Abstract
Objective
To repor the study of a multigenerational Swiss family with dopa-responsive dystonia (DRD).
Methods
Clinical investigation was made of available family members, including historical and chart reviews. Subject examinations were video recorded. Genetic analysis included a genome-wide linkage study with microsatellite markers (STR), GTP cyclohydrolase I (GCH1) gene sequencing, and dosage analysis.
Results
We evaluated 32 individuals, of whom 6 were clinically diagnosed with DRD, with childhood-onset progressive foot dystonia, later generalizing, followed by parkinsonism in the two older patients. The response to levodopa was very good. Two additional patients had late onset dopa-responsive parkinsonism. Three other subjects had DRD symptoms on historical grounds. We found suggestive linkage to the previously reported DYT14 locus, which excluded GCH1. However, further study with more stringent criteria for disease status attribution showed linkage to a larger region, which included GCH1. No mutation was found in GCH1 by gene sequencing but dosage methods identified a novel heterozygous deletion of exons 3 to 6 of GCH1. The mutation was found in seven subjects. One of the patients with dystonia represented a phenocopy.
Conclusions
This study rules out the previously reported DYT14 locus as a cause of disease, as a novel multiexonic deletion was identified in GCH1. This work highlights the necessity of an accurate clinical diagnosis in linkage studies as well as the need for appropriate allele frequencies, penetrance, and phenocopy estimates. Comprehensive sequencing and dosage analysis of known genes is recommended prior to genome-wide linkage analysis.
Dopa-responsive dystonia (DRD) is a rare disorder characterized by childhood-onset fluctuating foot dystonia, which later generalizes and becomes associated with tremor and parkinsonism.1–3 Response to small doses of levodopa is dramatic. In its classic form, also known as Segawa disease, the transmission is autosomal dominant, with variable penetrance. Most of the cases are due to a mutation in the GTP cyclohydrolase I gene (GCH1; DYT5; MIM: 600225) on chromosome 14q22.1-q22.2, the product of which is an enzyme needed for tetrahydrobiopterin (BH4) synthesis.4 BH4 is a cofactor of tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine synthesis. A recessive form of DRD can be caused by mutations in the TH gene.5
Although close to 100 mutations have been described in GCH1,6 comprehensive screening fails to identify the responsible mutation in 40 to 50% of the families.7 Recently, families have been described with heterozygous exonic deletions in GCH1, which were not detected by conventional screening techniques.8–11
In this study, we report more comprehensive clinical and genetic investigations of a multigenerational DRD family, in which a previous study had found linkage to a novel locus nominated DYT14 on chromosome 14q13, adjacent to the DYT5 locus.12 Expanded data allowed us to exclude DYT14, and to identify a novel deletion in GCH1 as the disease-causing mutation in this family.
METHODS
Genealogy and clinical evaluations
Information on the pedigree was collected by means of historical material, family records, and medical charts review, along with interviews with elderly family members.
The project was approved by the local ethics committee. After signing an informed consent, subjects underwent a detailed videotaped neurologic evaluation. A clinical diagnosis of definite DRD was established in patients with childhood onset foot dystonia, and in patients with late onset dopa-responsive parkinsonism with a history of childhood onset foot dystonia. A clinical diagnosis of probable DRD was established in obligate carriers with mild symptoms, and in subjects with suggestive but insufficient history or phenotype. Multiple evaluations were performed with a maximum follow-up of 7 years. Videotapes were reviewed by at least two out of five neurologists trained in movement disorders (C.W., M.H., A.S., J.G., F.V.), and a consensus was reached regarding clinical diagnoses.
In individuals who were confirmed to harbor the mutation, the diagnosis was changed to genetically proven DRD.
Genetic investigations
Since previous data had pointed to a locus outside GCH1, and direct sequencing of GCH1 exons and flanking intronic sequence showed no mutation,12 our initial approach included a whole-genome search using single nucleotide polymorphisms (SNP) and follow-up studies using microsatellite markers.
SNP genotyping
All family members with DNA available were genotyped using the Affymetrix 10K GeneChip Mapping array with previously described protocols.13 Linkage analysis was performed using Genespring GT software, with analysis settings as described below for the microsatellite genotyping.
Microsatellite genotyping
Short tandem repeats (STR) markers over chromosome 14 were chosen using both existing STR panels (Applied Biosystems, Foster City, CA) and the NCBI database. PCR reactions were performed, resulting products were analyzed, and genotype calls were made as previously described.14 Two-point analysis was performed using a modified version of AUTOSCAN/MLINK15 under an autosomal dominant model with the disease allele frequency set at 0.001. Two different penetrance models were used. First, as DRD is twice as penetrant in females than males within this pedigree and in prior DRD pedigrees,16 sex-specific penetrance classes of 0.8 for females and 0.4 for males were considered. Second, a more conservative affecteds only analysis was performed by modeling the penetrance at 0.01. Marker allele frequencies were set as equally frequent in all analyses. Subsequent analysis systematically dropped individuals designated as affected in the pedigree to at risk, and recalculated evidence for linkage. Model-based multipoint linkage analysis was also performed using SIM-WALK217 with the same settings as for two-point analysis.
Candidate gene sequencing
All coding exons of GCH1 were amplified by PCR using primers designed to flanking intronic sequence (primers sequences available upon request). PCR reactions and sequence analysis were performed as previously described.18
GCH1 dosage
Duplex semiquantitative PCR was performed, amplifying all six GCH1 exons (primers sequences available upon request) along with one of three different diploid internal controls, of 222, 280, and 328 bp. PCR conditions were empirically determined to remain in the log-linear range with 1 minute denaturation at 94 °C, followed by 25 cycles consisting of 30 seconds of denaturation at 94 °C, 30 seconds of annealing at 55 °C, and 2 minutes of extension at 72 °C, with a final period of extension at 72 °C for 5 minutes. We used 20 ng of DNA, with 0.02U Taq polymerase (CLP, San Diego, CA), 5% Q solution, and 1× Mg-containing buffer (both from Qiagen) in a 15 μL volume. All forward primers were labeled with fluorescein (6-FAM). The PCR products were run on an ABI 3730 genetic analyzer (Applied Biosystems), and the results were analyzed with GeneMap-per v. 4.0 (Applied Biosystems). We calculated the ratio of exon peak height to control peak heights in affected patient samples, and compared values to standards obtained from normal, diploid subjects under the same experimental conditions. Three independent amplifications were performed for each subject/exon, the mean ratio and SD was used to define heterozygous deletions or duplications.
Deletion mapping
For exact mapping of the deletion, a stepwise procedure was used. First, two markers were identified that flanked the breakpoints of the deleted region, delineating genomic intervals at each extremity. The 5′ telomeric and 3′ centromeric intervals were re-iteratively divided into progressively smaller genomic segments; new primers were designed to amplify new amplicons at evenly spaced intervals (primers sequence available upon request). Genomic dosage was subsequently assessed in the log-linear range, as described above. When the region expected to harbor the genomic breakpoint was <3 kb, long-range PCR was attempted using appropriate primers and the Expand Long Template PCR System (Roche Diagnostics, Mannheim, Germany), using the recommended protocol. The PCR product was sequenced as detailed above. Bioinformatic analysis within and flanking the deleted region was performed in silico using the Censor software tool.19
Other genetic analyses
Subjects who displayed only parkinsonism were assessed for the most frequent dominantly inherited missense mutations in SCNA (A53T, E46K, A30P) and LRRK2 (G2019S, R1441C).20
Imaging
The proband (VI-1) had brain MRI, employing standard techniques.
RESULTS
Genealogy
The family originates from the region of Bern, in the western part of Switzerland. It is a seven-generation pedigree with 132 individuals (figure 1), in which dystonia has an autosomal dominant mode of transmission. After the proband came to our attention in 1995, we were able to investigate 32 subjects.
Figure 1. Abbreviated pedigree drawing, showing the clinical and the genetic affection status, with the STR markers haplotypes.
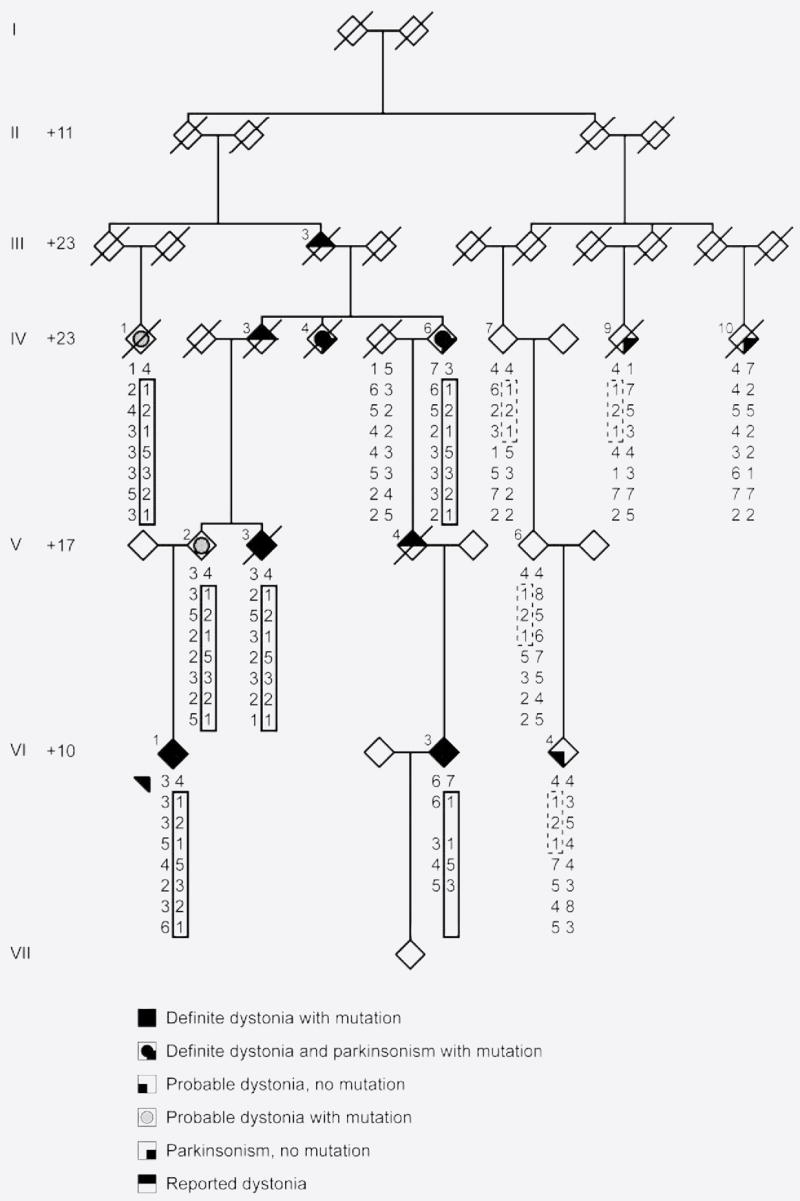
Generations are numbered using Roman numerals I through VII. To protect family members, individuals are represented as diamonds, and the + numbers at the beginning of each generation line indicate how many individuals were not included in the pedigree drawing. Shared haplotype in solid, apparent shared haplotype in dashed lines. Arrow: index case. Diagonal line: deceased individual.
Clinical description
Detailed clinical description of the proband is available at appendix E-1 on the Neurology® Web site at www.neurology.org.
In six patients, a clinical diagnosis of definite DRD was established with childhood onset dystonia that led to major motor impairment in three subjects (see figure 1 and table). Of these six patients, three were evaluated as young adults (VI-1, VI-3, VI-4), two with pure dystonia (VI-1, VI-3) and one with atypical features (VI-4, see below). Three patients were seen in later life, two with severe parkinsonism and dystonia (IV-4, IV-6) (untreated) and one with mild dystonia (V-3) (treated). Response to levodopa was very good in the four subjects in whom it was introduced (two patients refused treatment), with a mean daily intake of 234 mg.
Table.
Demographic and clinical data of the seven genetically proven patients with dopa-responsive dystonia (DRD)
| Subject | Birth | Death | Onset age, y | First symptom | Age seen, y | Clinical picture | Response to levodopa* (age, y) dose mg |
|---|---|---|---|---|---|---|---|
| VI-1 | 1971 | Alive | 2 | Foot D | 23 | Severe dystonia of feet, UL, axial, cervical (retrocolis), laryngeal | Excellent (23) 62.5 tid |
| IV-6 | 1914 | Alive | 8 | Foot D | 75 | Mild parkinsonism (A and T), generalized dystonia | Very good (50) 125 bid |
| IV-4 | 1923 | 2000 | 3 | Foot D | 73 | Severe parkinsonism (A and T), generalized dystonia | Very good (73) 125 tid |
| V-3 | 1942 | 1998 | Child | Foot D | 55 | Mild dystonia of feet and UL (treated) | Excellent (adult) 62.5 bid |
| VI-3 | 1978 | Alive | 5 | Foot D | 27 | Moderate foot dystonia, mild UL dystonia | NA |
| IV-1† | 1921 | 2001 | 15 | Gait difficulties | 78 | Axial rigidity, cervical dystonia (fixed to the right), hypomimia, clumsiness | NA |
| V-2† | 1946 | Alive | 13 | Foot eversion | 56 | Mild clumsiness and rigidity, intermittent exercise induced right foot inversion | NA |
Individual VI-4 did not have the mutation and is therefore not included.
Patients took levodopa/benserazide (Madopar).
Individuals IV-1 and V-2 were initially diagnosed with probable DRD.
D = dystonia; UL = upper limb; A = akinesia; T = tremor; NA = not assessed.
Two patients were diagnosed with probable DRD (IV-1 and V-2). In contrast, two patients from a more distant branch of the family (IV-9 and IV-10) presented with late-onset (60 and 63 years) asymmetric dopa-responsive parkinsonism.
Three subjects had DRD-suggestive symptoms on historical grounds (IV-3, V-4, III-3). They were probably affected with the same disease, first because of the pedigree structure (each of them was the father of an affected individual, with an obligate carrier status), and second because at least two family members recalled them having symptoms similar to their affected sons or daughters.
Among the unaffected subjects, eight had bilateral hip dysplasia, of which four had to undergo surgical hip replacement. Two affected and two unaffected subjects presented with dorsolum-bar scoliosis, but only in the proband (VI-1) was spine surgery performed.
Genetics
Linkage analysis using both SNP and microsatellite data confirmed the previous chromosome 14q locus, DYT14,12 as harboring the disease gene with a maximum multipoint lod score of 3.6. The shared haplotype spanned approximately 24 cM (D14S283-D14S70), and excluded GCH1. However, systemic re-analysis of linkage results was performed, specifying “affected” individuals as “at risk.” Reclassification of person VI-4 alone decreased the maximum multipoint lod score from 3.6 to 1.8 and the maximum gender-specific two-point lod score from 2.03 to 0.99 (lod score tables are available at appendix E-1), but expanded the chromosome 14q disease-associated haplotype to greater than 110 cM (D14S283-D14S292) which now included GCH1. The most parsimonious haplotype consistent with microsatellite genotyping and information on chromosomal phase is illustrated on the pedigree (figure 1). With this new analysis, we re-sequenced GCH1 in affected family members but no mutation was identified, suggesting that if GCH1 harbored a mutation it would likely be noncoding or quantitative.
Recently, mutations have been described in patients with DRD that create null alleles through genomic deletions of one or more GCH1 exons.8–11 Therefore we decided to screen for a similar deletion in our family.
Subsequent dosage analysis, higher resolution mapping, and sequencing identified a heterozygous 54,148 bp deletion of GCH1 in seven subjects, in whom a diagnosis of genetically proven DRD was established (table and figure 1). The deletion started in intron 2, included exons 3 to 6, and ended between GCH1 and the neighboring gene, SAMD4A (figure 2). The Affymetrix 10K SNP chip had no SNPs within the deleted interval, therefore the SNP data alone would not have identified this region as harboring a deletion.
Figure 2. Deletion in GCH1.
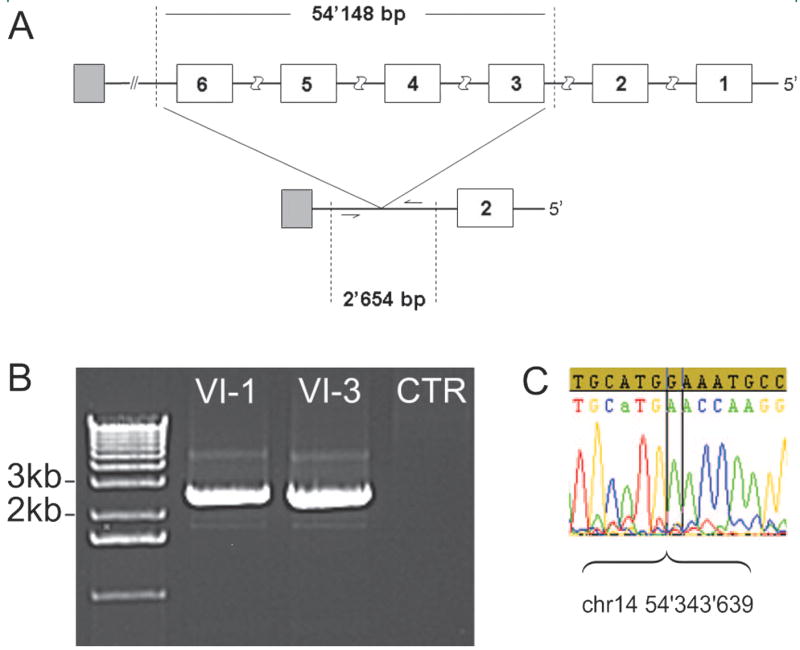
(A) The upper panel shows the wild type gene with GCH1 exons numbered 1 to 6 (in white), the deleted segment (vertical dashed lines), and the neighboring gene SAMD4A (in gray); the lower panel shows the mutant gene with the deletion, delineating the fragment (dashed lines) amplified in (B) in individuals VI-1 and VI-3. (C) Sequence chromatogram showing where the deletion starts (sample sequence not matching the wild type sequence). Note the CATG preceding the breakpoint, which may map to either the 5′or the 3′ end of the deletion.
The GCH1 deletion was not found in subjects on the right-hand branch of the pedigree. However, individuals IV-7, V-6, IV-4, and IV-9 shared three STR genotypes with the affected subjects of the left-hand branch of the pedigree (figure 1), which did not encompass GCH1. In the CEPH database, the reported frequency for the alleles of the first two markers D14S283 and D14S275 was 25 and 30%, but the allele of the third marker D14S70 was not reported, suggesting that it is rare.
Coding mutations in SNCA and LRRK2 were excluded in Patients IV-9 and IV-10 with parkinsonism.
Clinical data in light of the genetic results
Two patients who were clinically diagnosed with probable DRD were shown to harbor the mutation and thus have genetically proven DRD (IV-1 and V-2).
In one of the patients initially diagnosed with clinically definite DRD (VI-4), our genetic evaluation failed to identify a GCH1 mutation. This patient had a complex clinical presentation: he not only exhibited dystonia, but he was also mildly mentally retarded, had had epileptic seizures, and had been treated with antipsychotics for behavioral troubles.
DISCUSSION
Our Swiss family displayed a dominantly inherited DRD phenotype, which affected seven genetically proven patients spanning three of the seven generations. We found a large genomic deletion in GCH1 that was sufficient to explain neurologic symptoms in the majority of family members. Genome-wide SNP linkage analysis failed to identify any other chromosomal region that segregated as well with the disease.
The phenotype in our seven patients was consistent with that reported in families with dominant DRD.21 The clinical picture ranged from minor effort-induced dystonic symptoms that were stable or even improved over 50 years, to patients with severe parkinsonism and dystonia requiring the use of a wheelchair.16,22,23 Parkinsonism was most pronounced in the elderly patients, in good agreement with previous reports.21 Gender seemed to have a major influence on phenotypic severity, since all three severely affected patients were women, and the fourth woman with milder symptoms had been treated with levodopa for a long time prior to examination. In contrast, the three male mutation carriers did not have significant functional impairment and as a result none was treated with levodopa. A penetrance 2.3 times higher in women than in men has been reported for DRD, and the female-to-male ratio is generally found to be around four.24 Previous studies have shown that basal GCH1 mRNA25–27 and protein28 expression levels are lower in nigrostriatal dopaminergic neurons as compared to neurons producing serotonin and norepinephrine. Moreover, expression was significantly lower in female than in male mice,26 suggesting that DRD may result from neuron-specific nigrostriatal GCH1 haploinsufficiency, which is more pronounced in females due to lower basal GCH1 gene expression.29
Response to low doses of levodopa was dramatic in all the young patients in whom it was assessed, without any dyskinesia upon treatment initiation.30 The beneficial effect of levodopa remained complete after more than 7 years, in agreement with previous reports.21 This suggests that levodopa treatment may prevent worsening of motor impairment, and underscores the importance of evaluating clinical improvement with levodopa in every dystonic patient.
Several reports have found a high prevalence of psychiatric manifestations in the form of depression, anxiety, or obsessive compulsive disorders.23,31 Although our evaluation protocol did not specifically focus on the detection of such symptoms, we did not find overt psychiatric disease. Also, our patients did not complain of significant sleep difficulties, as reported in prior studies.31
We identified a novel mutation in this DRD kindred, a 54.1 kb deletion in GCH1, spanning exons 3 to 6. The deletion may be explained by the presence of repetitive sequence elements, non-long terminal repeats retro-transposons, which we found to flank the deleted region. Our results show the previously described DYT14 locus in this family is erroneous, and call for its removal from the list of loci linked to DRD.12 Linkage to this region, which we reproduced in our first round of genetic investigations, probably resulted from misclassification of one subject as affected (VI-4). Careful reanalysis with more stringent criteria showed the haplotype segregating with the disease to include GCH1. Patient VI-4 was initially diagnosed with clinically definite DRD, and he later proved to be a phenocopy. Among the atypical features he presented, mental retardation may occur in DRD, although it is not a feature of the classic form of the disease.21 The shared alleles, which initially suggested linkage to the wrong locus, DYT14, could be relatively common among subjects from this specific region, even though some are not reported in the CEPH database.
Genetic testing allowed diagnosis to be established in two subjects who were previously only regarded as probably affected. In one of them, the very mild phenotype precluded a definite diagnosis, whereas in the other, diagnostic uncertainty came from the association with a second functionally impairing disease. Further illustrating the difficulty of correctly assigning affection status in this disease, two patients, without the mutation in GCH1, received a diagnosis of late onset dopa-responsive parkinsonism for which the cause remains to be identified.
In keeping with the clinically typical DRD findings in our family, brain MRI was normal in the index case (C.W., personal communication), and brain pathology confirmed severe hypomelanization in the substantia nigra without cell loss.12
Only eight DRD families with exon deletions in GCH1 have been described so far.8–11 This type of mutation probably causes disease phenotype by a mechanism of haploinsufficiency, with the enzyme produced from the intact gene not reaching sufficient amounts for normal dopamine metabolism.
This study rules out the previously reported DYT14 locus as a cause of DRD, and instead implicates the DYT5 locus (GCH1). In light of the previous linkage to the DYT14 locus, this study illustrates the major impact an individual’s affection status attribution can have in generating false-positive results. Prior linkage simulations studies, using appropriate model parameters, may highlight this pitfall. Our study also supports the view that gene dosage analysis is required to exclude mutations in GCH1 in DRD.
Acknowledgments
Supported by the Swiss National Science Foundation (C.W.), by Parkinson Switzerland (C.W.), and by the Robert and Clarice Smith Foundation (S.M., C.W.) through fellowships grants. S.M. was funded by NRSA postdoctoral fellowship #AG24030. G.K., M.J.F., J.M.K., and S.A.C. are supported by National Institute of Neurological Disorders and Stroke R01 # NS26.
The authors thank the patients and their families for their cooperation in this project.
GLOSSARY
- DRD
dopa-responsive dystonia
- GCH1
GTP cyclohydrolase I
- SNP
single nucleotide polymorphisms
- STR
short tandem repeats
Footnotes
e-Pub ahead of print at www.neurology.org.
Disclosure: The authors report no conflicts of interest.
References
- 1.Segawa M, Ohmi K, Itoh S, Aoyama M, Hayakawa H. Childhood basal ganglia disease with remarkable response to L-dopa, hereditary basal ganglia disease with marked diurnal fluctuation. Shinryo (Tokyo) 1971;24:667–672. [Google Scholar]
- 2.Segawa M, Hosaka A, Miyagawa F, Nomura Y, Imai H. Hereditary progressive dystonia with marked diurnal fluctuation. Adv Neurol. 1976;14:215–233. [PubMed] [Google Scholar]
- 3.Nygaard TG, Marsden CD, Duvoisin RC. Dopa-responsive dystonia. Adv Neurol. 1988;50:377–384. [PubMed] [Google Scholar]
- 4.Ichinose H, Ohye T, Takahashi E, et al. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat Genet. 1994;8:236–242. doi: 10.1038/ng1194-236. [DOI] [PubMed] [Google Scholar]
- 5.Knappskog PM, Flatmark T, Mallet J, Ludecke B, Bartholome K. Recessively inherited L-DOPA-responsive dystonia caused by a point mutation (Q381K) in the tyrosine hydroxylase gene. Hum Mol Genet. 1995;4:1209–1212. doi: 10.1093/hmg/4.7.1209. [DOI] [PubMed] [Google Scholar]
- 6.Thony B, Blau N. Mutations in the BH4-metabolizing genes GTP cyclohydrolase I, 6-pyruvoyl-tetrahydropterin synthase, sepiapterin reductase, carbinolamine-4a-dehydratase, and dihydropteridine reductase. Hum Mutat. 2006;27:870–878. doi: 10.1002/humu.20366. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa Y. Update on dopa-responsive dystonia: locus heterogeneity and biochemical features. Adv Neurol. 2004;94:127–138. [PubMed] [Google Scholar]
- 8.Furukawa Y, Guttman M, Sparagana SP, et al. Dopa-responsive dystonia due to a large deletion in the GTP cyclohydrolase I gene. Ann Neurol. 2000;47:517–520. [PubMed] [Google Scholar]
- 9.Klein C, Hedrich K, Kabakci K, et al. Exon deletions in the GCHI gene in two of four Turkish families with dopa-responsive dystonia. Neurology. 2002;59:1783–1786. doi: 10.1212/01.wnl.0000035629.04791.3f. [DOI] [PubMed] [Google Scholar]
- 10.Hagenah J, Saunders-Pullman R, Hedrich K, et al. High mutation rate in dopa-responsive dystonia: detection with comprehensive GCHI screening. Neurology. 2005;64:908–911. doi: 10.1212/01.WNL.0000152839.50258.A2. [DOI] [PubMed] [Google Scholar]
- 11.Steinberger D, Trubenbach J, Zirn B, Leube B, Wildhardt G, Muller U. Utility of MLPA in deletion analysis of GCH1 in dopa-responsive dystonia. Neuro-genetics. 2007;8:51–55. doi: 10.1007/s10048-006-0069-6. [DOI] [PubMed] [Google Scholar]
- 12.Grotzsch H, Pizzolato GP, Ghika J, et al. Neuropathology of a case of dopa-responsive dystonia associated with a new genetic locus, DYT14. Neurology. 2002;58:1839–1842. doi: 10.1212/wnl.58.12.1839. [DOI] [PubMed] [Google Scholar]
- 13.Puffenberger EG, Hu-Lince D, Parod JM, et al. Mapping of sudden infant death with dysgenesis of the testes syndrome (SIDDT) by a SNP genome scan and identification of TSPYL loss of function. Proc Natl Acad Sci USA. 2004;101:11689–11694. doi: 10.1073/pnas.0401194101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackenzie IR, Baker M, West G, et al. A family with tau-negative frontotemporal dementia and neuronal intranuclear inclusions linked to chromosome 17. Brain. 2006;129:853–867. doi: 10.1093/brain/awh724. [DOI] [PubMed] [Google Scholar]
- 15.Hiekkalinna T, Terwilliger JD, Sammalisto S, Peltonen L, Perola M. AUTOGSCAN: powerful tools for automated genome-wide linkage and linkage disequilibrium analysis. Twin Res Hum Genet. 2005;8:16–21. doi: 10.1375/1832427053435382. [DOI] [PubMed] [Google Scholar]
- 16.Steinberger D, Weber Y, Korinthenberg R, et al. High penetrance and pronounced variation in expressivity of GCH1 mutations in five families with dopa-responsive dystonia. Ann Neurol. 1998;43:634–639. doi: 10.1002/ana.410430512. [DOI] [PubMed] [Google Scholar]
- 17.Sobel E, Sengul H, Weeks DE. Multipoint estimation of identity-by-descent probabilities at arbitrary positions among marker loci on general pedigrees. Hum Hered. 2001;52:121–131. doi: 10.1159/000053366. [DOI] [PubMed] [Google Scholar]
- 18.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative fronto-temporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 19.Kohany O, Gentles A, Hankus L, Jurka I. Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinformatics. 2006;7:474. doi: 10.1186/1471-2105-7-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 21.Segawa M, Nomura Y, Nishiyama N. Autosomal dominant guanosine triphosphate cyclohydrolase I deficiency (Segawa disease) Ann Neurol. 2003;54:S32–45. doi: 10.1002/ana.10630. [DOI] [PubMed] [Google Scholar]
- 22.Nygaard TG, Trugman JM, de Yebenes JG, Fahn S. Dopa-responsive dystonia: the spectrum of clinical manifestations in a large North American family. Neurology. 1990;40:66–69. doi: 10.1212/wnl.40.1.66. [DOI] [PubMed] [Google Scholar]
- 23.Hahn H, Trant MR, Brownstein MJ, Harper RA, Milstien S, Butler IJ. Neurologic and psychiatric manifestations in a family with a mutation in exon 2 of the guanosine triphosphate-cyclohydrolase gene. Arch Neurol. 2001;58:749–755. doi: 10.1001/archneur.58.5.749. [DOI] [PubMed] [Google Scholar]
- 24.Furukawa Y, Lang AE, Trugman JM, et al. Gender-related penetrance and de novo GTP-cyclohydrolase I gene mutations in dopa-responsive dystonia. Neurology. 1998;50:1015–1020. doi: 10.1212/wnl.50.4.1015. [DOI] [PubMed] [Google Scholar]
- 25.Lentz SI, Kapatos G. Tetrahydrobiopterin biosynthesis in the rat brain: heterogeneity of GTP cyclohydrolase I mRNA expression in monoamine-containing neurons. Neurochem Int. 1996;28:5–6. 569–582. doi: 10.1016/0197-0186(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 26.Shimoji M, Hirayama K, Hyland K, Kapatos G. GTP cyclohydrolase I gene expression in the brains of male and female hph-1 mice. J Neurochem. 1999;72:757–764. doi: 10.1046/j.1471-4159.1999.0720757.x. [DOI] [PubMed] [Google Scholar]
- 27.Hirayama K, Kapatos G. Human nigrostriatal dopamine neurons express low levels of GTP cyclohydrolase I mRNA. In: Milstien S, Kapatos G, Levine RA, Shane B, editors. Chemistry and biology of pteridines and folates Proceedings of the 12th International Symposium on Pteridines and Folates. Boston: Kluwer Academic Publishers; 2002. pp. 291–295. [Google Scholar]
- 28.Hirayama K, Kapatos G. Nigrostriatal dopamine neurons express low levels of GTP cyclohydrolase I protein. J Neurochem. 1998;70:164–170. doi: 10.1046/j.1471-4159.1998.70010164.x. [DOI] [PubMed] [Google Scholar]
- 29.Kapatos G, Hirayama K. GTP cyclohydrolase I Gene expression and catecholamine synthesis. In: Nabeshima T, McCarty R, Goldstein DS, editors. Catecholamine research: from molecular insights to clinical medicine. Boston: Kluwer Academic Publishers; 2002. pp. 143–146. [Google Scholar]
- 30.Segawa M, Nomura Y, Yamashita S, et al. Long-term effects of L-dopa on hereditary progressive dystonia with marked diurnal fluctuation. In: Berardelli A, Benecke R, Manfredi M, Marsden CD, editors. Motor disturbances II. London: Academic Press; 1990. pp. 305–318. [Google Scholar]
- 31.Van Hove JL, Steyaert J, Matthijs G, et al. Expanded motor and psychiatric phenotype in autosomal dominant Segawa syndrome due to GTP cyclohydrolase deficiency. J Neurol Neurosurg Psychiatry. 2006;77:18–23. doi: 10.1136/jnnp.2004.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]


