Monoclinic crystals of NovP, an O-methyltransferase from S. spheroides, were obtained and native X-ray data to 1.4 Å resolution were recorded.
Keywords: NovP, O-methyltransferase, Streptomyces, novobiocin, antibiotic biosynthesis
Abstract
Crystals of recombinant NovP (subunit MW = 29 967 Da; 262 amino acids), an S-adenosyl-l-methionine-dependent O-methyltransferase from Streptomyces spheroides, were grown by vapour diffusion. The protein crystallized in space group P2, with unit-cell parameters a = 51.81, b = 46.04, c = 61.22 Å, β = 104.97°. Native data to a maximum resolution of 1.4 Å were collected from a single crystal at the synchrotron. NovP is involved in the biosynthesis of the aminocoumarin antibiotic novobiocin that targets the essential bacterial enzyme DNA gyrase.
1. Introduction
The aminocoumarin antibiotics competitively inhibit the ATPase activity of DNA gyrase, a validated drug target (Maxwell, 1997 ▶; Maxwell & Lawson, 2003 ▶), and thus have considerable therapeutic potential. The three main compounds novobiocin, clorobiocin and coumermycin A1 share common structural features, namely a 3-amino-4,7-dihydroxycoumarin moiety, an l-noviosyl sugar and an aromatic acyl component attached to the amino group of the aminocoumarin moiety. Crystallographic studies on aminocoumarin complexes of DNA gyrase (Lewis et al., 1996 ▶; Holdgate et al., 1997 ▶; Tsai et al., 1997 ▶) have shown that methyl groups form important hydrophobic interactions with the target enzyme and thus may have profound effects on the potency of these antibiotics. Here, we report the crystallization and preliminary X-ray analysis of NovP, an S-adenosyl-l-methionine-dependent O-methyltransferase from Streptomyces spheroides responsible for the methylation of the 4′-hydroxyl group of the noviose sugar, which represents the penultimate step in novobiocin biosynthesis (Freel Meyers et al., 2004 ▶).
2. Materials and methods
2.1. Protein expression, purification and crystallization
The S. spheroides novP gene was amplified by PCR using S. spheroides genomic DNA as a template. The amplified DNA was cloned into the vector pET28a (Novagen) to give a plasmid encoding a polypeptide with a thrombin-cleavable N-terminal hexahistidine tag. This added a further 20 residues to the native protein (with sequence MGSSHHHHHHSSGLVPRGSH), giving a total deduced molecular weight of 32 130 Da. This plasmid was transformed into Escherichia coli strain BL21 (DE3) (Studier & Moffatt, 1986 ▶) and a 10 ml overnight culture of the cells harbouring the novP-pET28a construct was used to inoculate a 1 l culture of Luria–Bertani medium containing 50 mg kanamycin. This was left shaking for 24 h at 298 K without induction by isopropyl β-d-thiogalactopyranoside. Harvested cells were resuspended in buffer A [25 mM Tris–HCl pH 8.0, 400 mM NaCl, 10%(v/v) glycerol] and lysed by passage through a French press at 6.9 MPa. The cell lysate obtained by centrifugation at 15 000g for 30 min was bound onto approximately 3 ml Ni2+-charged Hi-trap metal-chelation resin for 2 h. Unbound proteins were removed by washing with 2 mM imidazole in 25 mM Tris–HCl pH 8.0, 400 mM NaCl, 10%(v/v) glycerol. His-tagged NovP bound to the resin was eluted stepwise with 20–500 mM imidazole in 25 mM Tris–HCl pH 8.0, 400 mM NaCl, 10%(v/v) glycerol. The fractions containing NovP were pooled and dialysed against buffer A. The His tag was not cleaved. The purified protein was stored under liquid nitrogen in 30 µl aliquots and subsequently shipped on dry ice for crystallization trials.
Prior to crystallization, approximately 1 ml purified NovP was buffer-exchanged into 20 mM Tris–HCl pH 7.5, 2 mM DTT and concentrated to around 10 mg ml−1 using an Ultrafree 10 kDa cutoff concentrator (Millipore). Dynamic light scattering (DLS) was used to monitor the solution properties of the protein. For this purpose, approximately 30 µl of sample was centrifuged through a 0.1 µm Ultrafree filter (Millipore) to remove particulate material before introduction into a 12 µl microsampling cell. The latter was then inserted into a Dynapro-MSTC molecular-sizing instrument at 293 K (Protein Solutions Inc.). A minimum of 15 scattering measurements were taken and the resulting data were analysed using the DYNAMICS software package (Protein Solutions Inc.).
Crystallization trials were carried out by vapour diffusion in hanging drops using a variety of in-house and commercially available screens (Hampton Research) at a constant temperature of 291 K. Drops consisted of 1 µl protein solution mixed with 1 µl well solution and the well volume was 1.0 ml; the protein concentration was approximately 10 mg ml−1. Trials were performed both with and without 1 mM S-adenosyl-l-homocysteine (SAH). Improved crystals were subsequently obtained by refining the successful conditions from the initial screens.
2.2. Cryoprotection and X-ray data collection
Prior to cryogenic data collection, crystals were briefly (<30 s) soaked in cryoprotectant, which corresponded to the crystallization solution with the addition of 25%(v/v) ethylene glycol in place of an equivalent volume of buffer. Crystals were routinely transferred from one solution to another and ultimately mounted for X-ray data collection using cryoloops (Hampton Research).
For data collection, a crystal was flash-cooled by plunging into liquid nitrogen and stored prior to transport to the synchrotron. The crystal was subsequently transferred to the goniostat on station PX10.1 at the Daresbury Synchrotron Radiation Source and maintained at 100 K with a Cryojet cryocooler (Oxford Instruments). Diffraction data were recorded using a MAR 225 CCD detector (MAR USA) with the wavelength set to 1.488 Å. The diffraction data were processed using the HKL-2000 suite (Otwinowski & Minor, 1997 ▶). Preliminary analysis of the data set was performed using programs from the CCP4 suite (Collaborative Computational Project, Number 4, 1994 ▶).
3. Results and discussion
NovP was overexpressed and purified with an approximate yield of 20 mg of protein from 1 l culture and was judged to be greater than 95% pure from SDS–PAGE analysis. DLS analysis gave a monomodal distribution with a relatively low polydispersity of 17.1%. From these results, the molecular size was estimated at 32.8 kDa, being very close to the value expected for a His-tagged NovP monomer (32.1 kDa).
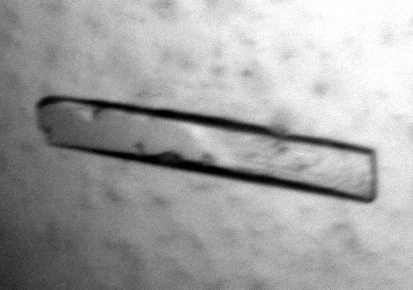
No crystallization conditions could be established for the NovP protein alone, whilst the sample containing SAH yielded crystals that were either plates or needles. The largest crystals were approximately 300 × 50 × 20 µm in size (Fig. 1 ▶) and took up to one month to grow. The optimized precipitant was 2 M ammonium sulfate in 100 mM HEPES buffer pH 7.0.
Figure 1.
Single crystal of S. spheroides NovP of approximately 300 × 50 × 20 µm in size.
Native X-ray data were collected from a single NovP crystal in two passes, the first at low resolution and the second at high resolution. For the low-resolution pass, 100 × 1.5° oscillation images were recorded to 2.46 Å resolution. The high-resolution pass was recorded to a maximum resolution of 1.35 Å and consisted of 150 × 1° oscillation images. The symmetry was established as primitive monoclinic, with unit-cell parameters a = 51.81, b = 46.04, c = 61.22 Å, β = 104.97°. After merging the two passes, the data set was 93.4% complete to 1.35 Å, but only 32.0% complete in the outer resolution shell. Reprocessing to a maximum of 1.40 Å resolution gave overall and outer shell completeness values of 98.8 and 89.5%, respectively (see Table 1 ▶). Inspection of pseudo-precession plots generated by the program HKLVIEW clearly indicated that the space group was P2. Estimation of the content of the asymmetric unit based on a single His-tagged protomer (32 130 Da) gave a crystal-packing parameter (V M) of 2.19 Å3 Da−1, with a corresponding solvent content of 43.9% (Matthews, 1968 ▶).
Table 1. Summary of X-ray data for NovP.
Values in parentheses are for the outer resolution shell.
| Data set | High-resolution pass | Low-resolution pass | Merged data set |
|---|---|---|---|
| Resolution range (Å) | 13.5–1.40 (1.42–1.40) | 36.3–2.46 (2.55–2.46) | 36.3–1.40 (1.42–1.40) |
| Unique reflections | 53540 | 10190 | 54638 |
| Completeness (%) | 97.1 (90.2) | 99.1 (95.4) | 98.8 (89.5) |
| Redundancy | 2.9 | 3.1 | 3.4 |
| Rmerge† | 0.044 (0.166) | 0.035 (0.054) | 0.057 (0.164) |
| 〈I/σ(I)〉 | 14.7 (4.2) | 36.6 (23.6) | 18.1 (4.2) |
| Wilson B value (Å2) | 16.9 | 30.7 | 17.6 |
R
merge = 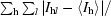
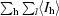 , where I
l is the lth observation of reflection h and 〈I
h〉 is the weighted average intensity for all observations l of reflection h.
, where I
l is the lth observation of reflection h and 〈I
h〉 is the weighted average intensity for all observations l of reflection h.
Analysis of the NovP sequence using the FUGUE server (http://www-cryst.bioc.cam.ac.uk/~fugue/prfsearch.html; Shi et al., 2001 ▶) indicated that there were no suitable templates for molecular replacement in the Protein Data Bank; although three ‘certain’ hits were identified (with Z scores ranging from 11.4 to 9.3), their sequence identities with NovP did not exceed 18%. Thus, we will use isomorphous replacement methods to solve the structure of NovP.
Acknowledgments
CEMS and DML would like to thank the BBSRC for financial support through responsive mode funding (ref. B19400) and the Core Strategic Grant to the John Innes Centre. CLFM and CTW were funded by NIH grants F32 AI054007 and GM 49338, respectively. R. Chen is acknowledged for isolation of S. spheroides genomic DNA. We are also grateful to W. Holsgrave for help with crystallization and to M. Cianci, M. Ellis and R. Strange for assistance with data collection at the SRS (Daresbury).
References
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Freel Meyers, C. L., Oberthur, M., Xu, H., Heide, L., Kahne, D. & Walsh, C. T. (2004). Angew. Chem. Int. Ed. Engl.43, 67–70. [DOI] [PubMed] [Google Scholar]
- Holdgate, G. A., Tunnicliffe, A., Ward, W. H., Weston, S. A., Rosenbrock, G., Barth, P. T., Taylor, I. W., Pauptit, R. A. & Timms, D. (1997). Biochemistry, 36, 9663–9673. [DOI] [PubMed] [Google Scholar]
- Lewis, R. J., Singh, O. M., Smith, C. V., Skarzynski, T., Maxwell, A., Wonacott, A. J. & Wigley, D. B. (1996). EMBO J.15, 1412–1420. [PMC free article] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Maxwell, A. (1997). Trends Microbiol.5, 102–109. [DOI] [PubMed] [Google Scholar]
- Maxwell, A. & Lawson, D. M. (2003). Curr. Top. Med. Chem.3, 283–303. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Shi, J., Blundell, T. L. & Mizuguchi, K. (2001). J. Mol. Biol.310, 243–257. [DOI] [PubMed] [Google Scholar]
- Studier, F. W. & Moffatt, B. A. (1986). J. Mol. Biol.189, 113–130. [DOI] [PubMed] [Google Scholar]
- Tsai, F. T., Singh, O. M., Skarzynski, T., Wonacott, A. J., Weston, S., Tucker, A., Pauptit, R. A., Breeze, A. L., Poyser, J. P., O’Brien, R., Ladbury, J. E. & Wigley, D. B. (1997). Proteins, 28, 41–52. [PubMed] [Google Scholar]