Abstract
Background
The early visual-evoked gamma oscillation (VGO) elicited by Gestalt stimuli is reduced in schizophrenia patients compared to healthy individuals, but it is unknown whether this effect is specific to these particular stimuli and task. In contrast, the early auditory-evoked gamma oscillation (AGO) was reported to be unaffected in a sample of unmedicated, mostly first-episode schizophrenics (Gallinat et al, 2004, Clin Neurophysiol), but it is unknown whether this oscillation is abnormal in chronic, medicated patients. We investigated these issues by examining the VGO and AGO in chronic schizophrenics (SZ) and matched healthy controls (HC).
Methods
Subjects (21 HC, 23 SZ) performed visual and auditory oddball tasks. Visual stimuli were letters, and auditory stimuli were simple tones. Event-related spectral measures (phase locking factor and evoked power) were computed on Morlet wavelet-transformed single epochs from the standard trials.
Results
VGO phase locking at occipital electrodes was reduced in SZ compared to HC. In contrast, AGO phase locking and evoked power did not differ between groups.
Conclusions
The VGO deficit may be a general phenomenon in schizophrenia, while the AGO evoked by simple tone stimuli does not appear to be abnormal in chronic, medicated schizophrenics.
Keywords: schizophrenia, EEG, gamma oscillation, evoked potentials
Oscillatory neuronal synchronization in the gamma band (~30–100 Hz) of the electroencephalogram (EEG) has been proposed to play an important role in neural information coding (1). Gamma abnormalities have been hypothesized to reflect core neural circuit abnormalities and cognitive deficits in schizophrenia (2–7). Among the gamma oscillations reported in humans, the most reliably found are the early sensory-evoked oscillations (8). The visual-evoked gamma oscillation (VGO) occurs at 25–45 Hz and ~100 ms and is sensitive to attentional and perceptual factors (9). The auditory-evoked gamma oscillation (AGO) occurs at 30–60 Hz and ~50 ms and is also sensitive to attentional factors (10).
Previously we reported that VGO phase locking was reduced in chronic schizophrenia patients compared to healthy individuals in a Gestalt perception task (6, 11), consistent with electrophysiological and behavioral observations of dysfunctional early visual processing in schizophrenia (e.g., 12–15). However, it is unclear whether this deficit is specific to that particular task and/or stimuli, or occurs generally for visual stimuli independently of the task. In contrast, Gallinat et al. (16) observed no abnormalities of the AGO in schizophrenics, which is surprising since abnormalities of auditory cortex and early auditory processes have been consistently reported in schizophrenia (e.g., 17–19). Since Gallinat et al. examined only unmedicated patients, most of whom were first-episode, it is possible that an AGO deficit would be apparent in chronic, medicated schizophrenics.
Here we sought to determine whether: 1) the VGO deficit in schizophrenia is specific to the Gestalt perception task used previously, or can be found with other stimuli and tasks; and 2) the AGO is abnormal in chronic, medicated schizophrenics.
Method
Subjects
The study was approved by the Institutional Review Boards of the VA Boston Healthcare System and Harvard Medical School. Informed consent was obtained from all subjects, who were paid for their participation. Subjects (all male) were 21 healthy controls (HC) and 23 chronic schizophrenia patients (SZ). HC were recruited from the community and were free of Axis I or II disorders (20, 21), as well as a history of Axis I disorders in first-degree relatives. SZ were diagnosed with schizophrenia according to DSM-IV criteria (22). Subjects were selected without regard for ethnicity and met our standard inclusion/exclusion criteria: 1) right-handed (23); 2) no history of electroconvulsive treatment or neurological illness; 3) no alcohol or drug dependence within the last 5 years (DSM-IV criteria); 4) no present medication for medical disorders that would have deleterious EEG, neurological, or cognitive consequences; and 5) estimated verbal IQ above 75.
Demographic and clinical data and inter-group comparisons are summarized in the Table. All patients received atypical antipsychotics. None of the electrophysiological measures were correlated with antipsychotic dosage (2-tailed Spearman’s ρ < 0.252, p > 0.299), calculated as chlorpromazine equivalents (25, 26).
Stimuli and Experimental Design
Subjects performed oddball tasks in which they counted rare target stimuli (30/180 stimuli, 1200 ms stimulus onset asynchrony). In the Visual task, stimuli (2° height by 1.8° width) were presented at central fixation in white on a black background for 100 ms. Targets and standards were the letters “X” and “Y”, respectively. In the Auditory task, tones (70 ms duration, 10 ms rise/fall) were presented through headphones (70 dB sound pressure level) while subjects fixated a cross in the center of the monitor. The target and standard tone frequencies were 1200 and 1000 Hz, respectively. Task order was counterbalanced across subjects.
Electrophysiological Recording and Analysis
The EEG was recorded (0.01-100 Hz, 500 Hz digitization) with sintered Ag/Ag-Cl electrodes in an electrode cap at 60 scalp sites, nosetip, and left earlobe, referenced to the right earlobe, grounded at AFz. Bipolar vertical and horizontal electro-oculograms were recorded from electrodes above and below the right eye and at the left and right outer canthi, respectively. Electrode impedances were <10 kΩ. Single-trial epochs were extracted from −250 to 850 ms relative to stimulus onset and corrected for eye movements and blinks (27). Epochs containing artifacts were removed.
We examined the gamma oscillations evoked by standard stimuli, which were unconfounded by target-related processing. The Morlet wavelet transform was applied to single epochs in 1 Hz steps from 20–100 Hz and −250 to 772 ms. Event-related spectral measures were computed on the wavelet-transformed epochs at each time point and wavelet frequency to yield time-frequency maps (28). Phase locking factor (PLF) measures the variance of phase across single trials, independent of power. PLF is computed as one minus the circular variance of phases and ranges from 0 (random distribution) to 1 (perfect phase locking). Evoked power measures the power of the average evoked potential, in which the contribution of non-stimulus-locked activity is minimized. Average baseline values were subtracted from each time-frequency map (−150 to 0 ms).
In ANOVAs the Greenhouse-Geisser correction for inhomogeneity of variance (29) was applied for factors with more than 2 levels, and is reflected in the reported p values.
Further methodological details are given in the Supplementary Material.
Results
SZ and HC had highly accurate target counts in both oddball tasks (see Table). Evoked potential data are presented in the Supplementary Material.
Table.
Comparison of demographic and clinical variables for the HC and SZ groups. Mean +/− standard deviation are given for each variable.
| HC | SZ | Statistic | p | |
|---|---|---|---|---|
| Age (years) | 42.2 +/− 9.4 | 40.7 +/− 10.6 | F[1,42] = 0.245 | 0.623 |
| Parental socioeconomic status (ref. 24) | 2.8 +/− 1.3 | 2.7 +/− 1.2 | F[1,38] = 0.143 | 0.707 |
| Handedness | 0.75 +/− 0.17 | 0.72 +/− 0.24 | F[1,42] = 0.180 | 0.674 |
| Age of onset (years) | 22.6 +/− 5.8 | |||
| Medication dosage | 340 +/− 267 | |||
| (CPZ equivalents) | range 0–900 | |||
| Target count: visual | 29.7 +/− 3.4 | 29.3 +/− 1.6 | F[1,42] = 0.261 | 0.612 |
| Target count: auditory | 30.4 +/− 1.6 | 29.6 +/− 2.4 | F[1,42] = 1.72 | 0.197 |
The VGO (Fig. 1A) was apparent in the PLF but not the evoked power data, as we reported previously (6, 11), so evoked power was not further analyzed. PLF was measured between 30–38 Hz and 96–130 ms. The ANOVA design was Group X Hemisphere X Electrode. As previously observed, the VGO had components at frontal and occipital electrode sites (30). Occipital VGO PLF was measured at electrodes P1/2, PO1/2, PO7/8, and O1/2, and was significantly reduced in SZ compared to HC (F[1,42] = 4.76, p < 0.05). Frontal VGO PLF was measured at electrodes F1/2, F3/4, FC1/2, FC3/4, FC5/6, and C1/2, and did not differ between groups (F[1,42] = 1.09, p = 0.303).
Figure 1.
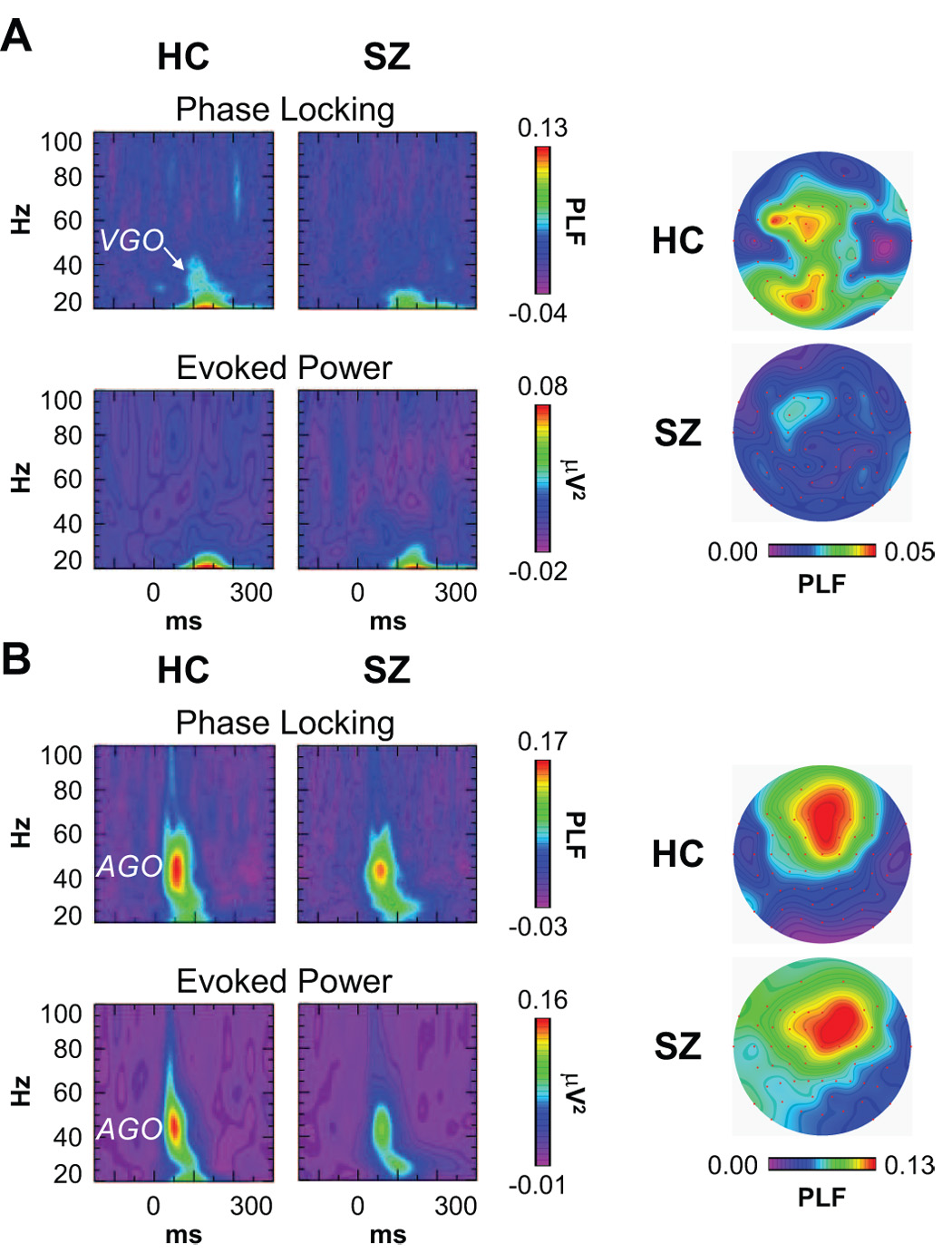
The visual- and auditory-evoked gamma oscillations (VGO/AGO) in healthy controls (HC) and chronic schizophrenia patients (SZ). (A) Visual data: Phase locking factor (PLF) and evoked power time-frequency maps at electrode O1 (left) and topographic maps of VGO PLF (right). The occipital VGO is present in HC but not SZ. (B) Auditory data: PLF and evoked power time-frequency maps at electrode Fz (left) and topographic maps of AGO PLF (right). There were no significant overall differences in either measure of the AGO between HC and SZ.
The AGO (Fig. 1B) was measured at fronto-central electrode sites (F1/2, F3/4, F5/6, FC1/2, FC3/4, FC5/6, C1/2, C3/4, and C5/6) between 34–54 Hz, in the 30–74 ms range for HC and 38–82 ms for SZ. The ANOVA design was Group X Hemisphere X Midline (Frontal/Fronto-central/Central) X Lateral Site (1/2, 3/4, 5/6). AGO PLF (F[1,42] = 0.404, p = 0.528) and evoked power (F[1,42] = 0.398, p = 0.531) did not differ between SZ and HC.
Discussion
VGO phase locking evoked by standard stimuli in an oddball task was reduced in SZ. Previously we reported that Gestalt stimuli evoked the VGO in healthy individuals but not in chronic schizophrenia patients (6, 11). One possible explanation was that this VGO deficit reflected a specific failure of synchronization to Gestalt stimuli. The present results rule this out because the stimulus used here was a letter.
Another explanation was that the VGO deficit reflected a failure of synchronization to “target-like” stimuli, as the VGO can be influenced by the degree to which a particular stimulus matches the target stimulus in a discrimination task (31). However, here the VGO was evoked in HC by standard stimuli in an oddball task that were not associated with a manual response, rather than target stimuli as in the Gestalt perception task. Thus, the VGO deficit has been found in two different tasks for different stimuli with differing response requirements. These results suggest that the VGO deficit may be a general phenomenon in schizophrenia, independent of task and stimulus type.
The origin of the VGO deficit is not clear. One possibility is some neural circuitry abnormality in visual cortex that prevents gamma-frequency synchronization from occurring. Another possibility is deficient attentional modulation from the dorsolateral prefrontal cortex (DLPFC) (32). In healthy individuals the VGO is influenced by attentional demands (33) and target-match effects (31, 34), so the failure of an attentional signal to enhance neuronal synchronization in visual cortex could impair the VGO. For instance, Barceló et al (35) found that attention-sensitive visual evoked potentials were reduced over the hemisphere ipsilateral to a DLPFC lesion. The VGO deficit could represent a similar failure of attentional modulation of sensory processing, given the evidence of DLPFC neural circuitry abnormalities (4, 36, 37) and attentional dysfunction (38) in schizophrenia.
The AGO was not abnormal in SZ, consistent with the finding of Gallinat et al. (16) that AGO evoked power did not differ between healthy individuals and unmedicated, mainly first-episode schizophrenics. Here the patients were chronic and medicated, so together these studies suggest that the AGO is generally not affected in schizophrenia. (Nor was an auditory N1 deficit found; see Supplementary Material.) The absence of an AGO deficit is surprising given the abundance of structural and functional abnormalities of the auditory system in schizophrenia (17–19), especially since the gamma-band auditory steady-state response is reduced in schizophrenia (39–41). But since our study and that of Gallinat et al. used oddball tasks with simple tone stimuli, it remains to be determined whether the absence of an AGO deficit is specific to this particular type of task and/or stimuli.
While none of the electrophysiological measures was correlated with antipsychotic medication dosage, possible influences of medication cannot be ruled out, given the inexact nature of chlorpromazine equivalency and the complex effects of atypical antipsychotics on neural activity.
Supplementary Material
Acknowledgments
Supported by a Department of Veterans Affairs Research Enhancement Award Program and Schizophrenia Center (RWM); National Institute of Mental Health grants R01 40799 (RWM) and R03 076760 (KMS); and a Young Investigator Award from the National Alliance for Research in Schizophrenia and Depression (KMS). The authors are grateful to Emily Fisher, Meredith Klump, Matthew Koskowski, Elizabeth Lewis, and Lisa Lucia for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Drs. Spencer, Niznikiewicz, Shenton, and McCarley report no conflicts of interest.
References
- 1.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 2.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical {gamma} synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Rev. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- 4.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 5.Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci. 2003;26:65–82. doi: 10.1017/s0140525x03000025. [DOI] [PubMed] [Google Scholar]
- 6.Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cog Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann CS, Munk MHJ, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cog Sci. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Näätänen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- 11.Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999;56:149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- 14.Clementz BA, Keil A, Kissler J. Aberrant brain dynamics in schizophrenia: delayed buildup and prolonged decay of the visual steady-state response. Cogn Brain Res. 2004;18:121–129. doi: 10.1016/j.cogbrainres.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, Bockbrader MA, O’Donnell BF. Steady state visual evoked potential abnormalities in schizophrenia. Clin Neurophysiol. 2005;116:614–624. doi: 10.1016/j.clinph.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57:692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry. 1995;52:550–558. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- 19.Rojas DC, Bawn SD, Carlson JP, Arciniegas DB, Teale PD, Reite ML. Alterations in tonotopy and auditory cerebral asymmetry in schizophrenia. Biol Psychiatry. 2002;52:32–39. doi: 10.1016/s0006-3223(01)01365-8. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R Non-Patient Edition (SCID-NP, Version 1.0) Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- 21.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P, Version 2.0) New York, NY: New York State Psychiatric Institute; 1995. [Google Scholar]
- 23.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 24.Hollingshead AB. Two Factor Index of Social Position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- 25.Stoll AL. The Psychopharmacology Reference Card. Belmont, MA: McLean Hospital; 2001. [Google Scholar]
- 26.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 27.Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr clin Neurophysiol. 1983;75:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 28.Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keselman HJ, Rogan JC. Repeated measures F tests and psychophysiological research: controlling the number of false positives. Psychophysiology. 1980;17:499–503. doi: 10.1111/j.1469-8986.1980.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 30.Tallon-Baudry C, Bertrand O, Wienbruch C, Ross B, Pantev C. Combined EEG and MEG recordings of visual 40 Hz responses to illusory triangles in human. NeuroReport. 1997;8:1103–1107. doi: 10.1097/00001756-199703240-00008. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann CS, Mecklinger A. Gamma activity in human EEG is related to high-speed memory comparisons during object selective attention. Vis Cognition. 2001;8:593–608. [Google Scholar]
- 32.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 33.Senkowski D, Herrmann CS. Effects of task difficulty on evoked gamma activity and ERPs in a visual discrimination task. Clin Neurophysiol. 2002;113:1742–1753. doi: 10.1016/s1388-2457(02)00266-3. [DOI] [PubMed] [Google Scholar]
- 34.Busch NA, Schadow J, Fründ I, Herrmann CS. Time-frequency analysis of target detection reveals an early interface between bottom-up and top-down processes in the gamma-band. NeuroImage. 2006;29:1106–1116. doi: 10.1016/j.neuroimage.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Barceló F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- 36.Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Rev. 2000;31:138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- 37.Selemon LD, Mrzljak J, Kleinman JE, Herman MM, Goldman-Rakic PS. Regional specificity in the neuropathologic substrates of schizophrenia: a morphometric analysis of Broca’s area 44 and area 9. Arch Gen Psychiatry. 2003;60:69–77. doi: 10.1001/archpsyc.60.1.69. [DOI] [PubMed] [Google Scholar]
- 38.Nestor PG, O’Donnell BF. The mind adrift: Attentional dysregulation in schizophrenia. In: Parasuraman R, editor. The Attentive Brain. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 39.Brenner CA, Sporns O, Lysaker PH, O'Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- 40.Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1995;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band EEG oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


