion area was 0.64 cm2. Ag/AgCl electrode wires 2 mm in diameter (obtained from In Vivo Metric, Heraldsburg, CA) made in the form of circular ring were placed 2 mm away from the skin in both the sampling and the reservoir compartments. The upper sampling compartment and the reservoir compartment were filled with 0.4 and 5 ml PBS, respectively. The electrical resistance of the epidermis was measured by placing a load resistor RL (100 kΩ) in series with the epidermis. Voltage drop across the whole circuit (VO) and across the epidermis (VS) were measured using a multimeter (Agilent Technologies, Santa Clara, CA). The epidermis resistance in kΩ was approximated from the following formula:
| (1) |
where RS is the epidermis resistance and RL is the load resistor in kΩ. The piece of porcine epidermis with a resistance greater than 20 kΩ·cm2 was used for the experiment.
In Vitro Glucose Sampling by Electroporation
The upper sampling compartment was filled with 0.4 ml of PBS, pH 7.4, and the lower reservoir compartment was filled with 5 ml of glucose solution in PBS concentrations between 50 and 400 mg/dl. Electroporation was carried out using an ECM 830 electrosquare porator (BTX Harvard Apparatus, Holliston, MA). The electroporation protocol was 30 pulses each of 1 ms duration at 120 V/cm2 of active diffusion area. PBS from the sampling compartment was withdrawn 15 minutes after the application of electrical pulses, and the amount of glucose was measured using the glucose assay kit by ultraviolet light at 340 nm. The in vitro permeability coefficient, Pin vitro (cm/min) was calculated using the formula:
| (2) |
In Vivo Experimental Arrangement
The in vivo experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Mississippi (Protocol #07-028). In vivo studies were carried out in Sprague–Dawley rats (Harlan Sprague–Dawley, Indianapolis, IN) (200–224 g) under ketamine (80 mg/kg) and xylazine (10 mg/kg) anesthesia. The moisture content in the epidermal layers and in the dermis of each rat was measured using the Delfin moisture meter-SC and the Delfin moisture meter-D (Delfin Technologies Ltd., Kuopio, Finland) before sampling to ensure the absence of edema or inflammation.10
Glucose sampling by ETS was carried out using a custom-made sampling cell. The back portion of rats was shaved, and a custom-made in vivo electroporation cell was fixed using an adhesive (Krazy Glue, Elmers Products Inc., Columbus, OH). The cell contains a sample collection chamber in which one of the Ag/AgCl electrodes was placed and the other electrode, which acts as a counterelectrode, was fixed just adjacent to the cell on the surface of the skin using micropore surgical tape (3M Healthcare, St. Paul, MN) (Figure 1). The skin was hydrated with 100 μl of saline for 5 minutes before each sampling and was replaced with 400 μl of sampling buffer. Thirty electrical pulses each of 1 ms duration at 120 V/cm2 were applied, and the sampling fluid remained in the chamber for 15 minutes after pulsing. The sampling fluid was withdrawn, and the amount of glucose present was measured using the glucose assay kit (the limits of detection were 0.1–5 mg/dl).
Figure 1.
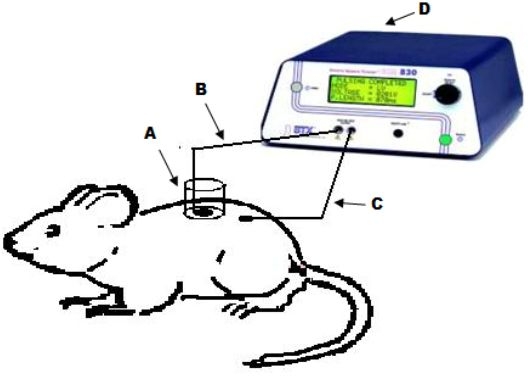
Diagram representing the in vivo experimental setup. A sampling chamber (A) was glued on the skin surface of a Sprague–Dawley rat. Ag/AgCl electrodes (B and C) were placed in the sampling chamber and secured on the skin surface, respectively, and 0.4 ml of sampling buffer was placed in the chamber. The two electrodes were connected to the BTX 830M electrosquare porator and electrical pulses were applied. The sampling buffer was collected after 15 minutes and the amount of glucose present was measured.
In Vivo Transcutaneous Permeability Coefficient
The calibration used to convert sampling chamber glucose to venous equivalent values is nothing but determination of the permeability coefficient in rats having steady normal glucose levels. Constant venous glucose levels were confirmed by triplicate samples. Venous glucose levels and corresponding ETS glucose levels of 18 data points in normal rats were used for calibration. The normal venous glucose range in Sprague–Dawley rats is ∼150–180 mg/dl.11 The in vivo permeability coefficient, Pin vivo (cm/min), of rat skin was calculated using the formula:
| (3) |
Sampling in Diabetes-Induced Rats
Nine rats were used, of which three were in the control group and six were in the test group. Transcutaneous sampling of glucose by ETS was carried out in the test group only. In the control group, transcutaneous sampling was carried out without the application of electrical pulses. All nine rats were injected with alloxan (200 mg/kg in saline) intraperitoneally. Generally the duration required to induce irreversible diabetes with alloxan is ∼24 hours.12 Blood and transcutaneous samples were obtained in all rats before injecting alloxan and also after 24 and 36 hours after the injection of alloxan. The venous blood glucose was measured using a glucose meter (True Track Smart System).
From the ETS glucose levels, venous blood glucose concentrations are predicted using the formula:
| (4) |
Data Analysis
The clinical utility of the method was determined based on the plot of data on a Clarke error grid and determining the percentage of points located in the clinically acceptable A and B zones. Mean absolute relative error was also calculated to estimate the clinical accuracy10 using the formula:
| (5) |
Results and Discussion
Blood glucose is in homoeostasis with the peripheral tissue extracellular fluid. Therefore, any change in the blood glucose is reflected in glucose levels in the tissue extracellular fluid. The transdermal permeability of a polar diffusant such as glucose is limited because of the barrier properties of stratum corneum. Therefore, transcutaneous sampling of glucose requires a technique that can reversibly permeabilize the stratum corneum and facilitate the rapid diffusion of glucose from dermal fluid into the sampling fluid. In vitro diffusion studies were carried out across the porcine epidermis to assess the feasibility of ETS for sampling glucose. Sampling without the application of electrical pulses (control) was not possible, as the glucose transfer by passive diffusion was below detectable levels, whereas a significant amount of glucose diffused following electroporation of the porcine epidermis. The electrical resistance of the epidermis dropped by an average of 70 ± 6% by the application of electrical pulses, indicating permeabilization of the skin. In vitro ETS data are represented in Figure 2. Along with other factors, the rate of diffusion is governed predominantly by the concentration of glucose in the dermal extracellular fluid (presuming that the concentration of glucose in the sampling compartment is negligible compared to the total glucose concentration in the reservoir compartment. Therefore, the amount of glucose sampled by ETS was proportional to the concentration of glucose in the reservoir compartment fluid (R2 = 0.93). The in vitro permeability coefficient (Pin vitro) of porcine epidermis permeabilized by electroporation was 1.9 ± 0.3 × 10−4 cm/min.
Figure 2.
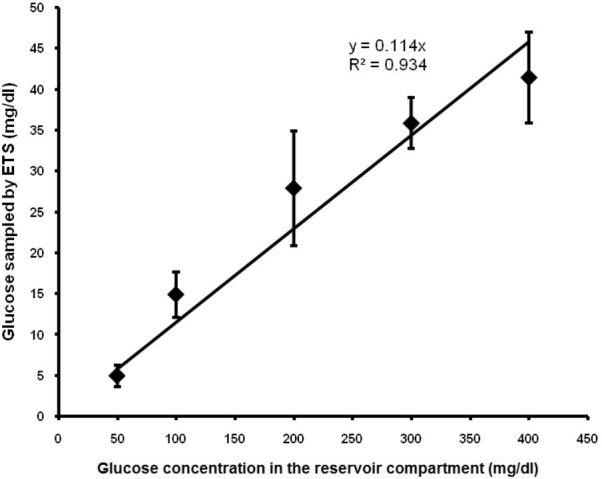
Relationship between glucose concentration in the reservoir compartment and glucose sampled by ETS across porcine epidermis in vitro.
Preclinical studies were carried out in a diabetes-induced Sprague–Dawley rat model to assess the workability of the technique in vivo. The moisture content in the rat skin was measured to ensure that there was no edema/inflammation10 in the region of glucose sampling. The measurement also confirms the uniformity of degree of hydration of epidermal layers of skin. The Delfin moisture meters measure the skin moisture contents in terms of dielectric constant of the skin. The average dielectric constant values from moisture meters SC and D were 9.94 ± 1.65 and 20.67 ± 3.31, respectively. The in vivo permeability coefficient (Pin vivo) of rat skin for glucose was found to be 1.95 ± 0.15 × 10−5 cm/min. Again a good linear correlation between the venous glucose levels and the amount of glucose sampled by ETS was observed (R2 = 0.87).
Generally, Clarke error grid analysis is carried out to assess the clinical utility of glucose monitoring devices. The analysis divides the reference and measured glucose into five zones—A, B, C, D, and E. Values in zones A and B are considered clinically acceptable, whereas values in zones C, D, and E lead to significant errors.13 At this stage we do not know if one can translate results from rat model experiments to human application. However, assessing preclinical data from a clinical perspective would provide some insight into the clinical applicability of the technique. The venous blood glucose determined by blood sampling (x axis) (reference values) and that predicted by the ETS techniques (y axis) are shown in Figure 3. All data points were within Clarke error grid A and B zones (Figure 3). A mean absolute relative error of 12.8% was found for all measurements (n = 18) from in vivo data.
Figure 3.
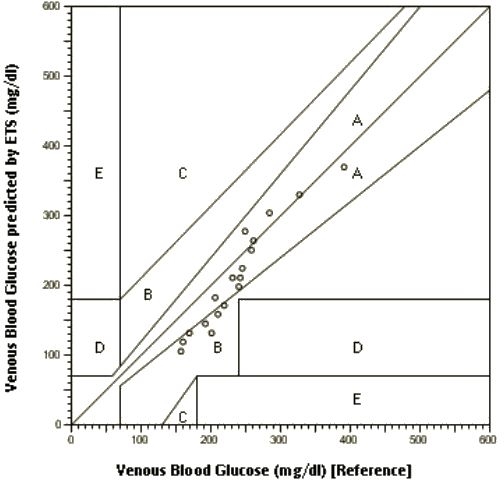
Clarke error grid analysis of venous blood glucose (reference) and venous blood glucose predicted by ETS in Sprague–Dawley rats.
Conclusion
Results support our hypothesis that the concentration of glucose sampled by ETS would be proportional to the concentration of glucose in the dermal extracellular fluid. ETS could be developed as a noninvasive method of blood sampling for glucose monitoring in people with diabetes. However, clinical studies need to be carried out to assess the workability of the ETS technique for human applications.
Acknowledgements
We acknowledge “The Epsilon Group,” Virginia for their support in carrying out Clarke error grid analysis. We also acknowledge Delfin Technologies Ltd., Kuopio, Finland, for lending us the moisture meters.
Abbreviations
- ETS
electroporation and transcutaneous sampling
- PBS
phosphate-buffered saline
References
- 1.Lee S, Nayak V, Dodds J, Pishko M, Smith NB. Glucose measurements with sensors and ultrasound. Ultrasound Med Biol. 2005;31(7):971–977. doi: 10.1016/j.ultrasmedbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Sieg A, Guy RH, Delgado-Charro MB. Noninvasive glucose monitoring by reverse iontophoresis in vivo: application of the internal standard concept. Clin Chem. 2004;50(8):1383–1390. doi: 10.1373/clinchem.2004.032862. [DOI] [PubMed] [Google Scholar]
- 3.Sieg A, Guy RH, Delgado-Charro MB. Reverse iontophoresis for noninvasive glucose monitoring: the internal standard concept. J Pharm Sci. 2003;92(11):2295–2302. doi: 10.1002/jps.10492. [DOI] [PubMed] [Google Scholar]
- 4.Klonoff DC. Noninvasive blood glucose monitoring. Diabetes Care. 1997;20(3):433–437. doi: 10.2337/diacare.20.3.433. [DOI] [PubMed] [Google Scholar]
- 5.Sieg A, Guy RH, Delgado-Charro MB. Noninvasive and minimally invasive methods for transdermal glucose monitoring. Diabetes Technol Ther. 2005;7(1):174–196. doi: 10.1089/dia.2005.7.174. [DOI] [PubMed] [Google Scholar]
- 6.Rong L, Bin D, Wenliang C, Kevin X. Next step of noninvasive glucose monitor by NIR technique from the well controlled measuring condition and results. Opt Quant Electron. 2005;37:1305–1317. [Google Scholar]
- 7.Mitragotri S, Coleman M, Kost J, Langer R. Analysis of ultrasonically extracted interstitial fluid as a predictor of blood glucose levels. J Appl Physiol. 2000;89(3):961–966. doi: 10.1152/jappl.2000.89.3.961. [DOI] [PubMed] [Google Scholar]
- 8.Wang PM, Cornwell M, Prausnitz MR. Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol Ther. 2005;7(1):131–141. doi: 10.1089/dia.2005.7.131. [DOI] [PubMed] [Google Scholar]
- 9.Murthy SN, Zhao YL, Hui SW, Sen A. Electroporation and transcutaneous extraction (ETE) for pharmacokinetic studies of drugs. J Control Release. 2005;105(1–2):132–141. doi: 10.1016/j.jconrel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Monkkonen J, Lahtinen MR, Nuutinen J, Lahtinen T. Measurement of edema in irritant-exposed skin by a dielectric technique. Skin Res Technol. 2006;12(4):235–240. doi: 10.1111/j.0909-752X.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 11.Brands MW, Hopkins TE. Poor glycemic control induces hypertension in diabetes mellitus. Hypertension. 1996;27(3 Pt 2):735–739. doi: 10.1161/01.hyp.27.3.735. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho EN, Carvalho NA, Ferreira LM. Experimental model of induction of diabetes mellitus in rats. Acta Cir Bras. 2003;18 [Google Scholar]
- 13.Tamada JA, Bohannon NJ, Potts RO. Measurement of glucose in diabetic subjects using noninvasive transdermal extraction. Nat Med. 1995;1(11):1198–1201. doi: 10.1038/nm1195-1198. [DOI] [PubMed] [Google Scholar]


