Abstract
The brain vesicular monoamine transporter (VMAT2) pumps monoamine neurotransmitters and Parkinsonism-inducing dopamine neurotoxins such as 1-methyl-4-phenyl-phenypyridinium (MPP+) from neuronal cytoplasm into synaptic vesicles, from which amphetamines cause their release. Amphetamines and MPP+ each also act at nonvesicular sites, providing current uncertainties about the contributions of vesicular actions to their in vivo effects. To assess vesicular contributions to amphetamine-induced locomotion, amphetamine-induced reward, and sequestration and resistance to dopaminergic neurotoxins, we have constructed transgenic VMAT2 knockout mice. Heterozygous VMAT2 knockouts are viable into adult life and display VMAT2 levels one-half that of wild-type values, accompanied by smaller changes in monoaminergic markers, heart rate, and blood pressure. Weight gain, fertility, habituation, passive avoidance, and locomotor activities are similar to wild-type littermates. In these heterozygotes, amphetamine produces enhanced locomotion but diminished behavioral reward, as measured by conditioned place preference. Administration of the MPP+ precursor N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine to heterozygotes produces more than twice the dopamine cell losses found in wild-type mice. These mice provide novel information about the contributions of synaptic vesicular actions of monoaminergic drugs and neurotoxins and suggest that intact synaptic vesicle function may contribute more to amphetamine-conditioned reward than to amphetamine-induced locomotion.
The brain vesicular monoamine transporter (VMAT2) is a proton-dependent transporter that accumulates monoamine neurotransmitters including dopamine, serotonin, norepinephrine, and histamine from neuronal cytoplasm into synaptic vesicles (1–4). Normal vesicular monoamine release through calcium-dependent vesicle fusion with presynaptic membranes is thus dependent on normal function of VMAT2.
Vesicular monoamine stores accumulated by normal VMAT2 function may play significant roles in the locomotor stimulation and/or the behavioral reward produced by amphetamines, drugs increasingly abused in certain areas of the United States (5–7). Amphetamines dissipate proton gradients across the membranes of synaptic vesicles, disrupt VMAT2 function, enhance cytoplasmic monoamine concentrations, and cause calcium-independent, nonvesicular monoamine release into synapses (7–9). They also act like cocaine in blocking the plasma membrane neurotransmitter transporters that use transmembrane sodium and chloride gradients to pump dopamine, norepinephrine, and serotonin from extracellular spaces into presynaptic neurons (7, 10–12). There is currently little evidence to document which amphetamine action provides which contribution to amphetamine-induced locomotion or behavioral reward. Recent data from knockout mice with interruptions in the gene encoding the plasma membrane dopamine transporter (DAT) do suggest that DAT is required for amphetamine-induced locomotion (13).
Losses of dopaminergic neurons are characteristic of both normal aging and of Parkinson disease, in which more intense losses from the substantia nigra pars compacta than from other dopaminergic cell groups are noted (14, 15). Although mechanisms for these selective dopaminergic neuronal losses are unknown in both cases, in vitro overexpression of VMAT2 reduces 1-methyl-4-phenyl-phenypyridinium (MPP+) toxicity, whereas overexpression of the plasma membrane transporter DAT enhances this toxicity (16, 17). Elevated VMAT2 and reduced DAT expression are each found in less vulnerable dopaminergic neuronal cell groups (18, 19). These data are consistent with the idea that variations in monoamine compartmentalization induced by different levels of expression of these two transporters could contribute to the cell-type selectivity of dopaminergic neurodegeneration in Parkinsonian brains.
To assess roles for amphetamine action at monoaminergic vesicles in psychomotor behavioral effects and to test in vivo roles for vesicular storage of dopaminergic neurotoxins, we have constructed transgenic VMAT2 knockout mice, assessed their basal physiologic, behavioral, and neurochemical features, and studied the effects of the reduced VMAT2 expression found in the heterozygous knockout mice on responses to amphetamine and MPP+.
MATERIALS AND METHODS
Targeted Disruption of the Murine VMAT2 Gene.
VMAT2 knockout transgenic mice were produced by standard techniques. To obtain a murine VMAT2 cDNA hybridization probe, single-stranded cDNA was synthesized from 1 μg of total mouse mid-brain RNA using the oligonucleotides 5′-ATGGCCCTGAGCGATCTGGTGCTCTGCG-3′ and 5′-TGCTGGTAGCCTTGTGTGACTGCCCTCCTGG-3′, corresponding to previously reported rat VMAT2 cDNA sequences (3, 4) by PCR, radiolabeled, and used to identify a 20-kb VMAT2 genomic fragment from a λ-FIX II genomic library prepared from the 129 mouse strain (Stratagene). The 20-kb fragment containing 9 kb of 5′ and 8 kb of 3′ sequences flanking the first three exons of the murine VMAT2 gene (N.T. and G.R.U., unpublished work) was subcloned into pBluescript (pBS, Stratagene) to produce pBSVMATG1. A VMAT2 targeting vector was constructed using a 4.5-kb XbaI–PstI fragment of pBSVMATG1 containing sequences 5′ to exon 1 and a 4.5-kb SphI–BglII fragment with sequences 3′ to exon 3 subcloned into pPGKneo (20) to produce pPGKneoV2KO (Fig. 1A). Twenty-five micrograms of pPGKneoV2KO DNA was linearized with NotI and transfected by electroporation into 107 AB1 embryonic stem cells derived from 129/SvEv mice (ref. 20, kindly supplied by Allan Bradley, Baylor College of Medicine, Houston). AB1 cells were selected for homologous recombination by culture in DMEM containing 15% fetal bovine serum (HyClone), 0.1 mM 2-mercaptoethanol, 500 μg/ml G418, and 2 μM gancyclovir (a generous gift of Syntex, Palo Alto, CA) on days 2–7 after transfection. G418- and gancyclovir-resistant colonies were picked on day 8. KpnI digests of DNA prepared from 1,200 resistant clones were screened by Southern blot analyses using a 168-bp KpnI–XbaI fragment of pBSVMATG1 radiolabeled by random priming as a hybridization probe. Eight positive cell lines from the doubly resistant colonies displayed the 4.5-kb KpnI fragment anticipated of wild-type readily distinguishable from the 12-kb fragment obtained from homologous recombinants DNA (Fig. 1 A and B). Chimeric mice were generated by injecting 15–20 homologous recombinant embryonic stem cells into blastocysts harvested from C57BL/6J mice and implanting the blastocysts into uteri of pseudopregnant CD-1 mice (Charles River Breeding Laboratories) 2.5 days postcoitus (21). Southern blot analyses of DNA extracted from tail-tip specimens of the offspring of the chimeras revealed that germ-line transmission was achieved from crosses between three chimeric animals, 22D7, 31A2, and 51D9. Forty-five percent of the 86 offspring of matings between 22D7 and C57BL/6J females revealed disrupted VMAT2 alleles. F2 homozygous, heterozygous, and wild-type offspring of F1 × F1 intercrosses were used for further biochemical and behavioral testing.
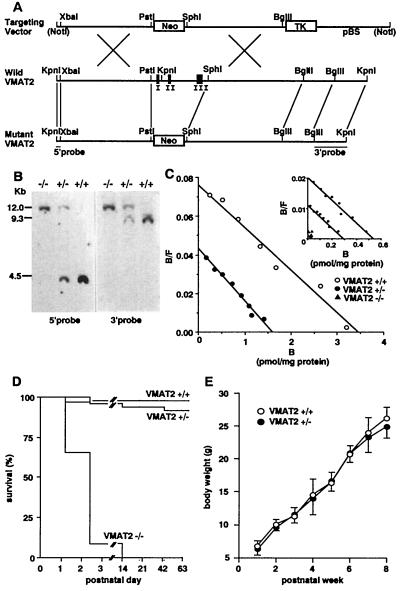
Figure 1.
Construction and characterization of VMAT2 knockout mice. (A) Representation of the wild-type and predicted knockout genomic sequences, with prominent restriction endonuclease sites, neomycin-resistance sequences, and sequences recognized by the pVMAT2ko5′ and pVMAT2ko3′ hybridization probes as indicated. Construction of pBSneoV2KO targeting vector indicating prominent restriction endonuclease cleavage sites and sites for the neomycin resistance (Neo) and of the thymidine kinase (TK) sequences. (B) Southern blot analyses of hybridization of radiolabeled pVMAT2ko5′ (Left) and pVMAT2ko3′ (Right) hybridization probes with genomic DNA extracted from the tails of wild-type (+/+), heterozygote (+/−), and homozygote (−/−) mice. Longer fragments result from homologous recombination replacing VMAT2 sequences including exons I–III with the neomycin-resistance cassette. (C) Scatchard analyses of saturation radioligand binding data using [3H]dihydrotetrabenazine and striatal membranes prepared from 6-week-old wild-type and heterozygous knockout mice and from whole-brain membranes prepared from postnatal day 1 wild-type, heterozygote, and homozygote knockout animals (Inset). (D) Kaplan–Meier survival plots of wild-type, heterozygous, and homozygous knockout mice. Approximately 15 mice of each genotype were followed for 2 months. (E) Weight gains of wild-type (+/+) and heterozygote (+/−) knockout mice.
Immunohistochemical Analysis.
VMAT2 immunohistochemistry was performed as described (22) using a 1:500 dilution of an affinity-purified rabbit polyclonal antiserum with antibodies raised against an N-terminally hemocyanin-conjugated 20 amino acid peptide corresponding to the murine VMAT2 C terminus (hemocyanin-CTQNNVQPYPVGDDEESESD; R.S.R., N.T., and G.R.U., unpublished work). Specificity was evident in ELISA assays, preadsorbtion tests, and in the anatomic distribution of immunoreactivity (data not shown).
Radioligand Binding Studies.
Washed membranes were prepared from brain regions and were incubated with tritiated ligands as described (23–30). Reactions were terminated by the addition of ice-cold buffered solutions as described (23–30), and membrane-associated ligand estimated after rapid filtration over Whatman GF/B filters using a Brandel apparatus. VMAT2, serotonin transporter, norepinephrine transporter, dopamine transporter, dopamine D1 and D2 receptor, serotonin 5HT1 and 5HT2 receptor, and α1 and β-adrenergic receptor densities were analyzed by saturation binding of [3H]dihydrotetrabenazine (146 Ci/mmol, 1 Ci = 37 GBq; Amersham), [3H]paroxetine (19 Ci/mmol; American Radiolabeled Chemicals, St. Louis), [3H]nisoxetine (80 Ci/mmol; NEN/DuPont), [3H]-WIN35428 (83.5 Ci/mmol; NEN/DuPont), [3H]-SCH23390 (88 Ci/mmol; Amersham), [3H]-YM-09151–2 (82 Ci/mmol; NEN/DuPont), [3H]-8-OH DPAT (22 Ci/mmol; Amersham), [3H]ketanserin (81 Ci/mmol; NEN/DuPont), [3H]dihydroalprenalol (82 Ci/mmol; Amersham), [3H]prazosin (20 Ci/mmol; Amersham) binding profiles, respectively, using results from parallel incubations with 1 mM unlabeled dihydrotetrabenazine, 0.1 mM citalopram, 0.1 mM desipramine, 0.1 mM cocaine, 10 μM unlabeled SCH23390, 10 μM sulpiride, 10 μM unlabeled serotonin, 10 μM methylsergide, 10 μM unlabeled alprenalol, and 10 μM phentolamine to estimate nonspecific binding, respectively. Protein concentrations were determined by the Bradford method (Bio-Rad). Results were analyzed using macligand.
Measurement of Blood Pressure and Heart Rates.
Blood pressure and heart rates were measured in nembutal-anesthetized mice using a catheter inserted into the femoral artery, strain gage, and recorder (N.T., L.L.M., P. Jose, and G.R.U., unpublished work).
Measurement of Levels of Neurotransmitters and MPP+.
HPLC with electrochemical detection was used to measure levels of monoamines, metabolites, and γ-aminobutyric acid (GABA). Mice were sacrificed by decapitation, brain regions rapidly dissected out, immediately frozen at −80°C, sonicated in 50 vol (wt/vol) of 0.1 M perchloric acid and centrifuged (15,000 × g, 15 min, 4°C). One-half of the supernatant was used for determination of monoamine and metabolite levels, and the other one-half for GABA. Levels of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), 3-methoxytyramine, norepinephrine, methoxyhydroxylphenylglycol, serotonin, and hydroxyindolacetic acid were quantitated by HPLC as described (31), except that a linear methanol gradient (2–20% over 20 min) and an ESA (Bedford, MA) CoulArray 8-electrode detector with potentials ranging from 0 to 420 mV in 60 mV steps were used. Levels of GABA, derivatized with O-phtaldialdehyde/ter-butylthiol, were determined by HPLC using a C18 column (HR-80, ESA), elution at 1 ml/min with 5% ethyl acetate/40% methanol/55% sodium phosphate (pH 6.9), and electrodetection using a Coulochem 5100A detector (GC = 600 mV, E1 = 250 mV, E2 = 650 mV; ESA) (32). Levels of MPP+ were estimated using a UV absorbance assay as described (31, 33).
Northern Blot Analysis.
Total RNA were extracted from midbrains of 6-week-old wild-type and heterozygote mice using RNAzolB (Tel-Test, Friendswood, TX) and transferred to Hybond-N nylon membranes (Amersham). The membranes were hybridized with a radiolabeled tyrosine hydroxylase (TH) cDNA corresponding to bases 1001–1246 of the murine TH cDNA (34) and washed under standard conditions. Hybridizing bands were detected by phosphorimaging, and values normalized for RNA loading by comparing ratios of hybridization intensity of TH mRNA to the intensity of subsequent hybridization to a radiolabeled β-actin cDNA corresponding to bases 622–977 of the murine β-actin cDNA (35).
Behavioral Tests.
All mice were housed in microisolator cages with free access to food and water and maintained on a 12-hr light/12-hr dark cycle with lights on at 0700 in accordance with the American Association for Laboratory Animal Care guidelines. Rotarod tests, screen tests, spontaneous locomotor activity tests, and passive avoidance tests were performed as described (36). As one measure of “emotionality” (37), the number of fecal boli found after this initial exposure was recorded manually.
Conditioned place preference was assessed in an apparatus with two 18 × 18 × 18 cm compartments distinguished by corncob bedding on one and wire mesh (2 mm) flooring in the other compartment. During a 20-min preconditioning session to determine side preference, a 5-cm opening allowed access to both compartments, and both locomotor activity and time spent on each side were determined by placing the cages in Optovarimex monitors. During 2 days of twice-daily conditioning sessions, animals were restricted to one side of the two-sided compartment and injected intraperitoneally with either amphetamine or saline. A single conditioned place preference assessment session followed the last conditioning session experienced by each mouse by 24 hr. In these sessions, mice had access to both compartments, the proportion of the 20 min session spent on each side was recorded, and results compared with the proportion of time spent on that side in preconditioning sessions. To test whether observed preference changes were due to positive drug effects or decreased aversive effects of the environment, place preference was also elicited by pairing 1 mg/kg doses of amphetamine to the initially preferred side of the apparatus and saline with the opposite side in additional animals. Locomotor stimulation induced by amphetamine doses was determined by the Optovarimex activity monitor results during the first amphetamine conditioning session. Data analyses used ANOVA to compare amphetamine-induced locomotion and conditioned place preference across genotypes.
Quantitative Assessments of Dopamine Neurons.
Mice were treated with four doses of either saline or 16 mg/kg N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (free base; Research Biochemicals, Natick, MA; intraperitoneal injections 2 hr apart on 1 day), doses previously identified as producing moderate levels of dopaminergic cell killing in wild-type mice, and sacrificed 7 days after the last injection as previously described (33). To quantitate substantia nigra dopaminergic neurons, consecutive 20-μm cryostat sections were cut through the entire midbrains of paraformaldehyde-perfused mouse brains, immunostained using specific antibodies directed against TH (1:1,000 dilution; Eugene Tech, Ridgefield Park, NJ), and cell counts for TH-positive nigral neurons were performed by an investigator unaware of the genotype or treatment status of the animal, as previously described (31).
RESULTS AND DISCUSSION
Generation of the VMAT2 Knockout Mice.
Expression of VMAT2 protein in neonatal brains, measured by saturation analyses of [3H]dihydrotetrabenazine binding, was decreased approximately 50% in heterozygotes compared with wild-type mice, and was undetectable in homozygous mice (newborn values: 510 ± 78, 296 ± 17, and 0 for wild-type, heterozygotes, and homozygotes, respectively; Fig. 1C Inset). Analyses of 93 offspring of matings between heterozygotes revealed 22 (24%) homozygous newborns, a fraction indistinguishable from the 25% expected. However, homozygous mice were poorly viable postnatally. More than one-half died by the first postnatal day 1, and all died by postnatal 14 (Fig. 1D). Pathological examination of homozygous mice sacrificed on P1 revealed only reduced milk in the stomachs, with good subcutaneous brown fat and otherwise normal organs (data not shown). Nissl stains revealed no dramatic differences in the densities of neurons in sections through rostral and mid-substantia nigra compacta from wild-type and heterozygote mice in initial studies (data not shown). Heterozygote VMAT2 knockout mice were histologically normal, viable into adult life, gained weight at rates similar to their wild-type littermate controls, and expressed [3H]dihydrotetrabenazine binding Bmax values about one-half those of wild-type mice when studied at 6 weeks of age (Fig. 1 C–E). These findings were consistent with the results of VMAT2 immunostaining. Striatal VMAT2 staining intensities in the VMAT2 heterozygous knockout mice were less than those of wild-type mice (data not shown). Although expression of at least some VMAT2 is thus necessary for viability past the immediate postnatal period, mice with one-half of wild-type levels of VMAT2 expression are able to develop and display many normal baseline behaviors that include complex reproductive behaviors.
Heart Rate, Blood Pressure, and Baseline Behaviors.
Heterozygous mice revealed moderately elevated heart rates as well as systolic, diastolic, and mean femoral arterial blood pressures greater than those of wild-type mice: values were 402 ± 18 vs. 365 ± 15, 117 ± 3 vs. 98 ± 5, 90 ± 3 vs. 65 ± 3, and 101 ± 3 vs. 80 ± 4, respectively (N.T., L.L.M., P. Jose, and G.R.U., unpublished work). However, they were similar to wild-type mice in expression of a previously conditioned passive avoidance habit, stress responses emitted in a stressful novel environment, the ability to hang onto an inverted screen, and gross locomotor activity measured under bright illumination. Heterozygotes did display a trend toward poorer motor performance in balancing on a rotating rod that did not reach statistical significance (data not shown).
Neurochemical Assessment of Heterozygote Mice.
Several neurochemical assessments of the heterozygote mice were similar to wild-type values. No significant alterations from wild-type values for striatal or frontal cortical levels of the principal inhibitory amino acid neurotransmitter GABA were found (Table 1). Bmax values and affinities for radioligand binding to the plasma membrane norepinephrine and serotonin transporters were similar to wild-type values. Affinity and Bmax values estimated from radioligand binding to dopamine D1 and D2, serotonin 5HT1A and 5HT2 subtypes, and β-adrenergic receptors were also similar to wild-type values. These data are consistent with the normal expression of several presynaptic and postsynaptic elements important for neurotransmission in the brains of heterozygotes.
Table 1.
Neurochemical assessments of VMAT2 knockout mice
| Markers | Wild type | Heterozygous |
|---|---|---|
| VMAT2 (striatum) | 3,766 ± 15 | 1,580 ± 78* |
| Dopamine (striatum) | ||
| DA | 11,711 ± 986 | 15,385 ± 485* |
| DOPAC | 1,296 ± 144 | 2,005 ± 296* |
| 3-MT | 479 ± 29 | 470 ± 46 |
| DAT | 7,796 ± 334 | 6,869 ± 298** |
| D1R | 1,112 ± 82 | 1,204 ± 127 |
| D2R | 522 ± 20 | 512 ± 47 |
| TH | 100 ± 3 | 130 ± 6** |
| Serotonin (frontal cortex) | ||
| 5-HT | 539 ± 34 | 349 ± 56** |
| 5-HIAA | 380 ± 35 | 207 ± 34* |
| SERT | 643 ± 36 | 694 ± 26 |
| 5HT1AR | 326 ± 66 | 301 ± 46 |
| 5HT2R | 30 ± 4 | 31 ± 5 |
| Norepinephrine | ||
| MHPG | 18.1 ± 0.9 | 13.8 ± 1.1** |
| NET | 120 ± 18 | 123 ± 9 |
| α1AR | 370 ± 17 | 428 ± 59 |
| βAR | 296 ± 35 | 272 ± 22 |
| GABA (striatum) | ||
| GABA | 3.9 ± 0.4 | 3.6 ± 0.4 |
Values for VMAT, dopaminergic, serotoninergic, noradrenergic, and GABAergic markers assessed by HPLC and saturation analyses of radioligand binding to receptors and transporters from whole brain or indicated brain regions (mean ± SEM). DA, dopamine; DOPAC, 3,4-dihydroxyphenylacetic acid; 3MT, 3-methoxytyramine; D1R, dopamine D1 receptors; D2R, dopamine D2 receptors; DAT, dopamine transporter; TH, tyrosine hydroxylase mRNA; 5HT, serotonin; 5-HIAA, hydroxyindolacetic acid; SERT, serotonin transporter; 5HT1AR, serotonin 1A receptor; 5HT2R, serotonin 2 receptor; MHPG, methoxyhydroxylphenylglycol; NET, norepinephrine transporter; α1AR, α1-adrenergic receptor; βAR, β-adrenergic receptor; GABA, γ-amino butyric acid. GABA levels are expressed in μmol/g tissue, other neurochemical levels in ng/g tissue, receptors and transporters in fmol specific binding/mg membrane protein, mRNAs as fraction of hybridization density found in normalized wild-type values. There were no significant differences between wild-type and VMAT2 heterozygote affinity estimates, which were: 2.6 ± 0.3 vs. 2.3 ± 0.3, 3.1 ± 0.4 vs. 3.2 ± 0.4, 0.13 ± 0.03 vs. 0.12 ± 0.01, 17.2 ± 1.1 vs. 17.3 ± 1.0, 10.9 ± 1.1 vs. 10.2 ± 1.2, 0.080 ± 0.007 vs. 0.082 ± 0.005, 1.3 ± 0.2 vs. 1.3 ± 0.1, 2.20 ± 0.54 vs. 2.47 ± 0.49, 1.14 ± 0.26 vs. 0.94 ± 0.13, 0.55 ± 0.07 vs. 0.51 ± 0.05, and 1.05 ± 0.21 vs. 0.98 ± 0.13 nM for VMAT2 (adult), VMAT2 (P1), D1R, D2R, DAT, SERT, NET, 5HT1A, 5HT2, α1AR and βAR, respectively.
P < 0.01, compared with wild type.
P < 0.05, compared with wild type.
Frontal cortex and/or striatal contents of dopamine and its principal metabolite DOPAC were enhanced by 23–39% in heterozygotes (Table 1). These increases in tissue content were accompanied by elevated brainstem levels of the mRNA encoding TH, the rate-limiting enzyme for dopamine and norepinephrine synthesis, and by 16% lower Bmax values for plasma membrane dopamine transporter DAT expression (Table 1). Absence of changes in striatal levels of 3-methoxytyramine, a dopamine metabolite produced largely in extracellular compartments, or in either dopamine D1 or D2 receptors, are consistent with the idea that synaptic dopamine levels might be closer to normal than the values for altered tissue content might suggest (38). Conceivably, enhanced dopamine synthetic capacity due to increased TH activity and reduced re-uptake from extracellular pools with reduced DAT expression could help to drive synaptic dopamine levels toward more normal values in the face of impaired vesicular dopamine storage. Possibly, elevated intraneuronal dopamine levels might also exert less impact on synaptic levels due to reduced DAT-mediated reverse transport out of intracytoplasmic/extravesicular dopamine pools. Near normal synaptic dopamine levels in the heterozygotes could also fit with observations that many of the tested baseline behaviors, including levels of locomotion that can provide an index of substantial functional dopamine depletions, are near normal values.
Levels of the norepinephrine marker, its metabolite methoxyhydroxylphenylglycol), were reduced in heterozygotes despite elevated levels of the mRNA encoding TH, whereas levels of norepinephrine itself were not reliably above the detection limits of the assay system used here (Table 1 and data not shown). Levels of serotonin and its metabolite 5-hydroxyindolacetic acid were also reduced by 25–45% in frontal cortex and striatum of heterozygotes, whereas serotonin immunostaining was reduced over fiber/terminal and cell body areas (data not shown). Possible influences of poor vesicular compartmentalization on the retention of immunoreactive serotonin available for immunohistochemical detection in fixed tissue are unknown, providing a caution in interpretation of these histologic results. Although the reasons for the striking differential effects of VMAT2 gene dose reductions on dopamine, norepinephrine, and serotonin levels are not clear, differential compartmentalization of the synthetic capacities for these monoamines in cytosolic vs. vesicular compartments has been identified in several studies (38). These sorts of data could provide one explanation for the relative sparing of dopamine synthetic pathways, if more of such paths were independent of vesicular function. Alternatively, these data could provide incentives to consider the regulatory functions on gene expression that might occur due to possible elevations of cytoplasmic monoamine concentrations. In initial studies, levels of the mRNA-encoding dopamine β-hydroxylase were substantially reduced in adult VMAT2 heterozygotes, providing an alternative explanation for the retained dopamine levels and reduced norepinephrine levels (N.T. and G.U., unpublished observations). Normal serotonin and β-adrenergic receptor levels, however, are again compatible with the idea that synaptic serotonin and norepinephrine levels might be closer to normal than these altered tissue content assessments reflect.
Amphetamine-Induced Locomotion and Reward in Heterozygote Mice.
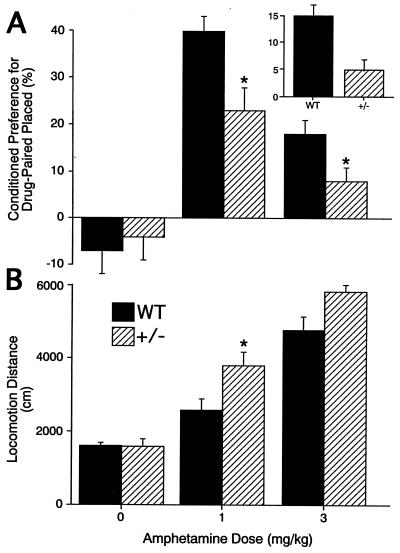
Encouraged by the heterozygotes’ nearly-normal values for several baseline behaviors, we examined responses to doses of amphetamine that produced locomotion and conditioned place preference, a prominent murine model for measuring aspects of drug reward and reinforcement (Fig. 2). Under bright illumination conditions, 1 mg/kg and 3 mg/kg intraperitoneal amphetamine doses enhanced locomotor activity in both wild-type and heterozygous mice. Significant heterozygote locomotor activity increases at 1 mg/kg were 146% of those in wild-type mice (Fig. 2B).
Figure 2.
Amphetamine effects in wild-type and heterozygous VMAT2 knockout mice. (A) Conditioned place preference induced by pairing indicated amphetamine doses with the initially nonpreferred side of the apparatus or pairing 1 mg/kg amphetamine with the initially preferred side of the apparatus (Inset) in heterozygote VMAT2 knockout mice or wild-type littermates. ∗, P < 0.05, comparing heterozygotes with wild-type littermates. (B) Locomotion induced by indicated amphetamine doses in heterozygote VMAT2 knockout mice or wild-type littermates. ∗, P < 0.05, comparing heterozygotes with wild-type littermates.
Conditioning trials for conditioned place preference studies revealed that wild-type and heterozygous mice both spent ≈65% of these trials on the initially preferred side. Fewer than 10%, 3 of the 47 tested heterozygote and 4 of the 47 tested wild-type mice, failed to spend more time on corncob bedding during the initial periods when they expressed their side preferences. Following conditioning with 1 mg/kg or 3 mg/kg amphetamine doses, both wild-type and heterozygote animals displayed conditioning for the initially nonpreferred place where they received the conditioning amphetamine doses (Fig. 2A). Heterozygote mice, however, displayed less conditioned place preference than wild-type control mice when they received either of these doses in the initially nonpreferred place (Fig. 2A). Heterozygotes also displayed less conditioned place preference when they received 1 mg/kg amphetamine at the initially preferred place (Fig. 2A Inset), suggesting that amphetamine pairing enhances the rewarding properties associated with a specific environment rather than reducing aversive features (39). Conversely, cocaine (5 mg/kg), a blocker of the plasma membrane dopamine transporter DAT devoid of significant activity on vesicular monoamine stores (40), induced conditioned place preferences in heterozygotes that were indistinguishable from those in wild-type mice (data not shown).
MPTP Toxicity in Mutant Mice.
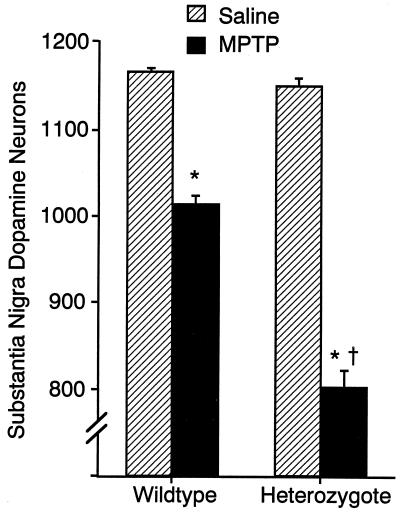
Counts of substantia nigra dopaminergic neurons marked by TH immunostaining in 6-week-old heterozygote mice were identical to those of wild-type animals (Fig. 3). MPTP treatments that led to 13% fewer TH immunoreactive neurons in wild-type mice led to 30% reductions in heterozygotes. Striatal TH immunostaining densities showed variability and trends in the same direction (data not shown). These data reveal the importance of normal levels of vesicular transport for MPP+ resistance, even in heterozygote animals with one-half of wild-type levels of VMAT2 expression. They are also remarkable, since the observed 16% reductions in heterozygote expression of the plasma membrane DAT dopamine transporter might otherwise be expected to decrease toxicity through reduced MPP+ uptake into dopaminergic neurons. Studies of brain MPP+ levels revealed no differences in concentrations found in wild-type and heterozygote mice sacrificed at any of several time points following this dose (data not shown).
Figure 3.
MPTP toxicity in wild-type and heterozygous VMAT2 knockout mice. Numbers of substantia nigra, pars compacta TH immunoreactive dopaminergic neurons in wild-type and heterozygote VMAT2 knockout mice treated with saline or MPTP (24). These doses produced similar brain levels of MPP+ in mice of both genotypes in preliminary studies. ∗, P < 0.05, compared with saline injection. †, MPTP effect significantly different from its effect in wild-type mice.
The results described here complement those obtained in DAT knockout mice, in which amphetamine-induced locomotor responses are ablated (ref. 13, and I.S. and G.R.U., unpublished observations). Taken together, the data from VMAT2 and DAT knockouts support the idea that plasma membrane transporter blockade by amphetamine contributes primarily to its locomotor stimulation, but that normal function of vesicular monoamine stores is necessary for full amphetamine-induced behavioral reward. Further studies will help us to determine what mechanisms are responsible for these differences. These data are also consistent with results from studies documenting differences between the effects of dopamine receptor knockouts on psychostimulant-induced locomotion and reward (41), and with the differences in dose-response relationships for amphetamine-induced locomotion and reward (42). These data, taken together, also fail to support substantial roles for other lower potency amphetamine actions, such as blockade of the inactivating/degrading enzyme monoamine oxidase (7), in the differences observed in the current studies.
Animals with reduced vesicular monoamine transporter expression thus provide valuable tools for dissections of molecular underpinnings of a number of features of monoaminergic and drug function and toxicity. Longer term studies of these animals will also allow exploration of the roles of the vesicular monoamine transporter in the differential compartmentalization of monoamine neurotransmitters of possible importance for age-related neuronal losses.
Acknowledgments
We thank A. Bradley and Y. Mishina for generously providing plasmids, AB1 embryonic stem cells, and lymphocyte-inhibitory factor (LIF)-secreting STO cells; P. Jose for assistance with blood pressure determinations; D. Togasaki and A. B. Naini for HPLC measurements; Y. Mishina, G. Koob, and D. Vandenbergh for helpful discussions; C. Brayton for help with pathological analysis; H.-F. Lui, N. Goodman, and S. Kinsey for technical contributions; L. Roggio and the Bionetics/Triad animal care staff for careful mouse care and breeding; and A. Flood and M. J. Robinson for assistance with the manuscript. This work was supported by the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health. S.P. and G.R.U. acknowledge support from the American Parkinson’s Disease Association (APDA); S.P. is a recipient of a Cotzias award of the APDA and is also supported by the Muscular Dystrophy Association, National Institute of Neurological Disorders and Stroke (NS01724), Lowenstein Foundation, and Irving A. Hansen Memorial Foundation; and V.J.-K. from the Parkinson’s Disease Foundation.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: VMAT2, brain synaptic vesicular monoamine transporter; DAT, dopamine transporter; MPP+, 1-methyl-4-phenyl-phenypyridinium; MPTP, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; GABA, γ-aminobutyric acid; TH, tyrosine hydroxylase.
References
- 1.Johnson R G. Physiol Rev. 1988;68:232–307. doi: 10.1152/physrev.1988.68.1.232. [DOI] [PubMed] [Google Scholar]
- 2.Henry J-P, Botton D, Sagne C, Isambert M-F, Desnos C, Blanchard V, Raisman-Vozari R, Krejci E, Massoulie J, Gasnier B. J Exp Biol. 1994;196:251–262. doi: 10.1242/jeb.196.1.251. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Peter D, Roghani A, Schuldiner S, Privé G G, Eisenberg D, Brecha N, Edwards R H. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- 4.Erickson J D, Eiden L E, Hoffman B J. Proc Natl Acad Sci USA. 1992;89:10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute of Justice. Annual Report on Adult and Juvenile Arrestees. Rockville, MD: Natl. Criminal Justice Ref. Serv.; 1995. [Google Scholar]
- 6.Kuczenski R, Segal D S. In: Amphetamine and its Analogs. Cho A K, Segal D S, editors. San Diego: Academic; 1994. pp. 81–113. [Google Scholar]
- 7.Seiden L S, Sabol K E, Ricaurte G A. Annu Rev Pharmacol Toxicol. 1993;32:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- 8.Sulzer D, Rayport S. Neuron. 1990;5:797–808. doi: 10.1016/0896-6273(90)90339-h. [DOI] [PubMed] [Google Scholar]
- 9.Rudnick G, Wall S C. Proc Natl Acad Sci USA. 1992;89:1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada S, Kitayama S, Lin C L, Patel A, Nanthakumar E, Gregor P, Kuhar M, Uhl G R. Science. 1991;254:576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- 11.Pacholczyk T, Blakely R D, Amara S G. Nature (London) 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- 12.Blakely R D, Berson H E, Fremeau R T, Jr, Caron M G, Peek M M, Prince H K, Bradley C C. Nature (London) 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- 13.Giros B, Jaber M, Jones S R, Wightman R M, Caron M G. Nature (London) 1996;379:606–616. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 14.McGeer P L, Itagaki S, Akiyama H, McGeer E G. Ann Neurol. 1988;24:574–576. doi: 10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- 15.Uhl G R, Hedreen J C, Price D L. Neurology. 1985;35:1215–1218. doi: 10.1212/wnl.35.8.1215. [DOI] [PubMed] [Google Scholar]
- 16.Kitayama S, Shimada S, Uhl G R. Ann Neurol. 1992;32:109–111. doi: 10.1002/ana.410320120. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Xu W, Harrington K A, Emson P C. Mol Brain Res. 1994;25:90–96. doi: 10.1016/0169-328x(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 18.Shimada S, Kitayama S, Walther D, Uhl G R. Mol Brain Res. 1992;13:359–362. doi: 10.1016/0169-328x(92)90220-6. [DOI] [PubMed] [Google Scholar]
- 19.Uhl G R, Walther D, Mash D, Faucheux B, Javoy-Agid F. Ann Neurol. 1994;35:494–498. doi: 10.1002/ana.410350421. [DOI] [PubMed] [Google Scholar]
- 20.Soriano P, Montgomery C, Geske R, Bradley A. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 21.Bradley A. In: Tetracarcinomas and Embryonic Stem Cells. Robertson E J, editor. Oxford: IRL; 1987. pp. 113–151. [Google Scholar]
- 22.Freed C, Revay R, Vaughan R A, Kriek E, Grant S, Uhl G R, Kuhar M J. J Comp Neurol. 1995;359:340–349. doi: 10.1002/cne.903590211. [DOI] [PubMed] [Google Scholar]
- 23.Boja J W, Carroll F I, Rahman M A, Philip A, Lewin A H, Kuhar M J. Eur J Pharmacol. 1990;184:329–332. doi: 10.1016/0014-2999(90)90627-i. [DOI] [PubMed] [Google Scholar]
- 24.Marcusson J O, Bergstrom M, Eriksson K, Ross S B. J Neurochem. 1988;50:1783–1790. doi: 10.1111/j.1471-4159.1988.tb02479.x. [DOI] [PubMed] [Google Scholar]
- 25.Sherman D. J Neurochem. 1986;47:331–339. doi: 10.1111/j.1471-4159.1986.tb04506.x. [DOI] [PubMed] [Google Scholar]
- 26.Tejani-Butt S M, Brunswick D J, Frazer A. Eur J Pharmacol. 1990;191:239–243. doi: 10.1016/0014-2999(90)94155-q. [DOI] [PubMed] [Google Scholar]
- 27.Ujike H, Akiyama K, Nishikawa H, Onoue T, Otsuki S. Brain Res. 1991;540:159–163. doi: 10.1016/0006-8993(91)90503-n. [DOI] [PubMed] [Google Scholar]
- 28.Terai M, Hidaka K, Nakamura Y. Eur J Pharmacol. 1989;173:177–182. doi: 10.1016/0014-2999(89)90516-5. [DOI] [PubMed] [Google Scholar]
- 29.Robinson M B, Anegawa N J, Gorry E, Qureshi I A, Coyle J T, Lucki I, Batshaw M L. J Neurochem. 1992;58:1016–1022. doi: 10.1111/j.1471-4159.1992.tb09356.x. [DOI] [PubMed] [Google Scholar]
- 30.Bylund D B, Snyder S H. Mol Pharmacol. 1976;12:568–580. [PubMed] [Google Scholar]
- 31.Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson V L, Dawson T M. Proc Natl Acad Sci USA. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naini A B, Vontzalidou E, Cote L J. Clin Chem. 1993;39:247–250. [PubMed] [Google Scholar]
- 33.Jackson-Lewis V, Jakowec M, Burke R E, Przedborski S. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 34.Ichikawa S, Sasaoka T, Nagatsu T. Biochem Biophys Res Commun. 1991;176:1610–1616. doi: 10.1016/0006-291x(91)90472-j. [DOI] [PubMed] [Google Scholar]
- 35.Alonso S, Minty A, Bourlet Y, Buckingham M. J Mol Evol. 1986;23:11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- 36.Sora I, Takahashi N, Funada M, Ujike H, Revay R S, Donovan D M, Miner L L, Uhl G R. Proc Natl Acad Sci USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flint J, Corley R, DeFries J C, Fulker D W, Gray J A, Miller S, Collins A C. Science. 1995;269:1432–1435. doi: 10.1126/science.7660127. [DOI] [PubMed] [Google Scholar]
- 38.Cooper J R, Bloom F E, Roth R H. The Biochemical Basis of Neuropharmacology. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 39.Carr G D, Fibiger H C, Phillips A G. In: Neuropharmacological Basis of Reward. Liebman J M, Copper S J, editors. New York: Oxford Univ. Press; 1984. pp. 264–319. [Google Scholar]
- 40.Amara S, Kuhar M J. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 41.Miner L L, Drago J, Chamberlain P M, Donovan D, Uhl G R. NeuroReport. 1995;6:2314–2316. doi: 10.1097/00001756-199511270-00011. [DOI] [PubMed] [Google Scholar]
- 42.Segal D S, Kuczenski R. In: Amphetamine and its Analogs. Cho A K, Segal D S, editors. San Diego: Academic; 1994. pp. 115–150. [Google Scholar]