Abstract
CD4+ helper T cells play a critical role in the production of the antinuclear autoantibodies that characterize systemic lupus erythematosus in mice and humans. A key issue is whether this help is derived from a diverse repertoire of autoreactive CD4+ T cells or from a select number of T cells of limited specificity. We used the chronic graft-versus-host disease model to define the diversity of the CD4+ T cell repertoire required to induce the autoantibody response. By transferring clonally restricted versus clonally diverse populations of MHC class II–reactive CD4+ T cells, we show that the loss of B cell tolerance to nuclear antigens has two distinct components with different CD4+ cell requirements. Activation of limited repertoires of CD4+ T cells was sufficient for the expansion of anergized anti–double-stranded DNA B cells and production of IgM autoantibodies. Unexpectedly, we found that CD4+ T cell diversity was necessary for CD4+ T cell trafficking into the follicle and for the generation of isotype-switched IgG autoantibodies. Importantly, combining two limited repertoires of T cells provides sufficient CD4+ T cell diversity to drive antinuclear Ab production. These data demonstrate that a diverse CD4+ T cell repertoire is required to generate a sustained effector B cell response capable of mediating systemic autoimmunity.
Introduction
Systemic lupus erythematosus (SLE), the prototypical systemic autoimmunity, is characterized in both mice and humans by the production of antinuclear Ab’s (ANAs). ANA-producing autoreactive B cells have undergone clonal expansion and somatic mutation, changes that suggest an antigen-driven, T cell–dependent process (1). A role for CD4+ T cells has been shown by the response of murine lupus to thymectomy, genetic deletion of MHC class II molecules, and anti-CD4 treatment (2–4). Although CD4+ T cells are clearly required for the development of murine lupus, their specific role in the loss of B cell tolerance has not been well defined.
The complexity of the CD4+ T cell repertoire required for autoantibody production has been examined. The T cell repertoire was restricted in T cell receptor–deficient (TCR-deficient) lupus-prone MRL-lpr mice to a single transgenic specificity directed against a foreign peptide (5). These mice failed to develop high-titer autoantibodies and immune complex–mediated kidney damage, suggesting that the autoreactivity requires some T cell diversity. In this case, however, disease progression could require either a broader T cell repertoire or an autoreactive T cell specificity. Therefore, we sought a more thorough understanding of the diversity of the MHC class II–reactive CD4+ T cell repertoire that drives the loss of B cell tolerance.
Mouse models of SLE include both inbred mice that spontaneously develop SLE-like disease and experimental induction of these manifestations in nonautoimmune mice. Chronic graft-versus-host disease (cGVHD) is induced in unirradiated, MHC-incompatible mice by the transfer of allogeneic CD4+ T cells and results in autoantibodies and renal disease similar to human SLE (6). cGVHD is induced in normal, nonautoimmune mice and is driven by T cell activation. Manipulation of both the T cell repertoire and the host permits a fine dissection of the T cell and B cell requirements for autoantibody production. Cohen, Eisenberg, and their colleagues have shown that, similar to inbred lupus-prone mice, cognate MHC class II–restricted interactions between donor CD4+ T cells and host B cells induce the host B cells to produce autoantibodies (7). CD4+ T cells play a critical role in the initiation and propagation of the disease, however, as in SLE, the T cell–B cell (T-B) interactions required for the production of ANAs remain poorly characterized.
To thoroughly characterize the T-B interactions required for the production of ANAs, we compared the ability of limited and diverse repertoires of MHC class II, I-Ab–reactive CD4+ T cells to induce ANAs in wild-type mice. We show that limited T cell repertoires activate autoreactive anti–double-stranded DNA (dsDNA) B cells; however, these repertoires were unable to induce detectable levels of IgG ANAs. This failure was associated with follicular exclusion of MHC class II–reactive CD4+ T cells and suggests that multiple, diverse T-B interactions leading to CD4+ T cell entry into the B cell follicle are required for the production of IgG ANAs.
Methods
Mice.
All mice used were between the ages of 2 and 3 months and were on an H-2b background. C57Bl/6J, B6.PL, and H-2bm12 (bm12) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). H2-DM–/– B6 mice were generously provided by Luc Van Kaer (Vanderbilt University, Nashville, Tennessee, USA) and were backcrossed onto the B6 background for six generations. K14-Aβb/Aβb–/– (K14) mice were backcrossed onto the B6 background for more than 20 generations. The 2-2-3 TCR transgene was backcrossed onto the K14 background for at least three generations. Previously, we described the development of a cutaneous autoimmune disease in approximately 20% of 2-2-3/K14 mice (8). Mice used in this study did not develop disease, and the CD4+ T cells were not activated. The 3H9.KI site-directed transgenic mice were generously provided by Martin Weigert (Princeton University, Princeton, New Jersey, USA) and were backcrossed onto the B6 background for at least eight generations; they were backcrossed to generate Thy1.1+/+ 3H9.KI+ mice. The 3H9.KI mice are on a B6 background (allotype IgHb); in some studies, we used flow cytometry to follow the 3H9 heavy chain by staining with allotype-specific IgDa (see below). Genotyping of all mice was determined by PCR amplification of tail DNA and/or phenotyping of peripheral blood lymphocytes in procedures described previously (14). All mice were bred and maintained at the animal facility at the University of Pennsylvania Medical Center (Philadelphia, Pennsylvania, USA).
CD4+ T cell isolation.
Spleen and all visible lymph nodes were removed from donor mice and mashed between the frosted ends of glass slides to generate single cell suspensions. Erythrocytes were lysed by hypotonic shock (0.83% NH4Cl) and CD4+ T cells isolated using a negative-selection magnetic-sorting approach. In brief, single cell suspensions were incubated with pretitrated volumes of anti-CD8 (2.43), anti-B220 (RA3-B220), anti-HSA (J11d), and anti-FcR (2.4G2) hybridoma tissue-culture supernatants were grown in our laboratory. Cells were washed and incubated with magnetic bead–conjugated goat anti-rat Ig (Polysciences Inc., Warrington, Pennsylvania, USA), and non-CD4+ T cells were removed utilizing a Bio-Mag magnetic stand (Polysciences Inc.). CD4+ T cell purity was generally greater than 80%.
CFSE.
CD4+ T cells were CFSE labeled according to procedures described previously (9, 10). Cells were washed and resuspended at a density of 2 × 107/ml in PBS. An equal volume of 5 μM CFSE (Molecular Probes Inc., Eugene, Oregon, USA) in PBS was added (5 minutes at room temperature). Labeling was stopped by addition of FBS, and cells were washed in PBS and resuspended in HBSS prior to intravenous injection. Forty-two hours later, spleen cells were stained for CD4 (see below), analyzed on a Becton Dickinson FACScalibur, and at least 1,000 live-gated CFSE+ events were collected.
Experimental cGVHD protocol.
Recipient mice on a B6 background were injected intraperitoneally with 10 × 106 purified CD4+ T cells in HBSS. Mice receiving the combination of the two limited T cell repertoires also received 10 × 106 total CD4+ T cells (5 × 106 2-2-3/K14 CD4+ T cells and 5 × 106 H2-DM–/– CD4+ T cells). Blood samples from experimental mice were obtained at 2- to 4-week intervals, serum isolated, and frozen at –20°C until analyzed. For some studies, experimental mice were sacrificed 7 days after cGVHD induction, and a small sample of the spleen was frozen in OCT for immunohistochemical analysis.
Anti-chromatin ELISA.
Bovine thymus chromatin was prepared as described previously (11). Flat-bottomed Immunolon II microtiter plates (ThermoLabsystems, Franklin, Massachusetts, USA) were coated with bovine thymus chromatin (1 μg/ml) for 2 hours and blocked with 3% BSA/0.1% gelatin/3 mM EDTA (4°C, overnight). Diluted serum samples (1:500) were added in duplicate and incubated 2 hours at room temperature. Plates were washed (0.1% Tween in PBS), and bound Ig was revealed with alkaline phosphatase–conjugated goat anti-mouse IgG or IgM (Southern Biotechnology Associates, Birmingham, Alabama, USA), using p-nitrophenyl phosphate (Sigma-Aldrich, St. Louis, Missouri, USA) as a substrate. The plates were read at 405–650 nm on a microplate reader (Molecular Devices, Berkshire, United Kingdom).
Anti-dsDNA ELISA.
The anti-dsDNA ELISA was carried out according to established methods (12, 13). In brief, Immunlon II microtiter plates were precoated with methylated BSA (Sigma-Aldrich), washed, and coated with 50 μg/ml dsDNA obtained from calf thymus (Sigma-Aldrich). Plates were blocked, samples added, and bound IgG detected as above.
Total IgG.
Total IgG was assessed by ELISA as described above. Plates were coated with goat anti-mouse IgG (Southern Biotechnology Associates) at 2 μg/ml. Diluted serum samples were added in duplicate, washed, and revealed with alkaline phosphatase–conjugated goat anti-mouse IgG using p-nitrophenyl phosphate as a substrate.
Antinuclear Ab assay.
The presence of antinuclear Ab’s was detected using permeabilized HEP-2 cells as the substrate (Antibodies Inc., Davis, California, USA). Serum was diluted 1:50 in 1% BSA. Antinuclear binding was revealed using FITC-conjugated goat anti-mouse IgG (Southern Biotechnology Associates). The samples were then visualized under a fluorescent microscope and scored blind.
Flow cytometry.
Live cells (106) were washed in PBS containing 0.5% BSA and 0.01% NaN3, and FcR was blocked with 2.4G2 (anti-FcγRII/III) tissue-culture supernatant. The following dye- or biotin-conjugated Ab’s were obtained from Pharmingen (BDBiosciences, San Diego, California, USA): CD4 (RM4-5), B220 (RA3-6B2), I-Ab (AF6-20.1), HSA (M1/69), Thy1.2 (53-2.1), B7.2 (GL1), λ1 light chain (R11-153), CD44 (Pgp-1), CD19 (1D3), CD21 (7G6), CD22.2 (Cy34.1), and IgDa (AMS9.1). Pretitrated doses of conjugated (FITC, phycoerythrin [PE], peridinin chlorophyll protein–cyanine 5.5 [PerCP-Cy5], APC, or biotinylated) Ab’s were added (30 minutes, 4°C). Cells were washed, and biotinylated Ab’s were revealed using streptavidin-allophycocyanin (SA-APC) (Pharmingen). Cells were analyzed on a Becton Dickinson Immunocytometry Systems (San Jose, California, USA) FACSCalibur using FlowJo software.
Immunohistochemistry.
Spleen samples were suspended in OCT and snap-frozen in 2-methyl-butane cooled with liquid nitrogen. Spleens were sectioned and fixed with acetone and stored at –20°C until stained. Samples were blocked with PBS/5% normal goat serum (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA)/0.1% Tween-20 and then incubated with Thy1.2-biotin (Thy1.2-bio) or λ1 light chain-bio. Sections were washed with PBS/0.1% Tween, and biotinylated Ab’s were revealed with Vectastain ABC kit conjugated to HRP (Vector Laboratories, Burlingame, California, USA); substrate for HRP was 3,3-diaminobenzidine. Sections were next incubated with B220-bio, washed, and incubated with Vectastain ABC conjugated to alkaline phosphatase; substrate for alkaline phosphatase was nitroblue tetrazdium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP). Slides were scored blind by multiple readers.
Statistical analysis.
Statistical significance was determined using the Student’s t test.
Results
T cell repertoires of limited and diverse specificities.
cGVHD is induced by the transfer of allogeneic T cells to unirradiated, nonautoimmune mice resulting in anti-dsDNA and ANA formation. We have examined the loss of B cell tolerance during cGVHD in B6 mice following the transfer of I-Ab–reactive CD4+ T cells from four different strains of mice. Specifically, we were interested in determining how the activation and expansion of I-Ab–reactive CD4+ T cell repertoires of limited and diverse specificities affected the induction of B cell autoimmunity in cGVHD.
We have described previously K14 mice in which the expression of MHC class II on thymic cortical epithelium results in syngeneic reactivity of CD4+ T cells to B6 APCs (14). We subsequently derived an I-Ab–reactive TCR transgenic, 2-2-3 (Vα1, Vβ5) from a K14 anti-B6 CD4+ T cell hybridoma and have shown previously that 2-2-3 TCR transgenic CD4 cells are selected on K14 thymic epithelium and maintain their syngeneic reactivity to B6 APCs (8). Although 2-2-3 CD4+ T cells can use endogenous α chains, the presence of the TCR transgene severely restricts the diversity of the CD4+ T cell repertoire.
In mice lacking H2-DM, the class II–like protein involved in removal of the CLIP peptide, the majority of I-Ab molecules are occupied with CLIP (15–17). Thymic selection on this very narrow array of self-peptides selects a population of CD4+ T cells that respond to the diverse array of self-peptides expressed on wild-type B6 APCs; however, the diversity of the CD4+ T cell repertoire is constrained (18, 19).
Finally, bm12 mice are a coisogenic strain of B6 mice with the I-A molecules differing by only three amino acids in the peptide-binding groove; this difference is sufficient to make bm12 alloreactive with B6.
Thus we have used two distinct repertoires of I-Ab–reactive CD4+ T cells of limited diversity (2-2-3/K14 and H2-DM–/–) and two repertoires of I-Ab–reactive CD4+ T cells of normal diversity (K14 and bm12). Both repertoires of diverse CD4+ T cells have been shown previously to be sufficient to induce cGVHD with hypergammaglobulinemia and ANA formation when transferred to naive B6 recipients (6, 20). Finally, in all experiments, transfer of syngeneic B6 CD4+ T cells served as a negative control.
I-Ab–reactive CD4+ T cell repertoires proliferate to B6 APCs in vivo.
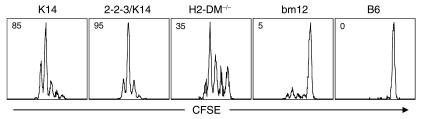
To verify the I-Ab reactivity of the different CD4+ T cell repertoires, we transferred CFSE-labeled CD4+ T cells into B6 mice. Figure 1 shows that all class II–reactive CD4+ T cells contained cells that proliferated, dividing at least four times by 42 hours after transfer. The individual repertoires clearly have different percentages of B6-reactive CD4+ T cells; however, this variability does not correlate with diversity. As such, the highest frequency of responding cells was the 2-2-3/K14 repertoire, whereas the lowest frequency was the bm12 repertoire. Importantly, subsequent results suggest that the precursor frequency of I-Ab–reactive CD4+ T cells does not determine the outcome of cGVHD.
Figure 1.
Proliferation of I-Ab–reactive CD4+ T cells in B6 mice. B6 mice were injected (intravenously) with 5 × 106 CFSE-labeled CD4+ T cells. After 42 hours, animals were euthanized and spleen cells stained with anti-CD4 and analyzed by flow cytometry. Histograms show the division history of gated live splenic CFSE+ CD4+ T cells. Percentage of CD4+ T cells responding to B6 APCs is shown in the upper-left corner of the histogram. The y axis is at least 100 events for each histogram. Representative results from three independent experiments are shown.
Polyclonal B cell activation during cGVHD does not lead to ANA production.
To ask if the diversity of the CD4+ cell repertoire alters the induction of cGVHD we followed B6 mice that received CD4+ T cells from 2-2-3/K14 and H2-DM–/– mice, the positive controls, K14 and bm12 mice, and syngeneic B6 mice as a negative control. Mice were serially bled and serum was assayed for IgG levels and ANA formation.
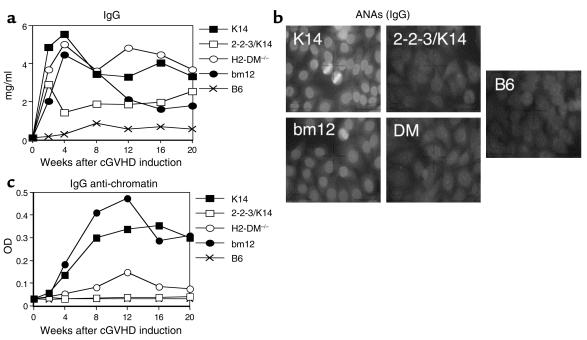
All I-Ab–reactive CD4+ T cells stimulated host B cells to secrete IgG (Figure 2a). The limited diversity repertoires, 2-2-3/K14 and H2-DM–/–, induced IgG levels comparable to that of the diverse K14- and bm12-positive controls. The kinetics and magnitude of IgG secretion appear different because 2-2-3/K14 and bm12 CD4+ T cells induce an early response that is not sustained in its initial intensity, whereas K14 and H2-DM–/– CD4+ T cells induce an early response that is sustained. These differences did not reach significance, however. Additionally, the differences in production of IgG do not correlate with T cell diversity. Therefore, all MHC class II–reactive CD4+ T cell repertoires induced polyclonal B cell activation.
Figure 2.
ANA production during cGVHD requires a diverse repertoire of CD4+ T cells. (a) cGVHD was induced in naive B6 mice. Serum was serially obtained and tested for IgG levels by ELISA. Each point represents the mean of 7–10 mice. All class II–reactive T cell repertoires stimulated a significant increase (P < 0.05) in total IgG at all time points tested as compared with B6 → B6. (b) Serum from mice 12 weeks after cGVHD induction was assayed for the production of IgG ANAs by indirect immunofluorescence of Hep-2 cells. B6→B6 serum failed to stain Hep-2 nuclei (zero of eight). cGVHD induction with K14 (eight of eight) and bm12 (seven of seven) CD4+ T cells induced a homogenous nuclear staining, whereas cGVHD induction with 2-2-3/K14 (zero of ten) and H2-DM–/– (one of ten) CD4+ T cells failed to stain the nucleus. Representative results are shown. (c) Serum from cGVHD mice was tested for the presence of IgG anti-chromatin autoantibodies by ELISA. K14 and bm12 CD4+ T cells induced a significant increase in anti-chromatin autoantibodies (P < 0.05). 2-2-3/K14 and H2-DM–/– CD4+ T cells failed to significantly increase (P > 0.05) autoantibody production at all time points tested compared with B6 → B6. Each point represents the mean of 7–10 mice.
To determine if polyclonal B cell activation was associated with autoimmunity, serum was assayed for IgG ANA production by indirect immunofluorescent staining of Hep-2 nuclei. Sera from B6 mice that received syngeneic B6 CD4+ T cells failed to stain Hep-2 nuclei (Figure 2b). As described previously, serum from K14 and bm12 CD4+ T cells induced a homogenous nuclear staining of Hep-2 nuclei indicative of anti-chromatin and anti-dsDNA Ab’s. Interestingly, transfer of 2-3-3/K14 and H2-DM–/– CD4+ T cells did not elicit any ANA production. The failure of 2-2-3/K14 and H2-DM–/– CD4+ T cells to induce ANAs was confirmed by an anti-chromatin (Figure 2c) and anti-dsDNA ELISA (data not shown). Importantly, the production of ANAs is independent of the precursor frequency of the inducing repertoires (see Figure 1); rather, it requires diversity within the transferred T cells. Thus the diverse bm12 CD4+ T cell repertoire had the lowest precursor frequency of class II–reactive cells, but it was sufficient to induce ANAs. On the other hand, the limited diversity 2-2-3/K14 repertoire had the highest frequency of class II–reactive cells, but it was not sufficient to induce ANAs. The observation that polyclonal B cell activation induced by limited diversity CD4+ T cells does not also yield ANAs demonstrates the demanding T cell requirements for breaking tolerance to nuclear antigens.
2-2-3/K14 and H2-DM–/– CD4+ T cells fail to induce ANAs in 3H9.KI mice.
To dissect the T and B cell requirements for ANA production, we used a site-directed transgenic mouse expressing the heavy chain from an anti-dsDNA Ab (3H9.KI) (21). “Knockin” of the 3H9 heavy chain into the endogenous locus allows endogenous light-chain rearrangements to generate a B cell repertoire with both anti-DNA and non-DNA specificities. Furthermore, the 3H9 heavy chain, when paired with the λ1 light chain, generates anti–dsDNA-specific B cells that can be tracked (22). The anti–DNA-binding B cells in nonautoimmune B6/3H9.KI mice are tolerant and do not spontaneously secrete anti-DNA Ab’s. Sekiguchi et al. have shown that cGVHD induction with bm12 CD4+ T cells results in anti-dsDNA Ab formation, including serum levels of λ1+ anti-dsDNA Ab’s (23).
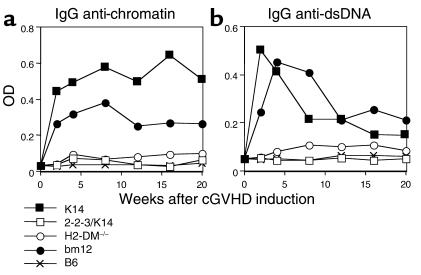
To determine if 3H9.KI mice recapitulated the phenotype of wild-type B6 mice, cGVHD was induced in 3H9.KI mice. Serum analysis of 3H9.KI mice undergoing cGVHD revealed that both K14 and bm12 CD4+ T cells stimulated host 3H9.KI B cells to secrete IgG anti-chromatin (Figure 3a) and anti-dsDNA Ab’s (Figure 3b), whereas B6 CD4+ T cells did not. As in wild-type B6 recipients, neither 2-2-3/K14 nor H2-DM–/– CD4+ T cells induced IgG anti-chromatin or anti-dsDNA Ab’s in 3H9.KI mice. Therefore, skewing the repertoire of B cells toward autoreactivity doesn’t change the requirements for a diverse T cell repertoire in generating ANAs.
Figure 3.
Introduction of an anti-dsDNA heavy chain does not alter the behavior of the class II–reactive T cell repertoires. (a) cGVHD was induced by injecting B6/3H9.KI mice with 10 × 106 of the indicated MHC class II–reactive CD4+ T cell repertoires. Serum samples were taken and tested for IgG anti-chromatin levels by ELISA. Limited T cell repertoires (2-2-3/K14 and H2-DM–/–) failed to stimulate a significant increase in the titers of IgG anti-chromatin autoantibodies as compared with B6 negative control at all time points tested (P > 0.05); the diverse T cell repertoires induced a significant increase at all time points (P < 0.05). The diverse T cell repertoires, K14 and bm12, induced a comparable (P > 0.1) elevation of IgG anti-chromatin autoantibodies. Each group includes at least eight experimental mice. (b) Serum samples from B6/3H9.KI mice that received the indicated MHC class II–reactive CD4+ T cell repertoires were tested for IgG anti-dsDNA levels by ELISA. Similar to the failure to induce IgG anti-chromatin autoantibodies, limited T cell repertories (2-2-3/K14 and H2-DM–/–) failed to stimulate a significant increase in IgG anti-dsDNA Ab production as compared with transfer of B6 CD4+ T cells at all time points tested (P > 0.05), whereas activation of the diverse T cell repertoires (K14 and bm12) induced a significant increase at all time points (P < 0.05). Each group contains at least eight experimental mice.
Activation of all class II–reactive T cell repertoires induces IgM ANAs with activation and follicular entry of anti-dsDNA B cells.
The behavior of anti-dsDNA–specific B cells in 3H9.KI mice can be monitored by focusing on λ1+ B cells. We have previously used this strategy to show that anti-dsDNA B cells are regulated by anergy and exclusion from the B cell follicle (24). On the autoimmune-prone MRL-lpr background, a CD4+ T cell–dependent process induces the activation and follicular entry of anti-dsDNA B cells associated with anti-dsDNA Ab formation (25, 26). To distinguish transferred I-Ab–reactive CD4+ T cells from host T cells, the Thy1.1 allele was introduced into 3H9.KI mice.
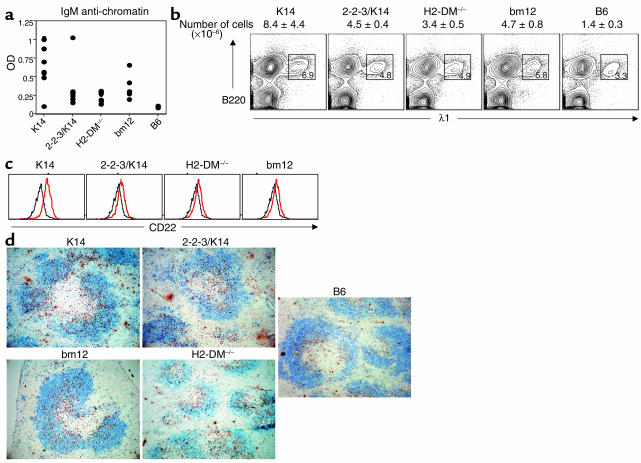
It has been difficult to detect the presence of IgM anti-chromatin Ab’s preceding the development of IgG in mouse models of SLE (27). The controlled induction of B cell autoreactivity during cGVHD allowed us to look for IgM production. Anti-chromatin IgGs can first be detected in the serum of 3H9.KI mice 14 days after transfer of a diverse repertoire of autoreactive T cells. Therefore, we examined IgM ANA production prior to the induction of IgG ANAs. At day 7 after cGVHD induction, we show that activation of a diverse repertoire of class II–reactive T cells induced IgM anti-chromatin (Figure 4a) and anti-dsDNA (data not shown) Ab. Interestingly, activation of the limited T cell repertoires also induced IgM anti-chromatin Ab. Mice receiving a diverse T cell repertoire did tend to have higher titers, although this difference did not reach significance. This IgM autoantibody response includes λ1+ anti–dsDNA-specific B cells because the serum contains λ1+ anti-chromatin Ab (data not shown), and flow-cytometric analysis revealed that the majority of λ1+ B cells are using the 3H9 heavy chain (data not shown). Thus, limited T cell repertoires induce an abortive autoantibody response that includes IgM but not IgG ANAs.
Figure 4.
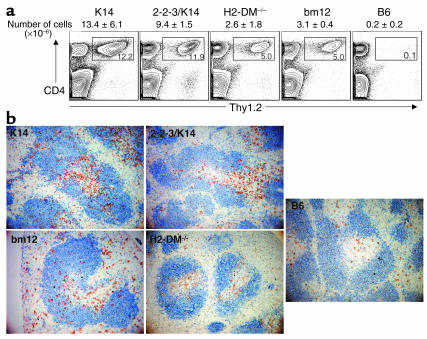
Activation of every class II–reactive T cell repertoire induced IgM ANAs with anti-dsDNA B cell activation and migration into the follicle. (a) Seven days after cGVHD induction, host sera were examined for the presence of IgM anti-chromatin autoantibodies. Scatter plots show IgM anti-chromatin autoantibody responses of individual mice. Recipients of every class II–reactive T cell repertoire had significantly more IgM anti-chromatin autoantibodies than 3H9.KI recipients of B6 CD4+ T cells (P < 0.05). The IgM autoantibody production was statistically similar among all recipients of class II–reactive CD4+ T cells (P > 0.05). (b) Seven days after cGVHD induction, splenic anti-dsDNA B cells of B6.PL/3H9.KI hosts were identified with Ab’s against B220 and λ1. The expansion in numbers of anti-dsDNA B cells was similar among all class II–reactive T cell recipients and significantly different from the B6-injected negative control (P < 0.05 for all groups). Representative results from four independent experiments are shown (c) CD22 expression on anti-dsDNA B cells (B220+ and λ1+). Histograms show the levels of CD22 for either B6-injected negative control (black lines) or following interaction with the indicated cell type (red lines). (d) Anti-dsDNA B cells were localized by staining spleen sections with Ab’s against B220 (blue) and Igλ1 (brown). In B6 → B6.PL/3H9.KI mice, λ1+ B cells localize to the T-B interface. Seven days after cGVHD induction, all class II–reactive repertoires induced the migration of λ1+ B cells into the follicle. Representative results from four separate experiments are shown.
To understand the T-B interactions associated with the anti-dsDNA B cell isotype switch to IgG production, we examined the regulation of anti-dsDNA B cells on day 7. Transfer of all class II–reactive CD4+ T cells induced an expansion in percentage and number of splenic B220+, λ1+ anti-dsDNA B cells to a similar extent among all groups (Figure 4b). Furthermore, these anti-dsDNA B cells are phenotypically activated. Flow-cytometric analysis of λ1+ anti-dsDNA B cells following cGVHD induction with all class II–reactive CD4+ T cell repertoires demonstrated an upregulation of CD22 (Figure 4c), B220, and I-Ab (data not shown). These data show that activation of any I-Ab–restricted CD4+ T cell repertoire leads to IgM anti-chromatin Ab production with activation and expansion of anti-dsDNA B cells.
Follicular exclusion of autoreactive B cells is a second mechanism for maintaining B cell tolerance to self-antigens. As such, anti-dsDNA B cell movement from the T-B interface into the follicle precedes seroconversion in lupus-prone strains. In addition, Marshak-Rothstein and her colleagues have found autoantibody-producing B cells in abnormal splenic localizations (28). To understand the splenic anatomy of ANA production which occurs during cGVHD with limited and diverse T cell repertoires, λ1+ B cells from B6.PL/3H9.KI mice undergoing cGVHD were localized by immunohistochemistry (IHC). In the nonautoimmune setting (transfer of B6 CD4+ T cells) λ1+ B cells are localized to the T-B interface of the spleen (Figure 4d). Activation of K14 and bm12 CD4+ T cells induced anti-dsDNA λ1+ B cells to scatter throughout the B cell follicle. Interestingly, activation of 2-2-3/K14 and H2-DM–/– CD4+ T cells also induced anti-dsDNA B cell migration into the follicle. Thus interaction of autoreactive anti-dsDNA B cells with any repertoire of autoreactive T cells leads to B cell migration into the follicle. Proliferation and activation of limited repertoires of 2-2-3/K14 and H2-DM–/– CD4+ T cells leads to activation, expansion, follicular entry of anti-dsDNA B cells, and even the production of IgM ANAs. However, none of these changes are sufficient to induce the production of detectable levels of IgG ANAs. Activation of a diverse repertoire of CD4+ T cells is specifically required for an isotype-switched autoimmune response.
2-2-3/K14 and H2-DM–/– CD4+ T cells are activated, but fail to enter the B cell follicle.
The production of IgG ANAs during cGVHD is directly determined by the population of CD4+ T cells studied. We therefore monitored CD4+ T cell responses in vivo by transferring CD4+ T cells to B6.PL/3H9.KI mice. The increase in percentage and number of I-Ab–restricted Thy1.2+ CD4+ T cells in 3H9.KI mice 7 days after transfer (Figure 5a) was comparable to that in wild-type B6 mice in vivo.
Figure 5.
Limited T cell repertoires are activated but fail to enter the B cell follicle. (a) Donor Thy1.2+ CD4 cells were identified 7 days after induction of cGVHD in B6.PL/3H9.KI mice by staining with Thy1.2 and CD4. Gated populations of the contour plots represent the expansion in percentage of transferred CD4+ T cells for the indicated cell population. The total number of recovered Thy1.2+ CD4+ splenocytes is indicated above the plot. Representative results from four experiments are shown. (b) Donor CD4+ cells were localized by staining spleen sections with Ab’s against Thy1.2 (brown) and B220 (blue). The few B6 CD4+ T cells detected by IHC were scattered throughout the T cell area. Activation of the class II–reactive T cell repertoires resulted in the majority of the cells migrating to T-B interface. The diverse T cell repertoires (K14 and bm12) contained T cells that were also evident in the B cell follicle; however, activation of the limited T cell repertoires (2-2-3/K14 and H2-DM–/–) did not result in the presence of transferred Thy1.2+ T cells in the follicle. Representative results of four experiments are shown.
We next examined the splenic localization of MHC class II–reactive CD4+ T cells. Antigen-specific priming of CD4+ T cells occurs in the T cell zone (29, 30). A subset of these primed CD4+ T cells then migrate to the follicle to help antigen-specific B cells produce high-affinity, isotype-switched Ab. In agreement with the flow-cytometric data, it was difficult to detect B6 CD4+ T cells by IHC (Figure 5b). The small number of persistent cells could be found scattered throughout the T cell zone. In contrast, activation of all class II–reactive T cell repertoires induced a significant expansion of cells in the T cell zone, with cells localizing to the T-B interface. A subset of K14 and bm12 CD4+ T cells had also translocated to the follicle, suggesting that these particular T cells may be the “helpers” of IgG ANA production. In contrast, 2-2-3/K14 and H2-DM–/– CD4+ T cells have expanded similarly within the T cell zone, but remain confined there.
To verify that the CD4+ T cells that entered the follicle were indeed diverse, we characterized their Vβ usage. Follicular entry of B cells is regulated by the chemokine receptor, CXCR5. CXCR5 is also expressed by a subset of CD4+ T cells, which provide “help” for Ab responses. The presence of follicular CD4+ T cells among the diverse T cell repertoires visualized by IHC correlated with an increased percentage of transferred cells expressing CXCR5 (data not shown). Importantly, the TCR Vβ usage by CXCR5+ K14 CD4+ T cells following cGVHD induction was identical to that of the naive CD4+ T cell repertoire (data not shown). Thus, the T cells that do enter the follicle are not obviously an oligoclonal subset of the cells that are activated but remain diverse. Importantly, the impaired ability to induce IgG ANAs with 2-2-3/K14 and H2-DM–/– CD4+ T cells is associated with a failure to induce a follicular-homing CD4+ T cell population, an event that is required for T cell–dependent IgG Ab production.
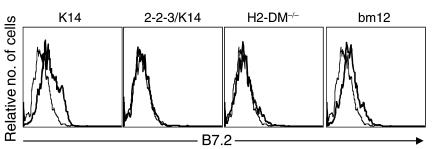
Follicular exclusion of 2-2-3/K14 and H2-DM–/– CD4+ T cells is associated with a failure to upregulate B7.2 expression on anti-dsDNA B cells.
Numerous TNF receptor and B7 family members are critical for T cell–dependent, class-switched Ab production; their genetic removal results in an abrogation of T cell–dependent Ab responses (31, 32). Thus, many candidates exist to describe a failure to generate class-switched Ab; however, we focused our study on B7/CD28. In fact, mice made deficient in B7/CD28 interactions do not generate a follicular-homing population of CD4+ T cells, which has been associated with a failure to produce class-switched Ab (33). Thus, we asked if the absence of CD4+ T cell migration into the follicle reflected an absence of B7/CD28 interactions. Anti-dsDNA λ1 B cells in recipients of K14 and bm12 CD4 cells had increased B7.2 expression 7 days after transfer. In contrast, the λ1 B cells in recipients of 2-2-3/K14 and H2-DM–/– do not upregulate B7.2 (Figure 6). Therefore, migration of T cells into the B cell follicle is associated with increased B cell expression of B7.2. Thus, activation of limited repertoires of autoreactive CD4+ T cells induces anti-dsDNA B cells to become activated and enter the follicle. These interactions, however, are not sufficient to increase B7.2 expression on anti-dsDNA B cells and induce the follicular entry of class II–reactive T cells and IgG ANA production.
Figure 6.
Loss of B cell tolerance is associated with B7.2 expression on anti-dsDNA B cells. Seven days after cGVHD induction in B6.PL/3H9.KI mice, spleen cells were stained for expression of B7.2, B220, and λ1. Histograms show B7.2 expression on gated B220+ λ1+ cells for mice receiving B6 (thin lines) or I-Ab–reactive CD4+ T cells (thick lines). Representative results of four experiments are shown.
Increasing CD4+ T cell diversity leads to the production of IgG ANAs.
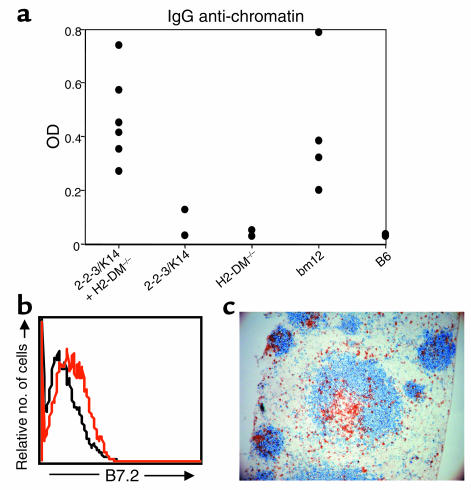
One interpretation of these data is that these two limited repertoires do not contain the particular CD4+ T cell specificities required to interact with an anti–dsDNA-specific B cell. Alternatively, IgG ANA production could require multiple, different cognate interactions that cannot be provided by a limited repertoire of CD4+ T cells. To distinguish these possibilities, we examined the effect of increasing the class II–reactive T cell diversity by combining the two limited repertoires. We induced cGVHD with an equivalent number of 2-2-3/K14 and H2-DM–/– CD4+ T cells together or 2-2-3/K14 and H2-DM–/– CD4+ T cells individually. As we described earlier, 2-2-3/K14 and H2-DM–/– CD4+ T cells individually failed to induce IgG anti-chromatin Ab’s (Figure 7a). Combining these limited T cell repertoires, however, induced IgG anti-chromatin (Figure 7a) and anti-dsDNA (data not shown) Ab’s to an extent comparable to that of the diverse bm12 repertoire. The combination of the two limited T cell repertoires induced a level of class II–reactive T cell expansion similar to the other groups (data not shown). Furthermore, by using a clonotypic Ab that recognizes the 2-2-3 TCR specificity, we determined that both limited repertoires of CD4+ T cells have expanded similarly and are therefore participating equivalently in the autoimmune response (data not shown). Thus, IgG ANA production requires a diverse array of cognate, class II–reactive T-B interactions.
Figure 7.
Increasing CD4+ T cell diversity is required for IgG ANA production. (a) The indicated CD4+ T cell repertoires were injected into 3H9.KI mice. Each recipient received a total of 10 × 106 cells. The scatter plots show IgG anti-chromatin autoantibody responses of individual mice 4 weeks after cGVHD induction. The IgG anti-chromatin autoantibody response elicited by 2-2-3/K14 plus H2-DM–/– CD4+ T cells was statistically similar to the response from recipients of bm12 CD4+ T cells (P > 0.05). (b) Seven days after cGVHD induction, mice were euthanized and splenocytes were stained with Ab’s against B220, λ1, and B7.2. Histograms show B7.2 expression on gated B220+ λ1+ anti-dsDNA B cells for the B6-transfer negative control (black line) or mice injected with the combination of the limited T cell repertoires (red line). Results are representative of three experiments. (c) Spleen sections from mice receiving the combination of the limited T cell repertoires were stained for Thy1.2 (brown) and B220 (blue). Note the presence of Thy1.2+ T cells in the B cell follicle. Results are representative of three experiments.
We next repeated our analysis at day 7 after cGVHD induction to determine if the loss of tolerance resulting from activating the two limited repertoires together was also associated with expression of B7.2 and follicular migration of T cells. Following activation with any repertoire, anti-dsDNA λ1+ B cells expanded in percentage and number of cells, upregulated expression of MHC class II I-Ab, CD22, and B220, and were scattered throughout the B cell follicle to an extent similar for all groups (data not shown). Importantly, combining the limited T cell repertoires increased B7.2 expression on anti-dsDNA B cells (Figure 7b) comparable to that seen with the diverse repertoire controls, whereas, individually, the limited T cell repertoires failed to upregulate B7.2 expression (Figure 6 and data not shown for this trial).
The splenic anatomy of class II–reactive T cell responses were again followed by IHC. The combination of the two limited T cell repertoires induced a follicular homing CD4+ T cell population (Figure 7c) comparable to the diverse repertoire controls, whereas, individually, these repertoires failed to migrate to the follicle (Figure 5 and data not shown for this trial). This result suggests that T cell migration into the follicle occurs following activation of a diverse repertoire of autoreactive T cells. Therefore, multiple, diverse T-B interactions are necessary for anti-dsDNA B cell expression of B7.2, T cell migration to the follicle, and IgG ANA production.
Discussion
To characterize the T cell requirements for the production of ANAs, we examined the ability of T cell repertoires of differing diversities to induce cGVHD. Transfer of autoreactive T cells of limited diversity into naive nonautoimmune mice induced high levels of total IgG in the recipients, but neither IgG ANAs nor anti-chromatin Ab’s; a diverse repertoire of autoreactive T cells was required to break B cell tolerance. Every activated repertoire of autoreactive T cells caused anti-dsDNA B cell expansion, activation, and entry into the B cell follicle. Activation of the limited T cell repertoires was associated with a failure to increase expression of B7.2 on anti-dsDNA B cells and induce CD4+ T cell movement into the follicle. Importantly, combining the two limited T cell repertoires to increase diversity was sufficient to induce B7.2 expression on anti-dsDNA B cells, CD4+ T cell entry into the B cell follicle, and IgG anti-chromatin Ab production. These results suggest that CD4+ T cell diversity is required in the cGVHD system to induce isotype-switching to IgG ANA production and this reflects the requirement for movement of CD4+ T cells into the B cell follicle.
Follicular localization of autoreactive T and B cells.
Follicular exclusion of autoreactive anti-dsDNA B cells maintains B cell tolerance in nonautoimmune mice (24). In lupus-prone strains, anti-dsDNA B cells can enter the B cell follicle in a T cell–dependent manner (25, 26); this process correlates with and precedes ANA production. Interestingly, the trafficking of B cells is accompanied by CD4+ T cells moving throughout the B cell follicle. However, our data show that follicular entry of B cells is not sufficient for the production of IgG ANAs. Rather, follicular entry of autoreactive B cells correlated with the production of IgM autoantibodies, production of isotype-switched IgG autoantibodies, and maintenance of the autoimmune response required the additional trafficking of autoreactive T cells into the follicle.
The CD4+ T cells from diverse repertoires localized by immunohistochemistry in the follicle also expressed the chemokine receptor, CXCR5 (data not shown), which mediates follicular entry of B cells and is also expressed by those CD4+ T cells that provide help for Ab responses. Preliminary analysis of these CXCR5-positive cells suggests that the CD4+ T cells that enter the follicle are not a restricted set of T cells because their TCR Vβ usage is as broad as that of the naive repertoire prior to activation. Thus, the diversity of the T cells within the follicle mirrors the diversity of the GVHD–inducing repertoire.
The role of B7.2 expression on anti-dsDNA B cells.
Recent studies have shown that mice made deficient in B7/CD28 interactions do not generate a follicular-homing population of CD4+ T cells (33). At present, we do not know if B7.2/CD28 interactions are sufficient to educate T cells to enter the follicle. In fact, numerous, additional, costimulatory molecules could be important for aiding T cell movement into the follicle. Removal of B7/CD28, OX40L/OX40, and B7RP-1/iCOS interactions (among many others) abrogates T cell–dependent Ab production (34–38). Therefore, B7.2 expression on anti-dsDNA B cells may be a “surrogate” for the failure to upregulate another costimulatory molecule. Alternatively, these costimulatory molecules may act collaboratively or sequentially for T cell–dependent Ab responses. It is tempting to speculate that B7.2 may be more than just a surrogate, however. As such, T cell–dependent Ab responses and maintenance of the germinal center require ongoing CD28/B7.2 interactions (39, 40). Furthermore, blockade of B7.2, but not B7.1, with mAb’s prevents autoantibody production in lupus-prone NZB × NZW F1 mice (41). Thus, it is possible that the upregulation of B7.2 on anti-dsDNA B cells may play an important role in instructing T cell migration into the follicle to maintain chronic signaling to autoreactive B cells.
Why must anti-dsDNA B cells express B7.2? Why is the interaction between autoreactive CD4+ T cells and B7.2-expressing DCs not sufficient to mediate T cell movement? These data mirror previous work suggesting that B cells elicit help from CD4+ T cells. Thus, Stockinger, Gray, and their colleagues have shown that B cell presentation of antigen to T cells enhanced the differentiation of T cells to drive heightened Ab responses (42). The authors hypothesize that a certain number of T-B interactions may be necessary to induce a B cell–helping T cell population; our data show that a similar threshold for T-B interactions also exists in the production of Ab’s to self-antigens. Thus, a self-enforcing positive-feedback loop exists between TCR/MHC class II, CD28/B7.2, and, probably, CD40/CD40L, to costimulate the T cell help required for the induction of a robust IgG autoantibody response.
What does a diverse repertoire of autoreactive CD4+ T cells provide?
Although the data presented here address the biological consequences of activation of a diverse repertoire of T cells, they do not directly explain the necessity for anti-dsDNA B cells to interact with a diverse array of T cells, nor do they directly address whether diversity reflects a requirement for a specific set of rare TCR specificities. We can think of many possibilities to explain the requirement for diversity. The first is that different CD4+ cell specificities have qualitatively different functions—in this model 2-2-3/K14 CD4s and H2-DM–/– CD4s would provide differing but additive functions to induce B cell activation. We note, however, that we have been unable to specifically identify any qualitative difference between these two populations of T cells. Importantly, cytokines such as IL-2, IL-4, IL-10, and IFN-γ are induced similarly among all groups following activation in B6 mice. An alternate interpretation of this model would be that activation of limited T cell repertoires induces a dominant suppression of the T and B cell autoreactivity. We have shown, however, that each of these repertoires have equivalent percentages of CD4+CD25+ regulatory T cells equally capable of suppressing an anti-B6 mixed-lymphocyte reaction (9). Furthermore, a dominant suppression model would predict that combining the two repertoires would not result in the generation of IgG ANAs. Thus, we feel that aberrant regulatory T cell responses cannot adequately explain our results.
The second possibility is that different CD4+ T cell specificities are required to supply the right set of TCRs necessary to interact with the different peptide/MHC complexes on B cells. It is possible that a particular set of rare CD4+ T cells can provide help to anti-dsDNA B cells in vivo. Such a model would be supported by Datta and his colleagues, who have identified histone-specific T cells, and Hahn and Wysocki’s groups, who have proposed that anti-dsDNA Ab induction is driven by T cells specific for autoantibody-derived peptides (43–47). In these models, diversity would be required to generate sufficient numbers of these rare CD4+ T cells. Indeed, it has been suggested that terminal deoxynucleotidyl transferase deficiency ameliorates murine lupus by decreasing CD4+ T cell diversity, either by decreasing the overall diversity or by a failure to generate particular TCR specificities (48). Thus, in some models of lupus, the function of CD4+ T cell diversity may reflect a requirement for specific uncommon T cell receptors. Our results suggest, however, that the Vβ repertoire of the CD4+ T cells that enter the follicle and provide help do not differ significantly from the starting population of cells, arguing against an oligoclonal response.
In any model, a diverse array of TCRs is required to interact with the different MHC-peptide epitopes expressed on an individual B cell. Therefore, T cell diversity could also be a “readout” for MHC-peptide epitope density, and the limiting factor would be the number of interactions the T cell and B cell could undergo. Similarly, the requirement for T cell diversity could arise if maintenance of autoantibody production requires epitope spreading of the T cell response (as has been seen in other T cell–dependent autoimmune syndromes) (49). T cell activation and differentiation could subsequently activate anti-dsDNA B cells resulting in the genesis of novel T cell epitopes. A diverse T cell repertoire would be necessary to recognize these epitopes and provide secondary CD4+ cell signals that are required for breaking anti-dsDNA B cell tolerance. These models are reminiscent of the model of Mamula, Shlomchik, and their colleagues who suggested that the peripheral T-B interactions in SLE constitute a positive-feedback loop that allows for a self-enforcing amplification of the autoantibody response (50). Thus, the authors proposed an important role for costimulation and epitope spreading during autoreactive T-B collaboration in SLE. In any event, we envision each additional T cell specificity providing the anti-dsDNA B cells with a sufficient array of CD4+ T cell–dependent signals to break anti-dsDNA B cell tolerance. Therefore, self-perpetuating signals between CD4+ T cells and B cells are necessary for anti-dsDNA Ab production.
cGVHD as a model system of SLE.
Do these results — elicited in nonautoimmune strains of mice — inform us about the loss of B cell tolerance and production of ANAs in lupus? Our results suggest that activation of a broad repertoire of autoreactive T cell specificities must necessarily precede the development and maintenance of B cell autoreactivity in nonautoimmune strains. An interesting corollary, however, is that one component of the genetic susceptibility to lupus may be a relaxation of this requirement for CD4+ T cell diversity — either on the B cell or the T cell side. Indeed, Wakeland, Mohan, and their colleagues have identified lupus-associated susceptibility alleles expressed in the B cell compartment that affect their activation threshold and ability to activate T cells (12, 51). We are currently determining if these susceptibility genes decrease the CD4+ T cell diversity required to induce autoantibody production during cGVHD.
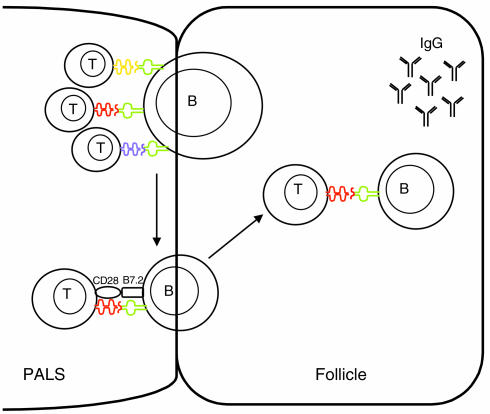
In summary, our results provide a model for how IgG ANAs are generated (Figure 8). Activation of autoreactive CD4+ T cells leads to the movement of autoreactive B cells into the follicle where B cells proliferate and differentiate to the production of IgM ANAs. A diverse array of cognate T-B interactions are necessary for B7.2 expression on anti-dsDNA B cells, which may educate CD4+ T cells to express a cell-surface receptor (such as CXCR5) important for movement into the follicle. Follicular T cells help the isotype switching and generation of high-affinity anti-dsDNA Ig’s. Therefore, these experiments permit us to divide the loss of B cell tolerance required for autoantibody production into two distinct components with different CD4+ T cell requirements: a nonstringent step in which T cell activation induces activation and proliferation of B cells, B cell movement into the follicle, and IgM secretion, and a second, stricter component in which ongoing T-B interactions lead to trafficking of T cells into the B cell follicle, isotype switching, and persistent Ab production. By identifying two different T cell–dependent components of the autoantibody response, we have provided a system in which to further investigate the pathways involved in the pathologic response.
Figure 8.
Activation of a diverse T cell repertoire is required for the production of IgG ANAs. Activation of autoreactive CD4+ T cells leads to the expansion of autoreactive anti-dsDNA B cells that enter the B cell follicle to produce IgM ANAs. Each individual anti-dsDNA B cell requires interaction with a diverse array of T cells. T cell diversity is necessary to provide a pool of cells that can interact with the different MHC-peptide epitopes expressed on each autoreactive B cell. These multiple T cell–derived interactions are necessary for B7.2 expression on anti-dsDNA B cells; B7.2 expression on anti-dsDNA B cells may educate the T cells to migrate into the follicle. In the follicle, T cells aid autoreactive follicular B cells to produce high-affinity, isotype-switched ANAs. T, T cells; B, B cells; PALS, peri-arteriolar lymphoid sheath.
Acknowledgments
We thank M. Weigert for generously providing 3H9.KI mice, L. Van Kaer for providing H2-DM–/– mice, and T. Lifsted for expert animal care. The authors also thank A. Bhandoola, J. Punt, M. Cancro, and members of the Laufer laboratory for helpful discussions. We thank R. Eisenberg for critical review of the manuscript. This work was supported by NIH grants (AI-48117 to T. Laufer and AI-32137 and AR-47913 to J. Erikson) and NIH training grant (5T32 EY-07131 to B. Busser). We also gratefully acknowledge the support of the Lupus Foundation of Southeastern Pennsylvania.
Footnotes
Brian W. Busser’s present address is:Department of Molecular Biology, Massachusetts General Hospital, Boston,Massachusetts, USA.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: systemic lupus erythematosus (SLE); antinuclear Ab (ANA); T cell receptor (TCR); chronic graft-versus-host disease (cGVHD); T cell–B cell (T-B);H-2bm12 (bm12); K14-Aβb/Aβb–/– (K14); double-stranded DNA (dsDNA); immunohistochemistry (IHC).
References
- 1.Shlomchik M, et al. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J. Exp. Med. 1990;171:265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wofsy D, Ledbetter JA, Hendler PL, Seaman WE. Treatment of murine lupus with monoclonal anti-T cell antibody. J. Immunol. 1985;134:852–857. [PubMed] [Google Scholar]
- 3.Jevnikar AM, Grusby MJ, Glimcher LH. Prevention of nephritis in major histocompatibility complex class II-deficient MRL-lpr mice. J. Exp. Med. 1994;179:1137–1143. doi: 10.1084/jem.179.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg AD, Roths JB, Murphy ED, Steinberg RT, Raveche ES. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J. Immunol. 1980;125:871–873. [PubMed] [Google Scholar]
- 5.Peng SL, Fatenejad S, Craft J. Induction of nonpathologic, humoral autoimmunity in lupus-prone mice by a class II-restricted, transgenic alpha beta T cell. Separation of autoantigen-specific and -nonspecific help. J. Immunol. 1996;157:5225–5230. [PubMed] [Google Scholar]
- 6.Morris SC, Cohen PL, Eisenberg RA. Experimental induction of systemic lupus erythematosus by recognition of foreign Ia. Clin. Immunol. Immunopathol. 1990;57:263–273. doi: 10.1016/0090-1229(90)90040-w. [DOI] [PubMed] [Google Scholar]
- 7.Morris SC, Cheek RL, Cohen PL, Eisenberg RA. Autoantibodies in chronic graft versus host result from cognate T-B interactions. J. Exp. Med. 1990;171:503–517. doi: 10.1084/jem.171.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan L, Busser BW, Lifsted T, Lo D, Laufer TM. Keratinocyte presentation of antigen directs autoimmune skin disease. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3386–3391. doi: 10.1073/pnas.0437899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J. Exp. Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J. Clin. Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burlingame RW, Rubin RL. Subnucleosome structures as substrates in enzyme-linked immunosorbent assays. J. Immunol. Methods. 1990;134:187–199. doi: 10.1016/0022-1759(90)90380-e. [DOI] [PubMed] [Google Scholar]
- 12.Mohan C, Alas E, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis. Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA subnucleosomes. J. Clin. Invest. 1998;101:1362–1372. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohan C, et al. Genetic dissection of lupus pathogenesis: a recipe for nephrophilic autoantibodies. J. Clin. Invest. 1999;103:1685–1695. doi: 10.1172/JCI5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki T, et al. Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell. 1996;84:531–541. doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 16.Fung-Leung WP, et al. Antigen presentation and T cell development in H2-M-deficient mice. Science. 1996;271:1278–1281. doi: 10.1126/science.271.5253.1278. [DOI] [PubMed] [Google Scholar]
- 17.Martin WD, et al. H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell. 1996;84:543–550. doi: 10.1016/s0092-8674(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 18.Sant’Angelo DB, et al. The imprint of intrathymic self-peptides on the mature T cell receptor repertoire. Immunity. 1997;7:517–524. doi: 10.1016/s1074-7613(00)80373-8. [DOI] [PubMed] [Google Scholar]
- 19.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 20.Laufer TM, Fan L, Glimcher LH. Self-reactive T cells selected on thymic cortical epithelium are polyclonal and are pathogenic in vivo. J. Immunol. 1999;162:5078–5084. [PubMed] [Google Scholar]
- 21.Chen C, Nagy Z, Prak EL, Weigert M. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity. 1995;3:747–755. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 22.Radic MZ, Mascelli MA, Erikson J, Shan H, Weigert M. Ig H and L chain contributions to autoimmune specificities. J. Immunol. 1991;146:176–182. [PubMed] [Google Scholar]
- 23.Sekiguchi DR, et al. Chronic graft-versus-host in Ig knockin transgenic mice abrogates B cell tolerance in anti-double-stranded DNA B cells. J. Immunol. 2002;168:4142–4153. doi: 10.4049/jimmunol.168.8.4142. [DOI] [PubMed] [Google Scholar]
- 24.Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, Erikson J. Regulation of anti-double-stranded DNA B cells in nonautoimmune mice: localization to the T-B interface of the splenic follicle. J. Exp. Med. 1997;186:1257–1267. doi: 10.1084/jem.186.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandik-Nayak L, et al. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J. Exp. Med. 1999;189:1799–1814. doi: 10.1084/jem.189.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo SJ, et al. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 27.Burlingame RW, Rubin RL, Balderas RS, Theofilopoulos AN. Genesis and evolution of antichromatin autoantibodies in murine lupus implicates T-dependent immunization with self antigen. J. Clin. Invest. 1993;91:1687–1696. doi: 10.1172/JCI116378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson BA, et al. Anatomy of autoantibody production: dominant localization of antibody-producing cells to T cell zones in Fas-deficient mice. Immunity. 1995;3:509–519. doi: 10.1016/1074-7613(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 29.Garside P, et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 30.Gulbranson-Judge A, MacLennan I. Sequential antigen-specific growth of T cells in the T zones and follicles in response to pigeon cytochrome c. Eur. J. Immunol. 1996;26:1830–1837. doi: 10.1002/eji.1830260825. [DOI] [PubMed] [Google Scholar]
- 31.Tivol EA, Schweitzer AN, Sharpe AH. Costimulation and autoimmunity. Curr. Opin. Immunol. 1996;8:822–830. doi: 10.1016/s0952-7915(96)80011-2. [DOI] [PubMed] [Google Scholar]
- 32.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 33.Walker LS, et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J. Exp. Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuber E, Strober W. The T cell-B cell interaction via OX40-OX40L is necessary for the T cell-dependent humoral immune response. J. Exp. Med. 1996;183:979–989. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tafuri A, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 36.McAdam AJ, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 37.Dong C, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 38.Borriello F, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 39.Han S, et al. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- 40.Nakajima A, et al. Requirement of CD28-CD86 co-stimulation in the interaction between antigen-primed T helper type 2 and B cells. Int. Immunol. 1997;9:637–644. doi: 10.1093/intimm/9.5.637. [DOI] [PubMed] [Google Scholar]
- 41.Nakajima A, et al. Preferential dependence of autoantibody production in murine lupus on CD86 costimulatory molecule. Eur. J. Immunol. 1995;25:3060–3069. doi: 10.1002/eji.1830251112. [DOI] [PubMed] [Google Scholar]
- 42.Stockinger B, Zal T, Zal A, Gray D. B cells solicit their own help from T cells. J. Exp. Med. 1996;183:891–899. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Smith DS, Guth A, Wysocki LJ. A receptor presentation hypothesis for T cell help that recruits autoreactive B cells. J. Immunol. 2001;166:1562–1571. doi: 10.4049/jimmunol.166.3.1562. [DOI] [PubMed] [Google Scholar]
- 44.Wang M, Desai D, Marion TN. T cells specific for DNA-binding peptides. Lupus. 1997;6:349–350. doi: 10.1177/096120339700600334. [DOI] [PubMed] [Google Scholar]
- 45.Ebling FM, Tsao BP, Singh RR, Sercarz E, Hahn BH. A peptide derived from an autoantibody can stimulate T cells in the (NZB × NZW) F1 mouse model of systemic lupus erythematosus. Arthritis. Rheum. 1993;36:355–364. doi: 10.1002/art.1780360311. [DOI] [PubMed] [Google Scholar]
- 46.Singh RR, et al. T cell determinants from autoantibodies to DNA can upregulate autoimmunity in murine systemic lupus erythematosus. J. Exp. Med. 1995;181:2017–2027. doi: 10.1084/jem.181.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J. Exp. Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feeney AJ, Lawson BR, Kono DH, Theofilopoulos AN. Terminal deoxynucleotidyl transferase deficiency decreases autoimmune disease in MRL-Fas(lpr) mice. J. Immunol. 2001;167:3486–3493. doi: 10.4049/jimmunol.167.6.3486. [DOI] [PubMed] [Google Scholar]
- 49.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 50.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat. Rev. Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 51.Mohan C, Morel L, Yang P, Wakeland EK. Genetic dissection of systemic lupus erythematosus pathogenesis: Sle2 on murine chromosome 4 leads to B cell hyperactivity. J. Immunol. 1997;159:454–465. [PubMed] [Google Scholar]