Abstract
Aminoacyl-tRNAs (aa-tRNAs) are the essential substrates for translation. Most aa-tRNAs are formed by direct aminoacylation of tRNA catalyzed by aminoacyl-tRNA synthetases. However, a smaller number of aa-tRNAs (Asn-tRNA, Gln-tRNA, Cys-tRNA and Sec-tRNA) are made by synthesizing the amino acid on the tRNA by first attaching a non-cognate amino acid to the tRNA, which is then converted to the cognate one catalyzed by tRNA-dependent modifying enzymes. Asn-tRNA or Gln-tRNA formation in most prokaryotes requires amidation of Asp-tRNA or Glu-tRNA by amidotransferases that couple an amidase or an asparaginase to liberate ammonia with a tRNA-dependent kinase. Both archaeal and eukaryotic Sec-tRNA biosynthesis and Cys-tRNA synthesis in methanogens require O-phosophoseryl-tRNA formation. For tRNA-dependent Cys biosynthesis, O-phosphoseryl-tRNA synthetase directly attaches the amino acid to the tRNA which is then converted to Cys by Sep-tRNA: Cys-tRNA synthase. In Sec-tRNA synthesis, O-phosphoseryl-tRNA kinase phosphorylates Ser-tRNA to form the intermediate which is then modified to Sec-tRNA by Sep-tRNA:Sec-tRNA synthase. Complex formation between enzymes in the same pathway may protect the fidelity of protein synthesis. How these tRNA-dependent amino acid biosynthetic routes are integrated into overall metabolism may explain why they are still retained in so many organisms.
INTRODUCTION
To maintain the fidelity of protein synthesis, pairing an amino acid with its cognate tRNA to form aminoacyl-tRNA (aa-tRNA) is essential. Usually, in cells, this is achieved by a group of enzymes known as aminoacyl-tRNA synthetases (aaRSs). Each aaRS specifically recognizes an amino acid and its corresponding set of tRNA isoacceptors in a cell. Accordingly, for each of the 20 canonical amino acids there is a matching aaRS found in nature. aa-tRNA formation catalyzed by the aaRSs occurs in a two-step process (1). First, the amino acid is activated in an ATP-dependent manner forming an enzyme-bound aminoacyl-adenylate. Second, the activated aa is transferred onto the 3′ terminal adenosine of the tRNA.
A full complement of all 20 aaRSs was thought to be essential for the survival of most species. Exceptions to the concept of 20 canonical amino acids/20 aaRSs in a species have been known about since 1968 (2). Only recently, functional genomic analyses confirmed by biochemical and genetic experiments have revealed only eukaryotes and a handful of bacteria have a full set of aaRSs (Table 1). The majority of bacterial and all known archaeal genomes do not encode a glutaminyl-tRNA synthetase (GlnRS) (3,4). Most prokaryotes do not have an asparaginyl-tRNA synthetase (AsnRS) (3–5) and a number of a methanogenic archaea lack a cysteinyl-tRNA synthetase (CysRS) (6–8). In addition, no aaRS exists to date for Sec, the 21st amino acid used in protein synthesis in a number of species across all three domains of life (9).
Table 1.
Prevalence of the indirect and direct pathways for the synthesis of the aa-tRNA species listed in all three domains of life
| aa-tRNA | Prevalence of | Refs. | |
|---|---|---|---|
| Indirect pathway | Direct pathway | ||
| Gln-tRNAGln | All known archaea, most bacteria, and chloroplasts | All known eukaryotes and a minority of bacteria | 3, 4, 17, 18 |
| Asn-tRNAAsn | Most bacteria and archaea | All known eukaryotes and a number of bacteria and archaea | 3–5, 38 |
| Cys-tRNACys | Methanogenic archaea (except M. smithii and M. stadtmanae) and A. fulgidus | All known eukaryotes and bacteria, and most archaea | 6–8, 11, 77, 80–82 |
| Sec-tRNASec | All known Sec-decoding eukaryotes, archaea and bacteria | None known | 107–110, 118 |
These organisms (Table 1) take advantage of indirect pathways to form the full complement of aa-tRNA species required for protein synthesis by synthesizing the amino acids (Gln, Asn, Cys and/or Sec) when bound to their cognate tRNAs (2,10–16). This is accomplished by relaxed-specificity aaRSs that form misacylated aa-tRNA species; they will be subsequently converted to the correct aa-tRNA by remarkable RNA-dependent modifying enzymes. By synthesizing the amino acids on the tRNA, these organisms are able to directly link amino acid metabolism with protein synthesis. The purpose of this article is to summarize recent advances in our understanding of the key enzymes involved in these tRNA-dependent amino acid transformation pathways.
tRNA-dependent transamidation
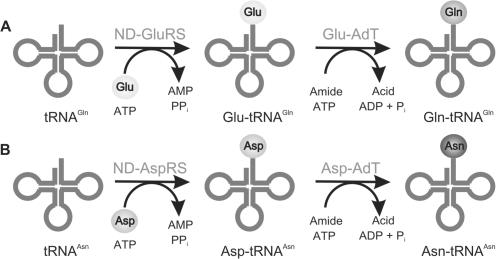
In all known archaea (3), most bacteria (4) and chloroplasts (17,18) GlnRS is absent and Gln-tRNAGln is formed by synthesizing Gln on the tRNA (Table 1) (Figure 1A). First, these organisms attach Glu to tRNAGln to form the mischarged species, Glu-tRNAGln, by taking advantage of a non-discriminating glutamyl-tRNA synthetase (ND-GluRS) (19), an aaRS with relaxed tRNA specificity (20). The Glu moiety on the tRNAGln is then amidated by a Glu-tRNAGln amidotransferase (Glu-AdT) in the presence of ATP and an amide donor to form the properly aminoacylated species, Gln-tRNAGln (2). Similarly, in the many bacteria and archaea lacking an AsnRS (Table 1) (3–5), Asn-tRNAAsn is synthesized by the concerted action of a non-discriminating aspartyl-tRNA synthetase (ND-AspRS) (20,21) and an aspartyl-tRNAAsn amidotransferase (Asp-AdT) (Figure 1B) (10,22).
Figure 1.
Indirect pathways for (A) Gln-tRNAGln and (B) Asn-tRNAAsn formation. (A) First a ND-GluRS glutamylates tRNAGln to form Glu-tRNAGln. The mischarged species is then amidated by a Glu-AdT to form Gln-tRNAGln. (B) First a ND-AspRS aspartylates tRNAAsn to form Asp-tRNAAsn. The mischarged species is then amidated by an Asp-AdT for form Asn-tRNAAsn.
In the mitochondria of many eukaryotes the indirect pathway may also be used to form Gln-tRNAGln. Homologs of AdT subunits are encoded in the nuclear genomes of numerous eukaryotes including Saccharomyces cerevisiae (23) and Homo sapiens (24). In S. cerevisiae, the homologs (Pet112 and YMR293C) are essential for mitochondrial function (25,26) and Glu-tRNAGln is found in the organelle (27). However, in vitro the yeast mitochondrial GluRS was unable to form the mischarged tRNA species (28). The presence of a Glu-AdT activity in yeast mitochondria was noticed already 30 years ago (29), and recent work characterizes this activity for mammalian (T. Suzuki, unpublished data) and plant mitochondria (A.M. Duchêne and H. Becker, unpublished data). In addition, it was shown that both cytoplasmic GlnRS and tRNAGln are imported into the S. cerevisiae mitochondrion (28) and that in H. sapiens tRNAGln is imported into the organelle (J. Alfonzo, unpublished data). The role (e.g. additional coding functionality) of such dual pathways of mitochondrial Gln-tRNAGln formation remains to be established.
Two different tRNA-dependent amidotransferases (AdT) exist, the heterotrimeric GatCAB (30) and the heterodimeric GatDE (3). The latter is an archaeal signature protein and serves as the Glu-AdT for Gln-tRNAGln synthesis in Archaea (3). GatCAB is found in both bacteria and archaea (3,30). In archaeal genomes, GatCAB is encoded only when an AsnRS is not (3,5). This and the fact that the Methanothermobacter thermautotrophicus GatCAB is unable to transamidate archaeal Glu-tRNAGln in vitro (31), strongly indicates that the role of the heterotrimeric AdT in Archaea is as an Asp-AdT.
All bacterial GatCAB enzymes studied to date are able to serve as both a Glu-AdT and an Asp-AdT in vitro (4,32–36). The exact activity/activities assumed by GatCAB in bacteria in vivo is determined by the presence and nature of the non-discriminating aaRS (ND-GluRS and/or ND-AspRS) in the organism. For example, bacteria like Bacillus subtilis (19) that have a ND-GluRS but lack a ND-AspRS use their GatCAB only as a Glu-AdT (30). In bacteria possessing a ND-AspRS but lacking a ND-GluRS (e.g. Pseudomonas aeruginosa, Neisseria meningitidis, Thermus thermophilus and Deinococcus radiodurans) GatCAB serves only as an Asp-AdT (21,22,32,33,37–40). In bacteria carrying both non-discriminating aaRSs [e.g. Chlamydia trachomatis (34) and Helicobacter pylori (41–43)], GatCAB serves a dual function as both a Glu-AdT and an Asp-AdT (4,34,36).
tRNA substrate recognition by the AdTs
The AdTs must discriminate their mischarged tRNA substrates (Glu-tRNAGln and/or Asp-tRNAAsn) from the cognate aa-tRNA species (Glu-tRNAGlu and Asp-tRNAAsp). Both AdTs achieve this without recognizing the anticodon of their tRNA substrates (Figure 2) (40,44–46). The elements that the bacterial GatCAB, GatDE and the archaeal GatCAB recognize in their aa-tRNA substrates are summarized here. The Staphylococcus aureus GatCAB positively recognizes the U1-A72 base pair in the acceptor stem of tRNAGln, while discriminating against tRNAGlu based on the presence of a supernumerary base in the D-loop of tRNAGlu (45). Biochemical studies with the N. meningitidis GatCAB revealed that the enzyme uses similar elements to distinguish Asp-tRNAAsn from Asp-tRNAAsp (40). These elements are conserved among bacteria using the indirect pathways for Gln-tRNAGln and/or Asn-tRNAAsn formation suggesting a general substrate discrimination mechanism for all bacterial GatCAB enzymes (40,45).
Figure 2.
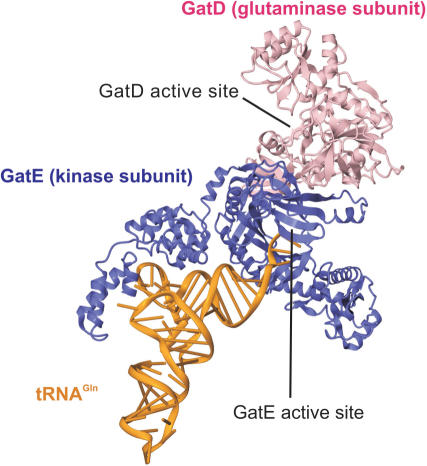
Crystal structure of the M. thermautotrophicus GatDE complexed with tRNAGln. The AdT forms an α2β2 tetramer, with two GatE subunits binding a GatD homodimer. Each GatE subunit binds one tRNAGln molecule. For clarity only one monomer of GatD and GatE are shown. The glutaminase active site of the D-subunit and the kinase active site of the E-subunit are connected by a 40 Å long molecular tunnel (44). Adapted from Polycarpo,C. et al. (2007). In Cavicchioli,R. (ed.) Archaea: Molecular and Cellular Biology. ASM Press, Washington, DC USA with permission from ASM Press.
The cocrystal structure of the M. thermautotrophicus GatDE with tRNAGln demonstrated that the enzyme makes contact with the acceptor stem, TΨC-stem/loop and D-loop of the tRNA (Figure 2) (44). The structural results coupled with biochemical analysis revealed GatDE recognizes the A1-U72 base pair found in the acceptor stem of archaeal Glu-tRNAGln and discriminates against Glu-tRNAGlu based on extra bases in the D-loop of tRNAGlu (44). In addition, the results indicated that the M. thermautotrophicus GatDE uses the first base-pair of the acceptor stem and positions 19 and 20 in the D-loop to distinguish Glu-tRNAGln from Asp-tRNAAsn (44).
In contrast to GatDE and bacterial GatCAB, archaeal GatCAB does not recognize the first base pair of the acceptor stem of its tRNA substrate, Asp-tRNAAsn (40,46). The M. thermautotrophicus GatCAB instead relies on antideterminants in tRNAAsp (the D-loop and position 49) to distinguish Asp-tRNAAsn from Asp-tRNAAsp (46). In addition, archaeal GatCAB discriminates Asp-tRNAAsn from archaeal Glu-tRNAGln and Asp-tRNAAsp based on the length of the variable loops of the tRNA species (40,46). Both the Methanosarcina barkeri and M. thermautotrophicus GatCAB enzymes were able to transamidate aa-tRNA species with variable loops five nucleotides in length, as is found in archaeal tRNAAsn isoacceptors, but not ones with variable loops four nucleotides in length, as is conserved in archaeal tRNAGln and tRNAAsp species (31,40,46). The differences in tRNA recognition by GatDE and archaeal GatCAB may enable archaea lacking a GlnRS and an AsnRS to encode one AdT (GatDE) as a Glu-AdT and the other (GatCAB) as an Asp-AdT (31). Why archaea lacking both aaRSs encode two AdTs (3), while bacteria in a same situation encode only GatCAB for synthesis of both Gln-tRNAGln and Asn-tRNAAsn (4,34,36) remains an open area of investigation.
AdTs couple a tRNA-dependent kinase with a glutaminase
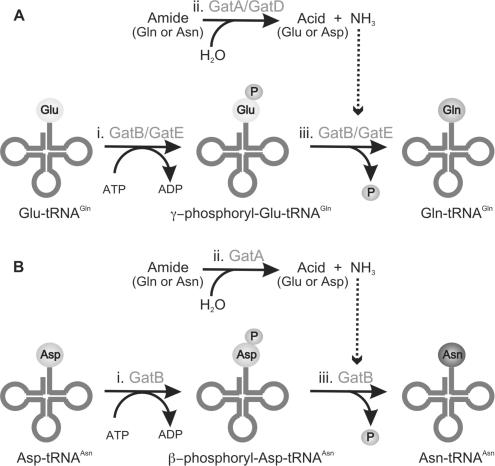
Regardless of the tRNA substrate recognized, both AdTs are thought to catalyze three distinct reactions to transamidate their mischarged substrates (Figure 3): (i) AdTs act as kinases to phosphorylate the Glu or Asp attached to the tRNA, forming an activated intermediate, γ-phosphoryl-Glu-tRNAGln (18,47–49) or β-phosphoryl-Asp-tRNAAsn, respectively. The formation of the latter is still speculative as this point as biochemical evidence for existence of β-phosphoryl-Asp-tRNAAsn has not been demonstrated as it has for γ-phosphoryl-Glu-tRNAGln (18,47–49) possibly due to the unstable nature of the putative β-phosphoryl-Asp-tRNAAsn (49); (ii) AdTs are glutaminases, liberating ammonia from amide donors such as Gln or Asn; (iii) AdTs amidate the activated intermediate using the liberated ammonia to form the properly aminoacylated species, Gln-tRNAGln or Asn-tRNAAsn. Thus, transamidation requires coupling of a tRNA-dependent kinase (GatB or GatE) with a glutaminase (GatA or GatD).
Figure 3.
Both GatCAB (A and B) and GatDE (A) catalyze three distinct reactions in order to transamidate their mischarged tRNA species, (A) Glu-tRNAGln and/or (B) Asp-tRNAAsn: (i) the kinase subunit of the respective AdT (GatB or GatE) phosphorylates the mischarged tRNA species to form an activated intermediate, (A) γ-phosphoryl-Glu-tRNAGln or (B) β-phosphoryl-Asp-tRNAAsn; (ii) the glutaminase subunit (GatA or GatD) hydrolyzes an amide donor such as Gln or Asn to release ammonia. A molecular tunnel connects the glutaminase and kinase active sites of the respective AdTs, allowing ammonia liberated from the glutamianse subunit (GatA or GatD) to flow to the kinase subunit (GatB or GatE) (denoted by the dashed arrow); (iii) the liberated ammonia is then used by the kinase subunit (GatB or GatE) to amidate the activated intermediate to form the product aa-tRNA, (A) Gln-tRNAGln or (B) Asn-tRNAAsn.
Recognition of the tRNA substrates of GatCAB and GatDE is the purview of the B and E-subunits of the respective AdTs (44,45,49). GatB and GatE are paralogs (24). The core of the two subunits belongs to an isolated protein family (3,44,45,50). Appended to the catalytic core of both subunits is a C-terminal extension that is homologous to the YqeY family of enzymes (24,44,45,51). The YqeY C-terminal extension of the D. radiodurans GlnRS enables the enzyme to productively bind to its tRNA substrate (51). For the AdTs, the YqeY-like domain serves a similar function interacting with the D-loop of the tRNA (44,45).
The 3′ terminal CCA end of the tRNA binds in the core of the kinase subunit (GatB or GatE) (44) placing the aminoacyl-moiety attached to the tRNA in the proper position in the active site to be phosphorylated (44,45,49). In the same active site, GatB and GatE catalyze the subsequent amidation of the activated intermediate to form the cognate aa-tRNA (Gln-tRNAGln or Asn-tRNAAsn) in their respective AdTs (Figure 3) (44,45). The phosphorylation step is thought to be Mg2+ dependent (44,45). Mutation of any of the three conserved residues that coordinate the Mg2+ in the catalytic pocket of the M. thermautotrophicus E-subunit (His15, Glu157 and Glu184) to Ala rendered the resulting GatDE mutant enzymes both as kinase and as transamidase inactive (44).
GatE pairs with GatD (3). The complex of the two is a α2β2 tetramer with two GatE polypeptides binding to a GatD homodimer (44,50). The D-subunit shares homology with type I l-asparaginases (3,50) and serves as the glutaminase domain for the heterodimeric AdT, liberating ammonia from an amide donor (Figure 3) (49). The M. thermautotrophicus GatDE is able to use Gln nearly as well as Asn as the donor (31). Like other l-asparaginases (52–55), GatD catalyzes the hydrolysis of Gln or Asn by making use of Thr as the nucleophile coupled with a Thr-Lys-Asp triad in the active site (49,50).
In the heterotrimeric AdT, the glutaminase subunit is GatA (4,45,56). The AdT subunit belongs to the amidase protein family (4,30,45,56) that uses a Ser-cisSer-Lys catalytic triad to hydrolyze amides (57). The cocrystal structure of S. aureus GatCAB with either Gln or Asn suggested Gln as a better substrate than Asn for GatA (45). Gln bound in the active site of the A-subunit forming a covalent bond with Ser178 (S. aureus GatA numbering), consistent with the predicted role of the residue as the nucleophile in the amidase catalytic triad (45). However, Asn bound into the same active site was unable to form the covalent intermediate, most likely due to the shorter length of the Asn side chain (45). Consistent with the structural results, the H. pylori GatCAB is 130-fold more efficient using Gln as the amide donor in transamidation than Asn, mostly due to a difference in kcat (4). Other mesophilic bacterial GatCAB enzymes studied have been shown to also be more active preferring Gln to Asn (18,58). On the other hand, the M. thermautotrophicus GatCAB is about equally efficient using Asn or Gln as amide donors suggesting that the active site of the archaeal GatA may differ slightly from that of S. aureus GatA (31). It appears that GatA requires a small helper protein, GatC (approximately 100 amino acids long), to fold properly (30,45) and to form a complex with GatB (45).
Gated tunnel couples ammonia release to transamidation
A molecular tunnel (30 Å and 40 Å long in GatCAB and GatDE, respectively) connects the glutaminase and kinase active sites in both AdTs (Figure 2) (44,45). It is proposed that the ammonia liberated by the glutaminase subunit of the AdT travels down the tunnel to the kinase active site where it is used to amidate the activated intermediate (44,45,50). Given that both tunnels are lined with hydrophilic residues it is speculated that ammonium is transported through the tunnel via a series of protonations and deprotonations (44,45), possibly akin to potassium transport through the potassium channel, KcsA (59).
The tunnel may provide the mechanism by which the glutaminase activity of GatA can be coupled to overall transamidation (45). Biochemical evidence has shown that the glutaminase activity of GatCAB is enhanced in the presence of mischarged substrate; it is further stimulated by ATP (4,60). In the S. aureus GatCAB structures (all in the absence of tRNA substrate), the tunnel is gated closed (45). It is plausible that upon binding to Glu-tRNAGln or Asp-tRNAAsn the tunnel opens, allowing ammonium to flow through it and in turn enhance the glutaminase activity of the A-subunit (45). Whether it is opening of the tunnel alone or if there are accompanying structural rearrangements awaits further investigations; in particular, a cocrystal structure of GatCAB with either Asp-tRNAAsn or Glu-tRNAGln along with ATP.
In the case of GatDE the coupling of the glutaminase activity of the enzyme with overall transamidation is tighter such that the AdT is glutaminase inactive in the absence of Glu-tRNAGln (49). In the crystal structure of the Pyrococcus abyssi, GatDE apo-enzyme not only is the molecular tunnel closed but the catalytically important Thr in the D-subunit is positioned 7 Å away from the subunit's active site (50). This Thr is located on a β-hairpin that molecular dynamic simulations predict to be flexible (50). It is hypothesized that Glu-tRNAGln binding to GatE induces conformational changes in the holoenzyme that open the molecular tunnel and also move the β-hairpin to correctly position the Thr into the GatD active site, thus enabling the subunit to catalyze the release of ammonia from an amide donor (50). Such a mechanism would prevent unproductive hydrolysis of Gln or Asn by GatDE in the absence of Glu-tRNAGln (49,50).
Substrate channeling between non-discriminating aaRS and AdT
Aminoacylation and transamidation may also be coupled. While a possible complex between ND-aaRS, AdT and tRNA was proposed almost two decades ago (17), it was only recently that evidence has emerged for such a complex that would enable substrate channeling (61) of a mischarged tRNA from the aaRS to the AdT (44,62,63). In the case of the T. thermophilus GatCAB and ND-AspRS, the complex between the AdT and ND-AspRS requires tRNAAsn (63). In vitro, the complex between ND-AspRS, AdT and tRNAAsn has been shown to enhance the aminoacylation activity of the aaRS (63). In addition, the complex appears to stabilize Asp-tRNAAsn and in particular Asn-tRNAAsn (62,63). The structural modeling of the complex suggests that the 3′ terminal CCA end of the tRNA flips from the aminoacylation site in the aaRS to the kinase center of the AdT after aminoacylation (63), analogous to the movements in the tRNA seen in certain aaRSs with editing domains (64,65). Thus, the Asp moiety of the mischarged tRNA formed by the aaRS can be rapidly amidated to Asn, de facto making this complex an AsnRS formed in trans. It is speculated that the complex between ND-AspRS, GatCAB and tRNAAsn enables formation of Asn-tRNAAsn without the risk of Asp-tRNAAsn being used in protein synthesis, while also protecting the product aa-tRNA from deacylation until it can be bound by EF-Tu to be used in translation (62,63).
Structural modeling also predicts a complex between ND-GluRS, tRNAGln and either Glu-AdT (GatCAB or GatDE) may also be possible (44,63). Given that AspRS, a class II aaRS, binds to the major groove side of the acceptor stem of the tRNA and GluRS, a class I aaRS, binds to the minor groove side indicate the complexes formed by GatCAB with ND-AspRS and tRNAAsn or ND-GluRS and tRNAGln will differ significantly from one another (63). In the case of GatDE, modeling suggests that the AdT would be able to form a complex with ND-GluRS but not ND-AspRS due to sterical clashes between the class II aaRS and an insertion domain found in GatE but not GatB (44). Whether such complexes exist between tRNAGln, ND-GluRS, and AdTs awaits further investigations.
Why have the indirect pathways for amide aa-tRNA formation been retained?
Both GlnRS and AsnRS were absent in the last universal communal ancestor (LUCA) and most likely Gln-tRNAGln and Asn-tRNAAsn were formed via the indirect pathways (5,66–69). Why the indirect pathways for amide aa-tRNA formation have been retained in so many prokaryotes remains an open question. The unique archaeal tRNAGln favored by GatDE may be a barrier to acquistion of GlnRS in Archaea (3,24) as neither the Escherichia coli nor S. cerevisiae GlnRS can aminoacylate archaeal tRNAGln (3). Recognition of tRNA though does not seem to be as a significant barrier preventing more bacteria from acquiring GlnRS. For example, the B. subtilis tRNAGln can serve as a substrate for the E. coli GlnRS (19).
It should be noted that glutamine is not only used in cells for translation. The synthesis of Gln from Glu by glutamine synthetase is the primary mechanism for ammonium assimilation in all free-living organisms and Gln serves as the primary amide donor for a variety of vital cellular biosynthetic pathways (70). In Firmicutes such as B. subtilis, glutamine is an important signaling molecule for the regulation of nitrogen metabolism through the transcriptional regulator TnrA (71) as Gln is an indicator of the nitrogen and Glu status of the cell (70,71). Therefore, retention of the indirect pathway for Gln-tRNAGln formation may constitute a regulatory link between central carbon metabolism (levels of α-ketoglutarate) and the level of nitrogen availability, both key parameters in regulation of protein synthesis. In addition, a number of bacteria that use GatCAB as a Glu-AdT have elevated cellular levels of Glu (72). It is currently unknown how well a GlnRS under such in vivo conditions could discriminate Gln from Glu but it is conceivable that the indirect pathway is retained in these organisms to maintain translational fidelity (30).
Metabolic reasons may also explain why so many prokaryotes have retained the indirect paths for Asn-tRNAAsn formation. For example, T. thermophilus and D. radiodurans encode an AsnRS in their genomes (73,74), while also retaining a ND-AspRS and GatCAB (21,22,32,33,37,38). The indirect path is the sole route for Asn biosynthesis in these species as they lack both synthetases responsible for free Asn formation (AsnA and AsnB) (38) a situation found in numerous other bacteria (4,38). Interestingly, all known bacterial and archaeal genomes encoding AnsA, the ammonia-dependent asparagine synthetase, use AsnRS and not GatCAB to form Asn-tRNAAsn (4,5) enabling these organisms to form Asn-tRNAAsn in a glutamine-independent fashion. How amide aa-tRNA formation is tied into other cellular needs for the amide amino acids is still unclear (70), but such investigations will likely enable a greater understanding into why the indirect pathways for Gln-tRNAGln and Asn-tRNAAsn are retained in so many bacteria and archaea.
tRNA-dependent cysteine biosynthesis
In certain archaea, a canonical class I CysRS is either absent or dispensable (75,76). In addition, some of these archaea lack homologs of enzymes involved in cysteine biosynthesis in bacteria and eukaryotes. Biochemical investigation and genetic analysis revealed that a tRNA-dependent indirect pathway is responsible for providing Cys-tRNACys for ribosomal translation as well as free cysteine biosynthesis in these organisms (Table 1) (11). This process couples protein synthesis with cysteine production and is mostly observed in a large subset of the Euryarchaeota (77).
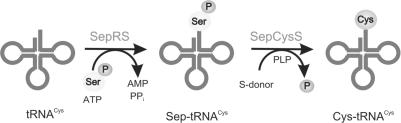
The tRNA-dependent pathway consists of a two-step pretranslational amino acid transformation on tRNACys (Figure 4). The tRNACys is initially aminoacylated with O-phosphoserine (Sep) by O-phosphoseryl-tRNA synthetase (SepRS). The Sep moiety is subsequently transformed to tRNA-bound cysteine by Sep-tRNA: Cys-tRNA synthase (SepCysS) (11). Sulfide was shown to be a sufficient sulfur donor in vitro. However, the nature of the sulfur donor in vivo awaits further investigation. This finding confirms an earlier report that Sep is the source of the carbon framework for cysteine biosynthesis in M. jannaschii (78). Furthermore, an archaeal Sep biosynthetic pathway was characterized and proven to provide a sufficient amount of Sep for serine, cystathionine and tRNA-dependent cysteine formation (79).
Figure 4.
Indirect pathway for Cys-tRNACys formation. First, SepRS aminoacylates tRNACys with Sep to form Sep-tRNACys. The Sep bound to the tRNA is then converted to Cys by SepCysS in the presence of a sulfur donor to form Cys-tRNACys.
Homologs of SepRS and SepCysS are identifiable in the genomes of the sulfate reducing archaeon Archaeoglobus fulgidus (80) and all known methanogenic archaea (77) except Methanobrevibacter smithii (81) and Methanosphaera stadtmanae (82). In the majority of these species, the indirect SepRS/SepCysS pathway coexists with the direct pathway catalyzed by CysRS (77). In many of these organisms, the enzymes required for tRNA-independent cysteine biosynthetic pathways are not encoded in their genomes (11,77). Consequently, the tRNA-dependent SepRS/SepCysS-mediated pathway provides the only means for the de novo production of cysteine in these species. This was demonstrated for Methanococcus maripaludis where the deletion of SepRS resulted in cysteine auxotrophy (11).
SepRS aminoacylates tRNACys with Sep
SepRS belongs to the subclass IIc of aaRSs and is most closely related to the α-subunit of PheRS based on its amino acid sequence and structural organization (11,83). Phylogenetic analyses show that SepRS and α-PheRS share a common ancestor (77). Crystal structures of SepRS from M. maripaludis (84), M. jannaschii, and A. fulgidus (85) supported by biophysical analyses in solution demonstrate that SepRS is a homotetrameric enzyme. The quaternary structure of the core region of this α4 assembly, consisting mostly of the four catalytic domains, resembles closely the core region of PheRS (84).
In spite of the structural similarity of their active sites, amino acid recognition differs significantly in SepRS relative to PheRS (85). Sep, unlike any of the 20 canonical amino acids, has a highly negatively charged side chain; therefore, it is not surprising that its phosphate moiety is extensively recognized by SepRS. Each of the three non-bridging oxygen atoms of the phosphate group forms two hydrogen bonds to residues in the amino acid binding pocket and mutation of the corresponding residues in M. maripaludis SepRS resulted in complete loss of enzymatic activity (84). Two non-bridging oxygen atoms in the phosphate group are hydrogen-bonding with the α-amino group of active site residues. The recognition of the side chain of a substrate amino acid by protein main chain groups is unique to SepRS. Furthermore, structural data suggests that the polar side chain of Sep is stabilized by dipole interactions with the positively charged N-terminal end of a central α-helix in the active site (85). This amino acid recognition mechanism including the organization of the substrate side-chain binding pocket is an exceptional characteristic of SepRS, and is not observed in any of the canonical aaRSs (85).
Unlike PheRS, where the catalytically active domain and the tRNA anticodon recognition site are located on different subunits, each SepRS monomer harbors both sites and tRNA recognition and aminoacylation occur in cis. However, the crystal structure of the A. fulgidus SepRS:tRNACys complex indicates that two tRNACys molecules are bound per SepRS α4 tetramer (85). The stoichiometry of tRNA and SepRS in solution is unclear. The binding of tRNA does not induce significant conformational changes in SepRS based on the crystal structure and four tRNAs per α4 complex can also be accommodated in silico (85).
A comparative biochemical study revealed that M. maripaludis SepRS and CysRS both recognize the same set of major identity elements in tRNACys: the discriminator base U73 and the anticodon (G34, C35 and A36) (86). In addition, nucleotides G15 and A47 serve as minor identity elements for both enzymes, whereas G37, A59 and the base pairs G1:C72 and G10:C25 are minor identity elements for SepRS but do not affect aminoacylation by CysRS. While SepRS approaches the tRNA from the major groove, CysRS approaches it from the minor groove side, and it has been speculated that a ternary complex of SepRS:tRNACys:CysRS could be formed (85). Class II aaRSs generally aminoacylate the 3′-terminal adenosine of their cognate tRNA at the 3′-OH group, with PheRS being a notable exception (87).
tRNA-dependent transformation of Sep to Cys
The conversion of Sep-tRNACys to Cys-tRNACys by SepCysS is pyridoxal phosphate (PLP)-dependent (11). In the resting state, the PLP cofactor was shown to covalently attach to the conserved lysine residue in the active site of SepCysS through a Schiff-base linkage (88). The side chain of a conserved asparagine rather than an aspartate residue observed in most other PLP enzymes, hydrogen-bonds with the nitrogen atom in the ring structure of PLP (88). The PLP cofactor is expected to be intimately involved in the catalysis of the β-replacement reaction by stabilizing the negative charge developed in the transition state as described in the basic mechanism for almost all PLP-dependent proteins (89).
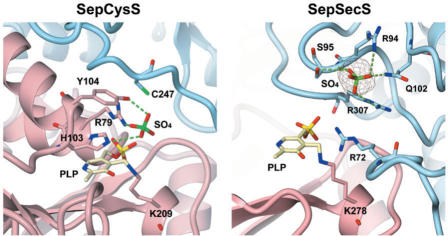
The crystal structure (2.4 Å resolution) of A. fulgidus SepCysS shows that the enzyme forms a homodimer (88). Conserved residues from both subunits form the catalytic center located deep within the large, basic cleft near the dimer interface. Structurally, SepCysS belongs to the fold type I family (90). Each monomer contains two domains and the N-terminal large domain has a characteristic seven-stranded β-sheet for this family of protein. A model of the complex between SepCysS with its substrate Sep-tRNACys, suggested that three conserved amino acid residues (Arg79, His103 and Tyr104) interact with the phosphate moiety of the Sep in the substrate and one of the three highly conserved cysteine residues in A. fulgidus SepCysS may be essential in the catalysis by carrying persulfide sulfur for cysteine formation (Figure 5) (88). The detailed mechanism for SepCysS catalyzing tRNA-dependent conversion of Sep to Cys awaits further biochemical investigation.
Figure 5.
The crystal structures of the active sites of A. fulgidus SepCysS and M. maripaludis SepSecS. In both, the different monomers of the respective enzyme are colored pink and blue. PLP and residues in the catalytic centers are shown as ball-and-stick models (adapted from 136).
In M. maripaludis, two pathways are present for Cys-tRNACys formation: the indirect SepRS/SepCysS pathway mentioned above and the direct charging of tRNACys by CysRS. The indirect pathway is also the sole route for free cysteine formation. Interestingly, when the organism is grown in the presence of exogenous cysteine, SepRS can be deleted (11) while SepCysS is still indispensable (T. Major, M. Hohn, D. Su, W.B. Whitman, unpublished data) suggesting SepCysS may possess an alternative essential function in M. maripaludis.
Cysteine biosynthesis in archaea and the evolutionary view of the indirect pathway
To date, four different routes are shown to be present for cysteine biosynthesis in archaea: the eukaryotic pathway with cystathionine as the precursor (91), the bacterial pathway with O-acetylserine as the precursor (92–94), the modified bacterial pathway with O-phosphoserine as the precursor (95,96) and the tRNA-dependent pathway (11). Free cysteine has been shown to be the sulfur source for multiple biosynthetic processes, including Fe-S cluster formation, tRNA modification and cofactor biosynthesis in bacteria (97). Methanogens encode an unusually high number of Fe-S cluster proteins (98). Organisms that rely on the tRNA-dependent pathway for cysteine biosynthesis are thought to regulate the amount of free cysteine versus tRNA-bound cysteine by controlling the deacylation of Cys-tRNACys, in order to maintain the balance between protein synthesis and other sulfur-related biosynthetic processes. Alternatively, considering that the natural habitat of methanogens is rich in reduced sulfur compounds, these organisms may have evolved to use inorganic sulfur directly for other sulfur-related biosynthetic processes.
The discovery of the SepRS/SepCysS pathway raised questions as to whether this mechanism is a recent evolutionary invention or if it is more ancient and possibly predates direct tRNA charging by CysRS. Phylogenetic analyses, using structure-based amino acid alignments (77,83) demonstrate that the indirect aminoacylation pathway for tRNACys and the direct pathway mediated by CysRS were already present at the time of LUCA. Although both pathways developed contemporarily, CysRS was initially only vertically inherited in the bacterial lineage, whereas SepRS was vertically inherited in Archaea. Later in evolution, CysRS was horizontally transferred to Archaea and replaced the indirect mechanism in some archaeal lineages, whereas in the methanogenic archaea the indirect pathway either was retained or coexisted with CysRS (77). The question why the SepRS/SepCysS pathway has been preserved in some archaea is still unclear. It has been speculated that some yet unknown link between indirect cysteine formation, sulfur metabolism and methanogenesis might exist (77). Further experimental evidence in this direction is required.
tRNA-dependent selenocysteine biosynthesis
Selenium, an essential dietary trace element, has a beneficial effect on the functions of four major organ systems in the human body as well as is involved in cancer prevention in a dose-dependent manner (99,100). Sec, the major biological form of selenium, is cotranslationally incorporated into proteins as the 21st amino acid in a number of organisms from all three domains of life (9,101). Under physiological conditions (pH 7), Sec is more stable in its ionized form due to the lower redox potential of selenium and thus the lower pKa of the selenol group compared to the thiol group in Cys (5.2 versus 8.5) (102). The extra electrons in the side chain of selenocysteine make it an extraordinary nucleophile in the catalytic center of selenoproteins for oxidation–reduction reactions.
The ribosomal selenoprotein biosynthesis uses selenocysteinyl-tRNASec (Sec-tRNASec) as a substrate. To date, a selenocysteinyl-tRNASec synthetase (SecRS), an enzyme that could directly aminoacylate tRNASec with Sec, has not been identified in any organism (Table 1). Free selenocysteine can be activated and ligated to tRNACys by CysRS from plants (103) and bacteria (104). To prevent mis-incorporation of Sec in Cys codons and the consequent malfunction of proteins (105), the free Sec concentration most likely has to be well controlled and kept at a low level. In addition, a Sec to Cys mutation in a selenoprotein can result in a drastic reduction of enzyme activity (106). Due to the lack of mechanisms differentiating Cys and Sec, the invention of SecRS might be undesirable. Therefore, a pathway to synthesize Sec on tRNA using selenophosphate as the selenium source rather than directly charging tRNA with Sec (Figure 6) may help to maintain the accuracy of Sec and Cys decoding (107).
Figure 6.
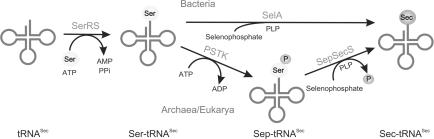
Indirect pathways for Sec-tRNASec formation. In all known Sec-decoding organisms, first SerRS aminoacylates tRNASec with Ser to form Ser-tRNASec. In Sec-decoding bacteria, the Ser bound to the tRNA is directly converted to Sec in the presence of selenophosphate by SelA to form Sec-tRNASec. In Sec-decoding eukaryotes and Archaea, the Ser-moiety on tRNASec is first phosphorylated by PSTK to form Sep-tRNASec. The Sep bound to the tRNA is then converted to Sec in the presence of selenophosphate by SepSecS to form Sec-tRNASec.
In all Sec-decoding organisms (108–110), the first step in the Sec biosynthetic pathway is the serylation of tRNASec with serine by seryl-tRNA synthetase (111–113). In bacteria, Sec biosynthesis and its incorporation into proteins have been extensively worked out in the nineties (9). Selenocysteine synthase (SelA) converts Ser-tRNASec to Sec-tRNASec, which is then incorporated into proteins in the presence of a RNA element (facilitating UGA recognition) and Sec-specific elongation factor SelB (9). In Archaea and eukaryotes, Ser-tRNASec is not directly converted to the final product Sec-tRNASec (Figure 4). Instead, it is first converted to Sep-tRNASec by O-phosphoseryl-tRNA kinase (PSTK) (114), and then the resulting tRNA-bound Sep is transformed to Sec by Sep-tRNA:Sec-tRNA synthase (SepSecS) in the presence of selenophosphate (15,16).
Both SelA and SepSecS are PLP-dependent proteins. They use the same selenium donor, selenophosphate, to produce Sec in vitro and in vivo (15,16,115–117). The bacterial SelA also converts Sep-tRNASec to selenocysteine in vitro (16), which may not be biologically relevant as PSTK, the enzyme producing Sep-tRNASec, is only found in Archaea and eukaryotes (15). In contrast, SepSecS only recognizes Sep-tRNASec but not Ser-tRNASec as a substrate. SepSecS genes are always copresent with PSTK genes in the complete genomes of all known Sec decoding archaea and eukaryotes (118). Sep-tRNASec has a more stable carboxyl ester bond between the amino acid and the tRNA than Ser-tRNASec (114). Additionally, the phosphate group in the side chain of Sep is expected to be a better leaving group compared to the hydroxyl group in the serine side chain. Therefore, the increased stability of the substrate and the decrease of activation energy make Sep-tRNASec a better precursor for Sec formation.
PSTK phosphorylates serine in a tRNASec-dependent manner
The enzymatic phosphorylation of Ser-tRNASec was first observed in rat and rooster liver and lactating bovine mammary gland in the 1970s (119,120). A partially purified enzyme from bovine liver was characterized and shown to have high affinity for tRNASec (121). It was not until recently that the protein responsible for this activity was identified in mouse as PSTK (114). Later, the archaeal homolog of mouse PSTK was also shown to have the same activity in vitro (122). PSTK catalyzes the transfer of the γ-phosphate of ATP to the serine moiety in the presence of magnesium ions (114,118). The N-terminal kinase domain of PSTK consists of a phosphate-binding loop (P-loop), a Walker B and RxxxR motif which are generally conserved in the P-loop kinase superfamily (118,123). Like T4 polynucleotide kinase (124), PSTK has relaxed NTP specificity with a preference for ATP in vitro (118).
Comprehensive biochemical studies of wild type and mutant M. jannaschii PSTK also uncovered several unique aspects of this protein (118). Most notably, PSTK binds to unacylated tRNASec and its substrate Ser-tRNASec with similar affinities (Kd ≅ 40 nM) (118). As the in vivo concentration of tRNASec is much lower than that of other tRNA species (e.g. it is <10% of tRNASer) (125,126), it is not surprising that PSTK has about 20-fold higher affinity for its substrate than aaRSs. The equally high affinity for unacylated tRNASec suggests a scavenger role of PSTK for tRNASec; this may assist other enzymatic reactions in the pathway such as the misacylation by SerRS and the final conversion step by SepSecS (118). PSTK may also prevent mis-incorporation by sequestering the misacylated tRNASec intermediates and channeling them to SepSecS for the cognate Sec-tRNASec production (118). Moreover, the tRNA-dependent activation of ATPase activity in PSTK (118) is another example of substrate-assisted catalysis in this kinase superfamily (123).
The recognition of Sep-tRNASec is different in Archaea and eukaryotes
The tRNASec has distinct structural features relative to other tRNA molecules: an elongated acceptor arm (acceptor stem + T-stem) and D-stem (127,128). Bacterial tRNASec has 8 bp and 5 bp in the acceptor stem and the T-stem, respectively, while archaeal and eukaryotic tRNASec has a 9 bp and 4 bp arrangement (127,129–131). For the serylation reaction, tRNASec shares the same major identity element as tRNASer, namely the long variable arm and the discriminatory base for SerRS recognition (132). A different set of identity elements in tRNASec for the phosphorylation reaction catalyzed by PSTK is observed in archaea relative to eukaryotes. In eukaryotes, the major identity element for PSTK recognition is the length and the conformation of the D-stem (133), which is rather a minor identity element in archaea. Instead, the second and the third base pair (G2-C71 and C3-G70) in the acceptor stem of tRNASec serve as the major identity elements for archaeal PSTK phosphorylation (134). Interestingly, PSTK phylogeny shows a deep divide between the archaeal and the eukaryotic type enzyme (118). These findings may suggest the co-evolution of the kinase and its substrate tRNASec (134).
Despite the differences in the selenocysteine incorporation machinery between the bacterial system and the archaeal and eukaryotic systems, SepSecS and PSTK recognize E. coli tRNASec in vivo (15). Similarly, previous reports also showed that human tRNASec complements a tRNASec gene deletion in E. coli and that the eukaryotic tRNASec can be serylated and converted to Sec-tRNASec by E. coli enzymes in vitro (135). These findings suggest that tRNASec is functionally conserved between the two different selenocysteine incorporation systems.
SepSecS catalyzes the tRNA-dependent transformation of Sep to Sec
The archaeal and eukaryotic pathway for selenocysteine formation is reminiscent of the indirect pathway for cysteine biosynthesis in archaea, most notably in the final conversion step where Sep-tRNA is the immediate precursor for cysteine or selenocysteine production by SepCysS or SepSecS in the presence of a sulfur or selenium donor (Figures 4 and 6). SepSecS can also use thiophosphate as a substrate and produce Cys-tRNASec in vitro (136). The inability of differentiating selenophosphate versus thiophosphate by SepSecS indicates that the phosphate group in the selenium donor is the major recognition moiety by the enzyme.
Analogous to SepCysS, SepSecS catalyzes the β-replacement of phosphoserine to form selenocysteine in the presence of selenophosphate in a tRNA-dependent manner. Recently, the crystal structure of SepSecS from M. maripaludis (136) and mouse (137) were solved at high resolution. Both structures revealed a homotetrameric state (α2)2 of SepSecS with two active sites per dimer (136,137). Each active site located at the dimer interface consists of catalytically crucial residues from both subunits (Figure 5). The tetramerization is mediated by the N-terminal extension of each SepSecS monomer. The elongated shape resulting from tetramerization together with the large patch of positive electronic potential on the surface of SepSecS are proposed to form an effective tRNASec binding surface (137). It appears that the disruption of the oligomeric state by deleting the N-terminal region abolishes the catalytic function of SepSecS (136). Similar to SepCysS (88), a conserved lysine and asparagine are involved in PLP binding by SepSecS (136). Conserved arginines, glutamine and serine are proposed to be essential for binding to the phosphate moiety of the substrates (Sep-tRNASec and/or selenophosphate) (Figure 5) (136). Mutations of these residues significantly reduce the catalytic activity of SepSecS in vivo and in vitro (136). An interesting filtering mechanism was proposed for excluding free amino acid including free Sep as a substrate, where the side chain of a conserved glutamate repels the carboxyl group of the free amino acid (137). Distinct from SepCysS, a perselenide intermediate is unlikely to be present in the reaction catalyzed by archaeal SepSecS due to the absence of a conserved cysteine residue near the active site. A structural phylogeny shows SepSecS is not closely related to other PLP-dependent proteins with solved structures in the fold type I family, while SepCysS belongs to the cysteine desulfurase group (136).
Cysteine and selenocysteine biosynthetic pathways are both ancient
Phylogenetic analysis demonstrated that bacterial, archaeal and eukaryotic selenocysteine incorporation machineries already existed at the time of LUCA (15) and so did the indirect pathway for cysteine formation (77). SepSecS and PSTK are strictly archaeal and eukaryotic enzymes and distinct from the bacterial SelA. The PSTK/SepSecS pathway for synthesizing selenocysteinyl-tRNA is reminiscent of the strictly archaeal tRNA-dependent pathway for cysteine formation in various aspects mentioned earlier. As both SepCysS and SepSecS use tRNA-bound phosphoserine as a substrate, it will be interesting to investigate if the functions of these two enzymes are interchangeable under certain conditions. Considering that most selenoproteins have Cys-containing homologs and a similar genetic code for Sec and Cys (UGA versus UGC/UGU), a dynamic evolutionary relationship may exist between selenocysteine and cysteine (138).
OUTLOOK
As discussed throughout this article, synthesis of an amino acid on its cognate tRNA requires the formation of a mischarged tRNA substrate. What prevents these misacylated tRNA species from being used in protein synthesis? While elongation factors (EF-Tu and SelB) have been shown in vitro to have higher affinity for cognate aa-tRNA pairs over certain mischarged tRNA species (22,139–143), it still remains unclear the level of discrimination by the elongation factors in vivo (63,144). Substrate channeling of the misacylated tRNA from an aaRS to a tRNA-dependent modifying enzyme would provide an additional mechanism to maintain the fidelity of protein synthesis despite formation of the mischarged tRNA species (17,44,62,63,88). Intriguingly, structural modeling does suggest complexes between ND-GluRS, Glu-AdTs and tRNAGln, and SepRS, SepCysS and tRNACys are possible (44,63,88). Whether these enzymes do form complexes similar to that found for ND-AspRS, GatCAB and tRNAAsn awaits further investigations, as do their cellular implications.
It is tempting to speculate that such ancient complexes allowed Gln, Asn, Cys and Sec to be added to the genetic code (44,145), but that should be tempered by the fact that the indirect pathway for Cys-tRNACys formation may not predate the direct one (77). It may well be that use of tRNA-dependent amino acid biosynthetic routes arose and have been retained to maintain the fidelity of protein synthesis in environments in which an aaRS alone could not reliably discriminate between two similar amino acids (146), e.g. CysRS with Cys and Sec (103,104). Delving into such speculation will require a better understanding as to how these indirect pathways for aa-tRNA formation are integrated into overall metabolism and regulated under different cellular conditions.
ACKNOWLEDGEMENTS
We thank all members of the Söll laboratory for helpful comments and discussions. We are grateful to Juan Alfonzo (Ohio State University), Anne-Marie Duchêne and Hubert Becker (Université Louis Pasteur de Strasbourg, France), Tsutomu Suzuki (University of Tokyo, Japan), and William B. Whitman (University of Georgia) for communicating data prior to publication. Work in the authors’ laboratories was supported by grants from the European Commission (Grant QLG2-CT-1999–01455) and Enterprise Ireland SC/02/109 (to K.M.D.), and grants from the Department of Energy, The National Institute of General Medical Sciences and the National Science Foundation (to D.S.). Funding to pay the Open Access publication charges for this article was provided by NIH grant GM22854.
Conflict of interest statement. None declared.
REFERENCES
- 1.Ibba M, Söll D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000;69:617–650. doi: 10.1146/annurev.biochem.69.1.617. [DOI] [PubMed] [Google Scholar]
- 2.Wilcox M, Nirenberg M. Transfer RNA as a cofactor coupling amino acid synthesis with that of protein. Proc. Natl Acad. Sci. USA. 1968;61:229–236. doi: 10.1073/pnas.61.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tumbula DL, Becker HD, Chang WZ, Söll D. Domain-specific recruitment of amide amino acids for protein synthesis. Nature. 2000;407:106–110. doi: 10.1038/35024120. [DOI] [PubMed] [Google Scholar]
- 4.Sheppard K, Akochy PM, Salazar JC, Söll D. The Helicobacter pylori amidotransferase GatCAB is equally efficient in glutamine-dependent transamidation of Asp-tRNAAsn and Glu-tRNAGln. J. Biol. Chem. 2007;282:11866–11873. doi: 10.1074/jbc.M700398200. [DOI] [PubMed] [Google Scholar]
- 5.Roy H, Becker HD, Reinbolt J, Kern D. When contemporary aminoacyl-tRNA synthetases invent their cognate amino acid metabolism. Proc. Natl Acad. Sci. USA. 2003;100:9837–9842. doi: 10.1073/pnas.1632156100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bult CJ, White O, Olsen GJ, Zhou L, Fleischmann RD, Sutton GG, Blake JA, FitzGerald LM, Clayton RA, Gocayne JD, et al. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 7.Smith DR, Doucette-Stamm LA, Deloughery C, Lee H, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, et al. Complete genome sequence of Methanobacterium thermoautotrophicum deltaH: functional analysis and comparative genomics. J. Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slesarev AI, Mezhevaya KV, Makarova KS, Polushin NN, Shcherbinina OV, Shakhova VV, Belova GI, Aravind L, Natale DA, Rogozin IB, et al. The complete genome of hyperthermophile Methanopyrus kandleri AV19 and monophyly of archaeal methanogens. Proc. Natl Acad. Sci. USA. 2002;99:4644–4649. doi: 10.1073/pnas.032671499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Böck A, Thanbichler M, Rother M, Resch A. Selenocysteine. In: Ibba M, Francklyn C, Cusak S, editors. The Aminoacyl-tRNA Synthetases. Georgetown, Texas, USA: Landes Bioscience; 2005. pp. 320–327. [Google Scholar]
- 10.Curnow AW, Ibba M, Söll D. tRNA-dependent asparagine formation. Nature. 1996;382:589–590. doi: 10.1038/382589b0. [DOI] [PubMed] [Google Scholar]
- 11.Sauerwald A, Zhu W, Major TA, Roy H, Palioura S, Jahn D, Whitman WB, Yates J.R.,, 3rd, Ibba M, Söll D. RNA-dependent cysteine biosynthesis in archaea. Science. 2005;307:1969–1972. doi: 10.1126/science.1108329. [DOI] [PubMed] [Google Scholar]
- 12.Forchhammer K, Boesmiller K, Böck A. The function of selenocysteine synthase and SELB in the synthesis and incorporation of selenocysteine. Biochimie. 1991;73:1481–1486. doi: 10.1016/0300-9084(91)90181-y. [DOI] [PubMed] [Google Scholar]
- 13.Forchhammer K, Böck A. Selenocysteine synthase from Escherichia coli. Analysis of the reaction sequence. J. Biol. Chem. 1991;266:6324–6328. [PubMed] [Google Scholar]
- 14.Forchhammer K, Leinfelder W, Boesmiller K, Veprek B, Böck A. Selenocysteine synthase from Escherichia coli. Nucleotide sequence of the gene (selA) and purification of the protein. J. Biol. Chem. 1991;266:6318–6323. [PubMed] [Google Scholar]
- 15.Yuan J, Palioura S, Salazar JC, Su D, O’Donoghue P, Hohn MJ, Cardoso AM, Whitman WB, Söll D. RNA-dependent conversion of phosphoserine forms selenocysteine in eukaryotes and archaea. Proc. Natl Acad. Sci. USA. 2006;103:18923–18927. doi: 10.1073/pnas.0609703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu XM, Carlson BA, Mix H, Zhang Y, Kazima S, Glass RS, Berry MJ, Gladyshev VN, Hatfield DL. Biosynthesis of Seloncysteine on its tRNA in eukaryotes. PLoS Biol. 2007;5:96–105. doi: 10.1371/journal.pbio.0050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schön A, Kannangara CG, Gough S, Söll D. Protein biosynthesis in organelles requires misaminoacylation of tRNA. Nature. 1988;331:187–190. doi: 10.1038/331187a0. [DOI] [PubMed] [Google Scholar]
- 18.Jahn D, Kim YC, Ishino Y, Chen MW, Söll D. Purification and functional characterization of the Glu-tRNA(Gln) amidotransferase from Chlamydomonas reinhardtii. J. Biol. Chem. 1990;265:8059–8064. [PubMed] [Google Scholar]
- 19.Lapointe J, Duplain L, Proulx M. A single glutamyl-tRNA synthetase aminoacylates tRNAGlu and tRNAGln in Bacillus subtilis and efficiently misacylates Escherichia coli tRNAGln1 in vitro. J. Bacteriol. 1986;165:88–93. doi: 10.1128/jb.165.1.88-93.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cathopoulis T, Chuawong P, Hendrickson TL. Novel tRNA aminoacylation mechanisms. Molecular Biosyst. 2007;3:408–418. doi: 10.1039/b618899k. [DOI] [PubMed] [Google Scholar]
- 21.Becker HD, Reinbolt J, Kreutzer R, Giege R, Kern D. Existence of two distinct aspartyl-tRNA synthetases in Thermus thermophilus. Structural and biochemical properties of the two enzymes. Biochemistry. 1997;36:8785–8797. doi: 10.1021/bi970392v. [DOI] [PubMed] [Google Scholar]
- 22.Becker HD, Kern D. Thermus thermophilus: a link in evolution of the tRNA-dependent amino acid amidation pathways. Proc. Natl Acad. Sci. USA. 1998;95:12832–12837. doi: 10.1073/pnas.95.22.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SI, Stange-Thomann N, Martins O, Hong KW, Söll D, Fox TD. A nuclear genetic lesion affecting Saccharomyces cerevisiae mitochondrial translation is complemented by a homologous Bacillus gene. J. Bact. 1997;179:5625–5627. doi: 10.1128/jb.179.17.5625-5627.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheppard K, Söll D. On the evolution of the tRNA-dependent amidotransferases,GatCAB and GatDE. J. Mol. Biol. 2008 doi: 10.1016/j.jmb.2008.01.016. doi: 10.1016/j.jmb.2008.01.016 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulero JJ, Rosenthal JK, Fox TL. PET112, a Saccharomyces cerevisiae nuclear gene required to maintain rho+ mitochondrial DNA. Current Genetics. 1994;25:299–304. doi: 10.1007/BF00351481. [DOI] [PubMed] [Google Scholar]
- 26.Hughes TR, Marton MJ, Jones AR, Roberts CJ, Stoughton R, Armour CD, Bennett HA, Coffey E, Dai H, He YD, et al. Functional discovery via a compendium of expression profiles. Cell. 2000;102:109–126. doi: 10.1016/s0092-8674(00)00015-5. [DOI] [PubMed] [Google Scholar]
- 27.Martin NC, Rabinowitz M, Fukuhara H. Yeast mitochondrial DNA specifies tRNA for 19 amino acids. Deletion mapping of the tRNA genes. Biochemistry. 1977;16:4672–4677. doi: 10.1021/bi00640a022. [DOI] [PubMed] [Google Scholar]
- 28.Rinehart J, Krett B, Rubio MA, Alfonzo JD, Söll D. Saccharomyces cerevisiae imports the cytosolic pathway for Gln-tRNA synthesis into the mitochondrion. Genes Dev. 2005;19:583–592. doi: 10.1101/gad.1269305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dirheimer D, Keith G, Sibler AP, Martin RP. The primary structure of tRNAs and their rare nucleosides. In: Schimmel PR, Söll D, Abelson JN, editors. Transfer RNA: Structure, Properties, and Recognition. New York, USA: Cold Spring Harbor; 1979. pp. 19–41. [Google Scholar]
- 30.Curnow AW, Hong K, Yuan R, Kim S, Martins O, Winkler W, Henkin TM, Söll D. Glu-tRNAGln amidotransferase: a novel heterotrimeric enzyme required for correct decoding of glutamine codons during translation. Proc. Natl Acad. Sci. USA. 1997;94:11819–11826. doi: 10.1073/pnas.94.22.11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheppard K, Sherrer RL, Söll D. Methanothermobacter thermautotrophicus tRNAGln confines the admidotransferase GatCAB to asparaginyl-tRNAAsn formation. J. Mol. Biol. 2008 doi: 10.1016/j.jmb.2008.01.064. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curnow AW, Tumbula DL, Pelaschier JT, Min B, Söll D. Glutamyl-tRNAGln amidotransferase in Deinococcus radiodurans may be confined to asparagine biosynthesis. Proc. Natl Acad. Sci. USA. 1998;95:12838–12843. doi: 10.1073/pnas.95.22.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker HD, Min B, Jacobi C, Raczniak G, Pelaschier J, Roy H, Klein S, Kern D, Söll D. The heterotrimeric Thermus thermophilus Asp-tRNA(Asn) amidotransferase can also generate Gln-tRNA(Gln) FEBS Lett. 2000;476:140–144. doi: 10.1016/s0014-5793(00)01697-5. [DOI] [PubMed] [Google Scholar]
- 34.Raczniak G, Becker HD, Min B, Söll D. A single amidotransferase forms asparaginyl-tRNA and glutaminyl-tRNA in Chlamydia trachomatis. J. Biol. Chem. 2001;276:45862–45867. doi: 10.1074/jbc.M109494200. [DOI] [PubMed] [Google Scholar]
- 35.Salazar JC, Zuniga R, Raczniak G, Becker H, Söll D, Orellana O. A dual-specific Glu-tRNA(Gln) and Asp-tRNA(Asn) amidotransferase is involved in decoding glutamine and asparagine codons in Acidithiobacillus ferrooxidans. FEBS Lett. 2001;500:129–131. doi: 10.1016/s0014-5793(01)02600-x. [DOI] [PubMed] [Google Scholar]
- 36.Cathopoulis TJ, Chuawong P, Hendrickson TL. A thin-layer electrophoretic assay for Asp-tRNA(Asn)/Glu-tRNA(Gln) amidotransferase. Anal. Biochem. 2007;360:151–153. doi: 10.1016/j.ab.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker HD, Roy H, Moulinier L, Mazauric MH, Keith G, Kern D. Thermus thermophilus contains an eubacterial and an archaebacterial aspartyl-tRNA synthetase. Biochemistry. 2000;39:3216–3230. doi: 10.1021/bi992573y. [DOI] [PubMed] [Google Scholar]
- 38.Min B, Pelaschier JT, Graham DE, Tumbula-Hansen D, Söll D. Transfer RNA-dependent amino acid biosynthesis: an essential route to asparagine formation. Proc. Natl Acad. Sci. USA. 2002;99:2678–2683. doi: 10.1073/pnas.012027399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akochy PM, Bernard D, Roy PH, Lapointe J. Direct glutaminyl-tRNA biosynthesis and indirect asparaginyl-tRNA biosynthesis in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2004;186:767–776. doi: 10.1128/JB.186.3.767-776.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailly M, Giannouli S, Blaise M, Stathopoulos C, Kern D, Becker HD. A single tRNA base pair mediates bacterial tRNA-dependent biosynthesis of asparagine. Nucleic Acids Res. 2006;34:6083–6094. doi: 10.1093/nar/gkl622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skouloubris S, Ribas de Pouplana L, De Reuse H, Hendrickson TL. A noncognate aminoacyl-tRNA synthetase that may resolve a missing link in protein evolution. Proc. Natl Acad. Sci. USA. 2003;100:11297–11302. doi: 10.1073/pnas.1932482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salazar JC, Ahel I, Orellana O, Tumbula-Hansen D, Krieger R, Daniels L, Söll D. Coevolution of an aminoacyl-tRNA synthetase with its tRNA substrates. Proc. Natl Acad. Sci. USA. 2003;100:13863–13868. doi: 10.1073/pnas.1936123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chuawong P, Hendrickson TL. The nondiscriminating aspartyl-tRNA synthetase from Helicobacter pylori: anticodon-binding domain mutations that impact tRNA specificity and heterologous toxicity. Biochemistry. 2006;45:8079–8087. doi: 10.1021/bi060189c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oshikane H, Sheppard K, Fukai S, Nakamura Y, Ishitani R, Numata T, Sherrer RL, Feng L, Schmitt E, Panvert M, et al. Structural basis of RNA-dependent recruitment of glutamine to the genetic code. Science. 2006;312:1950–1954. doi: 10.1126/science.1128470. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura A, Yao M, Chimnaronk S, Sakai N, Tanaka I. Ammonia channel couples glutaminase with transamidase reactions in GatCAB. Science. 2006;312:1954–1958. doi: 10.1126/science.1127156. [DOI] [PubMed] [Google Scholar]
- 46.Namgoong S, Sheppard K, Sherrer RL, Söll D. Co-evolution of the archaeal tRNA-dependent amidotransferase GatCAB with tRNA(Asn) FEBS Lett. 2007;581:309–314. doi: 10.1016/j.febslet.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilcox M. Gamma-phosphoryl ester of Glu-tRNAGln as an intermediate in Bacillus subtilis glutaminyl-tRNA synthesis. Cold Spring Harb. Symp. Quant. Biol. 1969;34:521–528. doi: 10.1101/sqb.1969.034.01.059. [DOI] [PubMed] [Google Scholar]
- 48.Wilcox M. Gamma-glutamyl phosphate attached to glutamine-specific tRNA. A precursor of glutaminyl-tRNA in Bacillus subtilis. Eur. J. Biochem. 1969;11:405–412. doi: 10.1111/j.1432-1033.1969.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 49.Feng L, Sheppard K, Tumbula-Hansen D, Söll D. Gln-tRNAGln formation from Glu-tRNAGln requires cooperation of an asparaginase and a Glu-tRNAGln kinase. J. Biol. Chem. 2005;280:8150–8155. doi: 10.1074/jbc.M411098200. [DOI] [PubMed] [Google Scholar]
- 50.Schmitt E, Panvert M, Blanquet S, Mechulam Y. Structural basis for tRNA-dependent amidotransferase function. Structure. 2005;13:1421–1433. doi: 10.1016/j.str.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 51.Deniziak M, Sauter C, Becker HD, Paulus CA, Giege R, Kern D. Deinococcus glutaminyl-tRNA synthetase is a chimer between proteins from an ancient and the modern pathways of aminoacyl-tRNA formation. Nucleic Acids Res. 2007;35:1421–1431. doi: 10.1093/nar/gkl1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swain AL, Jaskolski M, Housset D, Rao JK, Wlodawer A. Crystal structure of Escherichia coli L-asparaginase,an enzyme used in cancer therapy. Proc. Natl Acad. Sci. USA. 1993;90:1474–1478. doi: 10.1073/pnas.90.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palm GJ, Lubkowski J, Derst C, Schleper S, Rohm KH, Wlodawer A. A covalently bound catalytic intermediate in Escherichia coli asparaginase: crystal structure of a Thr-89-Val mutant. FEBS Lett. 1996;390:211–216. doi: 10.1016/0014-5793(96)00660-6. [DOI] [PubMed] [Google Scholar]
- 54.Ortlund E, Lacount MW, Lewinski K, Lebioda L. Reactions of Pseudomonas 7A glutaminase-asparaginase with diazo analogues of glutamine and asparagine result in unexpected covalent inhibitions and suggests an unusual catalytic triad Thr-Tyr-Glu. Biochemistry. 2000;39:1199–1204. doi: 10.1021/bi991797d. [DOI] [PubMed] [Google Scholar]
- 55.Aghaiypour K, Wlodawer A, Lubkowski J. Structural basis for the activity and substrate specificity of Erwinia chrysanthemi L-asparaginase. Biochemistry. 2001;40:5655–5664. doi: 10.1021/bi0029595. [DOI] [PubMed] [Google Scholar]
- 56.Harpel MR, Horiuchi KY, Luo Y, Shen L, Jiang W, Nelson DJ, Rogers KC, Decicco CP, Copeland RA. Mutagenesis and mechanism-based inhibition of Streptococcus pyogenes Glu-tRNAGln amidotransferase implicate a serine-based glutaminase site. Biochemistry. 2002;41:6398–6407. doi: 10.1021/bi012126u. [DOI] [PubMed] [Google Scholar]
- 57.Shin S, Yun YS, Koo HM, Kim YS, Choi KY, Oh BH. Characterization of a novel Ser-cisSer-Lys catalytic triad in comparison with the classical Ser-His-Asp triad. J. Biol. Chem. 2003;278:24937–24943. doi: 10.1074/jbc.M302156200. [DOI] [PubMed] [Google Scholar]
- 58.Strauch MA, Zalkin H, Aronson AI. Characterization of the glutamyl-tRNA(Gln)-to-glutaminyl-tRNA(Gln) amidotransferase reaction of Bacillus subtilis. J. Bacteriol. 1988;170:916–920. doi: 10.1128/jb.170.2.916-920.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morais-Cabral JH, Zhou Y, MacKinnon R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- 60.Horiuchi KY, Harpel MR, Shen L, Luo Y, Rogers KC, Copeland RA. Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase. Biochemistry. 2001;40:6450–6457. doi: 10.1021/bi002599l. [DOI] [PubMed] [Google Scholar]
- 61.Srivastava DK, Bernhard SA. Metabolite transfer via enzyme-enzyme complexes. Science. 1986;234:1081–1086. doi: 10.1126/science.3775377. [DOI] [PubMed] [Google Scholar]
- 62.Huot JL, Balg C, Jahn D, Moser J, Emond A, Blais SP, Chenevert R, Lapointe J. Mechanism of a GatCAB amidotransferase: aspartyl-tRNA synthetase increases its affinity for Asp-tRNA(Asn) and novel aminoacyl-tRNA analogues are competitive inhibitors. Biochemistry. 2007;46:13190–13199. doi: 10.1021/bi700602n. [DOI] [PubMed] [Google Scholar]
- 63.Bailly M, Blaise M, Lorber B, Becker HD, Kern D. The transamidosome: a dynamic ribonucleoprotein particle dedicated to prokaryotic tRNA-dependent asparagine biosynthesis. Mol. Cell. 2007;28:228–239. doi: 10.1016/j.molcel.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 64.Nureki O, Vassylyev DG, Tateno M, Shimada A, Nakama T, Fukai S, Konno M, Hendrickson TL, Schimmel P, Yokoyama S. Enzyme structure with two catalytic sites for double-sieve selection of substrate. Science. 1998;280:578–582. doi: 10.1126/science.280.5363.578. [DOI] [PubMed] [Google Scholar]
- 65.Silvian LF, Wang J, Steitz TA. Insights into editing from an Ile-tRNA synthetase structure with tRNAIle and mupirocin. Science. 1999;285:1074–1077. [PubMed] [Google Scholar]
- 66.Lamour V, Quevillon S, Diriong S, N’Guyen VC, Lipinski M, Mirande M. Evolution of the Glx-tRNA synthetase family: the glutaminyl enzyme as a case of horizontal gene transfer. Proc. Natl Acad. Sci. USA. 1994;91:8670–8674. doi: 10.1073/pnas.91.18.8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woese CR, Olsen GJ, Ibba M, Söll D. Aminoacyl-tRNA synthetases,the genetic code,and the evolutionary process. Microbiol. Mol. Biol. Rev. 2000;64:202–236. doi: 10.1128/mmbr.64.1.202-236.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Donoghue P, Luthey-Schulten Z. On the evolution of structure in aminoacyl-tRNA synthetases. Microbiol. Mol. Biol. Rev. 2003;67:550–573. doi: 10.1128/MMBR.67.4.550-573.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Donoghue P, Luthey-Schulten Z. Evolutionary profiles derived from the QR factorization of multiple structural alignments gives an economy of information. J. Mol. Biol. 2005;346:875–894. doi: 10.1016/j.jmb.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 70.Forchhammer K. Glutamine signalling in bacteria. Front. Biosci. 2007;12:358–370. doi: 10.2741/2069. [DOI] [PubMed] [Google Scholar]
- 71.Sonenshein AL. Control of key metabolic intersections in Bacillus subtilis. Nature Rev. 2007;5:917–927. doi: 10.1038/nrmicro1772. [DOI] [PubMed] [Google Scholar]
- 72.Tempest DW, Meers JL, Brown CM. Influence of environment on the content and composition of microbial free amino acid pools. J. Gen. Microbiol. 1970;64:171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]
- 73.White O, Eisen JA, Heidelberg JF, Hickey EK, Peterson JD, Dodson RJ, Haft DH, Gwinn ML, Nelson WC, Richardson DL, et al. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henne A, Bruggemann H, Raasch C, Wiezer A, Hartsch T, Liesegang H, Johann A, Lienard T, Gohl O, Martinez-Arias R, et al. The genome sequence of the extreme thermophile Thermus thermophilus. Nat. Biotech. 2004;22:547–553. doi: 10.1038/nbt956. [DOI] [PubMed] [Google Scholar]
- 75.Li T, Graham DE, Stathopoulos C, Haney PJ, Kim HS, Vothknecht U, Kitabatake M, Hong KW, Eggertsson G, Curnow AW, et al. Cysteinyl-tRNA formation: the last puzzle of aminoacyl-tRNA synthesis. FEBS Lett. 1999;462:302–306. doi: 10.1016/s0014-5793(99)01550-1. [DOI] [PubMed] [Google Scholar]
- 76.Stathopoulos C, Kim W, Li T, Anderson I, Deutsch B, Palioura S, Whitman W, Söll D. Cysteinyl-tRNA synthetase is not essential for viability of the archaeon Methanococcus maripaludis. Proc. Natl Acad. Sci. USA. 2001;98:14292–14297. doi: 10.1073/pnas.201540498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Donoghue P, Sethi A, Woese CR, Luthey-Schulten ZA. The evolutionary history of Cys-tRNACys formation. Proc. Natl Acad. Sci. USA. 2005;102:19003–19008. doi: 10.1073/pnas.0509617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.White RH. The biosynthesis of cysteine and homocysteine in Methanococcus jannaschii. Biochim. Biophys. Acta. 2003;1624:46–53. doi: 10.1016/j.bbagen.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 79.Helgadottir S, Rosas-Sandoval G, Söll D, Graham DE. Biosynthesis of phosphoserine in the Methanococcales. J. Bacteriol. 2007;189:575–582. doi: 10.1128/JB.01269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klenk HP, Clayton RA, Tomb JF, White O, Nelson KE, Ketchum KA, Dodson RJ, Gwinn M, Hickey EK, Peterson JD, et al. The complete genome sequence of the hyperthermophilic,sulphate-reducing archaeon Archaeoglobus fulgidus. Nature. 1997;390:364–370. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 81.Samuel BS, Hansen EE, Manchester JK, Coutinho PM, Henrissat B, Fulton R, Latreille P, Kim K, Wilson RK, Gordon JI. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc. Natl Acad. Sci. USA. 2007;104:10643–10648. doi: 10.1073/pnas.0704189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fricke WF, Seedorf H, Henne A, Kruer M, Liesegang H, Hedderich R, Gottschalk G, Thauer RK. The genome sequence of Methanosphaera stadtmanae reveals why this human intestinal archaeon is restricted to methanol and H2 for methane formation and ATP synthesis. J. Bacteriol. 2006;188:642–658. doi: 10.1128/JB.188.2.642-658.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kavran JM, Gundllapalli S, O’Donoghue P, Englert M, Söll D, Steitz TA. Structure of pyrrolysyl-tRNA synthetase,an archaeal enzyme for genetic code innovation. Proc. Natl Acad. Sci. USA. 2007;104:11268–11273. doi: 10.1073/pnas.0704769104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamtekar S, Hohn MJ, Park HS, Schnitzbauer M, Sauerwald A, Söll D, Steitz TA. Toward understanding phosphoseryl-tRNACys formation: the crystal structure of Methanococcus maripaludis phosphoseryl-tRNA synthetase. Proc. Natl Acad. Sci. USA. 2007;104:2620–2625. doi: 10.1073/pnas.0611504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fukunaga R, Yokoyama S. Structural insights into the first step of RNA-dependent cysteine biosynthesis in Archaea. Nat. Struct. Mol. Biol. 2007;14:272–279. doi: 10.1038/nsmb1219. [DOI] [PubMed] [Google Scholar]
- 86.Hohn MJ, Park HS, O’Donoghue P, Schnitzbauer M, Söll D. Emergence of the universal genetic code imprinted in an RNA record. Proc. Natl Acad. Sci. USA. 2006;103:18095–18100. doi: 10.1073/pnas.0608762103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sprinzl M, Cramer F. Site of aminoacylation of tRNAs from Escherichia coli with respect to the 2′- or 3′-hydroxyl group of the terminal adenosine. Proc. Natl Acad. Sci. USA. 1975;72:3049–3053. doi: 10.1073/pnas.72.8.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukunaga R, Yokoyama S. Structural insights into the second step of RNA-dependent cysteine biosynthesis in Archaea: crystal structure of Sep-tRNA:Cys-tRNA synthase from Archaeoglobus fulgidus. J. Mol. Biol. 2007;370:128–141. doi: 10.1016/j.jmb.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 89.Eliot AC, Kirsch JF. Pyridoxal phosphate enzymes: mechanistic,structural,and evolutionary considerations. Annu. Rev. Biochem. 2004;73:383–415. doi: 10.1146/annurev.biochem.73.011303.074021. [DOI] [PubMed] [Google Scholar]
- 90.Schneider G, Kack H, Lindqvist Y. The manifold of vitamin B6 dependent enzymes. Structure. 2000;8:R1–6. doi: 10.1016/s0969-2126(00)00085-x. [DOI] [PubMed] [Google Scholar]
- 91.Zhou D, White RH. Transsulfuration in archaebacteria. J. Bacteriol. 1991;173:3250–3251. doi: 10.1128/jb.173.10.3250-3251.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kitabatake M, So MW, Tumbula DL, Söll D. Cysteine biosynthesis pathway in the archaeon Methanosarcina barkeri encoded by acquired bacterial genes? J. Bacteriol. 2000;182:143–145. doi: 10.1128/jb.182.1.143-145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Borup B, Ferry JG. Cysteine biosynthesis in the Archaea: Methanosarcina thermophila utilizes O-acetylserine sulfhydrylase. FEMS Microbiol. Lett. 2000;189:205–210. doi: 10.1111/j.1574-6968.2000.tb09231.x. [DOI] [PubMed] [Google Scholar]
- 94.Borup B, Ferry JG. O-Acetylserine sulfhydrylase from Methanosarcina thermophila. J. Bacteriol. 2000;182:45–50. doi: 10.1128/jb.182.1.45-50.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mino K, Ishikawa K. Characterization of a novel thermostable O-acetylserine sulfhydrylase from Aeropyrum pernix K1. J. Bacteriol. 2003;185:2277–2284. doi: 10.1128/JB.185.7.2277-2284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mino K, Ishikawa K. A novel O-phospho-L-serine sulfhydrylation reaction catalyzed by O-acetylserine sulfhydrylase from Aeropyrum pernix K1. FEBS Lett. 2003;551:133–138. doi: 10.1016/s0014-5793(03)00913-x. [DOI] [PubMed] [Google Scholar]
- 97.Mihara H, Esaki N. Bacterial cysteine desulfurases: their function and mechanisms. Appl. Microbiol. Biotechnol. 2002;60:12–23. doi: 10.1007/s00253-002-1107-4. [DOI] [PubMed] [Google Scholar]
- 98.Major TA, Burd H, Whitman WB. Abundance of 4Fe-4S motifs in the genomes of methanogens and other prokaryotes. FEMS Microbiol. Lett. 2004;239:117–123. doi: 10.1016/j.femsle.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 99.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 100.Moghadaszadeh B, Beggs AH. Selenoproteins and their impact on human health through diverse physiological pathways. Physiology. 2006;21:307–315. doi: 10.1152/physiol.00021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hatfield DL, Carlson BA, Xu XM, Mix H, Gladyshev VN. Selenocysteine incorporation machinery and the role of selenoproteins in development and health. Prog. Nucleic Acid Res. Mol. Biol. 2006;81:97–142. doi: 10.1016/S0079-6603(06)81003-2. [DOI] [PubMed] [Google Scholar]
- 102.Huber RE, Criddle RS. Comparison of the chemical properties of selenocysteine and selenocystine with their sulfur analogs. Arch. Biochem. Biophys. 1967;122:164–173. doi: 10.1016/0003-9861(67)90136-1. [DOI] [PubMed] [Google Scholar]
- 103.Shrift A, Bechard D, Harcup C. Utilization of Selenocysteine by a cysteinyl-tRNA synthetase from Phaseolus aureus. Plant Physiol. 1976;58:248–252. doi: 10.1104/pp.58.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Young PA, Kaiser II. Aminoacylation of Escherichia coli cysteine tRNA by selenocysteine. Arch Biochem. Biophys. 1975;171:483–489. doi: 10.1016/0003-9861(75)90057-0. [DOI] [PubMed] [Google Scholar]
- 105.Müller S, Senn H, Gsell B, Vetter W, Baron C, Böck A. The formation of diselenide bridges in proteins by incorporation of selenocysteine residues: biosynthesis and characterization of (Se)2-thioredoxin. Biochemistry. 1994;33:3404–3412. doi: 10.1021/bi00177a034. [DOI] [PubMed] [Google Scholar]
- 106.Johansson L, Gafvelin G, Arner ES. Selenocysteine in proteins-properties and biotechnological use. Biochim. Biophys. Acta. 2005;1726:1–13. doi: 10.1016/j.bbagen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 107.Böck A, Forchhammer K, Heider J, Leinfelder W, Sawers G, Veprek B, Zinoni F. Selenocysteine: the 21st amino acid. Mol. Microbiol. 1991;5:515–520. doi: 10.1111/j.1365-2958.1991.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 108.Gladyshev VN, Hatfield DL. Selenocysteine-containing proteins in mammals. J. Biomed. Sci. 1999;6:151–160. doi: 10.1007/BF02255899. [DOI] [PubMed] [Google Scholar]
- 109.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 110.Kryukov GV, Gladyshev VN. The prokaryotic selenoproteome. EMBO Rep. 2004;5:538–543. doi: 10.1038/sj.embor.7400126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bilokapic S, Korencic D, Söll D, Weygand-Durasevic I. The unusual methanogenic seryl-tRNA synthetase recognizes tRNASer species from all three kingdoms of life. Eur. J. Biochem. 2004;271:694–702. doi: 10.1111/j.1432-1033.2003.03971.x. [DOI] [PubMed] [Google Scholar]
- 112.Mizutani T, Narihara T, Hashimoto A. Purification and properties of bovine liver seryl-tRNA synthetase. Eur. J. Biochem. 1984;143:9–13. doi: 10.1111/j.1432-1033.1984.tb08331.x. [DOI] [PubMed] [Google Scholar]
- 113.Leinfelder W, Zehelein E, Mandrand-Berthelot MA, Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988;331:723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- 114.Carlson BA, Xu XM, Kryukov GV, Rao M, Berry MJ, Gladyshev VN, Hatfield DL. Identification and characterization of phosphoseryl-tRNA[Ser]Sec kinase. Proc. Natl Acad. Sci. USA. 2004;101:12848–12853. doi: 10.1073/pnas.0402636101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leinfelder W, Forchhammer K, Veprek B, Zehelein E, Böck A. In vitro synthesis of selenocysteinyl-tRNA(UCA) from seryl-tRNA(UCA): involvement and characterization of the selD gene product. Proc. Natl Acad. Sci. USA. 1990;87:543–547. doi: 10.1073/pnas.87.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leinfelder W, Forchhammer K, Zinoni F, Sawers G, Mandrand-Berthelot MA, Böck A. Escherichia coli genes whose products are involved in selenium metabolism. J. Bacteriol. 1988;170:540–546. doi: 10.1128/jb.170.2.540-546.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu XM, Carlson BA, Irons R, Mix H, Zhong N, Gladyshev VN, Hatfield DL. Selenophosphate synthetase 2 is essential for selenoprotein biosynthesis. Biochem. J. 2007;404:115–120. doi: 10.1042/BJ20070165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sherrer RL, O’Donoghue P, Söll D. Characterization and evolutionary history of an archaeal kinase involved in selenocysteinyl-tRNA formation. Nucleic Acids Res. 2008 doi: 10.1093/nar/gkm1134. doi:10.1093/nar/gkm1134 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mäenpää PH, Bernfield MR. A specific hepatic transfer RNA for phosphoserine. Proc. Natl Acad. Sci. USA. 1970;67:688–695. doi: 10.1073/pnas.67.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sharp SJ, Stewart TS. The characterization of phosphoseryl tRNA from lactating bovine mammary gland. Nucleic Acids Res. 1977;4:2123–2136. doi: 10.1093/nar/4.7.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mizutani T, Hashimoto A. Purification and properties of suppressor seryl-tRNA: ATP phosphotransferase from bovine liver. FEBS Lett. 1984;169:319–322. doi: 10.1016/0014-5793(84)80342-7. [DOI] [PubMed] [Google Scholar]
- 122.Kaiser JT, Gromadski K, Rother M, Engelhardt H, Rodnina MV, Wahl MC. Structural and functional investigation of a putative archaeal selenocysteine synthase. Biochemistry. 2005;44:13315–13327. doi: 10.1021/bi051110r. [DOI] [PubMed] [Google Scholar]
- 123.Leipe DD, Koonin EV, Aravind L. Evolution and classification of P-loop kinases and related proteins. J. Mol. Biol. 2003;333:781–815. doi: 10.1016/j.jmb.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 124.Novogrodsky A, Tal M, Traub A, Hurwitz J. The enzymatic phosphorylation of ribonucleic acid and deoxyribonucleic acid. II. Further properties of the 5′-hydroxyl polynucleotide kinase. J. Biol. Chem. 1966;241:2933–2943. [PubMed] [Google Scholar]
- 125.Hatfield D, Lee BJ, Hampton L, Diamond AM. Selenium induces changes in the selenocysteine tRNA[Ser]Sec population in mammalian cells. Nucleic Acids Res. 1991;19:939–943. doi: 10.1093/nar/19.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dong H, Nilsson L, Kurland CG. Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J. Mol. Biol. 1996;260:649–663. doi: 10.1006/jmbi.1996.0428. [DOI] [PubMed] [Google Scholar]
- 127.Sturchler C, Westhof E, Carbon P, Krol A. Unique secondary and tertiary structural features of the eucaryotic selenocysteine tRNA(Sec) Nucleic Acids Res. 1993;21:1073–1079. doi: 10.1093/nar/21.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schön A, Böck A, Ott G, Sprinzl M, Söll D. The selenocysteine-inserting opal suppressor serine tRNA from E. coli is highly unusual in structure and modification. Nucleic Acids Res. 1989;17:7159–7165. doi: 10.1093/nar/17.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baron C, Westhof E, Böck A, Giege R. Solution structure of selenocysteine-inserting tRNASec from Escherichia coli. J. Mol. Biol. 1993;231:274–292. doi: 10.1006/jmbi.1993.1282. [DOI] [PubMed] [Google Scholar]
- 130.Hubert N, Sturchler C, Westhof E, Carbon P, Krol A. The 9/4 secondary structure of eukaryotic selenocysteine tRNA: more pieces of evidence. RNA. 1998;4:1029–1033. doi: 10.1017/s1355838298980888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ioudovitch A, Steinberg SV. Structural compenstation in an archaeal selenocysteine transfer RNA. J. Mol. Biol. 1999;290:365–371. doi: 10.1006/jmbi.1999.2901. [DOI] [PubMed] [Google Scholar]
- 132.Wu XQ, Gross HJ. The long extra arms of human tRNA((Ser)Sec) and tRNA(Ser) function as major identify elements for serylation in an orientation-dependent,but not sequence-specific manner. Nucleic Acids Res. 1993;21:5589–5594. doi: 10.1093/nar/21.24.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu XQ, Gross HJ. The length and the secondary structure of the D-stem of human selenocysteine tRNA are the major identity determinants for serine phosphorylation. EMBO J. 1994;13:241–248. doi: 10.1002/j.1460-2075.1994.tb06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sherrer RL, Ho J, Söll D. Divergence of selenocysteine tRNA recognition by archaeal and eukaryotic O-phosphoseryl-tRNASec kinase. Nucleic Acids Res. 2008 doi: 10.1093/nar/gkn036. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baron C, Sturchler C, Wu XQ, Gross HJ, Krol A, Böck A. Eukaryotic selenocysteine inserting tRNA species support selenoprotein synthesis in Escherichia coli. Nucleic Acids Res. 1994;22:2228–2233. doi: 10.1093/nar/22.12.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Araiso Y, Palioura S, Ishitani R, Sherrer RL, O’Donoghue P, Yuan J, Oshikane H, Domae N, DeFranco J, Söll D, et al. Structural insights into RNA-dependent eukaryal and archaeal selenocysteine formation. Nucleic Acids Res. 2007 doi: 10.1093/nar/gkm1122. doi: 10.1093/nar/gkml1122 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ganichkin OM, Xu XM, Carlson BA, Mix H, Hatfield DL, Gladyshev VN, Wahl MC. Structure and catalytic mechanism of eukaryotic selenocysteine synthase. J. Biol. Chem. 2007 doi: 10.1074/jbc.M709342200. doi:10.1074/jbc/M709342200 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 138.Zhang Y, Romero H, Salinas G, Gladyshev VN. Dynamic evolution of selenocysteine utilization in bacteria: a balance between selenoprotein loss and evolution of selenocysteine from redox active cysteine residues. Genome Biol. 2006;7:R94. doi: 10.1186/gb-2006-7-10-r94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stanzel M, Schön A, Sprinzl M. Discrimination against misacylated tRNA by chloroplast elongation factor Tu. Eur. J. Biochem. 1994;219:435–439. doi: 10.1111/j.1432-1033.1994.tb19956.x. [DOI] [PubMed] [Google Scholar]
- 140.Rother M, Wilting R, Commans S, Böck A. Identification and characterisation of the selenocysteine-specific translation factor SelB from the archaeon Methanococcus jannaschii. J. Mol. Biol. 2000;299:351–358. doi: 10.1006/jmbi.2000.3756. [DOI] [PubMed] [Google Scholar]
- 141.LaRiviere FJ, Wolfson AD, Uhlenbeck OC. Uniform binding of aminoacyl-tRNAs to elongation factor Tu by thermodynamic compensation. Science. 2001;294:165–168. doi: 10.1126/science.1064242. [DOI] [PubMed] [Google Scholar]
- 142.Asahara H, Uhlenbeck OC. The tRNA specificity of Thermus thermophilus EF-Tu. Proc. Natl Acad. Sci. USA. 2002;99:3499–3504. doi: 10.1073/pnas.052028599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roy H, Becker HD, Mazauric MH, Kern D. Structural elements defining elongation factor Tu mediated suppression of codon ambiguity. Nucleic Acids Res. 2007;35:3420–3430. doi: 10.1093/nar/gkm211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Min B, Kitabatake M, Polycarpo C, Pelaschier J, Raczniak G, Ruan B, Kobayashi H, Namgoong S, Söll D. Protein synthesis in Escherichia coli with mischarged tRNA. J. Bacteriol. 2003;185:3524–3526. doi: 10.1128/JB.185.12.3524-3526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Di Giulio M. The origin of the genetic code: theories and their relationships,a review. BioSystems. 2005;80:175–184. doi: 10.1016/j.biosystems.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 146.Ibba M, Curnow AW, Söll D. Aminoacyl-tRNA synthesis: divergent routes to a common goal. Trends Biochem. Sci. 1997;22:39–42. doi: 10.1016/s0968-0004(96)20033-7. [DOI] [PubMed] [Google Scholar]
- 147.Polycarpo C, Sheppard K, Randau L, Ambrogelly A, Cardoso AM, Fukai S, Herring S, Hohn M, Nakamura Y, Oshikane H, et al. Features of aminoacyl-tRNA synthesis unique to Archaea. In: Cavicchioli R, editor. Archaea: Molecular and Cellular Biology. Washington, DC, USA: ASM Press; 2007. pp. 198–208. [Google Scholar]