Abstract
Background
Phase synchronization of neural activity preceding a motor act may reflect an “efference copy” of the motor plan and its expected sensory consequences (“corollary discharge”), which is sent to sensory cortex to herald the arrival of self-generated sensations and dampen the resulting sensory experience. We performed time-frequency decomposition of response-locked EEG to examine phase synchronization of oscillations across trials (phase-locking factor, PLF) to self-paced button-presses. If pre-press PLF reflects the activity in motor cortex, it should be contra-lateralized. If it reflects the action of the efference copy, it should be related to subsequent sensory suppression. If efference copy/corollary discharge mechanisms are abnormal in schizophrenia, it should be reduced in patients with schizophrenia.
Method
EEG was collected while 23 patients (20 schizophrenia; 3 schizoaffective) and 25 age-matched controls pressed a button, at will, every 1–2s. PLF preceding and following button-presses was calculated from single-trial EEG; averaging single trials yielded response-locked event-related potentials (ERPs) to the tactile response associated with button-pressing.
Results
Consistent with its hypothesized reflection of efference copy/corollary discharge signals, pre-press gamma band neural synchrony was 1) maximal over the contra-lateral sensory-motor cortex in healthy subjects, 2) correlated with the ipsi-lateralized somatosensory ERP amplitude evoked by the press, and 3) reduced in patients. Pre-press neural synchrony in the beta band was also reduced in patients, especially those with avolition/apathy.
Conclusions
These data are consistent with dysfunction of forward model circuitry in schizophrenia and suggest that the specific motor-sensory system affected is selectively linked to symptoms involving that system.
Keywords: Schizophrenia, corollary discharge, EEG, neural oscillations, motor
Introduction
A forward model system involving transmission of an “efference copy” of motor commands to sensory cortex to generate ”corollary discharges” that prepare it for impending sensory consequences of self-initiated motor acts has been described as a mechanism that helps us unconsciously disregard sensations resulting from our own actions as we maneuver through our environment. Helmholtz first described a forward model mechanism that allows us to discriminate between moving objects and movements on the retina resulting from eye movements(1). Von Holst and Mittelstadt(2) and Sperry(3) later suggested that a motor action is accompanied by an “efference copy” of the action that produces a “corollary discharge” representation of its expected sensory consequences in sensory cortex.
Although not always couched in terms of a forward model, evidence is emerging that perception is accomplished by a dynamic matching of sensory expectations with sensory input in a combined top-down/bottom-up fashion(4). Coordination between neural populations or cortical regions may be “self-organizing”(5), with temporal synchronization between firing rates of spatially segregated and distributed neuronal assemblies being an “emergent property” of neural networks. Thus, top-down processes such as selective attention(6) enhance the phase locking of neural oscillations at specific frequency bands to incoming stimuli, promoting enhanced perception.
Similarly, the forward model may involve a dynamic matching of incoming sensory signals with experience-shaped “libraries” of percepts. It has been suggested that the synchronization of ongoing neural oscillations by the stimulus may reflect “binding” of separately processed, and neuro-anatomically segregated, stimulus features with each other and with expectations(e.g., 7). Perceptual enhancement associated with attention is adaptive, whereby organisms can quickly evaluate and categorize potentially important environmental events. However, unlike the post-stimulus synchronization of neural oscillations that occur in response to environmentally-generated sensory input, the sensory expectancies or corollary discharges associated with motor acts precede the self-generated re-afferent inputs to sensory cortex and are “subtracted” from them once they arrive(8).
Because the forward model mechanism involves coordinated motor-sensory communication, enhancement of neural synchrony might be evident prior to execution of motor acts. In fact, local field potential recordings from somatosensory cells in rats showed neural synchrony preceding exploratory whisking in the 7–12Hz(9) and 30–35Hz(10) bands, perhaps triggered by the transfer of an efference copy of motor preparation to somatosensory cortex, seen as oscillations occurring several hundred milliseconds before the action.
Failures of the corollary discharge(11) or forward model mechanism(12) may contribute to self-monitoring deficits and some symptoms of schizophrenia. If efference copies of willed intentions do not produce corollary discharges of their expected sensory consequences, patients with schizophrenia may fail to recognize that they are the source of their own actions, resulting in feelings of being controlled by outside forces. On a more basic level, this failure could also result in poor on-line, millisecond-to-millisecond correction of movements, and motor awkwardness typical of the illness might result(e.g., 13). Ultimately, abnormal kinesthetic experiences may diminish the motivation for action.
Behavioral and electrophysiological evidence for corollary discharge dysfunction in schizophrenia is growing. Using the N1 component of the event-related potential (ERP), we showed that auditory cortical response dampening observed in controls during talking was not evident in patients with schizophrenia (14). Deficits in self-monitoring of action may also underlie the failure of schizophrenic patients to correct action errors when only proprioceptive feedback is available(15). The normal attenuation of sensation from self-produced touch was not as evident in patients with hallucinations and/or passivity experiences(16), patients with negative symptoms who had failures of willed actions(17), and patients demonstrating sensory prediction deficits during movements(18). All could result from a dysfunction of the corollary discharge system and/or associated insufficient suppression of the sensory re-afference.
Given the close temporal proximity of corollary discharge signals and the subsequent sensory re-afference, real-time measures of neural signals, such as EEG and ERPs, are needed to examine neural activity preceding, during, and following the action. Recently, time-frequency decomposition of event-locked single-trial EEG epochs has been developed for finer-grained examination of neural oscillations across both frequency and time domains. Phase-locking factor (PLF) (19) estimates phase synchronization of neural oscillations across individual event-locked EEG epochs, at specific frequencies, independent of power. It reflects “temporal phase coherence” of oscillations with respect to an event, across trials, and is different from “spatial EEG coherence” (see 20), or spatial phase coherence(21), which are calculated between different electrode sites. Neural synchrony may be involved in mechanisms of sensorimotor integration and provide voluntary motor systems with continually updated feedback on performance(22).
Also of interest in studies of the corollary discharge is suppression of the neural response to the sensory re-afference following a self-initiated action. While phase resetting to the onset of an external stimulus may reflect sensation(23), early components of the averaged ERP are traditionally used to assess “gating” of sensation preceding and during motor movements(24–31). For example, Rosini et al noted that early (<50ms) somatosensory ERPs to median nerve shocks are reduced contra-laterally, but not ipsi-laterally, during motor movement conditions compared to conditions with no-motor movement, suggesting that the gating of sensation is specific to the contra-lateral hemisphere. Thus, ample evidence shows that the somatosensory ERP is relatively ipsi-lateralized when a somatosensory stimulus is delivered to a limb during self-generated movement, compared to the contra-lateralized response observed when the stimulus is delivered to a stationary limb. On the basis of this literature and the theoretical argument that contra-lateral suppression of the somatosensory ERP during movement reflects the action of an efference copy/corollary discharge mechanism, we subtracted the post-button press somatosensory ERP measured over sites contra-lateral to the response hand from the ERP measured ipsi-lateral to the response hand and reasoned that the degree of ipsi-lateralization (i.e., reduced contra-lateralization) reflects the degree of corollary discharge-mediated somatosensory suppression. If true, we hypothesized that this relative ipsi-lateralization would be less evident in schizophrenia (due to their presumed corollary discharge dysfunction) and would be directly related to the strength of pre-press measures of the efference copy, at least in normal subjects. Thus, by positing on theoretical grounds that a pre-press efference copy signal should be directly related to the ipsi-lateralization of the post-press ERP, we set criteria for evaluating our hypothesis that pre-press neural synchrony reflects the efference copy signal that is instrumental in predicting and dampening the (somato)sensory consequences of our own motor acts.
Current Approach
Our first goal in this study was to quantify the synchrony of neural events associated with the hypothesized efference copy during initiating a button-press. Because of the contralateral organization of the sensorimotor system, we hypothesized that an efference copy of a planned action should arise in contra-lateral, but not ipsi-lateral, motor cortex, and be apparent before but not after an action. Accordingly, we compared trial-to-trial synchronous neural activity preceding and following a button-press, from contra- and ipsi-lateral sites.
Our second goal was to relate possible measures of the efference copy to subsequent suppression of sensory re-afference. If the contra-lateralized pre-press neural synchrony were a reflection of the efference copy, it should be related to post-press suppression of the tactile experience associated with pressing the button. Accordingly, we related pre-press synchrony to post-press ERP suppression.
Our third goal was to assess deficits in the efference copy in patients with schizophrenia. To the extent that pre-press neural synchrony reflects the efference copy, and to the extent that patients have abnormalities in the efference copy, we hypothesized that they would have deficits in pre-press neural synchrony. We compared pre- and post-press synchrony, from contra- and ipsi-lateral sites in controls and patients with schizophrenia.
Our fourth goal was to relate motor system efference copy abnormalities to symptoms in the motor domain. To the extent that pre-press neural synchrony reflects the efference copy in the motor system, we hypothesized that pre-press neural synchrony would be especially reduced in patients with deficits in the initiation of actions, such as avolition and apathy, and delusions that their motor actions were not their own, such as delusions of control.
Methods and Materials
Participants
EEG data were acquired from 24 patients and 26 healthy comparison subjects. All gave written informed consent after procedures had been fully described. Data from one patient and one control contained high frequency noise and were dropped from the analysis. Demographic and clinical data for the remaining subjects are summarized in Table 1.
Table 1.
Demographics of populations studied
| Normal Control Subjects n=25 | Schizophrenic Patients n=23 | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | min | max | Mean | SD | min | max |
| Age (years) | 42.20 | 10.80 | 25 | 72 | 42.04 | 10.59 | 21.0 | 67.0 |
| Controls vs. patients, p=.96 | ||||||||
| Education (years) | 15.44 | 1.66 | 12.0 | 19.0 | 14.02 | 2.10 | 11.0 | 18.0 |
| Controls vs. patients, p=.01 | ||||||||
| Average Parental Socioeconomic Status | 38.10 | 15.32 | 9.5 | 77.0 | 36.80 | 9.87 | 22.0 | 58.0 |
| Controls vs. patients, p=.74 | ||||||||
| Mean Symptom Scores | ||||||||
| Global Avolition/Apathy (SANS*) | 1.32 | 0.82 | 0 | 3 | ||||
| Hallucinatory Behavior (BPRS**) | 3.07 | 1.69 | 1 | 6 | ||||
| Delusions of Being Controled (SAPS*) | 1.38 | 1.43 | 0 | 3.5 | ||||
| Handedness | All right handed | All right handed | ||||||
| Gender | 18 men, 7 women | 21 men, 2 women | ||||||
| Diagnosis | 11 Undifferentiated Schizophrenia | |||||||
| 7 Paranoid Schizophrenia | ||||||||
| 3 Schizo-Affective | ||||||||
| 2 Residual | ||||||||
| Medication type | 21 Atypical, 2 Typical | |||||||
| Medication dose (chlorpromazine equivalents in mg)*** | 682.00 | 572.00 | 100.00 | 2451.00 | ||||
Scoring on SANS and SAPS: 0=not present, 6=extremely severe
Scoring on BPRS: 1=not present, 7=extremely severe
Dose missing for one patient
Patients were recruited from community mental health centers and inpatient and outpatient services of the Veterans Affairs Healthcare System in Palo Alto and San Francisco, CA. All were on stable doses of antipsychotic medications and met DSM-IV (32) criteria for schizophrenia (n=20) or schizoaffective disorder (n=3) based on a Structured Clinical Interview for DSM-IV (SCID; (33)) conducted by a clinical psychologist, or a SCID conducted by a clinically trained research assistant followed by a clinical interview with a clinical psychologist. Patients meeting DSM-IV criteria for alcohol or drug abuse within 30 days prior to study were excluded. In addition, patient and control participants were excluded for significant head injury, neurological disorders, or other medical illnesses compromising the central nervous system. Hallucinatory Behavior was rated using the Brief Psychiatric Rating Scale (BPRS)(34), Avolition/Apathy with the Scale for Assessment of Negative Symptoms (SANS)(35), and Delusions of Control with the Scale for Assessment of Positive Symptoms(36). For correlations reported here, symptom ratings were derived from the mean of two independent raters, for all but one of the patients. There were reasonably high intra-class correlations for Hallucinatory Behavior (.95), Avolition/Apathy (.77), and Delusions of Control (.93).
Comparison subjects were recruited by newspaper advertisements and word-of-mouth, screened by telephone using questions from the SCID(33) non-patient screening module, and excluded for history of Axis I psychiatric illness.
Task
Subjects were asked to press a button with the index finger of their right hand, at will, every 1–2 seconds. They were stopped after about 2 minutes of pressing, or after about 50–60 presses. This was a control task for auditory experiments described elsewhere(37). The button was fabricated to minimize clicking, and subjects wore sound-occluding headphones.
EEG / ERP Acquisition
EEG data were acquired (0.05–100Hz bandpass filter, 1000Hz analog-to-digital conversion rate) from 42 scalp sites (including mastoids) referenced to the nose, and re-referenced to linked mastoids. Vertical and horizontal electro-oculograms (VEOG, HEOG) were recorded from the outer canthi of both eyes and above and below the right eye. Button-presses inserted event codes into the data. Following correction for eye movements and blinks using VEOG and HEOG(38), trials containing voltages > ± 100µV in ventral (FC5, FC6) and dorsal (FC3, FC4) frontal-central sites were rejected. EEG epochs spanned 1060ms centered on the button-press, as there were some very short inter-press intervals (<500ms), due to the self-paced nature of the experiment. In controls, an average of 52.2 trials remained after artifact rejection, and 60.6 remained for the patients.
EEG Analysis
The time-frequency analysis was done with a Morlet wavelet decomposition using FieldTrip software(39). Its Gaussian shape was defined by a constant ratio (σf = f/7) and wavelet duration (4σt), where f is the center frequency and σt = 1/(2πσf). At 40Hz, the wavelet duration is over four cycles (4σt = 111.4ms) with a spectral bandwidth of 4σf = 22.8179Hz. Typical wavelet decompositions convolve the EEG signal with complex wavelets for all frequencies of interest, moving sample-by-sample in the time domain. FieldTrip achieves the same result by multiplying the Fast Fourier Transform (FFT) of the wavelet by the FFT of the EEG signal. The inverse FFT of the resultant is then adjusted so that the time course of the data corresponds to the time course of the original signal. This series of calculations progresses in 1Hz steps in the frequency domain and is equivalent (40) and computationally more efficient than convolution in the time domain.
After applying this method to every trial for the frequencies between 8–80Hz at FC3, FC4, FC5, and FC6, the PLF was calculated, as 1 minus the phase variance. This calculation is identical to that used by others(39). High phase locking at a specific frequency and within a specific time window indicates that oscillations have become phase-synchronized across trials with respect to event onset. Although slow activity is of theoretical interest to us, our major focus was on distinguishing pre- and post-press activity, best done at higher frequencies where the mother wavelets have a shorter duration.
EEG measures of efference copy
As can be seen in Figure 1, controls had increased synchrony between −150 and −75ms preceding the (right-handed) button-press over contralateral (i.e., left hemisphere) sites around 40Hz (gamma band: 36–45Hz) and 20Hz (beta band: 16–24Hz). PLF values within these frequency bands were averaged into three 25ms time bins between −150 and −75ms (pre-press) and a comparable post-press interval (75 to 150ms). This was done for two contra-lateral (FC3, FC5) and two ipsi-lateral sites (FC4, FC6). Data were subjected to a 6-way analysis of variance (ANOVA) for Group (2), Band (2), Pre-Post Press (2), Dorsal/Ventral Area (2), Hemisphere (2), and Time (3).
Figure 1.
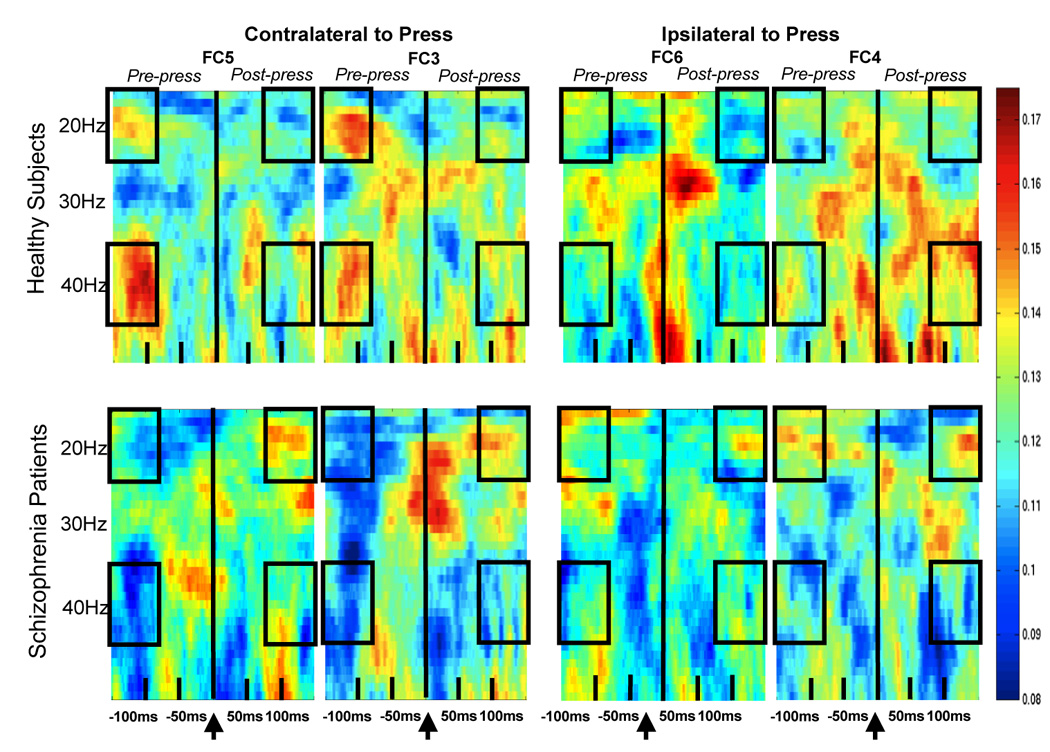
Phase-locking factor plots from healthy comparison subjects are shown for FC5, FC3 (contra-lateral to the press) on the upper left and for FC6, FC4 (ipsi-lateral to the press) on the upper right. The same is shown for patients with schizophrenia, below. EEG frequency is indicated on the y-axis and spans 16–50Hz. Time is indicated on the x-axis and spans −150 to 150ms. The button-press occurred at 0ms. Greater trial-to-trial PLF, reflecting phase synchronization with respect to the button-press onset, is shown in hot colors, as indicated on the color scale located at the far right of the plots. Boxes indicate regions subjected to statistical analysis.
Other features of PLF
As can also be seen in Figure 1, there were bursts of synchronous activity that spanned the moment of button pressing in both groups, at different sites and frequencies. Because the activity in these regions did not significantly distinguish pre- from post-press activity, blurring the distinction between efference copy and re-afference signals, it is not discussed here. Not apparent in our (short-epoch, high frequency) analysis is the possible desynchronization of the mu rhythm (8–14Hz), typically seen 1500ms before movements in human subjects(41).
ERP Analysis
As can be seen in Figure 2, the evoked activity following the button press has a contralateral distribution over somatosensory areas in the patients (CP3>CP4; C3>C4), as if a tactile stimulus had been externally applied. In the controls, the contra-lateral distribution is minimized, perhaps due to the suppressing effects on sensation of the efference copy during the self-initiated motor action. After filtering the data (2–50Hz) to remove the slowest pre-motor potentials, ERP area was quantified between button press onset and 50ms, post-press, relative to a pre-press (−10 to 0ms) baseline. Data were subjected to a 3-way ANOVA for Group (2), Area (2), and Hemisphere (2).
Figure 2.
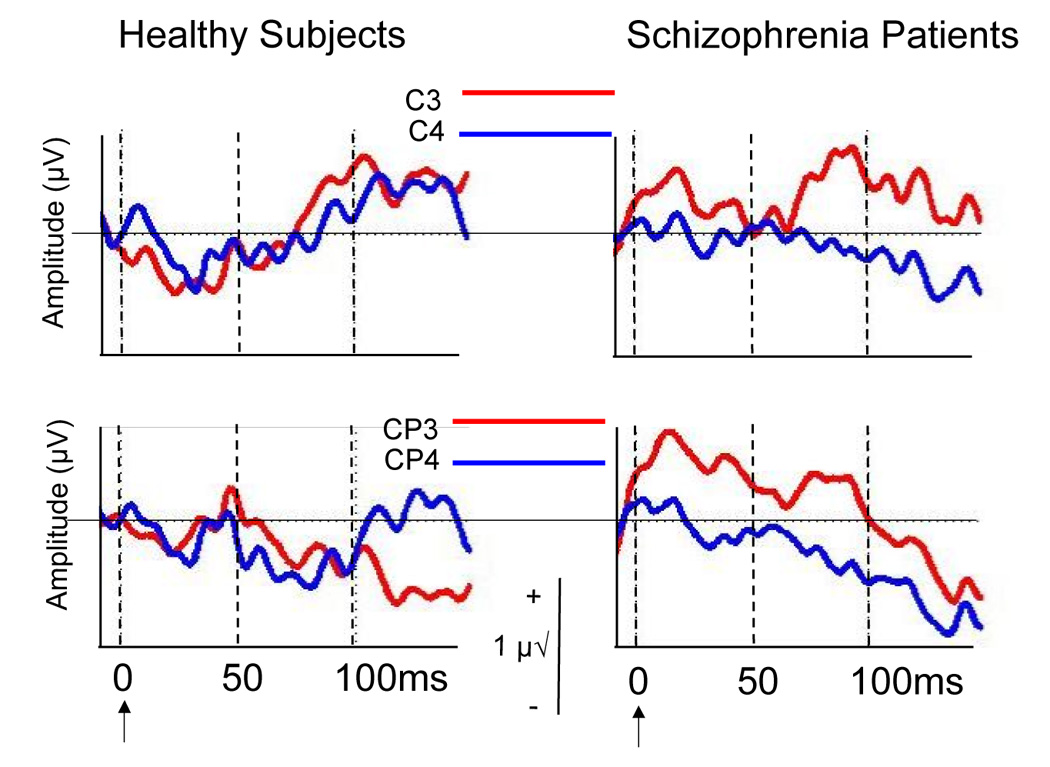
Somatosensory ERPs to the onset of the button press (arrow), with ERPs from C3 and C4 (top) and CP3 and CP4 (bottom) overlaid. Area from 0 to 50ms was analyzed. Time is indicated on the x-axis and spans −10 to 150ms. Amplitude is indicated on the y-axis, with negative voltages relative to mastoids, going down. Amplitude calibration bar is seen in center.
Relationships between Variables
Pre-press neural synchrony and sensory re-afference
Because the contra-lateralization of the somatosensory ERP elicited by index finger stimulation is suppressed during self-generated compared to externally-generated button-pressing(42), we presumed that contralateral suppression of the somatosensory ERP would be reflected in its relative ipsi-lateral distribution. To quantify this, we subtracted the voltage measured over left somatosensory cortex from that measured over the right (a larger value reflects suppression, or relative ipsi-lateralization). To assess relationships between pre-press neural synchrony and subsequent suppression of the post-press somatosensory re-afference, pre-press (−150 to −75ms) neural synchrony in both the gamma (36–45Hz) and beta bands (16–24Hz), averaged across the three 25ms time bins, were regressed on this right minus left area measure. To reduce the number of correlations, we averaged across C3/4 and CP3/4 for the somatosensory ERP area measure and FC3 and FC5 for the synchrony measure. Thus, one correlation analysis was done for gamma and one for beta. Overall amplitude of the somatosensory response was not correlated with the difference score for controls (r=.16, p=.45) or patients (r=.28, p=.21).
Pre-press neural synchrony and symptoms
Correlations were calculated to address the relationship between pre-press beta and gamma neural synchrony and Avolition/Apathy, Delusions of Control, and Hallucinatory Behavior. To reduce the number of tests, and because patients had greater synchrony at FC5 than FC3, we used only pre-press synchrony measures at FC5, collapsed across the three 25ms time bins in which this activity was evident. Because Delusions of Control were not normally distributed, we also used Spearman rank-order correlations.
Results
Number of Button Presses
Three patients pressed the button more than twice as fast as all the other subjects, affecting group differences on this variable. When these 3 were dropped from the behavioral analysis, schizophrenia patients did not press more often than controls, F(1, 43)=2.34, p=.13. Pressing the button quickly (inter-press interval) was not related to Apathy/Avolition (r=0.17, p=.48).
Neural Synchrony (PLF)
As seen in Figure 3, the predicted Hemisphere × Pre-post × Group interaction was significant (F(1,46)=9.18, p=.004), and this interaction was not affected by Area (p=.25) or Time (p=.34). We parsed this interaction by looking for a Group × Pre-post interaction separately for each hemisphere. The Group × Pre-post interaction was significant for the left (F(1,46)=7.65, p=.008) but not right (F(1,46)=.36, p=.55) hemisphere. Within the left hemisphere, we parsed the Group × Pre-post interaction by assessing the Pre-post effect in each group separately. In controls, left hemisphere pre-press synchrony was significantly greater than post-press (F(1,24)=5.53, p=.03), whereas in patients, a tendency for post-press synchrony to be greater than pre-press synchrony was not significant (F(1,22)=2.96, p=.09). This interaction was also parsed by looking for the Group × Hemisphere interaction in the pre-press and post-press epochs separately. It was significant in the pre-press epoch (F(1,14)=11.34, p<.001), but not in the post-press (F(1,46)=.72, p=.40). The Band × Group interaction (F(1,46)=4.04, p=.05) revealed controls had greater synchrony than patients in the gamma (F(1,46)=4.65, p=.036), but not in beta band (F(1,46)=0.01, p=.94). A Hemisphere × Area × Band × Group interaction (F(1,46)=5.66, p=.022) was parsed revealing an Area × Band × Group interaction over the right (F(1,46)=8.04, p=.007), but not left hemisphere (F(1,46)=1.17, p=.29). This was due to patients having more beta than gamma synchrony, at right dorsal sites (Area × Band: (F(1,22)=5.39, p=.03).i
Figure 3.
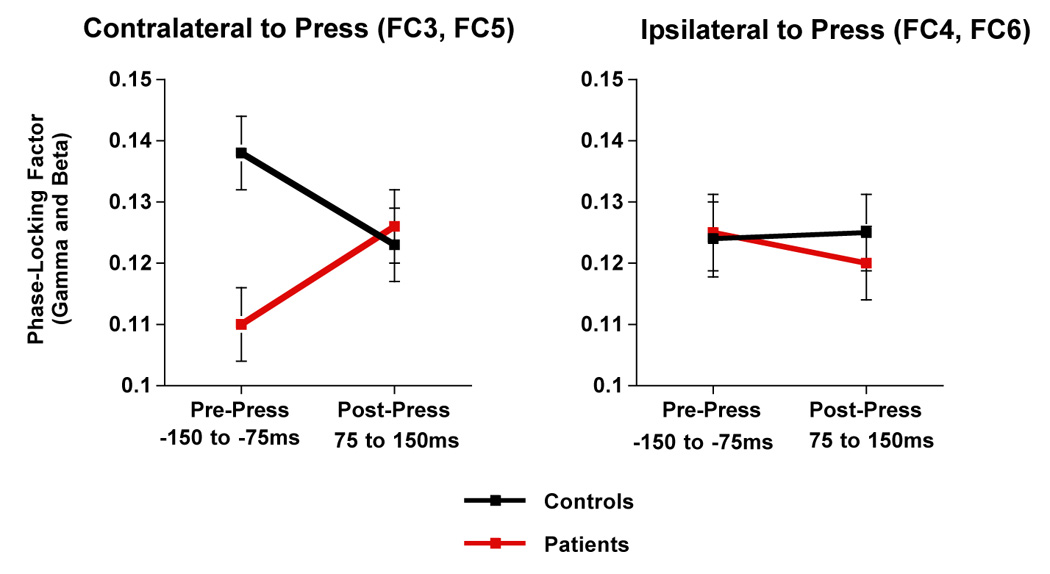
Means and standard error bars are plotted for the Hemisphere × Pre-Post × Group interaction for PLF. Area and Time are collapsed.
Sensory Re-afference: Somatosensory ERP
The somatosensory ERP was affected by a Hemisphere × Group interaction (F(1,46)=4.06, p=.05), being larger over left than right hemisphere in patients (F(1,22)=7.30, p=.013), but not in controls (F(1,24)=.103, p=.75).
Relationships between pre-press synchrony and re-afference
Pre-press gamma synchrony (−150 and −75ms) was directly related to the ipsi-lateralization of the somatosensory ERP area (Figure 4), in controls (r=.40, p<.05), but not patients (r=.05, p=.81). This was not seen for pre-press beta synchrony,
Figure 4.
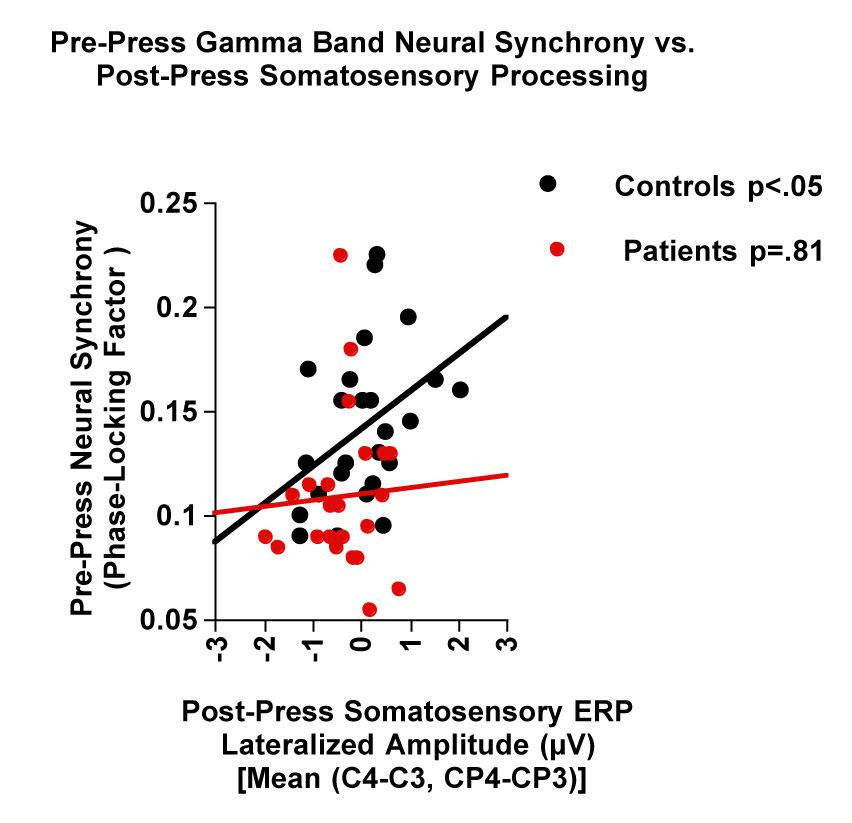
Scatter plots showing the relationship between pre-press neural synchrony (PLF) in the gamma band (−150 to −75ms) at FC3 + FC5 and post-press somatosensory ERP area between 0 and 50ms [right (C4, CP4) – left (C3, CP3]. Regression lines are drawn separately for controls and patients.
Pre-press neural synchrony and symptoms
Pre-press beta band synchrony over the left hemisphere (FC5), between −150 and −75ms, was significantly related to Avolition/Apathy (r=−.44, p<.04; rho=−0.49, p=.02) (Figure 5), but not Hallucinations (r=−.11, p=.6; rho=−.13, p=.55), or Delusions of Control (r=−.25, p=.25; rho=−.22, p=.30).
Figure 5.
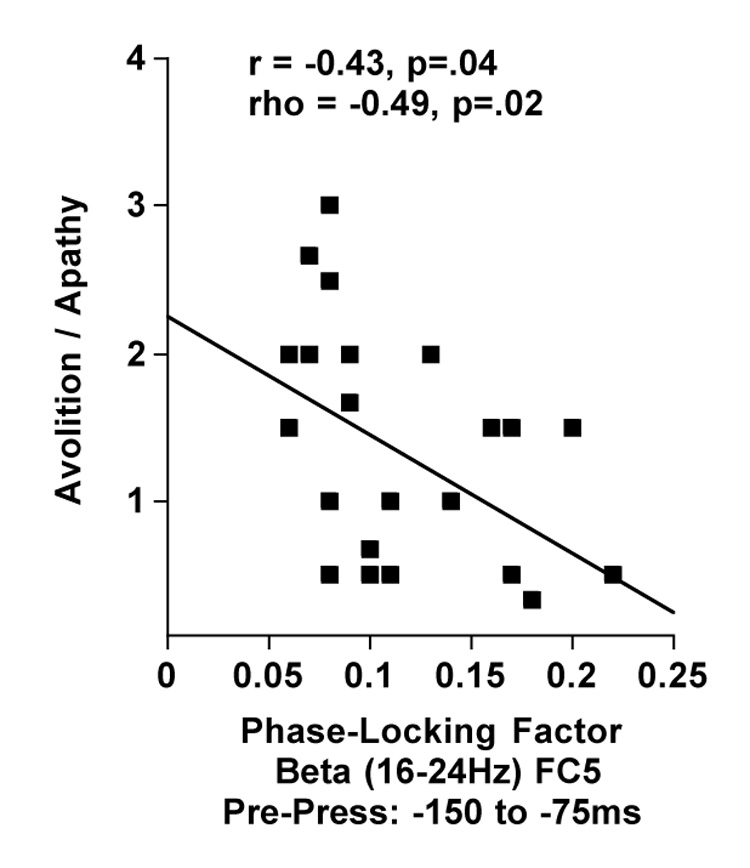
Scatter plots showing the relationship between avolition/apathy and neural synchrony (PLF) in the beta band over contra-lateral hemisphere (FC5) between −150 and −75ms before the press.
Gamma band synchrony was not related to Avolition/Apathy (r = −.15, p=.50; rho=−.12, p=.57), Hallucinations (r = −.04, p=.85; rho=−.13, p=.55), or Delusions of Control (r=−.39, p<.07; rho=−.25, p=.24).
Relationships between pre-press synchrony and medication dose
Because medications may have extra-pyramidal side effects affecting motor performance, we correlated chlorpromazine equivalents(43) and pre-press synchrony in the beta and gamma bands recorded from FC3 and FC5, averaged across the 3 time bins. Because there were 3 outliers on the medication variable, we used a non-parametric analysis. Antipsychotic dose was not related to pre-press synchrony in either the gamma (FC3: rho=0.21, p=.33; FC5: rho=.23, p=.29) or beta bands (FC3: rho=−0.012, p=.96; FC5: rho=−0.24, p=.27).
Discussion
Locally specialized brain functions must be coordinated with each other, and this coordination could be accomplished by synchronization of oscillatory activity among distributed neuronal assemblies(e.g., 44). The specific frequency of synchronous oscillations may identify neural populations as belonging to the same functional network(45). An emerging theoretical model of schizophrenia suggests dysfunctional regional coordination, communication, or connectivity(46), possibly associated with deficient synchronization of neuronal oscillations(e.g., 5,47), may be responsible for a wide-range of functional impairments, from psychotic experiences to cognitive deficits(47,48). Time-frequency analyses of EEG, time-locked to specific events, allow us to resolve changes in phase synchrony on a millisecond timescale to investigate integrated neural systems and their compromise in complex neuropsychiatric disorders.
Using time-frequency decomposition of EEG recorded during a simple self-paced button-pressing task, we examined phase synchrony of neural oscillations preceding the press. We found that healthy subjects showed an increase phase synchrony over the left hemisphere that was larger before a press than after it. This is consistent with the rodent literature, suggesting that this burst of synchronous pre-movement neural activity reflects the corollary discharge, preparing an organism’s central nervous system for the sensory consequences of its own actions(10). Based on the contra-lateral organization of the motor system, cortical reflections of motor acts should be contra-lateral to the movement, as seen in controls prior to the button-press.
Recently, we showed a strong negative correlation in healthy subjects between pre-speech neural synchrony in the beta band and suppression of the subsequent auditory ERP during talking, with listening as the control condition(14). We interpreted this as the efference copy suppressing the sensory re-afference. To the extent that pre-press synchrony reflects the efference copy of the action, we predicted a similar negative relationship with self-paced button-pressing. As described in the Introduction, the degree of ipsi-lateralization (i.e., reduced contra-lateralization) of the ERP during the button press was considered to reflect the degree of corollary discharge-mediated somatosensory suppression. Consistent with its reflecting an efference copy-related signal, pre-press gamma, but not beta, band synchrony was correlated with the degree of ipsi-lateralization of the post-press somatosensory ERP in healthy controls.
Consistent with the efference copy/corollary discharge mechanism being dysfunctional in patients(11,17), pre-press gamma synchrony was reduced in patients and was unrelated to ipsi-lateralization of the somatosensory ERP. Although we could not support pre-press beta synchrony as evidence of an efference copy in healthy controls, it was nevertheless reduced in patients especially those with avolition and apathy. We can only guess about the mechanism linking asynchrony in the beta band preceding motor acts to avolition, but we suggest that abnormal kinesthetic and proprioceptive experiences may ultimately diminish the motivation for action. Patients with schizophrenia show motor abnormalities in both brain imaging (49) and brain stimulation(50) studies. Furthermore, overt motor symptoms have been observed in neuroleptic naïve patients with schizophrenia(51), as well as during the premorbid and prodromal phases of the illness(13,52). Whether pre-movement asynchrony is related to rigorously assessed motor awkwardness and neurological soft signs remains to be addressed.
Notwithstanding the findings of others(16), our failure to find a relationship between auditory hallucinations and forward model-related signals in the sensorimotor system, coupled with our recent findings of such relationships when measures were obtained during talking(14), suggests that there may be some modality-specificity in the linkage of corollary discharge dysfunction to auditory hallucinations.
There are both limitations and benefits to using scalp recorded EEG measures of neural synchrony. Among the limitations is the lack of current understanding about what the different frequencies mean. For example, why does beta, but not gamma, synchrony correlate with avolition/apathy, and why does gamma synchrony, but not beta synchrony, correlate with subsequent somatosensory ERP amplitudes? Similarly, it is not immediately clear whether synchrony or de-synchrony of neural activity should reflect the sending of an efference copy from motor to sensory cortex. Among the advantages is the ease of translating human EEG data to animal research, and even to bench neuroscience. As basic neuroscience and clinical neuroscience converge, these questions can be addressed.
In conclusion, we propose that pre-movement asynchrony may reflect an elemental deficit in patients with schizophrenia, seen in both pre-speech and pre-button press data, reflecting dysfunction in the efference copy/corollary discharge mechanism across modalities.
Acknowledgments
We thank Drs. Alison Adcock and Sophia Vinogradov for referring patients to this study, Ryan Miller for help with data processing, and the patients and controls who participated.
This work was supported by the VA Schizophrenia Biological Research Center (JMF), VA Merit Review (JMF), NIMH (JMF, DHM), NARSAD (JMF, DHM), VA Career Award (DHM), Astra-Zeneca (DHM, JMF), Glaxo-Smith Kline (DHM).
This work was supported by grants from the VA Schizophrenia Biological Research Center, National Institute of Mental Health (MH40052, MH 58262, MH067967), the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD), and the Department of Veterans Affairs.
Footnotes
Evoked power and phase locking factor both reflect synchrony of activity across trials, but phase locking factor is independent of the amplitude of the signal, while evoked power depends on it. We calculated the evoked power surrounding the button press, and found a Group × Hemisphere × Pre-Post × Band interaction (F(1,46)=4.35, p=.04). We parsed this 4-way interaction, by looking for the Group × Hemisphere × Pre-Post interaction separately for beta and gamma band power. It was significant for beta power (F(1,46)=6.40, p=.015), but not for gamma. Although the pattern of means for PLF and power was similar, the Pre-Post × Group interaction for beta power over the left hemisphere, which we saw for PLF, was weaker and failed to reach significance for power (F(1,46)=3.28, p<.08). Failures to find significant effects for evoked power, which we were able to detect in the PLF, may be due to the fact that signals can be synchronized across trials, even if the power of that signal is too small to be detected in an analysis of evoked power.
Financial Disclosures During the last 2 years, Judith M. Ford has been funded by the VA, NIH, NARSAD, and Astra-Zeneca.
Brian J. Roach has no independent funding. He is paid from grants to Ford and Mathalon.
William O. Faustman has no independent funding. He is a clinical psychologist, paid by the Palo Alto VA.
During the last 2 years, Daniel H. Mathalon is funded by the VA, NIH, NARSAD, Astra-Zeneca, and Glaxo-Smith Kline.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Helmholtz H. Treatise on Physiological Optics. In: Southall JPC, editor. Optical Society of America. Rochester, NY; 1925. pp. 44–51. [Google Scholar]
- 2.Von Holst E, Mittelstaedt H. Das Reafferenzprinzip. Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- 3.Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. Journal of Comparative and Physiological Psychology. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- 4.Engel A, Fries P, Singer W. Dynamic predictions: oscillations and sychrony in top-down processing. Nat Rev Neurosci. 2001;10:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 5.Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- 6.Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- 7.Fries P, Neuenschwander S, Engel AK, Goebel R, Singer W. Rapid feature selective neuronal synchronization through correlated latency shifting. Nat Neurosci. 2001;4:194–200. doi: 10.1038/84032. [DOI] [PubMed] [Google Scholar]
- 8.Weiskrantz L, Elliott J, Darlington C. Preliminary observations on tickling oneself. Nature. 1971;230:598–599. doi: 10.1038/230598a0. [DOI] [PubMed] [Google Scholar]
- 9.Nicolelis MA, Baccala LA, Lin RC, Chapin JK. Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system. Science. 1995;268:1353–1358. doi: 10.1126/science.7761855. [DOI] [PubMed] [Google Scholar]
- 10.Hamada Y, Miyashita E, Tanaka H. Gamma-band oscillations in the "barrel cortex" precede rat's exploratory whisking. Neuroscience. 1999;88:667–671. doi: 10.1016/s0306-4522(98)00468-0. [DOI] [PubMed] [Google Scholar]
- 11.Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4:636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- 12.Frith CD, Blakemore S, Wolpert DM. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res Brain Res Rev. 2000;31:357–363. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 13.Cannon TD, Rosso IM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of neurodevelopmental processes in the genesis and epigenesis of schizophrenia. Dev Psychopathol. 1999;11:467–485. doi: 10.1017/s0954579499002163. [DOI] [PubMed] [Google Scholar]
- 14.Ford JM, Roach BJ, Faustman WO, Mathalon DH. Synch before you speak: Auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164:458–466. doi: 10.1176/ajp.2007.164.3.458. [DOI] [PubMed] [Google Scholar]
- 15.Turken AU, Vuilleumier P, Mathalon DH, Swick D, Ford JM. Are Impairments of Action Monitoring and Executive Control Dissociable Dysfunctions in Patients With Schizophrenia? Am J Psychiatry. 2003;160:1881–1883. doi: 10.1176/appi.ajp.160.10.1881. [DOI] [PubMed] [Google Scholar]
- 16.Blakemore SJ, Smith J, Steel S, Johnstone EC, Frith CD. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychol Med. 2000;30:1131–1139. doi: 10.1017/s0033291799002676. [DOI] [PubMed] [Google Scholar]
- 17.Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med. 1987;17:631–648. doi: 10.1017/s0033291700025873. [DOI] [PubMed] [Google Scholar]
- 18.Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry. 2005;162:2384–2386. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- 19.Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;21:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- 21.Uhlhaas PJ, Singer W. What do disturbances in neural synchrony tell us about autism? Biol Psychiatry. 2007;62:190–191. doi: 10.1016/j.biopsych.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav Brain Res. 2001;127:119–136. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- 23.Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- 24.Huttunen J, Homberg V. Modification of cortical somatosensory evoked potentials during tactile exploration and simple active and passive movements. Electroencephalogr Clin Neurophysiol. 1991;81:216–223. doi: 10.1016/0168-5597(91)90075-9. [DOI] [PubMed] [Google Scholar]
- 25.Jones SJ, Halonen JP, Shawkat F. Centrifugal and centripetal mechanisms involved in the 'gating' of cortical SEPs during movement. Electroencephalogr Clin Neurophysiol. 1989;74:36–45. doi: 10.1016/0168-5597(89)90049-x. [DOI] [PubMed] [Google Scholar]
- 26.Cheron G, Borenstein S. Specific gating of the early somatosensory evoked potentials during active movement. Electroencephalogr Clin Neurophysiol. 1987;67:537–548. doi: 10.1016/0013-4694(87)90056-3. [DOI] [PubMed] [Google Scholar]
- 27.Rossini PM, Caramia D, Bassetti MA, Pasqualetti P, Tecchio F, Bernardi G. Somatosensory evoked potentials during the ideation and execution of individual finger movements. Muscle Nerve. 1996;19:191–202. doi: 10.1002/(SICI)1097-4598(199602)19:2<191::AID-MUS11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 28.Tapia MC, Cohen LG, Starr A. Selectivity of attenuation (i.e., gating) of somatosensory potentials during voluntary movement in humans. Electroencephalogr Clin Neurophysiol. 1987;68:226–230. doi: 10.1016/0168-5597(87)90031-1. [DOI] [PubMed] [Google Scholar]
- 29.Cohen LG, Starr A. Localization, timing and specificity of gating of somatosensory evoked potentials during active movement in man. Brain. 1987;110(Pt 2):451–467. doi: 10.1093/brain/110.2.451. [DOI] [PubMed] [Google Scholar]
- 30.Rossini PM, Babiloni C, Babiloni F, Ambrosini A, Onorati P, Carducci F, Urbano A. "Gating" of human short-latency somatosensory evoked cortical responses during execution of movement. A high resolution electroencephalography study. Brain Res. 1999;843:161–170. doi: 10.1016/s0006-8993(99)01716-3. [DOI] [PubMed] [Google Scholar]
- 31.Wasaka T, Hoshiyama M, Nakata H, Nishihira Y, Kakigi R. Gating of somatosensory evoked magnetic fields during the preparatory period of self-initiated finger movement. Neuroimage. 2003;20:1830–1838. doi: 10.1016/s1053-8119(03)00442-7. [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association. Washington: American Psychiatric Association; Diagnostic and Statistical Manual of Mental Disorder (DSM-IV) 1994
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 34.Faustman WO, Overall JE. The Brief Psychiatric Rating Scale. In: Maruish M, editor. The Use of Psychological Testing For Treatment, Planning and Outcome Assessment. Second Edition. Lawrence Erlbaum Associates: Hillsdale, NJ; 1999. pp. 791–830. [Google Scholar]
- 35.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: University of Iowa; 1983. [Google Scholar]
- 36.Andreasen NC. Scale for the Assessment of Positive Symptoms. Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 37.Ford J, Gray M, Faustman W, Roach B, Mathalon D. Dissecting forward model dysfunction in schizophrenia. Psychophysiology. 2007;44:522–529. doi: 10.1111/j.1469-8986.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 38.Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 39.Tallon-Baudry C, Bertrand O, Delpuech C, Permier J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. J Neurosci. 1997;17:722–734. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyons R. Understanding Digital Signal Processing. Prentice Hall PTR; 2004. [Google Scholar]
- 41.Pfurtscheller G, Aranibar A. Evaluation of event-related desynchronization (ERD) preceding and following voluntary self-paced movement. Electroencephalogr Clin Neurophysiol. 1979;46:138–146. doi: 10.1016/0013-4694(79)90063-4. [DOI] [PubMed] [Google Scholar]
- 42.Mathalon DH, Colrain I, Gray EM, Ford JM. It's not my fault: ERPs to induced errors. In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts and the Brain Current Opinions on Performance Monitoring. Leipzig: MPI of Cognitive Neuroscience; 2004. pp. 27–35. [Google Scholar]
- 43.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 44.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 45.Bastiaansen MCM, Hagoort P. Oscillatory brain dynamics during language comprehension. In: Klimesch W, Neuper C, editors. Event-related dynamics of brain oscillations Progress in Brain Research Series. Elsevier; Amsterdam: 2006. [Google Scholar]
- 46.Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clinical Neurosciences. 1995;3:89–97. [PubMed] [Google Scholar]
- 47.Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci. 2003;26:65–82. doi: 10.1017/s0140525x03000025. discussion 82–137. [DOI] [PubMed] [Google Scholar]
- 49.Mattay VS, Callicott JH, Bertolino A, Santha AK, Tallent KA, Goldberg TE, Frank JA, Weinberger DR. Abnormal functional lateralization of the sensorimotor cortex in patients with schizophrenia. Neuroreport. 1997;8:2977–2984. doi: 10.1097/00001756-199709080-00034. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgerald PB, Brown TL, Marston NA, Oxley TJ, de Castella A, Daskalakis ZJ, Kulkarni J. A transcranial magnetic stimulation study of abnormal cortical inhibition in schizophrenia. Psychiatry Res. 2003;118:197–207. doi: 10.1016/s0165-1781(03)00094-5. [DOI] [PubMed] [Google Scholar]
- 51.Caligiuri MP, Lohr JB, Jeste DV. Parkinsonism in neuroleptic-naive schizophrenic patients. Am J Psychiatry. 1993;150:1343–1348. doi: 10.1176/ajp.150.9.1343. [DOI] [PubMed] [Google Scholar]
- 52.Walker E, Lewine RJ. Prediction of Adult-Onset Schizophrenia from Childhood Home Movies of the Patients. Am J Psychiatry. 1990;147:1052–1056. doi: 10.1176/ajp.147.8.1052. [DOI] [PubMed] [Google Scholar]


