Abstract
Anemopsis californica (Saururaceae) commonly called yerba mansa, is an important medicinal plant in many deserts in the southwestern region of North America. Populations of A. californica, collected throughout New Mexico, were examined for chemical variability in roots and rhizomes for select monocyclic (cymene, limonene, piperitone and thymol) and bicyclic (α-pinene, 1,8-cineole and myrtenol) monoterpenoid and phenylpropanoid (methyleugenol, isoeugenol and elemicin) derived essential oil components. Three distinct chemotypes were detected using a hierarchical clustering analysis on the concentration of 10 different analytes in three individuals from each of 17 populations. One chemotype was characterized by high elemicin concentrations, a second chemotype by high methyleugenol concentrations and the third by high piperitone and thymol concentrations. Steam distilled oil was used to screen for anticancer bioactivity. A. californica root oils demonstrated anti-proliferative activity against AN3CA and HeLa cells in vitro but no activity against lung, breast, prostate or colon cancer cells. The IC50 values for the root oil were 0.056% and 0.052% (v/v) for the AN3CA and HeLa cells respectively.
KEYWORD Index: Anemopsis californica, Sauraceae, yerba mansa, essential oil, chemotypes, supercritical fluid extraction, SFE, uterine cancer, cervical cancer
1. Introduction
Anemopsis californica (Nutt.) Hook. and Arn. (Houttuynia californica Benth.et Hook.) is one of five genera belonging to the Saururaceae family. The genus contains a single species, A. californica, commonly known as yerba mansa. Geographically, yerba mansa is found in the Southwestern region of the United States, and the northern regions of Mexico (Kelso, 1932; Caffey-Moquin, 1986). Although limited to desert biomes, yerba mansa is not a xerophyte. Populations of the plant are only found in wet, marshy and often alkaline or saline habitats surrounded by desert.
Anemopsis essential oil was studied extensively from the late 1950’s to the early 1970’s (Horton and Paul, 1957; Childs, 1962; Acharya and Chaubal, 1968; Sanvordeker and Chaubal, 1969; Tutupalli and Chaubal, 1971). The collective result of these works was the identification of 12 volatiles isolated from the roots and rhizomes of Anemopsis including methyleugenol (57%), thymol (13.8%) and piperitone (8%), and the isolation and identification of crystalline (+) asarinin. We have recently described the isolation and characterization of Anemopsis leaf volatiles (Medina et al., 2005). Thirty-eight compounds isolated by steam distillation or solid phase microextraction (SPME) were detected by GC/MS. Readily detectable compounds included α-pinene (1.9%), β-phellandrene (1.6%), 1,8-cineole (2.5%), piperitone (11.5%), methyleugenol (6.9%), (E)-caryophyllene (4.6%) and elemicin (53%).
Ethnographic information on yerba mansa is consistent. A variety of people from different cultural backgrounds and geographical areas have been interviewed and researchers report similar or related uses again and again: treatment of wounds, cold and flu symptoms, pain and inflammation, as well as lung, circulatory, urinary, and digestive tract ailments (Swank, 1932; Wyman and Harris, 1947; Bean and Saubel, 1972; Bocek, 1984; Moore, 1989; Artschwager-Kay, 1996; Davidow, 1999). Both aerial and root/rhizome tissues are used medicinally; Hippocratic screening of Anemopsis tissues demonstrates that root/rhizome, with a minimum lethal dose of 316 mg/kg, is more potent than aerial parts which showed no lethality even at the maximum dose administered 1 g/kg (Tutupalli et al., 1975). Tea made from A. californica leaves and roots is used to treat uterine cancer, ease menstrual cramps, induce conception, and staunch excessive bleeding after childbirth (Bocek, 1984; Artschwager-Kay, 1996); as a treatment for other gynecological conditions including yeast infection, and vaginitis (Moore, 1989; Davidow, 1999); or to treat venereal sores and ulcers (Bean and Saubel, 1972).
Chemical polymorphisms or chemotypes have been reported for many medicinal plants (Mockute et al., 2001; Russell and Southwell, 2003; Curado et al., 2006). Douglas et al. (2004) conducted a study to determine whether triketone rich chemotypes of Leptospermum scoparium are present in New Zealand and define the boundaries of chemotypic variation. Ten chemotypes were identified, two of which contained high levels of triketones. This analysis indicated a link between the two triketone rich populations although they were spatially very distant. In a related study based on the analysis of L. scoparium grown from seed collected at 15 sites around the country, they identified three chemotypes, and determined that oil composition was largely genetically controlled (Perry et al., 1997).
Rapid screening protocols have been developed for the purposes of identifying natural products with anticancer properties (Simon et al., 2000; Bjornsti, 2002). Assaying plants known to have useful or associated chemistries maximizes efficiency, as these are most likely to demonstrate activity (Balunas and Kinghorn, 2005). An herbal remedy, such as yerba mansa, is an excellent candidate for anticancer screening. The objective of this study was to determine if there were different chemotypes among the different populations of A. californica grown and used by herbalists in New Mexico. The bioactivity of root extracts was determined by screening for inhibition of growth of in vitro cultured cancer cell lines.
2. Results and Discussion
2.1 Characterization of Root Oil
The essential oil composition of New Mexico Anemopsis root tissue was characterized. Root tissue from one population, San Pedro in Rio Arriba Co. was selected for triplicate steam distillations (Table 1). This population was chosen for steam distillation based on the large amount of tissue available. The most abundant compounds in the oil were methyleugenol, thymol, and elemicin. This is the first published report of myrtenol, anethole and elemicin in Anemopsis root tissue. The level of the major component methyleugenol corresponds to previously published level in root oils characterized by Acharya and Chaubal (1968). In that study using plants from California, methyleugenol comprised 55% of the root oil, with thymol at 13% and piperitone at 5%. The reduced levels of piperitone and thymol in the San Pedro sample could reflect different genetic sources of the plant material.
Table 1.
Characterization of Anemopsis root essential oil. Triplicate steam distillations of root tissue were characterized using GC/MS, and abundant compounds identified by matching mass spectral data to the mass spectra of standard compounds. Quantities are expressed as % peak area, compounds are listed in order of increasing retention time.
| Compound | % Peak Area (avg ± sd) |
|---|---|
| α-pinene | 0.2 ± 0.0 |
| Cymene | 0.1 ± 0.1 |
| Limonene | 0.2 ± 0.0 |
| 1,8-cineole | 0.3 ± 0.0 |
| Myrtenol | 1.5 ± 0.3 |
| Anethole | 1.0 ± 0.1 |
| Piperitone | 0.4 ± 0.0 |
| Thymol | 4.2 ± 0.4 |
| Methyleugenol | 59 ± 2 |
| Elemicin | 2.7 ± 0.2 |
2.2 Chemotypic Variation in Anemopsis populations
Root tissue was collected in the Fall 2001 from 17 populations of Anemopsis. Plants were collected in 7 counties, from Northern to Southern New Mexico; populations were located at altitudes between 1198 and 1793 m (Fig. 1). This represents zones 4 through 8 on the United States Department of Agriculture plant hardiness map (http://www.usna.usda.gov/Hardzone/ushzmap.html).
Fig. 1.

A. californica population sites of collection in New Mexico. Approximate sites of collection are identified on the map as colored dots (red, yellow, blue and green) corresponding to the four chemotype designations determined in this study and numbers corresponding to the following names used throughout: 1, Pojoaque A; 2, Pojoaque B; 3, Velarde; 4, Alcalde field; 5, Alcalde wild; 6, San Juan; 7, Ranchitos; 8, San Pedro; 9, Española; 10, Faywood; 11, City of Rocks; 12, RGNC (Rio Grande Nature Center); 13, Chaparral HR (horse ranch); 14, Bosque; 15, Mesilla Valley; 16, Greenhouse; 17, Picacho.
The concentration of select monocyclic and bicyclic monoterpenoid and phenylpropanoid derived essential oil components in the 17 Anemopsis populations are presented in Tables 2–4. These extracts were prepared by standard SFE methods with trapping of the analytes in methanol and then the composition was determined using GC/MS. There was remarkable variability between populations across all compound classes. Of the ten compounds characterized only three, thymol, α-pinene and methyleugenol were found in all plants collected at each of the 17 sites. The abundances of monocyclic monoterpenoid compounds were reported in Table 2; thymol was usually the most abundant compound in this class, while cymene was the least abundant. The abundances of bicyclic monoterpenoid compounds were reported in Table 3; although 1,8-cineole and myrtenol were found in only 13 or 12 of the 17 populations respectively, in some populations these bicyclic monoterpenoids were found at higher concentrations than α-pinene. The abundances of phenylpropanoid derived essential oil components were reported in Table 4; with the exception of one population, San Pedro, methyleugenol was the most abundant compound in this class.
Table 2.
Chemical variability of Anemopsis populations in monocyclic monoterpenoid essential oil components, reported as average μg/g dry wt root. Populations are listed in order of descending elevation; values are reported as the average of three individuals, Tukey’s HSD separate means at 95% confidence are indicated by different letters within the columns.
| Population | Elevation (m) | Cymene | Limonene | Piperitone | Thymol |
|---|---|---|---|---|---|
| Pojoaque A | 1793 | 0.0 a | 24.5 ab | 11.4 a | 255.1 abc |
| Pojoaque B | 1788 | 0.0 a | 31.7 ab | 35.2 ab | 303.8 abcd |
| Velarde | 1764 | 0.0 a | 8.4 a | 360.7 c | 998.6 e |
| Alcalde field | 1748 | 0.0 a | 28.9 ab | 195.4 bc | 808.7 cde |
| Alcalde wild | 1727 | 0.0 a | 32.3 ab | 340.2 c | 884.4 de |
| Ranchitos | 1719 | 0.0 a | 13.4 a | 0.0 a | 150.5 a |
| San Juan | 1719 | 0.0 a | 15.5 ab | 11.1 a | 308.1 abcd |
| San Pedro | 1712 | 0.0 a | 7.8 a | 10.3 a | 222.6 abc |
| Española | 1709 | 0.0 a | 0.0 a | 0.0 a | 191.7 ab |
| Faywood | 1538 | 0.0 a | 8.1 a | 0.0 a | 423.2 abcde |
| City of Rocks | 1531 | 0.0 a | 0.0 a | 0.0 a | 170.0 ab |
| RGNC | 1520 | 0.0 a | 22.4 ab | 80.2 ab | 563.0 abcde |
| Chaparral | 1506 | 4.4 a | 8.9 a | 0.0 a | 442.7 abcde |
| Bosque | 1385 | 4.2 a | 16.2 ab | 50.1 ab | 404.5 abcde |
| Greenhouse | 1198 | 12.7 a | 65.2 b | 48.0 ab | 457.6 abcde |
| Mesilla | 1198 | 6.3 a | 49.3 ab | 67.2 ab | 717.2 abcde |
| Picacho | 1198 | 10.5 a | 49.0 ab | 12.7 a | 755.9 bcde |
Table 4.
Chemical variability of Anemopsis populations in phenylpropanoid essential oil components, reported as μg/g dry wt root. Populations are listed in order of descending elevation; values are reported as the average of three individuals, Tukey’s HSD separate means at 95% confidence are indicated by different letters within the columns.
| Population | Elevation (m) | Methyleugenol | Isoeugenol | Elemicin |
|---|---|---|---|---|
| Pojoaque A | 1793 | 21748.4 ab | 0.0 a | 1284.8 ab |
| Pojoaque B | 1788 | 23044.7 ab | 0.0 a | 1032.2 ab |
| Velarde | 1764 | 19831.2 ab | 0.0 a | 615.7 a |
| Alcalde field | 1748 | 11303.7 ab | 0.0 a | 229.0 a |
| Alcalde wild | 1727 | 14936.5 ab | 0.0 a | 526.7 a |
| Ranchitos | 1719 | 13343.8 ab | 0.0 a | 834.5 ab |
| San Juan | 1719 | 18546.3 ab | 0.0 a | 511.7 a |
| San Pedro | 1712 | 19045.0 ab | 0.0 a | 2169.0 b |
| Española | 1709 | 10155.7 ab | 0.0 a | 597.8 a |
| Faywood | 1538 | 20047.2 ab | 0.0 a | 0.0 a |
| City of Rocks | 1531 | 9538.5 a | 0.0 a | 0.0 a |
| RGNC | 1520 | 24879.8 ab | 0.0 a | 744.6 ab |
| Chaparral | 1506 | 18009.7 ab | 0.0 a | 550.1 a |
| Bosque | 1385 | 13306.8 ab | 0.0 a | 166.0 a |
| Greenhouse | 1198 | 32228.2 ab | 31.7 a | 0.0 a |
| Mesilla | 1198 | 38138.4 b | 0.0 a | 129.5 a |
| Picacho | 1198 | 22955.6 ab | 0.0 a | 67.4 a |
Table 3.
Chemical variability of Anemopsis populations in bicyclic monoterpenoid essential oil components, reported as μg/g dry wt root. Populations are listed in order of descending elevation; values are reported as the average of three individuals, Tukey’s HSD separate means at 95% confidence are indicated by different letters within the columns.
| Population | Elevation (m) | Pinene | 1,8-Cineole | Myrtenol |
|---|---|---|---|---|
| Pojoaque A | 1793 | 33.9 a | 119.8 abc | 10.7 a |
| Pojoaque B | 1788 | 50.6 a | 134.2 abc | 12.4 a |
| Velarde | 1764 | 45.4 a | 17.7 a | 0.0 a |
| Alcalde field | 1748 | 36.2 a | 121.4 abc | 7.9 a |
| Alcalde wild | 1727 | 86.8 a | 176.9 abc | 14.9 a |
| Ranchitos | 1719 | 10.4 a | 0.0 a | 9.6 a |
| San Juan | 1719 | 53.3 a | 115.7 abc | 0.0 a |
| San Pedro | 1712 | 8.6 a | 73.7 ab | 24.6 a |
| Española | 1709 | 9.2 a | 0.0 a | 0.0 a |
| Faywood | 1538 | 60.7 a | 0.0 a | 24.7 a |
| City of Rocks | 1531 | 35.6 a | 0.0 a | 0.0 a |
| RGNC | 1520 | 86.0 a | 173.0 abc | 30.1 a |
| Chaparral | 1506 | 45.4 a | 30.2 a | 14.7 a |
| Bosque | 1385 | 44.0 a | 117.8 abc | 10.4 a |
| Greenhouse | 1198 | 124.8 a | 325.3 bc | 44.2 a |
| Mesilla | 1198 | 141.5 a | 343.2 c | 343.2 b |
| Picacho | 1198 | 150.2 a | 355.8 c | 21.1 a |
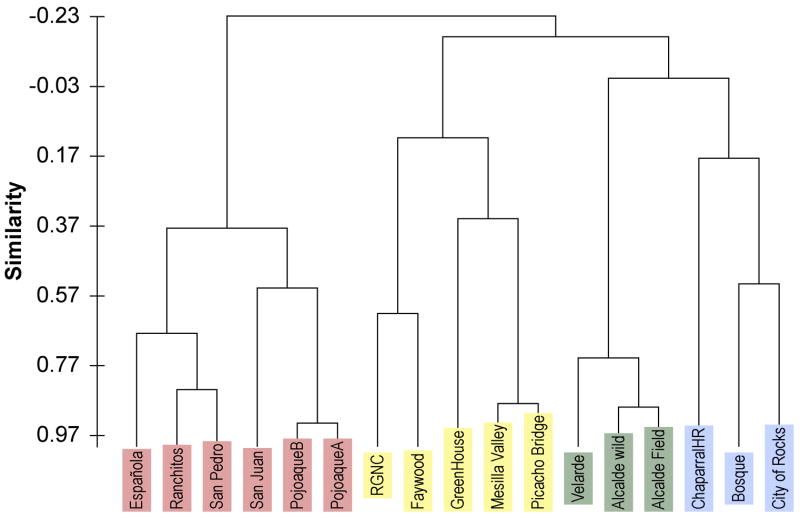
Statistical analyses were performed to assess the relationships among the different populations. We used the abundance of the 10 different chemical compounds (α-pinene, 1,8-cineole, myrtenol, cymene, limonene, piperitone, thymol, methyleugenol, isoeugenol, elemicin) for each population as input variables in principal component analyses to detect correlations among the populations, i.e. predict chemotypes. The correlation circle plotting the F1 and F2 axes is presented in Fig. 2. This projection accounts for 69.9% (F1 + F2) of the initial variability of the data. The projections for piperitone and thymol appear to be positively correlated with one another, and opposite to elemicin. The remaining compounds all have similar projections, clustered around methyeugenol. To further test whether the populations would distribute into statistically significant chemotypes we performed an agglomerative hierarchical clustering analysis. Using the Pearson similarity correlation coefficient we generated the dendrogram shown in Fig. 3. Again, the dataset consisted of the concentration of 10 essential oil analytes in extracts from three individuals from each of the 17 populations (51 samples). Four clusters resulted from this analysis; three corresponded to distinct chemotypes, with chemical abundances predicted by the PCA analysis in Fig. 2. Cluster 1, Elemicin Chemotype (depicted in red in Figs. 1 and 3) plants in this group had high elemicin levels, typically > 500 μg/g (Table 4) and low levels of thymol and piperitone (Table 2). The elemicin chemotype was restricted to northern New Mexico. Cluster 2, Methyleugenol Chemotype, (depicted in yellow in Figs. 1 and 3) plants in this group had high methyleugenol, typically >20000 μg/g (Table 4), high levels of α-pinene, (Table 3), and low levels of elemicin (Tables 4). The methyleugenol chemotype was mainly found in the southernmost counties sampled in New Mexico. However, one population in this cluster was located in central New Mexico (Fig. 1). Cluster 3, Piperitone/Thymol Chemotype, (depicted in green in Figs. 1 and 3) plants in this group had the highest levels of piperitone and thymol of any population, > 190 μg/g piperitone and more than 800 μg/g thymol (Table 2). The piperitone/thymol chemotype was found in the northernmost populations in New Mexico (Fig. 1).
Fig. 2.
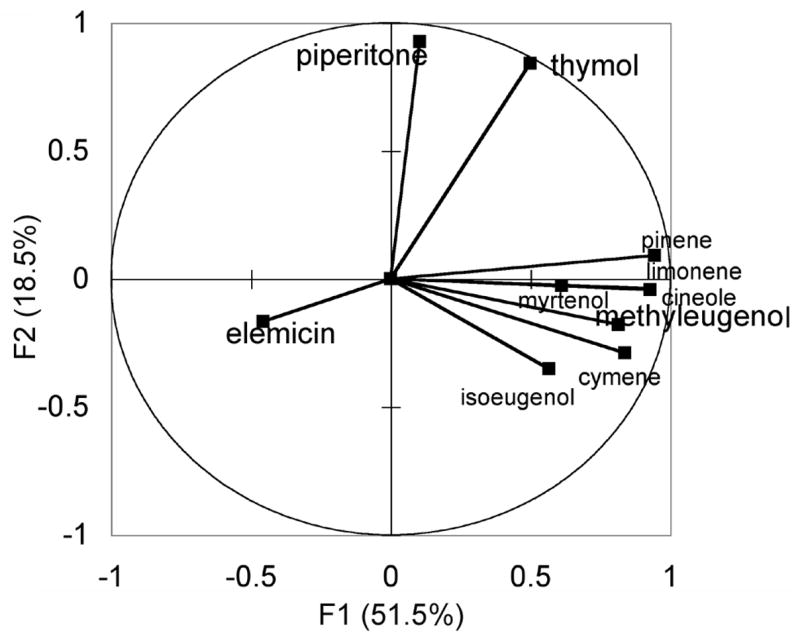
Correlation circle for prinicipal component analysis. The chemical abundances for 10 essential oil components determined in the 17 populations of A. californica were submitted for analysis by the PCA algorithm in Excel-XLSTAT, using Pearson correlation matrix (significance level, 5%). The values for factors 1 and 2 (F1 and F2) for each chemical are plotted.
Fig. 3.
Agglomerative hierarchical clustering analysis. The chemical abundances for 10 essential oil components determined in the 17 populations of A. californica were submitted for analysis by the agglomerative hierarchical clustering algorithm in Excel-XLSTAT, Pearson similarity coefficients. The chemical characteristics of the four clades or chemotypes, colored red, yellow, blue and green, are described in the text.
The chemical profile of Cluster 4 (depicted in blue in Figs. 1 and 3) was unremarkable (Tables 2–4). None of the populations in this cluster (Chaparral Horse Ranch, Bosque and City of Rocks) were significantly abundant in any specific compound. Individual populations in Cluster 4 had the lowest levels of limonene (8 μg/g), 1,8-cinole (49 μg/g) and methyleugenol (13618 μg/g) across all populations. The remaining compounds were only found at moderate levels in plants in this cluster.
A summary table of the abundances of the chemical classes, monocyclic and bicyclic monoterpenoids and phenylpropanoid compounds determined in each population is presented in Table 5. This table also lists the ratio of phenylpropanoid to monocyclic monoterpenoid compounds for each population. When these values are used to rank order the populations, the populations fall into the order of the chemotypes predicted by the clustering algorithm (Fig. 3). These results support the description of chemotypes 1 and 2 as relatively high in phenylpropanoid essential oil compounds, elemicin and methyleugenol respectively, and chemotype 3 as high in monocyclic monoterpenoids, piperitone and thymol.
Table 5.
Abundances of chemical classes of essential oil components in A. californica populations. Monocyclic monoterpenoids (total MMT), total bicyclic monoterpenoids (total BMT) and total phenylpropanoids (total Phe) in μg/g dry wt root are presented along with the ratio of total phenylpropanoids to total monocyclic monoterpenoids (Phe/MMT). The chemotypes predicted by the agglomerative hierarchical clustering analysis are listed: 1, elemicin chemotype; 2, methyleugenol chemotype; 3, piperitone/thymol chemotype. Populations listed in decreasing Phe/MMT.
| Population | Chemotypes | total MMT | total BMT | total Phe | Phe/MMT |
|---|---|---|---|---|---|
| San Pedro | 1 | 240.7 | 106.9 | 21214.0 | 88.1 |
| Ranchitos | 1 | 163.9 | 20.0 | 14178.3 | 86.5 |
| Pojoaque A | 1 | 291.0 | 164.5 | 23033.3 | 79.2 |
| Pojoaque B | 1 | 370.7 | 197.2 | 24076.9 | 64.9 |
| San Juan | 1 | 334.7 | 169.0 | 19058.0 | 56.9 |
| Española | 1 | 191.7 | 9.2 | 10753.4 | 56.1 |
| City of Rocks | n* | 170.0 | 35.6 | 9538.5 | 56.1 |
| Greenhouse | 2 | 583.5 | 494.3 | 32259.9 | 55.3 |
| Faywood | 2 | 431.3 | 85.4 | 20047.2 | 46.5 |
| Mesilla | 2 | 840.0 | 827.8 | 38267.9 | 45.6 |
| Chaparral | n | 455.9 | 90.2 | 18559.8 | 40.7 |
| RGNC | 2 | 665.6 | 289.1 | 25624.4 | 38.5 |
| Bosque | n | 474.9 | 172.3 | 13472.8 | 28.4 |
| Picacho | 2 | 828.1 | 527.1 | 23023.0 | 27.8 |
| Velarde | 3 | 1367.6 | 63.0 | 20446.9 | 15.0 |
| Alcalde wild | 3 | 1256.9 | 278.6 | 15463.2 | 12.3 |
| Alcalde field | 3 | 1033.1 | 165.4 | 11532.7 | 11.2 |
n, cluster 4, no chemotype detected.
This report is the most comprehensive study of A. californica to date. Three distinct chemotypes were identified in 17 populations of A. californica. A fourth cluster was identified by relatively low levels of all of the analytes examined. All four of the unique chemical profiles found in Anemopsis populations were geographically distinct. An analysis of influence of temperature, rainfall and elevation at the 17 sites of collection has been reported (Medina-Holguín et al., 2007). In short, temperature and elevation as environmental factors were inversely correlated with one another; mean annual temperatures increased with decreasing elevation. In New Mexico, the southern region was hot and dry (methyleugenol chemotype) while the northern regions were progressively cooler with increased precipitation, (elemicin chemotype, and further north, piperitone/thymol chemotype). It is interesting to note that unlike some plant species that are found in isolated places, all of the plants in this study were collected in areas that are heavily populated by people. For example, Anemopsis was collected near the natural hot springs in Faywood, NM. An as yet unsampled population is also growing near the sacred hot springs in Ojo Caliente, NM.
Many of the populations in this study were originally identified from herbarium specimens housed at New Mexico State University. The oldest specimen used to locate a population in this manner was collected by E.O. Wooten and dates back to June 25th, 1906. That these populations have remained unharmed for so many years in such heavily populated areas is evidence that they are valuable to the people in these locations and that their distribution may in fact be a result of anthropocory as suggested by Caffey-Moquin (1986). Anemopsis was regularly located near a water source, although water sources may dry out periodically throughout the year. The Apache report the of use mansa to treat horses for wounds and sore muscles, so it is interesting to note that 3 of the 14 wild populations (Velarde, Pojoaque A and Chapparal Horse Ranch) were collected in or near a horse pasture.
The three unique chemotypes detected among the seventeen populations are likely to be the result of genetic variability rather than environmental effects. The limited influence of the environment on chemical composition can be inferred in part from the data presented in Tables 2–4. Two different sets of populations each from a different chemotype represent clonally propagated plants managed under two different environments, controlled vs wild conditions. The population, Alcalde Field was clonally propagated from plants collected from the site for Alcalde Wild; while the population Greenhouse, represents plants clonally propagated from the population Mesilla Valley. Vegetative propagation of wild plants under cultivated conditions did not significantly alter the chemical profile of the root essential oil. There were no statistically significant differences between these pairs of populations for the abundances of the ten essential oil components with the exception of myrtenol in the Mesilla Valley-Greenhouse pair (Table 3).
In a recent report we have presented additional evidence that the environment plays a limited role in the chemical variability of essential oil composition in A. californica (Medina-Holguín et al., 2007). Similar chemical profiles were detected in root samples recollected after a three-year interval for four populations, suggesting retention of unique chemical profiles in different populations. The chemical concentrations for six essential oil components of A. californica roots were determined under field conditions with varying irrigation and N fertilizer regimens. The concentration of only two compounds, thymol and piperitone, were increased by increasing irrigation. The concentration of all other compounds, methyeugenol, elemicin, 1,8-cineole, and myrtenol, were independent of the irrigation rates and N fertilizer rates used in the study. Altogether, these results suggest that the chemical variability observed between different populations of A. californica is primarily genetically controlled.
2.3 Inhibition of Growth of Cancer Cell Lines
Steam distilled oils prepared from roots/rhizomes of Anemopsis californica were tested for growth inhibitory activity against a standard bank of several human cancer cell lines: A549 (lung), MCF7 (breast), PC3 (prostate), and HCT116 (colon). No inhibitory activity was observed in these cell cultures except a relatively weak inhibition of HCT116, approximately 50% growth inhibition at 0.1% oil (v/v). Since the ethnobotanical record reported uses of this plant for uterine cancer, Anemopsis root essential oil was tested against AN3CA (uterine) and HeLa (cervical) human cancer cell lines.
A. californica root essential oil inhibited the growth of AN3CA and HeLa cell lines (Fig. 4, solid line) with IC50 of 0.056 % v/v and 0.052% v/v respectively. The three most abundant compounds, present in the oil, were tested independently for growth inhibitory activity against AN3CA and HeLa cells. Thymol, piperitone and methyleugenol also inhibited cell growth, (Fig. 4, dashed lines). The IC50 for each of these compounds against each cell line was determined and compared with the concentration of these compounds in the root oil from A. californica. For example, piperitone is present at 3.97 ng/mL in the volume of oil that inhibits 50% growth of HeLa cells. The IC50 for pure piperitone is 177 ng/mL; piperitone is therefore unlikely to be responsible for the growth inhibitory properties of the root essential oil from Anemopsis californica. The IC50 for thymol, 34 ng/mL, is similar to its concentration (22 ng/mL) in the steam distilled oil that inhibits 50% growth of HeLa cells Similar results were obtained in the bioassays for the uterine cancer cell line AN3CA. The IC50 for thymol against AN3CA cells was 45 ng/mL; the concentration of thymol in the root oil at 50% inhibition was 24 ng/mL.
Fig. 4.
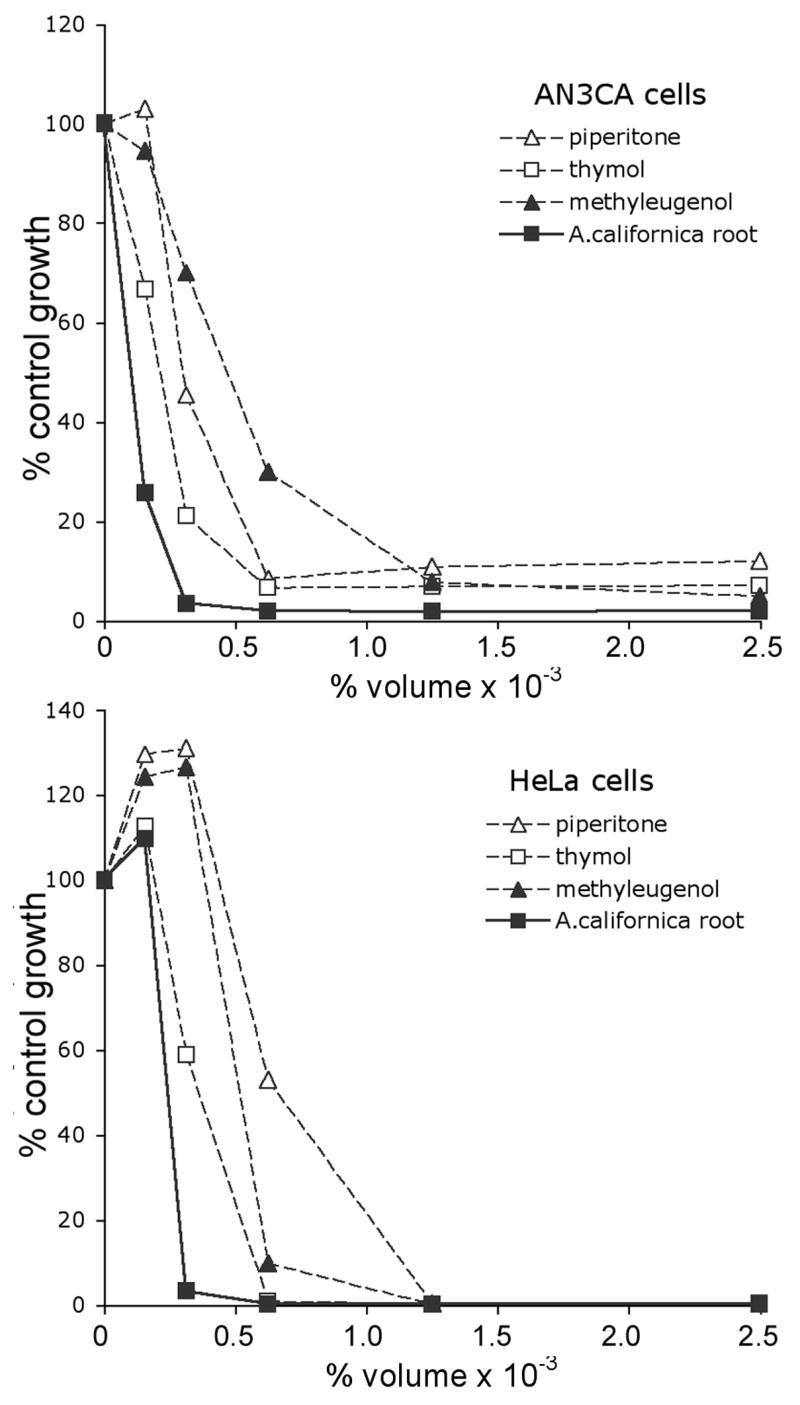
A. californica root essential oil inhibits cervical and uterine cancer cell growth. AN3CA or HeLa cells were treated with the indicated dilutions of the root essential oil or the volume dilutions of the pure oil components and compared with 1%, v/v DMSO, (control) for 4 days. The cells were separated from the media and then incubated with FCS supplemented with [methyl 3H] thymidine (2 μCi/mL). The plates were counted using a scintillation microplate reader. Results are shown as a percentage of control cells growth indicated by incorporation of 3H-thymidine.
Because piperitone and methyleugenol also inhibit the growth of AN3CA and HeLa cells, inhibition may be the result of a synergistic relationship between the combined abundant compounds. It is also possible that the bioactive compound is not piperitone, thymol, or methyleugenol but rather a minor component in the oil. Anomalously, at inhibiting concentrations of whole oil, methyleugenol is present at 1362 ng/μL (v/v). However the IC50 of pure methyleugenol is 447 ng/μL (v/v), approximately threefold lower. Perhaps, there are other compounds in the whole oil that reduce the bioavailability of methyleugenol and render it less effective.
Eugenol and isoeugenol have antiproliferative effects; one mechanism appears to be based on cell cycle arrest in G0/G1 phase (Kalmes et al., 2006). This mechanism is dependent on the arylhydrocarbon receptor, so that some cell types may be more sensitive to the antiproliferative effects than other cell lines. Eugenol and isoeugenol are present at low levels in A. californica root oil, less than 250 μg/g dried root (Medina-Holguín, 2006). Anticancer activity has been reported in essential oils from Croton flavens (Sylvestre et al., 2006), in this case, inhibiting the growth of human lung carcinomas and colon adenocarcinoma cell lines. The bioactive compound in the extract was not determined but several minor components in the oil are known cytotoxic agents. Clearly, determining the bioactive compound(s) in the A. californica root essential oil that inhibit uterine and cervical cancer cell lines will need further investigation.
2.6 Conclusions
Three distinct chemotypes based on the essential oil composition of roots and rhizome extracts of Anemopsis califormica were identified. Specific bioactivity against uterine and cervical cancer cell lines was demonstrated with steam-distilled oil of Anemopsis root tissue. These results support the traditional, cultural use of Anemopsis extracts to treat uterine cancer.
3. Experimental procedures
3.1 Plant material
Anemopsis plants from 17 populations growing in New Mexico were collected selectively in the Fall of either 1999, 2001 or 2004. Populations were identified by either personal communication, or by voucher specimens located in the New Mexico State University Herbarium or the Range Science Herbarium at New Mexico State University in Las Cruces, NM. Elevation, latitude and longitude were determined using a GPS Garmin eTrex personal navigator. Coordinates will not be published in the interest of protecting the populations.
A. californica from Dona Aña County, NM (elevation, 1198 m) was harvested and propagated by root division in August 1999. A voucher specimen was placed in the Range Science Herbarium at New Mexico State University in Las Cruces, NM (Collection number: Medina 6). Plants from this population were greenhouse cultivated in Metro Mix 360 (Greenhouse & Garden Supply Inc. Albuquerque, NM), fertilized with Osmocote™ 14-14-14, and watered daily using drip irrigation. Based on the large amount of root tissue available, San Pedro, a wild population growing in a colonia of Española, NM was selected for a comparative analysis of steam distillation and SFE. Three individuals were collected from each population, placed in plastic bags, and stored on ice for transport to the lab. The roots were gently washed with de-ionized water to remove loose soil, dried on seed germinating paper at room temperature, and weighed periodically until water loss was no longer detected. The intact dried root was then stored in a manila envelope until all roots were completely dry. The roots of plants from each population were then ground in an industrial blender. The ground tissue was placed in 50 mL culture tubes and stored at −80ºC until extraction.
3.2 Extractions
Triplicate steam distillations were carried out in a Likens-Nickerson apparatus using 20 g of dried root as previously described (Medina et al., 2005). SFE generated extracts were trapped in methanol as described earlier (Medina et al., 2005) and was used for the characterization of the different populations.
3.3 GC/MS
Extracts were analyzed by GC/MS using a Varian model 3400 GC with a DB-5 column (30 m × 0.25 mm fused silica capillary, 0.25 μm film thickness), coupled to an ion trap mass spectrometer (EI, 70 eV). Helium carrier gas flowed at 1 mL/min, and injector and transfer line temperatures were 220 and 260 °C, respectively. The initial column temperature was 60 °C, with a linear gradient of 3 °C/min programmed into each 65 min run. Comparisons of mass spectra and retention indices with literature data (Adams, 2001) or authentic standards were used to identify the peaks; extracts were spiked with hydrocarbons octane and heptadecane to standardize column retention times (Kováts, 1958).
Calibration curves with authentic standards were used to quantify the abundance of α-pinene, 1,8-cineole, thymol, methyleugenol, piperitone, and elemicin. Reference standards were obtained from Sigma-Aldrich, St. Louis, MO (1,8-cineole, methyleugenol, α-pinene, thymol) and from Pfaltz & Bauer, Waterbury, CT (piperitone). Elemicin was synthesized as previously described (Medina et al, 2005). Dry matter percent of greenhouse tissue was determined using AOAC procedures (Helrich, 1990).
3.4 Mammalian Cancer Cell Assays
In vitro anti-tumor cytotoxicity assays were performed against A549 (lung), MCF7 (breast), PC3 (prostate), HCT116 (colon), AN3CA (uterine) and HeLa (cervical) human cell lines. All cell lines were incubated in tissue culture flasks in Fetal Calf Serum (FCS) media in a humidified atmosphere with 5% CO2, and at 37 °C. Trypsinized (trypsin-EDTA, Gibco) cell cultures were washed with FCS media and diluted to deliver 1,000 live cells per 135 μL aliquot in each well of a 96 well plate. The plates were returned to the incubator for 24 h. The wells were then treated with 15 μL of plant extract/oil suspended in 1% dimethylsulfoxide (DMSO) in FCS medium; solvent control wells received 15 μL of 1% DMSO in FCS medium. The plates were incubated for 4 d at 37°C in 5% CO2. FCS medium was supplemented with [methyl 3H] thymidine at a concentration of 24 μM and cells were incubated for another 24 h. The cells were washed three times with phosphate buffered saline 100 μL/well. The wells were aspirated dry and 100 μL of Microscint 20 was added to each well. Each plate was then sealed using Topseal-A (Packard), and counted using a scintillation microplate reader (Packard, Topcount). All results were reported as percent growth of the averaged controls.
3.6 Statistical analysis
Means of compound abundances were calculated for each population, and significant differences between means were determined with Excel-XLSTAT2006 ANOVA (analysis of variance) using the Tukey’s HSD (Honestly Significantly Different) test. Principal component analyses, and agglomerative hierarchical clustering were performed with Excel-XLSTAT2006 using the means of the compound abundances for each of the populations.
Acknowledgments
The authors thank Charles Martin, NMSU for help in collection of selected plant populations, Rich Richins, NMSU for help with graphics. This work was supported in part by the NM Agricultural Experiment Station, United States Department of Agriculture CSREES grant 200634387-15885 and National Institutes of Health grants NIGMS GM61222 and NCI U56 CA96286.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya RN, Chaubal MG. Essential oil of Anemopsis californica. J Pharm Sci. 1968;57:1020–1022. doi: 10.1002/jps.2600570622. [DOI] [PubMed] [Google Scholar]
- Adams RP. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured Pub. Corp; Carol Stream, IL: 2001. [Google Scholar]
- Artschwager-Kay M. Healing with plants in the American and Mexican West. University of Arizona Press; Tucson, Arizona: 1996. pp. 94–96. [Google Scholar]
- Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Bean LJ, Saubel KS. Temalpakh (from the earth); Cahuilla Indian knowledge and usage of plants. Malki Museum Press; Banning, CA: 1972. [Google Scholar]
- Bjornsti MA. Cancer therapeutics in yeast. Cancer Cell. 2002;2:267–273. doi: 10.1016/s1535-6108(02)00160-5. [DOI] [PubMed] [Google Scholar]
- Bocek BR. Ethnobotany of Costanoan Indians, California, based on collections by John P. Harrington. Econ Bot. 1984;38:240–255. [Google Scholar]
- Caffey-Moquin M. Yerba del manso: an evaluation. Rocky Mountain Forest & Range Experiment Station, General Technical Report R.M. U.S. Department of Agriculture, Forest Service; Ft. Collins, Colorado. 1986. pp. 78–82. [Google Scholar]
- Childs RF. A preliminary phytochemical and pharmacological study of Anemopsis californica. Chemistry. 1962;63:3136. [Google Scholar]
- Curado MA, Oliveira CBA, Jesus JG, Santos SC, Searphin JC, Ferri PH. Environmental factors influence on chemical polymorphism of the essential oils of Lychnophora ericoides. Phytochemistry. 2006;67:2363–2369. doi: 10.1016/j.phytochem.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Davidow J. Infusions of Healing: a treasury of Mexican American herbal remedies. Fireside; New York, NY: 1999. [Google Scholar]
- Douglas MH, van Klink JW, Smallfield BM, Perry NB, Anderson RE, Johnstone P, Weavers RT. Essential oils from New Zealand manuka: triketone and other chemotypes of Leptospermum scoparium. Phytochemistry. 2004;65:1255–1264. doi: 10.1016/j.phytochem.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Helrich K. Official Methods of Analysis of the Association of Official Analytical Chemists: Agricultural Chemicals; Contaminants; Drugs. Association of Official Analytical Chemists, Inc; Arlington, VA: 1990. [Google Scholar]
- Horton WJ, Paul EG. 4-Allylveratrole from Anemopsis californica. J Amer Chem Soc. 1957;79:2264–2266. [Google Scholar]
- Kalmes M, Neumeyer A, Rio P, Hanenberg H, Fritsche E, Blomeke B. Impact of the arylhydrocarbon receptor on eugenol- and isoeugenol-induced cell cycle arrest in human immortalized keratinocytes (HaCaT) Biol Chem. 2006;367:1201–1207. doi: 10.1515/BC.2006.148. [DOI] [PubMed] [Google Scholar]
- Kelso L. A note on Anemopsis californica. Amer Midl Naturalist. 1932;13:110–113. [Google Scholar]
- Kováts E. Gas-chromatographische charakterisierung organischer verbindungen. Teil 1: retentionsindices aliphatischer halogenide, alkohole, aldehyde und ketone. Helv Chim Acta. 1958;41:1915–1932. [Google Scholar]
- Medina AL, Lucero ME, Holguín FO, Estell RE, Posakony JJ, Simon JA, O’Connell MA. Composition and antimicrobial activity of Anemopsis californica leaf oil. J Agric Food Chem. 2005;53:8694–8698. doi: 10.1021/jf0511244. [DOI] [PubMed] [Google Scholar]
- Medina-Holguín AL. Population analysis of Anemopsis californica in New Mexico: searching for anti-cancer activity in the desert (Dissertation) New Mexico State University; Las Cruces, New Mexico: 2006. [Google Scholar]
- Medina-Holguín AL, Martin C, Micheletto S, Holguín FO, Rodriguez J, O’Connell MA. Environmental influences on essential oils in roots of Anemopsis californica. HortScience. 2007;42:1–6. [Google Scholar]
- Mockute D, Bernotiene G, Judzentiene A. The essential oil of Origanum vulgare L. ssp vulgare growing wild in Vilnius district (Lithuania) Phytochemistry. 2001;57:65–69. doi: 10.1016/s0031-9422(00)00474-x. [DOI] [PubMed] [Google Scholar]
- Moore M. Medicinal plants of the desert and canyon west: a guide to identifying, preparing, and using traditional medicinal plants found in the deserts and canyons of the West and Southwest. Museum of New Mexico Press; Santa Fe, New Mexico: 1989. pp. 132–134. [Google Scholar]
- Perry NB, Brennan NJ, van Klink JW, Harris W, Douglas MH, McGimpsey JA, Smallfield BM, Anderson RE. Essential oils from New Zealand manuka and kanuka: chemotaxonomy of Leptospermum. Phytochemistry. 1997;44:1485–1494. [Google Scholar]
- Russell MF, Southwell IA. Monoterpenoid accumulation in 1,8-cineole, terpinolene and terpinen-4-ol chemotypes of Melaleuca alternifolia seedlings. Phytochemistry. 2003;62:683–689. doi: 10.1016/s0031-9422(02)00607-6. [DOI] [PubMed] [Google Scholar]
- Sanvordeker DR, Chaubal MG. Essential oil of Anemopsis californica. Part II: minor constituents. J Pharm Sci. 1969;58:1213–1217. doi: 10.1002/jps.2600581010. [DOI] [PubMed] [Google Scholar]
- Simon JA, Szankasi P, Nguyen DK, Ludlow C, Dunstan HM, Roberts CJ, Jensen EL, Hartwell LH, Friend SH. Differential toxicities of anticancer agents among DNA repair and checkpoint mutants of Saccharomyces cerevisiae. Cancer Res. 2000;60:328–333. [PubMed] [Google Scholar]
- Swank GR. Master’s Thesis. University of New Mexico; Albuquerque, NM: 1932. The Ethnobotany of the Acoma and Laguna Indians. [Google Scholar]
- Sylvestre M, Pichette A, Longtin A, Nagau F, Legault J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J Ethnopharmacol. 2006;103:99–102. doi: 10.1016/j.jep.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Tutupalli LV, Chaubal MG. Saururaceae. Constituents of Anemopsis californica. Phytochemistry. 1971;10:3331–3332. [Google Scholar]
- Tutupalli LV, Chaubal MG, Malone MH. Saururaceae. VI: Hippocratic screening of Anemopsis californica. Lloydia. 1975;38:352–354. [PubMed] [Google Scholar]
- Wyman LC, Harris SK. Bull Anthro Ser. Vol. 3. University of New Mexico; Albuquerque, NM: 1947. Navaho Indian Medical Ethnobotany; pp. 1–76. [Google Scholar]