Abstract
Selenocysteine (Sec) biosynthesis in archaea and eukaryotes requires three steps: serylation of tRNASec by seryl-tRNA synthetase (SerRS), phosphorylation of Ser-tRNASec by O-phosphoseryl-tRNASec kinase (PSTK), and conversion of O-phosphoseryl-tRNASec (Sep-tRNASec) by Sep-tRNA:Sec-tRNA synthase (SepSecS) to Sec-tRNASec. Although SerRS recognizes both tRNASec and tRNASer species, PSTK must discriminate Ser-tRNASec from Ser-tRNASer. Based on a comparison of the sequences and secondary structures of archaeal tRNASec and tRNASer, we introduced mutations into Methanococcus maripaludis tRNASec to investigate how Methanocaldococcus jannaschii PSTK distinguishes tRNASec from tRNASer. Unlike eukaryotic PSTK, the archaeal enzyme was found to recognize the acceptor stem rather than the length and secondary structure of the D-stem. While the D-arm and T-loop provide minor identity elements, the acceptor stem base pairs G2-C71 and C3-G70 in tRNASec were crucial for discrimination from tRNASer. Furthermore, the A5-U68 base pair in tRNASer has some antideterminant properties for PSTK. Transplantation of these identity elements into the tRNASerUGA scaffold resulted in phosphorylation of the chimeric Ser-tRNA. The chimera was able to stimulate the ATPase activity of PSTK albeit at a lower level than tRNASec, whereas tRNASer did not. Additionally, the seryl moiety of Ser-tRNASec is not required for enzyme recognition, as PSTK efficiently phosphorylated Thr-tRNASec.
INTRODUCTION
While UGA is typically a stop codon, selenocysteine is co-translationally inserted into proteins in response to in-frame Sec-decoding UGA codons in a limited number of organisms from all three domains of life (1). Sec is formed by a tRNA-dependent transformation of Ser to Sec. In Sec-decoding organisms, tRNASec is first aminoacylated with Ser by SerRS (2–4). Bacteria convert Ser to Sec in one step using selenocysteine synthase (SelA) in the presence of the selenium donor selenophosphate (1). Eukaryotes and archaea perform the Ser to Sec conversion using two enzymes: PSTK phosphorylates the serine moiety of Ser-tRNASec to Sep-tRNASec (5–8), and SepSecS catalyzes the Sep-tRNASec to Sec-tRNASec conversion (9,10).
About four decades ago the presence of a Sep-tRNA was discovered in rooster (11) and rat liver (12) and later in other eukaryotes and archaea (5,13–15). Also established early (14) was the Sep-tRNA synthesis requirement for two enzymes, SerRS and a ‘phosphotransferase activity’. The latter enzyme was purified from bovine liver (7), the human counterpart was characterized with regard to tRNA recognition (8) and finally the protein responsible was identified (5) as PSTK. Detailed characterizations of PSTK activity were subsequently performed on the mouse (5) and Methanocaldococcus jannaschii enzyme (15). PSTK transfers the γ-phosphate from ATP to Ser-tRNASec yielding Sep-tRNASec and ADP (5,15). tRNASec binds to PSTK with high affinity and specifically induces its ATPase activity (15). Although SerRS must be able to recognize and aminoacylate both tRNASer and tRNASec, proper interpretation of the genetic code requires that PSTK differentiate Ser-tRNASec from Ser-tRNASer. tRNASec species from all three domains of life are unusual in both length (>90 nt) and structure. While most tRNAs including tRNASer are in a 7/5 cloverleaf form (i.e. 7 bp in the acceptor stem and 5 in the TΨC arm), bacterial tRNASec is in an 8/5 form (16), while eukaryal tRNASec (17,18) and archaeal tRNASec (17,19) likely exist in a 9/4 clover leaf form. Besides the difference in acceptor stem length at 9 bp in archaeal and eukaryotic tRNASec versus 7 bp in tRNASer, several other features of tRNASec are significantly different from tRNASer. Eukaryotic and archaeal tRNASec species have 6 or 7 bp D-stems, respectively (17–19); in contrast tRNASer has a 3 to 4 bp D-stem. Molecular modeling suggested that a 7 bp D-stem in archaeal tRNASec would compensate for the short 4 bp T-stem (5 bp in tRNASer) thus allowing the normal interaction between the D- and T-loops (19). The sequence, length and orientation of the variable arm of tRNASec also vary from those of tRNASer.
Previous work with HeLa cell extracts (8) demonstrated that the length and secondary structure of the D-stem of human tRNASec are the major determinants for serine phosphorylation by a kinase activity (in the following called ‘human PSTK’). Here we present the basis of tRNASec discrimination from tRNASer by an archaeal PSTK from M. jannaschii. The G2-C71 and C3-G70 base pairs within the acceptor stem of Methanococcus maripaludis tRNASec are the major identity elements for tRNA-dependent serine phosphorylation by archaeal PSTK.
MATERIALS AND METHODS
Materials and reagents
All oligonucleotide synthesis and DNA sequencing were carried out by the Keck Foundation Biotechnology Research Laboratory at Yale University. L-[U-14C]Ser (163 mCi/mmol) was from Amersham Biosciences and [α-32P]-ATP (3000 Ci/mmol) was from GE Healthcare. L-[1-14C]Thr (50–60 mCi/mmol) was from American Radiolabeled Chemicals.
Expression and purification of enzymes
Since heterologous overexpression of the M. maripaludis PSTK gene in Escherichia coli was not successful, M. jannaschii PSTK-His6 (MJ1538) in pET20b (Novagen) was overproduced and purified as described (15). M. maripaludis SerRS was overproduced and purified as described (2).
Cloning, purification, transcription and 32P-labeling of tRNAs
All tRNAs were cloned into pUC19, expressed in E. coli DH5α, transcribed by T7 RNA polymerase, gel purified and folded as described previously (15). Refolded transcript was 32P-labeled on the 3' terminus using the E. coli CCA-adding enzyme and [α-32P]ATP as before (15). After phenol/chloroform extraction the reaction was passed over a Sephadex G25 Microspin column (Amersham Biosciences) to remove excess ATP.
Serylation and phosphorylation of tRNASec
These assays were carried out in 1× PSTK buffer [50 mM HEPES-KOH (pH 7.5), 10 mM MgCl2, 20 mM KCl, 1 mM DTT] with 1 mM L-Ser (Sigma), 5 mM ATP, 600 nM M. maripaludis SerRS, 50 nM M. jannaschii PSTK and 1 µM 32P-labeled transcript for 45 min at 37°C. Aliquots (2 µl) of each reaction were quenched on ice with 3 µl of 100 mM sodium citrate (pH 5.0) and 0.66 mg/ml nuclease P1 (Sigma) and incubated at room temperature for 35 min (20,21). Assays that included 100 µM [14C]-Ser were carried out in 1× PSTK buffer with 1 µM tRNASec transcript, 5 mM ATP, 1.2 µM SerRS and 200 nM PSTK for 45 min at 37°C. The reactions were stopped by phenol/chloroform extraction and purified over a G25 column to remove unincorporated [14C]-Ser, and aliquots (2 µl) of each reaction were quenched and digested as above. To separate Sep-AMP, Ser-AMP and AMP, 1 µl of quenched, digested sample was spotted on glass polyethyleneimine (PEI) cellulose 20 cm × 20 cm thin layer chromatography (TLC) plates (EMD) and developed for 75 min in 100 mM ammonium acetate and 5% acetic acid. The plates were exposed on an imaging plate (FujiFilms), scanned on a Molecular Dynamics Storm 860 PhosphorImager, and quantified using ImageQuant software.
Preparation of seryl-tRNA
tRNASec, chimera and tRNASer 32P-labeled transcripts were each aminoacylated in 1× PSTK buffer with 1 mM L-Ser (Sigma), 5 mM ATP, 3 µM M. maripaludis SerRS and 5 µM 32P-labeled transcript as described previously (15). Reactions were incubated at 37°C for 1 h followed by phenol/chloroform extraction, ethanol precipitation and resuspension in water. The samples were passed over Sephadex G25 Microspin columns (Amersham Biosciences) equilibrated with water. To check aminoacylation levels, 2 µl aliquots were removed at the end of the reactions, quenched on ice with 3 µl of 100 mM sodium citrate (pH 5.0) and 0.66 mg/ml nuclease P1 (Sigma), and analyzed as described above.
Phosphorylation of seryl-tRNA
These assays were carried out in 1× PSTK buffer with 1 µM 32P-labeled Ser-tRNA transcript, 5 mM ATP and 50 nM PSTK at 37°C. Reaction mixes were preincubated at 37°C and started by addition of enzyme. At each time point, 2 µl aliquots were taken and treated as described above.
ATPase activity measurement
ATPase activity was determined by measuring the amount of [α-32P]ATP converted to [α-32P]ADP as described before with modifications (15). These assays were carried out in a 12 µl reaction volume including 1× PSTK buffer with 130 nM cold ATP, 100 nM [α-32P]ATP and 1 µM enzyme at 37°C for 30 min. Unless noted otherwise, 1 µM unlabeled tRNA (tRNASec, G2-C71:C-G tRNASec, chimera tRNA or tRNASer) was included. At six time points, 0.75 µl aliquots were taken from each reaction and quenched by the addition of 9.25 µl ice-cold 55 mM EDTA. One microliter of each reaction mixture was spotted on PEI cellulose TLC plates (EMD) and developed in 1 M LiCl for 60 to 75 min. After separation, the [α-32P]ATP and [α-32P]ADP spots were quantified by PhosphorImager using ImageQuant software.
Secondary structure alignment of archaeal tRNAs
Archaeal tRNASec and tRNASer sequences were acquired from The Institute for Genomic Research, USCS Archaeal Genome Browser (22) and from the Microbial Genomes at the Joint Genome Institute. The secondary structures were determined by Aragorn 1.1 (23) and aligned manually by secondary structure.
Phosphorylation of threonyl-tRNASec
These assays were carried out in 1× PSTK buffer with 5 mM ATP, 2.5 mM threonine (Thr) (Fluka, 99.5% purity), 1 µM 32P-labeled tRNASec transcript, 1.2 µM SerRS and with or without 200 nM PSTK for 45 min at 37°C. The reactions were quenched, digested and analyzed by TLC as stated above.
RESULTS
A survey of tRNA identity elements of archaeal PSTK
We decided to undertake a preliminary survey for potential tRNA identity elements for phosphorylation by M. jannaschii PSTK. Our assay conditions (see Materials and Methods section and Table 1) guarantee achievement of plateau levels of Ser-tRNASec formation, while phosphorylation may or may not reach plateau values for all the different mutants. Such an approach will reveal major elements, but will miss some less important ones. Previous work established that Ser-tRNASec but not Ser-tRNASer is a substrate for phosphorylation by PSTK (7,8,12–15). Thus, the identity elements for phosphorylation must lie within tRNASec (Figure 1A) and be absent from tRNASer (Figure 1B), while any antideterminants would be found only in tRNASer. Based on a comparison of the sequences and secondary structures of archaeal tRNASec and tRNASer, we designed transplantation mutants of M. maripaludis tRNASec by replacing the acceptor stem + T-arm, D-arm, anticodon, anticodon stem–loop, variable arm, T-arm or T-loop of M. maripaludis tRNASec with those of M. maripaludis tRNASerUGA. Additionally, we made point mutations to tRNASec to localize the nucleotides recognized by PSTK. While the mutations did not overlap with previously identified identity elements for the archaeal Methanosarcina barkeri SerRS (the long variable arm, G1-C72, the discriminator base G73 and the anticodon stem base-pair G30-C40) (24) some of the mutants did not serylate efficiently (Table 1). Generally, tRNASec mutants that did not serylate efficiently would deacylate during purification of Ser-tRNA, leaving little charged tRNA to test for phosphorylation by PSTK. To circumvent this issue we tested the mutants in an activity assay similar to what was described previously (15), in which tRNA was serylated with SerRS and phosphorylated by PSTK in the same reaction. Thus, mutants of tRNASec that serylated poorly were phosphorylated by PSTK without a purification of the Ser-tRNASec prior to phosphorylation by PSTK. A similar approach for tRNA mutants that serylated inefficiently was implemented in a study of human PSTK in partially purified HeLa cell extracts (8). In our assay, after serylation by purified M. maripaludis SerRS and phosphorylation by purified M. jannaschii PSTK, the tRNASec mutants were digested with nuclease P1, and the Sep-[32P]AMP, [32P]AMP and Ser-[32P]AMP products were separated by TLC. The amounts of aminoacylated (serylated plus phosphorylated) and phosphorylated products were determined by PhosphorImager analysis (Table 1). Additionally, a filter-binding assay (15) was used to determine the affinity of PSTK for each tRNA mutant (Supplementary Table 1). We should note that in this study we used the transcript of the M. maripaludis tRNASec for our identity studies; SerRS serylated both M. jannaschii tRNASec and M. maripaludis tRNASec similarly (Table 1), but the M. jannaschii Ser-tRNASec deacylated during purification. Additionally, PSTK from M. jannaschii was used because we were able to obtain sufficient quantities of purified and active enzyme.
Table 1.
Phosphorylation of tRNASec mutants by M. jannaschii PSTK
| tRNAa | Base exchange or domain transplantation | Aminoacylationb (%) | Phosphorylationc (%) | Relative efficiencyd (%) |
|---|---|---|---|---|
| M. maripaludis tRNASec | Wild-type tRNASec | 80.3 ± 3.8 | 75.6 ± 3.8 | 100 |
| M. maripaludis tRNASer | Wild-type tRNASerUGA | 71.6 ± 8.1 | NDf | – |
| Acceptor stem | Acceptor stem + T arme | 20.1 ± 1.4 | 0.4 ± 0.1 | 2.1 |
| Δ 5a-67b (G-U) | 90.6 ± 0.3 | 87.0 ± 0.7 | 102.0 | |
| Δ 5b-67a (G-C) | 89.7 ± 0.9 | 85.8 ± 1.0 | 101.6 | |
| Δ 5a-67b and 5b-67a | 8.9 ± 0.4 | 4.6 ± 0.4 | 54.9 | |
| Δ G2-C71 | 73.4 ± 10.4 | 0.9 ± 0.3 | 1.3 | |
| G2-C71 →C-G | 63.4 ± 6.0 | 2.2 ± 0.3 | 3.7 | |
| →G-U | 75.6 ± 0.5 | 19.6 ± 1.4 | 27.5 | |
| →A-U | 47.2 ± 0.9 | 5.7 ± 1.6 | 12.8 | |
| →U-A | 35.3 ± 0.6 | 13.8 ± 2.7 | 41.5 | |
| C3-G70 →G-C | 64.7 ± 1.4 | 7.6 ± 1.1 | 12.5 | |
| →A-U | 58.3 ± 1.5 | 41.7 ± 1.7 | 75.9 | |
| →U-A | 70.6 ± 1.6 | 66.5 ± 1.5 | 100.0 | |
| →U-G | 66.1 ± 3.8 | 57.9 ± 4.6 | 93.0 | |
| C5-G68 →G-C | 41.6 ± 0.8 | 36.7 ± 0.9 | 93.7 | |
| →A-U | 65.6 ± 1.3 | 28.1 ± 0.7 | 45.5 | |
| →U-A | 50.6 ± 1.5 | 43.8 ± 1.3 | 91.9 | |
| →U-G | 61.3 ± 0.6 | 49.2 ± 4.7 | 85.3 | |
| D-arm | D-arme | 1.4 ± 0.4 | NDf | – |
| U16→A | 25.1 ± 0.14 | 18.4 ± 0.1 | 77.9 | |
| U16→G | 48.7 ± 7.9 | 32.0 ± 6.0 | 69.8 | |
| C15→G | 79.7 ± 1.0 | 70.9 ± 3.9 | 94.5 | |
| U16→A/C15→G | 75.8 ± 2.1 | 57.6 ± 1.5 | 80.7 | |
| A20a→G | 56.6 ± 2.2 | 50.2 ± 1.3 | 94.2 | |
| A20a→U | 71.3 ± 10.1 | 43.9 ± 2.1 | 65.4 | |
| A20a→U/U16→A | 57.8 ± 1.2 | 55.7 ± 1.1 | 102.3 | |
| A20a→C | 69.9 ± 2.7 | 63.6 ± 2.2 | 96.6 | |
| Anticodon stem | Anticodon stem-loope | 46.5 ± 0.2 | 40.3 ± 1.2 | 92.1 |
| Anticodon | UCA→UGAe | 84.9 ± 1.0 | 80.1 ± 0.4 | 100.2 |
| Variable arm | Variable arme | 52.8 ± 2.6 | 50.2 ± 2.5 | 101.0 |
| T-arm | T-arme | 37.9 ± 0.8 | 24.6 ± 1.9 | 68.9 |
| G50-C64 →C-G/G51-C63 →C-G | 52.6 ± 0.2 | 40.3 ± 1.2 | 81.4 | |
| T-loope | 44.9 ± 0.9 | 24.9 ± 6.0 | 58.9 | |
| G57→A | 74.2 ± 1.5 | 71.7 ± 0.9 | 102.6 | |
| U59→A | 70.2 ± 1.6 | 58.4 ± 4.3 | 88.4 | |
| M. jannaschii tRNASec | M. jannaschii tRNASec | 75.0 ± 0.5 | 65.3 ± 6.4 | 92.5 |
| Heterologous tRNASec | M. kandleri tRNASec | 50.4 ± 1.0 | 48.2 ± 0.9 | 101.6 |
| E. coli tRNASec | 53.3 ± 2.4 | 7.0 ± 2.1 | 13.9 | |
| H. sapiens tRNASec | 74.4 ± 3.1 | 12.8 ± 2.4 | 18.3 | |
| Chimeric tRNA | Transplant 1 | 4.8 ± 0.3 | 0.0001 ± 0.0006 | 0.002 |
| Transplant 2 | 9.6 ± 0.7 | 4.3 ± 0.4 | 47.6 | |
| Transplant 3 | 17.7 ± 1.7 | 5.2 ± 0.7 | 31.2 | |
| Chimera | 18.9 ± 0.1 | 12.1 ± 1.1 | 68.0 |
atRNA transcripts were used.
bThe percent aminoacylation (serylation plus phosphorylation) refers to the intensity of the Ser-[32P]AMP and Sep-[32P]AMP spots divided by the total intensity of the Ser-[32P]AMP, Sep-[32P]AMP, and [32P]AMP spots and phosphorylation refers to the intensity of the Sep-[32P]AMP spot divided by the total intensity of the Ser-[32P]AMP, Sep-[32P]AMP, and [32P]AMP spots (See ‘Materials and Methods’ section).
cThe assay was performed on all tRNAs in triplicate and the standard deviations for each are reported.
dThe efficiency of phosphorylation was calculated by dividing the percent of tRNA phosphorylated by the percent total aminoacylation. The relative efficiency is a comparison of the efficiency of phosphorylation of each mutant tRNA to that of wild-type M. maripaludis tRNASec calculated by dividing the percent efficiency of phosphorylation of each mutant by that of wild-type M. maripaludis tRNASec multiplied by 100%.
eDomain from M. maripaludis tRNASerUGA was transplanted onto the M. maripaludis tRNASec backbone.
fND, Activity not detectable.
Figure 1.
Conservation of archaeal tRNASec and tRNASer sequences and transplantation of tRNASec identity elements into tRNASer. The cloverleaf structures of M. maripaludis tRNASec (A) and tRNASerUGA (B) are shown. The secondary structures of presently available archaeal tRNASec (seven sequences) and tRNASer from Sec-decoding archaea (18 sequences) were compared (See Supplementary Figure 1). Bold and red nucleotides are invariant within each tRNASec or tRNASer. Bold and blue nucleotides are at least 70% conserved within each tRNASec or tRNASer. Blue nucleotides are at least 70% conserved within each tRNASec or tRNASer but not in the pictured M. maripaludis tRNAs. Gray shading shows residues conserved at >70% between tRNASec and tRNASer. Black boxes on G2-C71 and C3-G70 of tRNASec in (A) indicate identity elements transplanted into the tRNASer scaffold to produce the tRNA chimera pictured in (C). The black box on A5-U68 of tRNASer in (B) indicates an antideterminant that was mutated to C5-G68 in the tRNA chimera (C). The mutated nucleotides are boxed, bold and orange in the tRNA chimera. The numbering of tRNASec is according to Sturchler et al. (18), and the numbering of tRNASer is according to Sprinzl et al. (39).
The unusual D-arm provides a minor identity element for archaeal PSTK
The length and structure but not sequence of the D-stem of human tRNASec were shown to be the major identity elements for serine phosphorylation by human PSTK (8), but our investigation revealed the D-arm to be a minor identity element for archaeal PSTK. The D-arm of tRNASec likely has a 7-bp stem and a 4 nt loop, whereas in the isoacceptors of tRNASer the stem and loop are 3 to 4 bp and 9 to 13 nt, respectively. (Figure 1A and B). Replacing the D-arm of tRNASec with the D-arm from tRNASer resulted in a mutant (D-arm, Table 1) that serylated poorly (1.4%), which made assaying for phosphorylation difficult. The severe effects propagated by the D-arm mutant were possibly due to disruption of tertiary structure. More specific mutations of the D-arm disrupted 1 bp (U16-A20a or C15-G20b) or 2 bp (U16-A20a and C15-G20b) in the 7-bp D-stem: U16A, U16G, C15G, U16A/C15G, A20aU and A20aC. Disrupting 1 bp of the D-stem by mutation of U16 to A or G or A20aU had moderate effects on the relative phosphorylation efficiency of the mutant tRNAs compared to wild-type, while the C15G and A20aC mutations had little effect (Table 1). Compensatory mutations that retained or closed the 7-bp stem retained or restored phosphorylation by PSTK (A20aG, A20aU/U16A, Table 1). The relative phosphorylation efficiency of the U16A/C15G double mutant (disrupted 2 bp in the D-stem) was similar to the single U16 mutants, showing no added effect due to a shorter 5-bp D-stem (Table 1). Although the affinity of PSTK was about 10-fold lower for most of the D-stem mutants, the U16A/C15G double mutant did cause a 28.5-fold decrease over wild-type tRNASec, which is comparable to PSTK recognition of tRNASer (Supplementary Table 1). Thus, the D-stem of tRNASec does provide a minor identity element for PSTK recognition and phosphorylation but is not the signal for phosphorylation by archaeal PSTK.
PSTK recognition of the acceptor stem of archaeal tRNASec is essential for phosphorylation
The acceptor stems of archaeal tRNASec and tRNASer from Sec-decoding archaea differ in both length and sequence (Figure 1A and B and Supplementary Figure 1), which probably affects the secondary and tertiary structures of these tRNAs. The L-form tertiary structure of tRNA has two helical domains; domain I consists of the anticodon stem and the D-stem, and domain II consists of the T-stem and the acceptor stem (25–27). Domain II has 13 bp (9-bp acceptor stem and 4-bp T-stem) in archaeal tRNASec and 12 bp (7 bp acceptor stem and 5-bp T-stem) in tRNASer (Figure 1A and B). Thus to maintain the domain II structure as that of tRNASer, we transplanted both the acceptor stem and T-arm from tRNASerUGA to tRNASec, resulting in a 12-bp domain II. The mutant did not serylate efficiently (20.1%), and the relative phosphorylation efficiency was poor (2.1%) (acceptor stem + T arm, Table 1), suggesting that elements within the acceptor stem were required for phosphorylation by archaeal PSTK.
To determine whether the length of the acceptor stem was an important identity element for PSTK recognition of tRNASec, tRNASec mutants with deletions of base pairs within the acceptor stem were analyzed: Δ5a-67b, Δ5b-67a, Δ5a-67b/Δ5b-67a and ΔG2-C71. Deletion of 5a-67b or 5b-67a did not influence phosphorylation efficiency (Table 1). Deletion of both 5a-67b and 5b-67a resulted in mutant tRNASec that serylated poorly, similarly to the acceptor stem + T arm mutant (8.9% and 20.1%, respectively). Unlike the acceptor stem + T arm mutant, the relative phosphorylation efficiency was higher at 54.9% of the wild-type tRNASec level (Table 1). This may suggest that the length of the acceptor stem plays a role in phosphorylation. Additionally, mutant tRNASec with a G2-C71 deletion serylated efficiently (73.4%) but its relative phosphorylation efficiency was only 1.3% (Table 1), signifying a possible sequence-specific interaction with PSTK.
Archaeal tRNASec has invariant G2-C71 and C3-G70 base pairs and a well-conserved C5-G68 base pair in the acceptor stem (Figure 1A) (C5-A68 in Methanococcus voltae and G5-C68 in Methanopyrus kandleri, Supplementary Figure 1). We mutated each of these base pairs to determine their importance for PSTK recognition. Mutation of the invariant tRNASec G2-C71 bp was detrimental to phosphorylation with a C2-G71 mutation producing the most severe decrease in relative phosphorylation efficiency at 96.3% below wild-type level (Table 1); interestingly, C2-G71 is highly conserved in all archaeal tRNASer but invariant in tRNASer from Sec-decoding archaea (Supplementary Figure 1). Mutation of the invariant C3-G70 base pair of archaeal tRNASec to G3-C70 had a severe effect on phosphorylation with an 87.5% decrease in the relative efficiency, while mutation to A3-U70 had a moderate decrease (24.1%) (Table 1). G3-C70 and A3-C70 are the most common base pairs found at this position in tRNASer from Sec-decoding archaea although the isoacceptors of M. kandleri tRNASer have a conserved C3-G70 bp (Supplementary Figure 1). It should be noted that the genome of M. kandleri has an accelerated rate of evolution among the archaea (28). Mutation of C3-G70 to U3-A70 or U3-G70 had little to no influence on the relative phosphorylation efficiency (Table 1). Mutation of the well-conserved C5-G68 base pair of tRNASec to G5-C68, U5-A68, or U5-G68 had a minimal effect on phosphorylation while mutation to A5-U68, the most common base pair at that position in tRNASer from Sec-decoding archaea, caused a 54.5% decrease in phosphorylation over wild-type tRNASec (Table 1). Additionally, Ser-tRNASec from M. kandleri, which has a G5-C68 base pair, phosphorylates as efficiently as M. maripaludis Ser-tRNASec (Table 1). These data demonstrate that A5-U68 in tRNASer has some antideterminant value for PSTK recognition.
The anticodon stem–loop and long variable arm are not identity elements for phosphorylation by PSTK
The anticodon stem–loop is highly conserved in archaeal tRNASec compared to the anticodon stem–loops of tRNASer isoacceptors (with the anticodons UGA, GGA and GCU) found in Sec-decoding archaea (Figure 1 and Supplementary Figure 1). Neither a mutation of the anticodon of tRNASec from UCA to one of a tRNASer isoacceptor, UGA (anticodon UCA to UGA), nor replacing the entire anticodon stem–loop of tRNASec with that of tRNASerUGA (anticodon stem–loop) had a negative effect on the relative phosphorylation efficiency by PSTK (Table 1).
The variable arms of tRNASec and tRNASer differ in sequence, length and orientation (Figure 1A and B and Supplementary Figure 1). Replacement of the variable arm of tRNASec with that of tRNASerUGA did not influence the relative phosphorylation efficiency (variable arm, Table 1).
The T-loop of tRNASec is a minor identity element for phosphorylation
The structure of the T-arm of archaeal tRNASec is quite unusual in that the T-stem has 4 bp while most tRNAs, including tRNASer, have 5-bp T-stems (Figure 1A and B and Supplementary Figure 1). Unlike the T-stems of archaeal tRNASer isoacceptors, the sequences of the T-stems of archaeal tRNASec found to date are invariant (Figure 1A and B and Supplementary Figure 1). Replacement of the T-arm of tRNASec with that of tRNASerUGA resulted in a 31.1% decrease in relative phosphorylation efficiency compared to wild-type tRNASec (T-arm, Table 1) and a 29.1-fold decrease in the affinity of PSTK for the mutant tRNA (Supplementary Table 1). The T-arm mutation also had a significant effect on serylation, serylating at 37.9% as compared to 80.3% for wild-type tRNASec (Table 1), possibly due to disruption of the tertiary structure. Mutation of the first 2 bp of the T-stem from G50-C64 to C-G and G51-C63 to C-G had little effect on the relative phosphorylation efficiency (G50-C64 to C-G/G51-C63 to C-G, Table 1).
The T-loop size is conserved between archaeal tRNASec and tRNASer but the sequences are variant in two positions, 57 and 59 (Figure 1A and B and Supplementary Figure 1). A57 is invariant in the isoacceptors of tRNASer from Sec-decoding archaea, whereas G57 is found in most archaeal tRNASec. U59 is invariant in archaeal tRNASec, while A59 is highly conserved in archaeal tRNASer. Transplanting the T-loop from tRNASerUGA onto tRNASec which mutates G57A and U59A, causes a 41.1% decrease in the relative phosphorylation efficiency and a decrease in serylation to 44.9% compared to wild-type tRNASec (T-loop, Table 1); these results are similar to replacement of the entire T-arm with that of tRNASerUGA, suggesting that G57 and U59 might be responsible. Nevertheless, the single mutations G57A or U59A serylated efficiently and had minimal effects on the relative phosphorylation efficiency (Table 1). Consequently, although neither single mutation had a significant effect, the collective mutation of G57 and U59 in the T-loop mutant had a moderate effect on serylation, phosphorylation and binding (T-loop, Table 1 and Supplementary Table 1).
Transplantation of acceptor stem base pairs G2-C71, C3-G70 and C5-G68 into tRNASer allows robust phosphorylation by PSTK
Although mutation of G2-C71 in tRNASec resulted in a mutant that only phosphorylated at 3.7% of the wild-type level, transplantation of G2-C71 alone into the tRNASerUGA backbone was not sufficient to confer phosphorylation onto this mutant (transplant 1, Table 1). The archaeal tRNASec identity elements found at base pairs G2-C71 and C3-G70 were transplanted into the tRNASerUGA backbone, and the negative determinant A5-U68 was mutated to G5-C68 as found in tRNASec (Figure 1C). PSTK phosphorylated the chimera with a relative phosphorylation efficiency of 68% compared to wild-type tRNASec (chimera, Table 1). Furthermore, the affinity of PSTK for the chimeric tRNA was higher than that for tRNASerUGA (Supplementary Table 1). Phosphorylation of the chimeric Ser-tRNASer by PSTK demonstrated the admirable substrate qualities of the transplanted tRNASer species (Figure 2). Neither mutation of the D-arm in the tRNA chimera to add base pairs to the D-stem (transplant 2, Table 1), thus making a smaller D-loop as seen for tRNASec, nor addition of an additional base pair to the acceptor stem of the chimera to lengthen it from 7 to 8 bp improved phosphorylation (transplant 3, Table 1). Thus, the major identity elements for phosphorylation by archaeal PSTK are found in the acceptor stem at base pairs G2-C71 and C3-G70.
Figure 2.
In vitro conversion of Ser-tRNA to Sep-tRNA by M. jannaschii PSTK. One micromolar 32P-labeled Ser-tRNASec (70.8% serylated), chimera Ser-tRNA (64.5% serylated) or Ser-tRNASer (63.4% serylated) was incubated with 50 nM PSTK at 37°C for 15 min. Aliquots of the reactions were quenched with 100 mM sodium citrate, pH 5.0 and digested with 0.66 mg/ml nuclease P1 for 35 min at room temperature. Samples were then spotted onto a PEI-cellulose TLC plate and developed in 100 mM ammonium acetate, 5% acetic acid for 75 min. Following quantification of the intensities of Ser-[32P]AMP, [32P]AMP and Sep-[32P]AMP using ImageQuant, the fraction (%) of aminoacyl-tRNA formed at each time point was calculated by dividing the intensity of the Sep-[32P]AMP and Ser-[32P]AMP spots by the total intensity and the fraction (%) of Sep-tRNASec formed at each time point was calculated by dividing the intensity of the Sep-[32P]AMP spot by the total intensity. The phosphorylation efficiency was determined by dividing the percent Sep-tRNA by the percent aminoacyl-tRNA. Error bars represent the standard deviation of three separate experiments.
Chimeric tRNA induces the ATPase activity of PSTK
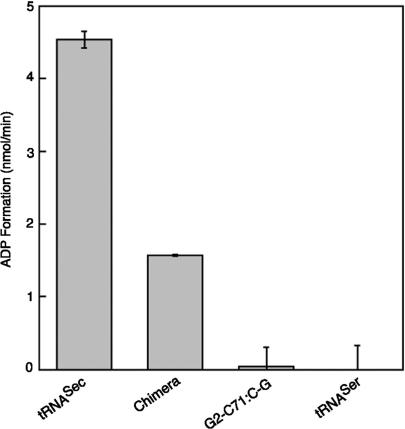
The ATPase activity of PSTK is specifically induced by tRNASec and there was minimal ATPase activity of the enzyme in the presence of tRNASer or in the absence of tRNA (15). When the ATPase activity of PSTK was tested in the presence of the G2-C71 to C-G tRNASec mutant, which was poorly phosphorylated (Table 1), the little ATPase activity detectable was similar to the ATPase activity in the presence of tRNASer (Figure 3). Yet, the chimeric tRNA (G2-C71, C3-G70, and C5-G68 of tRNASec transplanted into tRNASerUGA) (Figure 1C) significantly induced the ATPase activity of PSTK (Figure 3), further demonstrating the importance of these acceptor stem base pairs in phosphorylation by PSTK.
Figure 3.
tRNASer chimera with transplanted (tRNASec) identity elements induces the ATPase activity of PSTK. A graph is shown of the ratio of [α-32P]ATP converted to [α-32P]ADP by PSTK (1 µM) in the presence of 1 µM tRNASec, chimera tRNA, G2-C71:C-G tRNASec mutant or tRNASer. The minimal ATPase activity in the absence of tRNA was subtracted. Error bars represent the standard deviation of three separate experiments.
Divergence in tRNASec acceptor stems from Archaea, Eukarya and Bacteria
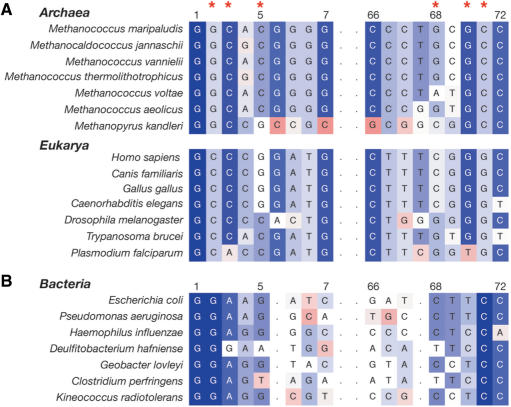
Alignment of archaeal and eukaryal tRNASec by secondary structure (Figure 4A) revealed insightful differences in the acceptor stems in light of the described identity elements for serine phosphorylation of M. maripaludis tRNASec by M. jannaschii PSTK and human tRNASec by human PSTK (8). As established by the phosphotransferase assay with M. jannaschii PSTK and M. maripaludis tRNASec, G2-C71 is a major identity element for phosphorylation (Table 1) and is invariant in archaeal tRNASec (Figures 1A and 4A). Eukaryal tRNASec, however, has a highly conserved C2-G71 bp at this position (Figure 4A and Supplementary Figure 2); only 2 out of 57 eukaryal tRNASec genes analyzed were variant at this position; tRNASec from the diatom Thalassiosira pseudonana has a U2-A71 bp (29). A BLAST search against the genome of the marsupial Monodelphus domestica identified a probable tRNASec (by secondary structure and sequence conservation), which has a U2-G71 bp at this position (Supplementary Figure 2). Archaeal tRNASec is invariant at bp C3-G70, and 84% of eukaryal tRNASec genes analyzed also have a C3-G70 bp (Figure 4A and Supplementary Figure 2). Of the remaining 16% of eukaryal tRNASec, only 3.5% have a base pair (G3-C70) that caused poor phosphorylation in the context of archaeal tRNASec by archaeal PSTK (Table 1). The A5-U68 with negative determinant properties in archaeal tRNASer is found in 4% of eukaryal tRNASec genes (Supplementary Figure 2). Although human tRNASec has a C2-G71 bp rather than the major identity element G2-C71 found in archaeal tRNASec, it retains the C3-G70 bp in the acceptor stem (Figure 4A), as well as other similar structural features such as the unusual D-arm, and archaeal PSTK is able to weakly phosphorylate human tRNASec (Table 1). Thus, eukaryal tRNASec has diverged at a critical identity element, G2-C71, for serine phosphorylation by archaeal PSTK.
Figure 4.
Comparative alignment of archaeal, eukaryotic and bacterial tRNASec acceptor stems. The secondary structures of archaeal (seven sequences, Supplementary Figure 1), eukaryotic (57 sequences, Supplementary Figure 2) and bacterial tRNASec (50 sequences) were aligned. (A) Alignment of the acceptor stems of all presently known archaeal tRNASec and representative eukaryotic tRNASec are shown. Nucleotides are colored according to sequence similarity (BLOSUM 50) between the archaeal and eukaryotic tRNASec. Red asterisks indicate key nucleotides in the acceptor stem of archaeal tRNASec for phosphorylation by PSTK. (B) Alignment of the acceptor stems of representative bacterial tRNASec to those of the archaeal and eukaryotic tRNASec. Nucleotides are colored according to sequence similarity (BLOSUM 50) among the bacterial tRNASec.
The acceptor stem of bacterial tRNASec consists of 8 bp (16) rather than 9 bp as found in archaeal and eukaryal tRNASec. Acceptor stems from 50 divergent Sec-decoding bacterial species were analyzed, and the G2-C71 bp is highly conserved if not invariant (Figure 4B). The third base pair in the acceptor stem, however, was 75% conserved as an A3-U70, 21% as a G3-C70 bp and 4% as a U3-A70 or C3-G70 bp. Most bacterial tRNASec analyzed did not have the antideterminant for archaeal PSTK, A5-U68. E. coli tRNASec has G2-C71, A3-U70 and G5-C68 bp in the acceptor stem (Figure 4B) and is phosphorylated weakly at a relative phosphorylation efficiency of 13.9% compared to wild-type M. maripaludis tRNASec (Table 1). Although E. coli tRNASec has the major identity element for phosphorylation by archaeal PSTK, G2-C71, other features such as the A3-U70 bp, the C59 in the T-loop, the 8/5 cloverleaf arrangement (8-bp acceptor stem and 5-bp T-stem), and other possible differences in tertiary structure could contribute to the poor phosphorylation and binding by archaeal PSTK (Table 1 and Supplementary Table 1).
Amino acid recognition by PSTK
It was observed earlier that M. barkeri SerRS misactivates threonine (30) and inefficiently forms Thr-tRNASer (I. Weygand-Durasevic, personal communication). Given that M. jannaschii PSTK has a similar affinity for tRNASec as Ser-tRNASec and thus does not seem to recognize the serine moiety on the tRNA (15), we considered whether PSTK could phosphorylate Thr-tRNASec. M. maripaludis [32P]tRNASec was mischarged with Thr by M. maripaludis SerRS, producing Thr-tRNASec (Figure 5, lane 1). Thr-tRNASec was subsequently phosphorylated with M. jannaschii PSTK (Figure 5, lane 2) and digested with nuclease P1. The products, [32P]AMP, Thr-[32P]AMP and phosphothreonyl-[32P]AMP were separated by TLC on PEI cellulose. While aminoacylation with Thr was poor (only 3.7%), PSTK converted 94.4% of the Thr to phosphothreonine, which migrated similarly to phosphoserine (Figure 5, compare lanes 2 and 4). This corresponds well to the 96.3% conversion of Ser to Sep by PSTK (Figure 5, lane 4). The experiment was performed with [14C]Thr to confirm the results (Supplementary Figure 3). Again, [14C]Thr-tRNASec was phosphorylated by PSTK (Supplementary Figure 3, compare lanes 2 and 4). The results provide further evidence that PSTK primarily recognizes the tRNA and not the amino acid, if any, that is attached to the 3' end of the tRNA.
Figure 5.
In vitro conversion of threonyl-tRNASec to phosphothreonyl-tRNASec by PSTK. One micromolar 32P-labeled tRNASec transcript was incubated with 600 nM SerRS and Thr (lane 1), 600 nM SerRS, 100 nM PSTK and Thr (lane 2), 600 nM SerRS and Ser (lane 3), or 600 nM SerRS, 100 nM PSTK and Ser (lane 4) at 37°C for 45 min. Aliquots of the reactions were quenched with 100 mM sodium citrate, pH 5.0 and digested with 0.66 mg/ml nuclease P1 for 35 min at room temperature. Samples were then spotted onto a PEI-cellulose TLC plate and developed in 100 mM ammonium acetate, 5% acetic acid for 75 min.
DISCUSSION
The pathway for selenocysteine formation differs in bacteria from that present in eukaryotes and archaea; however, tRNASec remains a common factor for selenocysteine formation among the three. The tRNA-dependent conversion of Ser to Sec in eukaryotes and archaea requires that the unusual tRNASec be recognized initially by enzymes that interact with many tRNAs such as processing and modifying enzymes and the CCA-adding enzyme prior to ‘mischarging’ with Ser by SerRS, phosphorylation of the resulting Ser-tRNASec by PSTK and conversion of Sep-tRNASec to Sec-tRNASec by SepSecS. Subsequently, the tRNASec-specific elongation factor SelB must recognize Sec-tRNASec specifically for selenoprotein synthesis (31–33). While we have speculated that a complex of the enzymes involved in selenocysteine biosynthesis might exist (15) as has been suggested and shown for the tRNA-dependent amidotransferases (34,35), it is not known which components of the pathway might exist in a complex or whether the enzymes might compete for the tRNASec substrate. Yet, PSTK provides the first line of defense in maintaining the fidelity of genetic code translation by binding with high affinity to Ser-tRNASec (7,15). This sequesters mischarged tRNA from use in translation and discriminates Ser-tRNASec from Ser-tRNASer.
A previous study detailed how a human PSTK discriminates human Ser-tRNASec from Ser-tRNASer by virtue of the atypical D-stem structure of tRNASec (8). While archaeal tRNASec retains a D-stem structure similar to that of eukaryal tRNASec, mutations that open the last two base pairs in the D-stem of M. maripaludis tRNASec—leading to the formation of a 5-bp rather than a 7-bp stem—did not result in a complete loss of serine phosphorylation by M. jannaschii PSTK as occurred when similar mutations were made to human tRNASec (phosphorylation by human PSTK) (8). Some mutations to the D-stem of M. maripaludis tRNASec (U16A, U16G, U16A/C15G and A20aU) caused a moderate decrease in phosphorylation but others (C15G and A20aC) did not, which brings into question whether a 7-bp stem has an effect on phosphorylation by archaeal PSTK. The intact 7-bp D-stem per se may not be recognized for phosphorylation; these data might suggest a sequence-specific interaction of archaeal PSTK with U16, but the compensatory mutation A20aU/U16A that keeps the D-stem intact phosphorylated efficiently even with a mutation of U16. Perhaps mutations of A20aU or of U16 perturb the tertiary structure of tRNASec, causing the decrease in phosphorylation efficiency by PSTK.
The T-loop of tRNASec also provides a minor identity element for archaeal PSTK phosphorylation. The combined mutation of G57A and U59A in the T-loop, which resulted in a T-loop identical to that found in tRNASer from Sec-decoding archaea, caused a moderate decrease in phosphorylation that was not completely attributable to a single mutation of either G57 or U59. Conceivably these mutations, specifically that of U59, could disrupt a tertiary interaction of the D- and T-loops between U17 and U59. A novel tertiary interaction was reported for E. coli tRNASec between C16 (located at the same position as U17 in archaeal tRNASec) in the D-loop and C59 (U59 in archaeal tRNASec) in the T-loop (16) although a similar interaction was not found between U16 (located at the same position as U17 in archaeal tRNASec) and U59 in a study of eukaryotic-type Xenopus laevis tRNASec (18). If archaeal tRNASec is able to form interactions common to all known tRNA crystal structures between the D-loop and T-loop (25–27) as was supported by molecular modeling of the archaeal-type M. jannaschii tRNASec (19), an alternative explanation is possible; nucleotide 57 stacks with other purines in an intercalation of bases from both the D- and T-loops, and nucleotide 59 stacks to the tertiary base pairs 15–48, playing a crucial role in fixing the juxtaposition of domains I (anticodon stem and D-stem) and II (T-stem and acceptor stem) (19). Although nucleotide 57 is typically conserved as a purine and nucleotide 59 can be any nucleotide, the invariant nature of U57 and highly conserved G59 may be essential for tertiary interactions in archaeal tRNASec. A future structural study of archaeal tRNASec would be helpful in making these determinations.
Despite the fact that at present we cannot conclusively state that the length of the acceptor stem is involved in phosphorylation of Ser-tRNASec by archaeal PSTK, our data definitively revealed that critical identity elements are found within the acceptor stem. This finding is quite unlike what was shown for eukaryotic-type PSTK and tRNASec where neither the sequence nor length of the acceptor stem was important for phosphorylation (8). Mutagenesis of M. maripaludis tRNASec at the G2-C71 and C3-G70 bp demonstrated their importance for phosphorylation, and transplantation of these base pairs into tRNASerUGA along with mutating the A5-U68 antideterminant endowed efficient phosphorylation to the chimeric tRNA. Additionally, we previously demonstrated that tRNASec stimulated the ATPase activity of PSTK by a potential induced fit mechanism (15); the fact that our chimeric tRNA unlike tRNASer stimulated the ATPase activity of PSTK is further confirmation of the essentiality of the G2-C71 and C3-G70 bp.
A fascinating aspect revealed by this study is the divergence in recognition of archaeal and eukaryal tRNASec by archaeal and eukaryal PSTKs; there is precedence for this as aminoacyl-tRNA synthetases from the three domains of life sometimes utilize variant tRNA identity elements (36), and additionally, the tRNA-dependent amidotransferase GatCAB from bacteria and archaea evolved to recognize different elements within tRNAAsn (37,38). While the length and structure of the D-stem of human tRNASec were essential for phosphorylation by human PSTK (8), the unusual D-stem of archaeal tRNASec only moderately affected phosphorylation by archaeal PSTK, possibly by means of maintenance of proper tertiary structure. On the contrary, invariant base pairs within the acceptor stem of archaeal tRNASec were vital for phosphorylation by archaeal PSTK. Moreover, the invariant G2-C71 of archaeal tRNASec was found to be highly conserved as C2-G71 in eukaryotic tRNASec, which resulted in minimal phosphorylation by archaeal PSTK in the context of archaeal tRNASec. This distinction between archaeal and eukaryotic tRNASec corresponds to a deep evolutionary divide between archaeal-type and eukaryotic-type PSTKs (15). While tRNASec transverses interactions with a number of enzymes in the selenocysteine biosynthesis pathway, that distinction may indicate co-evolution of archaeal and eukaryotic tRNASec with their respective PSTKs.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
We thank Sotiria Palioura, Lennart Randau, Kelly Sheppard and Jing Yuan for helpful discussions and critical reading of the paper. We appreciate the assistance of William B. Whitman in providing us with archaeal tRNASec sequences and Ivana Weygand-Durasevic for helpful advice. R.L.S. is the recipient of a Ruth L. Kirschstein National Research Service Award (F32 GM075602) from the National Institute of General Medical Sciences. J.H. holds an Edward A. Bouchet Undergraduate Fellowship from Yale University. This work was supported by grants from the Department of Energy and the National Institute of General Medical Sciences (to D.S.). Funding to pay the Open Access publication charges for this article was provided by National Institute of General Medical Sciences grant GM22854 (to D.S.).
Conflict of interest statement. None declared.
REFERENCES
- 1.Böck A, Thanbichler M, Rother M, Resch A. In: Aminoacyl-tRNA Synthetases. Ibba M, Francklyn C, Cusack S, editors. Georgetown, TX: Landes Bioscience; 2005. pp. 320–327. [Google Scholar]
- 2.Bilokapic S, Korencic D, Söll D, Weygand-Durasevic I. The unusual methanogenic seryl-tRNA synthetase recognizes tRNASer species from all three kingdoms of life. Eur. J. Biochem. 2004;271:694–702. doi: 10.1111/j.1432-1033.2003.03971.x. [DOI] [PubMed] [Google Scholar]
- 3.Leinfelder W, Zehelein E, Mandrand-Berthelot MA, Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988;331:723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- 4.Wu XQ, Gross HJ. The long extra arms of human tRNA((Ser)Sec) and tRNA(Ser) function as major identify elements for serylation in an orientation-dependent, but not sequence-specific manner. Nucleic Acids Res. 1993;21:5589–5594. doi: 10.1093/nar/21.24.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson BA, Xu XM, Kryukov GV, Rao M, Berry MJ, Gladyshev VN, Hatfield DL. Identification and characterization of phosphoseryl-tRNA[Ser]Sec kinase. Proc. Natl Acad. Sci. USA. 2004;101:12848–12853. doi: 10.1073/pnas.0402636101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaiser JT, Gromadski K, Rother M, Engelhardt H, Rodnina MV, Wahl MC. Structural and functional investigation of a putative archaeal selenocysteine synthase. Biochemistry. 2005;44:13315–13327. doi: 10.1021/bi051110r. [DOI] [PubMed] [Google Scholar]
- 7.Mizutani T, Hashimoto A. Purification and properties of suppressor seryl-tRNA: ATP phosphotransferase from bovine liver. FEBS Lett. 1984;169:319–322. doi: 10.1016/0014-5793(84)80342-7. [DOI] [PubMed] [Google Scholar]
- 8.Wu XQ, Gross HJ. The length and the secondary structure of the D-stem of human selenocysteine tRNA are the major identity determinants for serine phosphorylation. EMBO J. 1994;13:241–248. doi: 10.1002/j.1460-2075.1994.tb06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu XM, Carlson BA, Mix H, Zhang Y, Saira K, Glass RS, Berry MJ, Gladyshev VN, Hatfield DL. Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol. 2007;5:e4. doi: 10.1371/journal.pbio.0050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan J, Palioura S, Salazar JC, Su D, O’Donoghue P, Hohn MJ, Cardoso AM, Whitman WB, Söll D. RNA-dependent conversion of phosphoserine forms selenocysteine in eukaryotes and archaea. Proc. Natl Acad. Sci. USA. 2006;103:18923–18927. doi: 10.1073/pnas.0609703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlsen EN, Trelle GJ, Schjeide OA. Transfer ribonucleic acids. Nature. 1964;202:984–986. doi: 10.1038/202984a0. [DOI] [PubMed] [Google Scholar]
- 12.Mäenpää PH, Bernfield MR. A specific hepatic transfer RNA for phosphoserine. Proc. Natl Acad. Sci. USA. 1970;67:688–695. doi: 10.1073/pnas.67.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatfield D, Diamond A, Dudock B. Opal suppressor serine tRNAs from bovine liver form phosphoseryl-tRNA. Proc. Natl Acad. Sci. USA. 1982;79:6215–6219. doi: 10.1073/pnas.79.20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharp SJ, Stewart TS. The characterization of phosphoseryl tRNA from lactating bovine mammary gland. Nucleic Acids Res. 1977;4:2123–2136. doi: 10.1093/nar/4.7.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherrer RL, O’Donoghue P, Söll D. Characterization and evolutionary history of an archaeal kinase involved in selenocysteinyl-tRNASec formation. Nucleic Acids Res. 2008 doi: 10.1093/nar/gkm1134. doi:10.1093/nar/gkm1134 (Advance access published 3 January 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baron C, Westhof E, Böck A, Giege R. Solution structure of selenocysteine-inserting tRNASec from Escherichia coli. Comparison with canonical tRNASer. J. Mol. Biol. 1993;231:274–292. doi: 10.1006/jmbi.1993.1282. [DOI] [PubMed] [Google Scholar]
- 17.Hubert N, Sturchler C, Westhof E, Carbon P, Krol A. The 9/4 secondary structure of eukaryotic selenocysteine tRNA: more pieces of evidence. RNA. 1998;4:1029–1033. doi: 10.1017/s1355838298980888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sturchler C, Westhof E, Carbon P, Krol A. Unique secondary and tertiary structural features of the eucaryotic selenocysteine tRNASec. Nucleic Acids Res. 1993;21:1073–1079. doi: 10.1093/nar/21.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ioudovitch A, Steinberg SV. Structural compensation in an archaeal selenocysteine transfer RNA. J. Mol. Biol. 1999;290:365–371. doi: 10.1006/jmbi.1999.2901. [DOI] [PubMed] [Google Scholar]
- 20.Bullock TL, Uter N, Nissan TA, Perona JJ. Amino acid discrimination by a class I aminoacyl-tRNA synthetase specified by negative determinants. J. Mol. Biol. 2003;328:395–408. doi: 10.1016/s0022-2836(03)00305-x. [DOI] [PubMed] [Google Scholar]
- 21.Wolfson AD, Uhlenbeck OC. Modulation of tRNAAla identity by inorganic pyrophosphatase. Proc. Natl Acad. Sci. USA. 2002;99:5965–5970. doi: 10.1073/pnas.092152799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider KL, Pollard KS, Baertsch R, Pohl A, Lowe TM. The UCSC Archaeal Genome Browser. Nucleic Acids Res. 2006;34:D407–D410. doi: 10.1093/nar/gkj134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laslett D, Canback B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004;32:11–16. doi: 10.1093/nar/gkh152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korencic D, Polycarpo C, Weygand-Durasevic I, Söll D. Differential modes of transfer RNASer recognition in Methanosarcina barkeri. J. Biol. Chem. 2004;279:48780–48786. doi: 10.1074/jbc.M408753200. [DOI] [PubMed] [Google Scholar]
- 25.Ladner JE, Jack A, Robertus JD, Brown RS, Rhodes D, Clark BF, Klug A. Structure of yeast phenylalanine transfer RNA at 2.5 Å resolution. Proc. Natl Acad. Sci. USA. 1975;72:4414–4418. doi: 10.1073/pnas.72.11.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moras D, Comarmond MB, Fischer J, Weiss R, Thierry JC, Ebel JP, Giege R. Crystal structure of yeast tRNAAsp. Nature. 1980;288:669–674. doi: 10.1038/288669a0. [DOI] [PubMed] [Google Scholar]
- 27.Quigley GJ, Seeman NC, Wang AH, Suddath FL, Rich A. Yeast phenylalanine transfer RNA: atomic coordinates and torsion angles. Nucleic Acids Res. 1975;2:2329–2341. doi: 10.1093/nar/2.12.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brochier C, Forterre P, Gribaldo S. Archaeal phylogeny based on proteins of the transcription and translation machineries: tackling the Methanopyrus kandleri paradox. Genome Biol. 2004;5:R17. doi: 10.1186/gb-2004-5-3-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatfield DL, Lee BJ, Price NM, Stadtman TC. Selenocysteyl-tRNA occurs in the diatom Thalassiosira and in the ciliate Tetrahymena. Mol. Microbiol. 1991;5:1183–1186. doi: 10.1111/j.1365-2958.1991.tb01891.x. [DOI] [PubMed] [Google Scholar]
- 30.Bilokapic S, Maier T, Ahel D, Gruic-Sovulj I, Söll D, Weygand-Durasevic I, Ban N. Structure of the unusual seryl-tRNA synthetase reveals a distinct zinc-dependent mode of substrate recognition. EMBO J. 2006;25:2498–2509. doi: 10.1038/sj.emboj.7601129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fagegaltier D, Hubert N, Yamada K, Mizutani T, Carbon P, Krol A. Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation. EMBO J. 2000;19:4796–4805. doi: 10.1093/emboj/19.17.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forchhammer K, Leinfelder W, Böck A. Identification of a novel translation factor necessary for the incorporation of selenocysteine into protein. Nature. 1989;342:453–456. doi: 10.1038/342453a0. [DOI] [PubMed] [Google Scholar]
- 33.Rother M, Wilting R, Commans S, Böck A. Identification and characterisation of the selenocysteine-specific translation factor SelB from the archaeon Methanococcus jannaschii. J. Mol. Biol. 2000;299:351–358. doi: 10.1006/jmbi.2000.3756. [DOI] [PubMed] [Google Scholar]
- 34.Oshikane H, Sheppard K, Fukai S, Nakamura Y, Ishitani R, Numata T, Sherrer RL, Feng L, Schmitt E, Panvert M, et al. Structural basis of RNA-dependent recruitment of glutamine to the genetic code. Science. 2006;312:1950–1954. doi: 10.1126/science.1128470. [DOI] [PubMed] [Google Scholar]
- 35.Bailly M, Blaise M, Lorber B, Becker HD, Kern D. The transamidosome: a dynamic ribonucleoprotein particle dedicated to prokaryotic tRNA-dependent asparagine biosynthesis. Mol. Cell. 2007;28:228–239. doi: 10.1016/j.molcel.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Giege R, Sissler M, Florentz C. Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res. 1998;26:5017–5035. doi: 10.1093/nar/26.22.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailly M, Giannouli S, Blaise M, Stathopoulos C, Kern D, Becker HD. A single tRNA base pair mediates bacterial tRNA-dependent biosynthesis of asparagine. Nucleic Acids Res. 2006;34:6083–6094. doi: 10.1093/nar/gkl622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Namgoong S, Sheppard K, Sherrer RL, Söll D. Co-evolution of the archaeal tRNA-dependent amidotransferase GatCAB with tRNAAsn. FEBS Lett. 2007;581:309–314. doi: 10.1016/j.febslet.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sprinzl M, Dank N, Nock S, Schon A. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1991;19:2127–2171. doi: 10.1093/nar/19.suppl.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]