Abstract
Though simple cysts are easily identified using sonography, description and management of non-simple cysts remains uncertain. This study evaluated whether the correlation coefficient differences between breast tissue and lesions, obtained from 2D breast elastography, could potentially distinguish non-simple cysts from cancers and fibroadenomas. We hypothesized that correlation coefficients in cysts would be dramatically lower than surrounding tissue because noise, imaging artifacts, and particulate matter move randomly and decorrelate quickly under compression, compared with solid tissue. For this preliminary study, 18 breast lesions (7 non-simple cysts, 4 cancers, and 7 fibroadenomas) underwent imaging with 2D elastography at 7.5 MHz through a TPX (a polymethyl pentene copolymer) 2.5 mm mammographic paddle. Breasts were compressed similar to mammographic positioning and then further compressed for elastography by 1–7%. Images were correlated using 2D phase-sensitive speckle tracking algorithms and displacement estimates were accumulated. Correlation coefficient means and standard deviations were measured in the lesion and adjacent tissue, and the differential correlation coefficient (DCC) was introduced as the difference between these values normalized to the correlation coefficient of adjacent tissue. Mean DCC values in non-simple cysts were 24.2 ± 11.6%, 5.7 ± 6.3% for fibroadenomas, and 3.8 ± 2.9 % for cancers (p < 0.05). Some of the cysts appeared smaller in DCC images than grayscale images. These encouraging results demonstrate that characterization of non-simple breast cysts may be improved by using DCC values from 2D elastography, which could potentially change management options of these cysts from intervention to imaging follow-up. A dedicated clinical trial to fully assess the efficacy of this technique is recommended.
Keywords: breast, cyst, elastography, elasticity imaging, speckle tracking
Introduction and Literature
Breast ultrasound is well respected for its ability to differentiate simple cysts from solid lesions with 98–100% accuracy (Bassett and Kimme-Smith 1991; Hilton et al. 1986; Jackson 1995; Sickles et al. 1984). For a cyst to be characterized as “simple”, it must exhibit all four of the following criteria: anechoic, well circumscribed, imperceptible wall, and posterior acoustic enhancement (Berg et al. 2003; Hilton et al. 1986; Stavros 2004; Venta et al. 1999). Terminology of cysts which meet some but not all of these criteria varies, but includes complicated cysts, complex cysts, cluttered microcysts, cystic lesions with a thick (perceptible) wall and/or thick (> 0.5 mm) septations which are > 50% cystic, and predominantly solid lesions with eccentric cystic foci (Berg et al. 2003; Mendelson et al. 2001; Stavros 2004; Venta et al. 1999). The American College of Radiology (ACR) ultrasound breast imaging reporting and data system (BI-RADS) lexicon identifies a “complex cyst” as those cysts which contain some discrete solid component (ACR 2003). The ACR ultrasound BI-RADS system also defines “complicated cysts” as containing homogeneous low-level internal echoes and fluid-fluid or fluid-debris levels which can shift as the patient’s position changes (ACR 2003).
Non-simple cysts are common, and the percentage of cysts being classified as “complicated” or “complex” is increasing (Jackson 1990; Parker and Jobe 1993; Stavros 2004; Venta et al. 1999). In a recent study, Berg et al. (2003) classified only 11% (16 of 150) of cystic lesions as “simple”. This increase in clinically observed non-simple cysts is at least partly due to the use of high quality ultrasound scanners which can both image small particulate matter and which create artifacts, including reverberations, clutter, and sidelobes, in the cysts (Jackson 1990; Parker and Jobe 1993; Stavros 2004; Venta et al. 1999). An in vitro study by Helvie et al. (1996) reported that cyst fluid almost always exhibited internal echoes when imaged with high quality equipment, demonstrating that cysts are rarely anechoic and thus rarely simple. Therefore, finding a reliable imaging method to differentiate benign non-simple cysts from solid lesions is of great clinical importance.
Variability exists in how non-simple cysts should be managed, based on sonographic findings (many have suspicious sonographic appearances which can overlap with appearances of solid masses), patient risk factors for breast cancer, and patient anxiety, with management options including 6-month or 1-year imaging follow-up, aspiration, aspiration with possible core biopsy, and excisional biopsy (Mendelson et al. 2001; Stavros et al. 1995; Venta et al. 1999). Berg et al. (2003) reported a malignancy rate of 23% (18 of 79) for the complex cysts they imaged that contained some degree of discrete solid components, stating that biopsy would be required for these lesions. In contrast, the National Comprehensive Cancer Network (NCCN) reports that aspiration is the standard of care for non-simple cysts which do not contain solid components (NCCN 2006). Reports of cysts which do not meet the strict criteria of simple cysts, but do not have a solid component, show very low malignancy rates between 0 – 1.4% (3 of 744 total cysts, or 0.4% on average) (Berg et al. 2003; Buchberger et al. 1999; Kolb et al. 1998; Louie et al. 2003; Venta et al. 1999). This low malignancy rate reveals that non-simple cysts with no solid components could be characterized as probably benign and managed with follow-up imaging rather than aspiration or biopsy when accurately identified (Berg et al. 2003; Venta et al. 1999). It may be possible to circumvent follow-up entirely if these cysts could be characterized with certainty. Because many patients have multiple cysts in each breast, needle aspiration, biopsy, or close follow up cannot be performed on each cyst.
Methods used to improve the characterization of non-simple cysts include decreasing dynamic range, using tissue harmonic imaging, and using spatial compounding (Berg et al. 2003). Several groups have reported that the presence of internal echoes in a cyst precludes it from being classified as “simple”, however echoes which surround the anterior of the cyst are usually recognized as reverberations (Venta et al. 1999). Spatial compounding has been successfully used to reduce speckle and other noise in cysts, but it can also reduce posterior acoustic enhancement and its benefits decrease with increasing depth (Merritt et al. 2000).
Ultrasound elastography may prove to be a more viable tool to identify internal echoes created by noise and artifacts, as well as fluid-debris and particulate matter. Elastography is a diagnostic method that evaluates the viscoelastic properties of tissue, as changes in tissue elasticity are usually related to an abnormal, pathological process. During breast elastography, a surrogate to manual palpation, tissue is externally deformed to create internal displacements. Displacements are estimated by tracking speckle movement in the ultrasound images before and after compression. The gradient of these displacements in the direction of the compression is then calculated to create an elastogram which displays the relative induced strain in the image region.
Though breast elastography has demonstrated clinical utility in studies by Garra et al. (1997) and Hall et al. (2003), visualization of cysts in elastograms are often difficult. Garra et al. (1997) reported easy visualization of only 37% (3 of 8) cysts in their study, and even when visible, those cysts had ill-defined margins. Hall et al. (2003) also noted that because cysts rapidly decorrelate, they can appear relatively stiff or soft in elastograms, often depending on how much strain is applied. Because the elastogram cannot be reliably used in cyst characterization, the correlation coefficient derived from elastography could become a valuable tool in differentiating cysts from solid masses.
This study aims to exploit the rapid decorrelation of non-simple cysts observed in elastography as a way to differentiate them from solid breast lesions. Because cysts are filled with fluid, they contain little speckle which can be tracked for elastography. Thus, the correlation coefficient in that region will be markedly lower than in the rest of the tissue. One caveat is that signal from surrounding tissue, including reverberations, clutter, and sidelobes can contribute to signal in the cyst, increasing the correlation coefficient. Additionally, some breast cancers and fibroadenomas are highly hypoechoic, and the signal-to-noise ratio (SNR) in breasts is often low. This could cause these lesions to decorrelate as quickly as non-simple cysts even though these lesions contain true speckle. The extent to which non-simple cysts decorrelate relative to surrounding tissue in comparison with other breast lesions is explored. Whether improved cyst/solid mass differentiation could impact clinical management of non-simple cysts by allowing follow-up imaging rather than intervention is discussed.
Materials and Methods
Recruitment of Human Subjects
This study was approved by the institutional review board and informed consent was obtained on all human subjects. The study was compliant with the Health Insurance Portability and Accountability Act. Subject confidentiality was protected at all times.
Twenty-six consecutive human subjects were recruited in two groups for participation in this study over a 12 month period (02/01/2006 to 02/01/2007). The first group (N=16) were imaged immediately before core biopsy with lesions classified as BI-RADS assessment category 4 or 5 (ACR 2003). The second group (N=10) were subjects with known, benign lesions, diagnosed from previous biopsies (solid lesions) or previous clinical mammograms/ultrasounds (cysts). Participants in this study were required to have had previous clinical mammograms and all solid lesions were pathologically diagnosed. After imaging, 8 subjects were excluded from final analysis due to limitations introduced by our imaging system or post-processing technique, described below. These exclusion criteria will be detailed later in this section for clarity.
The final 18 lesions comprising the case group were characterized as 7 cysts, 4 cancers, and 7 fibroadenomas. Two cancers were diagnosed as invasive ductal carcinomas and two were lobular carcinomas. All cysts were classified as benign and 71% (5 of 7) were managed by previous mammogram(s) stability with 12-month imaging follow-ups while 29% (2 of 7) were aspirated. All cysts were classified as “non-simple” based on their sonographic appearance at the time of imaging because they only met some or none of the requirements for simple cysts (Table 1), but did not have solid components.
Table 1.
Sonographic characteristics and resulting differential correlation coefficients (DCC) of the non-simple cysts analyzed in this study. Characteristics which the cysts exhibited were marked with an “X”. Cysts must exhibit all four characteristics to be classified as “simple”.
| Cyst | Anechoic | Well-circumscribed | Imperceptible wall | Posterior acoustic enhancement | Differential Correlation Coefficient (DCC) (%) |
|---|---|---|---|---|---|
| 1 | X | X | X | 17.4 | |
| 2 | 34.0 | ||||
| 3 | 16.1 | ||||
| 4 | X | X | 38.5 | ||
| 5 | X | 12.1 | |||
| 6 | X | X | X | 36.7 | |
| 7 | X | 14.9 |
Overall, subjects had a mean age of 44.4 ± 7.7 (range 35–66) years. The mean age of women with cysts was 48.0 ± 4.8 years, with fibroadenomas was 39.6 ± 3.9 years, and with cancers was 46.5 ± 13 years.
Lesion depth was measured as the distance from the transducer to the center of the lesion, when the breast was under compression. The mean depth of the cyst lesions (26.5 ± 8.0, range 14.5–33.5 mm) was compared with the mean depth of the fibroadenoma lesions (26.7 ± 4.7, range 19.5–32 mm) and malignant lesions (24.0 ± 7.2, range 18–32.5 mm).
Lesion diameter was calculated from the grayscale ultrasound image of the compressed breast as the average of the transverse and longitudinal dimensions of the lesion. This measure was evaluated between cysts (μcysts = 12.0 ± 4.7 mm) and each of the solid lesion groups (μfibroadenomas = 13.0 ± 7.5 mm, μcancers = 9.0 ± 1.2 mm).
Imaging Methods
The elastography conducted in this study was completed as part of a larger study which combines 3D ultrasound and x-ray tomography for improved breast lesion characterization and diagnosis (Booi et al. 2005; Booi et al. 2007; Kapur et al. 2004).
Subjects were seated throughout the entire exam. A radiologist with 16 years of breast imaging experience initially located the lesions by manually scanning the transducer over the non-compressed breast and obtaining a 3D volume over the region-of-interest (ROI) using grayscale sonographic imaging. This scan was later used to assess the sonographic appearances of the cysts. After initial localization, the subject’s breast was compressed in our mammography-mimicking research device, co-built by General Electric (Schenectady, NY, USA) and University of Michigan researchers (Ann Arbor, MI, USA) in a manner similar to cranial-caudal (CC) mammography and the ultrasound transducer was attached to a holder on the unit (Fig 1a,b). Water was then added to the paddle to acoustically couple between the transducer and paddle. Hairspray (Got2BGlued, Schwarzkopf & Henkel, Irvine, CA, USA) achieved coupling between the paddle and breast with minimal signal degradation (Sinha et al. 2007). Preload, the amount of compression applied to the breast immediately before elastography was performed, was used to minimize chest wall motion in ultrasound images. This did not affect results because preload effects are a manifestation of the non-linearity of Young’s modulus and cysts are not a continuous medium in the mechanical sense. To further minimize motion, subjects held their breath during scans. All radiofrequency (RF) images were acquired with a LOGIQ 9 ultrasound scanner (GE Healthcare, Milwaukee, WI, USA) using a 1D linear array operating at 7.5 MHz through a TPX (a polymethyl pentene) paddle (speed of sound (c) = 2.22 mm/μs) 2.5 mm thick mammographic paddle (Booi et al. 2005; Booi et al. 2007; Kapur et al. 2004).
Figure 1.
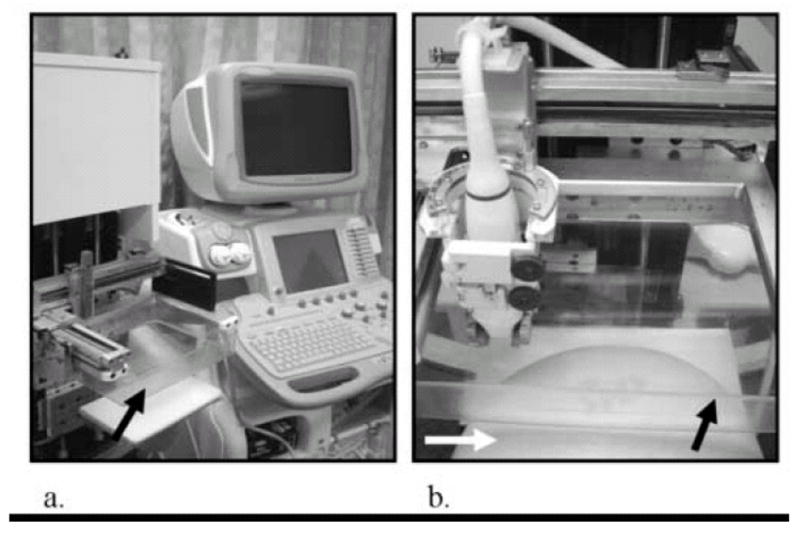
Mammography-mimicking research device (left) and GE LOGIQ 9 ultrasound scanner (right) used in all experiments in this study (a). After moderate pre-compression of the subject’s breast (white arrow) with the TPX (a polymethyl pentene) 2.5 mm thick mammographic paddle (black arrows) (b), additional compression was induced for elastography via stepper motor control. Water was poured in the paddle to provide acoustic coupling during scans (not pictured).
Compression for elastography was induced by automatically raising the lower compression paddle, which was mechanically damped to minimize vibration artifacts. This process was supervised from a nearby computer which communicated through its serial port to a VXM stepper motor controller (Velmex, Bloomfield, NY, USA) connected to the paddle. In this manner, 90 RF images for 2D elastography were continuously acquired by compressing at 2.5 mm/s for up to 2 s and 7% strain. Mean strain was measured as the change in compressed breast thickness after compression divided by the original compressed breast thickness, multiplied by 100. A preliminary experiment was conducted to determine that the differential correlation coefficient was approximately constant over the optimal strain imaging range, or 0.3–0.6% in this study. Data were decimated off-line to fall in this range.
2D Correlation-based Speckle Tracking
RF images were processed using conventional 2D phase-sensitive speckle tracking algorithms (Kaluzynski et al. 2001; Lubinski et al. 1999). These algorithms estimate tissue motion with correlation-based processing in the axial (along the ultrasound beam) and lateral (perpendicular to ultrasound beam) directions.
Calculation of the 2D correlation coefficients for elastography in this study follows. A 2D correlation kernel (spatially equivalent to one speckle spot) is defined around every pixel in the complex pre-deformation image. The size of the speckle in each direction is determined from the full width at half maximum (FWHM) of the 2D autocorrelation function of the image. Next, the kernel is cross-correlated with the complex post-deformation image. The resulting unit-normalized, complex, cross-correlation coefficient at pixels (x, y) as a function of lags (lx, ly) is described in Equation 1. In this equation, Gt(x, y) is the original pre-deformation image, Gt+1(x, y) is the post-deformation image, and Wij is a 2D hamming window over the 2D correlation kernel.
| (1) |
To improve SNR, the correlation coefficient was then filtered with a unity gain function ( ), twice the speckle size:
| (2) |
The 2D displacement vector was then estimated from the peak position of the complex correlation function described in Equation 2 in a two step process. First, both axial and lateral displacements were determined from the correlation lags which correspond to the maximum value of the magnitude of the correlation function. Next, the axial displacement estimate was refined using the position of the closest zero-crossing of the phase of the complex correlation function. The final outputs of this processing are the lateral displacement u(x, y), axial displacement v(x, y), and magnitude of the correlation coefficient |ρ(x, y)|.
Estimated displacements were accumulated over the total deformation range (O’Donnell et al. 1994). The final correlation coefficient image was then used to calculate differential correlation coefficient values.
Calculation of Differential Correlation Coefficients
After speckle tracking, the lesion was manually segmented in the grayscale ultrasound image, at least 1 mm within its border to minimize boundary effects and ensure that no outside tissue contributed to calculated lesion correlation coefficients. Correlation coefficient values in fat and glandular tissue were not independently calculated due to the dependence of depth on the correlation coefficient in fat tissue and the lack of sufficient glandular tissue in older breasts for a reliable measurement. Background tissue was considered to be all tissue in the 4 cm × 3.9 cm (axial × lateral) image outside the lesion ROI. The differential correlation coefficient was calculated according to:
| (3) |
where μρ,background is the mean correlation coefficient in the background ROI, and μρ, lesion is the mean correlation coefficient in the lesion ROI. In order to create DCC images, Equation 3 was applied to the correlation coefficient value of each pixel in the final correlation coefficient image.
Statistical Analysis
Due to the high variability of data included in this study, it could not be assumed that data followed a normal statistical distribution. Thus all statistical analysis was conducted using a Mann Whitney U-test because of its non-parametric assumptions in its calculations. Additionally, because this study had a small sample size, we chose to report an exact p-value given by the Mann Whitney test, which is based on a finite sample distribution. P < 0.05 was considered to indicate statistical significance. For completeness, we also conducted a 2-sample student t-test with unequal variances which confirmed statistical results in all cases. It should be noted, however, that the sample size of the study might be too small to observe some differences which may exist.
Exclusion Criteria for Human Subjects
Due to limitations introduced by our imaging methods and post-processing techniques, as well as pathology results which were only available after recruitment for this study, some examinations were retrospectively excluded from analysis. Lesions deeper than 4 cm when compressed were excluded because the SNR at those depths is too low for the speckle tracking algorithms to work reliably (N=1). Lesions compressed to < 1% total additional strain during the exam were also excluded from analysis because the deformation was too small (N=2) (Rubin et al. 2006). Finally, because the goal of this study is to differentiate non-simple cysts from solid breast masses, subjects whose pathology results did not characterize their lesions as a fibroadenoma or cancer and cysts characterized as simple were excluded from final analysis (N=5).
Results
Subject Population
Age differences between cyst and cancer groups were not statistically significant (P = 0.21), but differences between cyst and fibroadenoma group ages were (P = 0.006). No statistical differences were found between cysts and solid lesion groups regarding lesion depths and diameters (P ≫ 0.1).
2D Elastography
Mean and standard deviation of the DCC between cysts and tissue was 24.2 ± 11.6%, compared with 5.7 ± 6.3% (P = 0.002) for fibroadenomas and 3.8 ± 2.9% for cancers (P = 0.006) (Fig 2). Overall, much lower correlations (12–39%) were observed in cysts than in surrounding tissue, as expected. The DCC for each cyst, along with its sonographic characteristics, is listed in Table 1. No cyst exhibited a higher correlation coefficient than the background in this study, though one fibroadenoma did (DCC = −5.6%).
Figure 2.
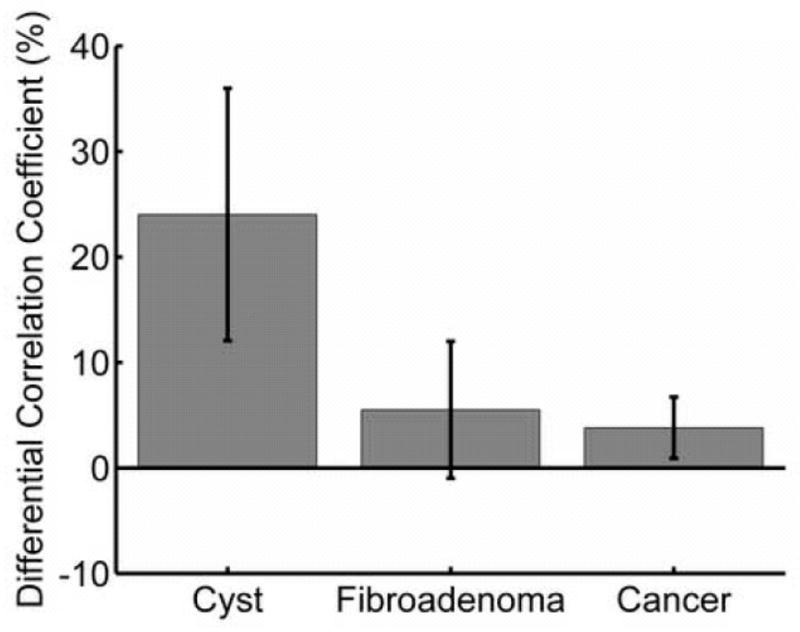
Mean and standard deviation differential correlation coefficient (DCC) values for each lesion classification. A Mann Whitney U-test (confirmed with a student t-test with unequal variances) verified that differences were significant between cysts and each of the other groups.
Fig 3a compares DCCs for three depth groups: <15 mm, 15–25 mm, and >25 mm. Though the size of these sub-groups is too small for statistical comparison, the smallest difference between DCCs is in the >25 mm depth range. To assess the effect of lesion size on DCC values, lesions were grouped into 3 regions based on their diameter: < 10, 10–20, and > 20 mm (Fig 3b). Though not enough lesions fell into each group to be statistically evaluated, relative DCC values between lesion groups were fairly consistent with respect to size.
Figure 3.

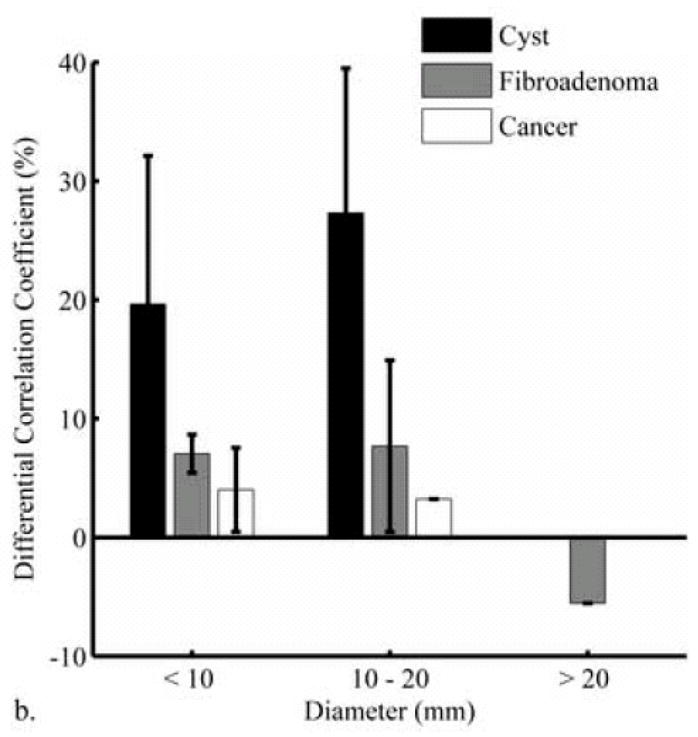
Analysis of differential correlation coefficient (DCC) values grouped according to lesion depth (a) and diameter (b). Statistical comparison was not possible due to the low number of lesions in each group. The most overlap between lesion types occurred at the greatest depths analyzed in this study, most likely due to the decorrelation being dominated by the low signal-to-noise (SNR) in the lesions. Characterization of small (< 10 mm) lesions may not be adversely affected by this technique, if partial volume averaging and sidelobe “fill in” do not dominate DCC values.
The appearance of the lesions in the DCC image, scaled from minimum possible value (white) to 15%, also varied with lesion type. The DCC in the cysts appeared mostly uniform and black (high) in those images with some random scattering of low DCC values in their interior (Fig 4). Only one cyst in this study exhibited a DCC less than 15% below the image mean (DCC = 12%). Some of the cysts appeared smaller in the DCC image than in the grayscale image (Fig 4).
Figure 4.
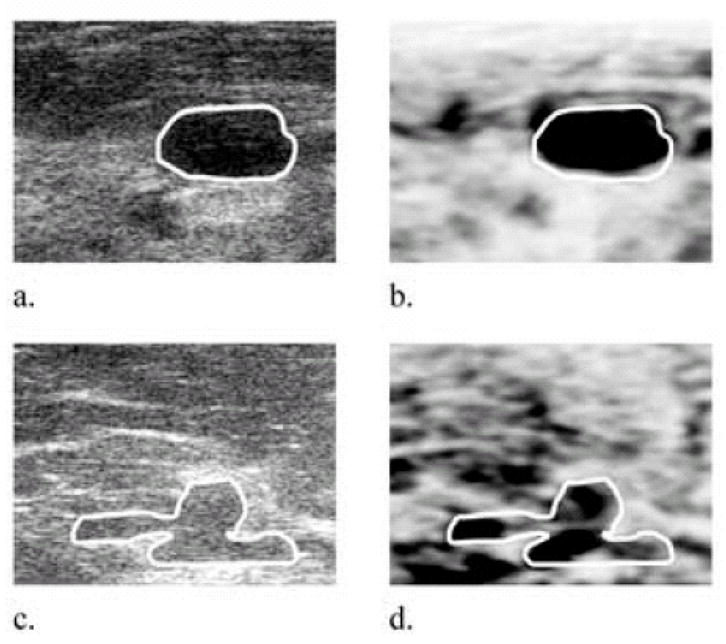
Representative cyst grayscale (a, c) and corresponding 2D differential correlation coefficient (DCC) (b, d) images, respectively. Cysts might be classified in DCC images according to their mainly black appearance, spotty interior, and slightly smaller size than in the grayscale image. The cyst shown in (c, d) was 3.3 cm deep (DCC = 16.1%). DCC images are plotted on a scale from minimum possible value (−6.4% in (b), −7.5% in (d)) to a maximum 15%. Black represents high DCC values in the image (highly decorrelating tissue), and white represents low DCC values. Image sizes are 25 mm × 25 mm.
Two (29%) fibroadenomas exhibited DCC values ≤ 2% (Fig 5a,b). Both of these were classified as “classic” fibroadenomas in their grayscale images: demonstrating characteristics such as wider than tall, elliptical in shape, and encapsulated by a thin, echogenic capsule (Stavros 2004). The DCC in the remaining 5 (71%) fibroadenomas was 5.3–15.4%. The standard deviation of the correlation coefficient in three (43%) of these fibroadenomas was visibly high, as seen in Fig 5d.
Figure 5.
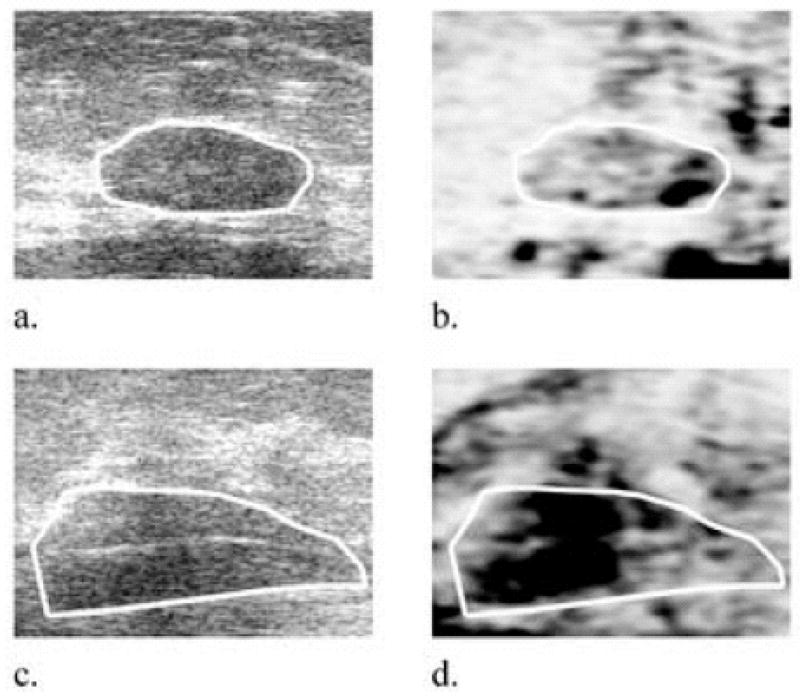
Representative fibroadenoma grayscale (a, c) and corresponding 2D differential correlation coefficient (DCC) (b, d) images, respectively. Fibroadenomas might be classified in DCC images according to their lower mean DCC values coupled with high standard deviations. The classic fibroadenoma in (a) exhibited a DCC = 3.2% in (b). The fibroadenoma in (c, d) exhibited the greatest DCC by a fibroadenoma in this study (15.4%, depth = 3.1 cm). DCC images are plotted on a scale from minimum possible value (−7.5% in (b), −9.9% in (d)) to a maximum 15%. Black represents high DCC values in the image (highly decorrelating tissue), and white represents low DCC values. Image sizes are 25 mm × 25 mm.
The DCC values from all 4 cancers analyzed in this study, two which are shown in Fig 6, were more homogeneous and more closely matched to the background tissue correlation coefficient than observed in the fibroadenomas (DCC = 0.0 – 6.7%). These measures were consistent between carcinoma types.
Figure 6.
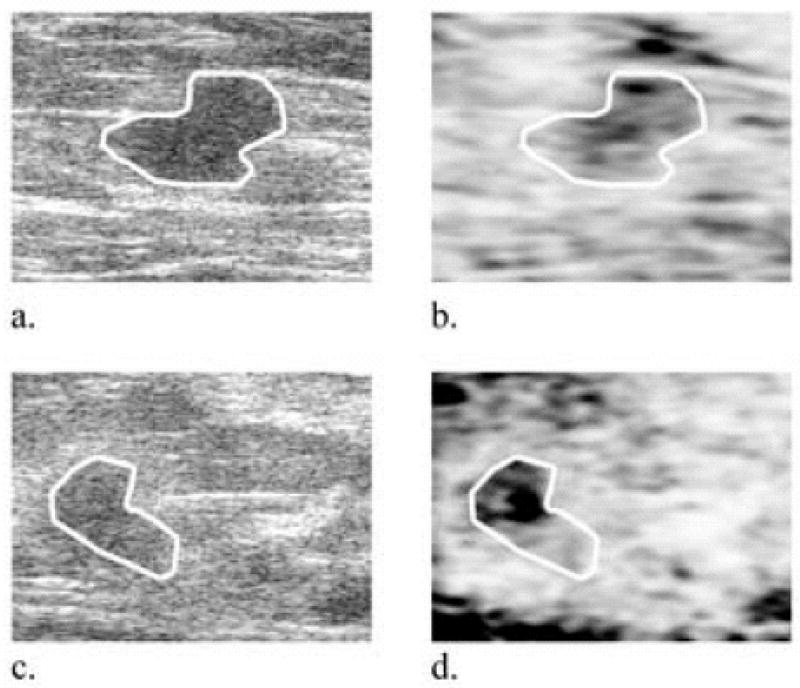
Representative cancer grayscale (a, c) and corresponding 2D differential correlation coefficient (DCC) (b, d) images, respectively. Cancers might be classified in DCC images according to both their lower mean DCC values and lower standard deviations as evidenced in (b). The cancer in (d) (DCC = 5.4%) was described clinically as having posterior acoustic enhancement, though it did not meet strict sonographic criteria for a cyst. Pathology results confirmed it was an invasive ductal carcinoma, Bloom-Richardson grade 2. DCC images are plotted on a scale from minimum possible value (−7.5% in (b) and (d)) to a maximum 15%. Black represents high DCC values in the image (highly decorrelating tissue), and white represents low DCC values. Image sizes are 25 mm × 25 mm.
Discussion
The high DCC values (12–39%) observed in the cysts in this study were due to the decorrelating nature of the noise, artifacts, fluid-debris, and particulate matter in the interior of cysts which move randomly when compressed and thus cannot be tracked using conventional elastography. Due to partial volume averaging, sidelobes, and multiple scattering, cyst edges stayed somewhat correlated between frames, “filling in” the cyst in the DCC image and causing them to appear slightly smaller than in the grayscale image. In larger cysts, this effect had little impact on the overall DCC. This finding might have diagnostic utility, being the reverse of cancers which frequently look larger in elasticity images than in grayscale (Hall et al. 2003). Lesion depth appeared to impact DCC values more than lesion size, most likely because lower SNR at greater depths reduces the efficacy of speckle tracking. The spotty and random highly correlated regions in the cyst were caused by particulate matter and reverberations which did not completely decorrelate. Sources of the reverberations were often easy to identify in the original grayscale ultrasound image, however, so these regions could be retrospectively removed from the DCC calculations.
The differential correlation coefficients measured in both fibroadenoma and cancer images were significantly lower than in cyst images. This was as expected because in most cases, even hypoechoic solid lesions contain more true speckle than cysts with no discrete solid components. These structures are solid so they have real elastic properties, which keep the speckle correlated. The high variability of DCC values in fibroadenomas is consistent with reports that fibroadenomas exhibit both soft and hard regions in elastograms, which can decorrelate to varying degrees (Hall et al. 2003).
The technique we have presented here is similar to the acoustic streaming method investigated by Nightingale et al. (1999). That study successfully used high intensity ultrasound to move low level internal echoes in cysts and detect them with Doppler methods to differentiate them from solid lesions. In our case, the force used to produce the deformation in elastograms can cause particles to move in the cyst. Thus, the deformation force is, in some sense, analogous in our method to the radiation force used by Nightingale et al (1999) and is independent of mechanical index (MI). Our differential correlation coefficient technique may be additionally efficacious in cysts which contain cells that are too large to be moved solely by the energy of the ultrasound beam, such as red blood cells, white blood cells, epithelial cells, and apocrine cells (Stavros 2004).
An additional advantage to this technique is that it should be independent of preload. Though tissue elasticity contrast decreases with preload due to the nonlinearity of tissue, the correlation coefficient in a cyst will be low regardless of preload. Any speckle in a cyst is either noise or moves randomly when compressed, independent of the level of pre-compression. This can further be extended to the true statement that any strain measured in a cyst must be noise.
Recall that conventional speckle tracking algorithms used in elasticity imaging maximize correlation coefficient values to estimate displacements after tissue deformation. In this way, differential correlation coefficient values are simultaneously calculated with elastograms without additional computations and strain evaluations of solid masses can be complemented with differential correlation coefficient imaging to determine if a mass is cystic or solid. Since clinical machines capable of performing strain imaging in real time are in clinical use (Janssen et al 2007, Thomas et al 2007), differential correlation coefficient images could be simultaneously displayed along with tissue strain in real time.
This preliminary study had several limitations. First, imaging with a mammography-mimicking system limited visualization of breast lesions to the CC view, which some times increased the imaging depth of the lesions. Because ultrasound SNR is low in most breast lesions and decreases with depth, low SNR could cause fibroadenomas and cancers to be mistaken for cysts in the ultrasound images, or to be obscured completely. Additionally, low SNR could dominate decorrelation and greatly increase the DCC in fibroadenomas and cancers. Both of these effects would increase the likelihood of false negatives in this study and were some times emphasized in this study by imaging through the mammographic paddle rather than the position which placed the lesion closest to the transducer. However, use of a horizontal rotational axis as on standard mammographic systems and our combined ultrasound/mammography system or use of free-hand ultrasound, as would generally be done clinically, on a real-time elastography scanner would eliminate this limitation for cases in which the CC mammographic view is not ideal. In addition, freehand scanning would be expected to only increase the amount of available signal in solid masses, thus improving the discrimination between cyst and solid beyond what we have detected. In some sense, we have defined a lower bound for the differentiation between cystic and solid masses.
As a preliminary study, we did not preferentially select any of the lesions for analysis. But future studies could preferentially select human subjects with cancers which are cystic in appearance (round, non-spiculated cancers) and hypoechoic to assess the full utility of this technique. Because some complex cysts can have solid components, future studies should also aspirate and/or biopsy all cysts after imaging in order to fully understand the DCC values in non-simple cysts with only fluid components versus cysts with mixed fluid/solid components.
In this regard, only two of sever simple cysts in this study were aspirated, and although it has not been proven that the remaining five masses were cysts, these masses were deemed benign cysts by our clinical institutional criteria (see Methods section). At our institution, all breast ultrasound is performed by breast imaging sub-specialized radiologists, and we believe this may result in greater specificity or accuracy in determining benign from malignant masses. In our experience, aspirating these cysts would have needlessly inflicted an interventional procedure on these five patients. Further, the five cysts that were not aspirated did not change over a year’s time. Because these cases had 12 month stability and were classified as benign, they are extremely unlikely (<2% risk) to have solid elements (Crystal et al 2003, Kolb et al 1998). . Thus, the technique appears in this very small sample to be able to distinguish clinically diagnosed benign complex cysts from malignant masses at our institution. However in other settings wherein breast ultrasound is performed by technologists, this differential correlation coefficient imaging can be an independent additional test providing the interpreting radiologist with greater certainty toward distinguishing a non-simple cyst from a solid mass and may indeed offer greater accuracy.
In the future, this technique could be applied with 3D elastography, which should further reduce the correlation coefficient in the cysts while maintaining the background correlation coefficient, causing the overall DCC in cysts to increase. Both the increased kernel size and the increased scanning time inherent in 3D imaging would contribute to these increased values.
Potential clinical implications
The goal of this technique, and of most clinical importance, is whether it can differentiate non-simple cysts from cancerous lesions. This differentiation could result in a reduction of the number of cysts that require aspiration or biopsy and could be especially efficacious in women with multiple lesions. Coupled with the potential to reduce interventions of benign masses is the opportunity to reduce costs and times associated with those procedures: because localized breast sonography is ¼ the cost of cyst aspiration, this could tremendously reduce clinical costs (Hilton et al. 1986).
Even with a small subject size (N=18), the DCC differences between cysts and cancers are statistically significant. If a threshold were set based on these values, equal to the mean DCC in the cancer plus three standard deviations, then the cutoff between benign and malignant lesions would be 12.5%. Because the greatest DCC value exhibited by a cancer in this study was 6.7%, no false-negatives would be created with this technique. Only one cyst fell below this threshold (DCC = 12.1%) and though one fibroadenoma in this study fell above this threshold (DCC = 15.4%), there are no negative clinical implications of mischaracterizing a fibroadenoma as a cyst.
Based on this promising preliminary data which suggests the feasibility of this technique, this study will hopefully lead the way toward larger, dedicated clinical trials. Additional factors of cyst characterization, such as signal level and increased through-transmission, could be combined with differential correlation coefficient values for improved cyst identification.
Summary
Cysts, fibroadenomas, and carcinomas are the most common masses in the female breast and reliable differentiation of these masses is of great clinical importance (Harper 1985; Hilton et al. 1986). The low malignancy rate of non-simple cysts which contain no solid components suggest that they can be monitored through 6-month or 1-year follow up imaging studies, rather than intervention when reliably characterized (Venta et al. 1999). As the number of non-simple cysts observed clinically continues to increase, non-invasive methods to distinguish these cysts from solid breast lesions, including cancers and fibroadenomas, will become increasingly valuable.
The goal of this very preliminary study was to demonstrate the potential for using differential correlation coefficients in distinguishing non-simple cysts from solid masses and to discuss the need for such differentiation and the possible clinical impact. Although the numbers in this study are small, the results are consistent with what one would expect from signal processing theory and continuum mechanics/generalized Hooke’s Law (Hall et al 2003, Chou and Pagano 1992). Clearly, a larger, dedicated trial must be conducted to ascertain the fidelity of this technique.
The differential correlation coefficient introduced in this study exploits the decorrelating nature of noise, artifacts, and particulate matter found in non-simple cysts to differentiate them from solid lesions or non-simple cystic lesions with solid components. Although ultrasound is generally excellent for cyst determination, non-simple breast cysts can be difficult to diagnose because of exactly these same artifacts, clutter, and particulate matter (Jackson 1990; Parker and Jobe 1993; Stavros 2004; Venta et al. 1999, Helvie et al 1996).
In contrast, solid tissue components, in solid masses or in cysts with solid components, contain true speckle which will stay correlated even when external compression is applied. This technique is essentially an independent imaging method for confirming a breast mass is a cyst. Though the sonographic appearance of non-simple cysts can overlap with that of solid breast lesions, the DCC values in cysts are statistically different from solid lesions. Thus, differential correlation coefficient measures have the potential to increase confidence that a lesion is 1) a cyst and 2) suggest that a lesion does not contain solid components. Hopefully, after more extensive investigations, differential correlation coefficient imaging would potentially support the conviction that suspicious lesions were indeed cysts, thus possibly reducing aspirations of non-symptomatic complicated cysts by changing the management to follow-up imaging.
Acknowledgments
This work was supported in part by NIH Grants R21–CA109440 and RO1–CA91713.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American College of Radiology (ACR) Breast imaging reporting and data system (BI-RADS) Reston, VA: American College of Radiology; 2003. [DOI] [PubMed] [Google Scholar]
- Bassett LW, Kimme-Smith C. Breast sonography. AJR Am J Roentgenol. 1991;156:449–455. doi: 10.2214/ajr.156.3.1899737. [DOI] [PubMed] [Google Scholar]
- Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: Sonographic-pathologic correlation. Radiology. 2003;227:183–191. doi: 10.1148/radiol.2272020660. [DOI] [PubMed] [Google Scholar]
- Booi RC, Carson PL, Erkamp RQ, Xie H, Kapur A, LeCarpentier GL, Roubidoux MA, Fowlkes JB, O’Donnell M. Applying in vitro elasticity imaging results to optimize in vivo breast lesion characterization using a combined 3D ultrasound/digital x-ray system. Proceedings of the IEEE Ultrasonics Symposium. 2005:727–730. [Google Scholar]
- Booi RC, Krucker JF, Goodsitt MM, O'Donnell M, Kapur A, LeCarpentier GL, Roubidoux MA, Fowlkes JB, Carson PL. Evaluating thin compression paddles for mammographically compatible ultrasound. Ultrasound Med Biol. 2007;33(3):472–482. doi: 10.1016/j.ultrasmedbio.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchberger W, DeKoekkoek-Doll P, Springer P, Obrist P, Dunser M. Incidental findings on sonography of the breast: clinical significance and diagnostic workup. AJR Am J Roentgenol. 1999;173:921–927. doi: 10.2214/ajr.173.4.10511149. [DOI] [PubMed] [Google Scholar]
- Chou PC, Pagano NJ. Elasticity – tensor, dyadic, and engineering approaches. New York: Dover Publications, Inc; 1992. pp. 54–60. [Google Scholar]
- Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. Am J Roentgenol. 2003;181:177–82. doi: 10.2214/ajr.181.1.1810177. [DOI] [PubMed] [Google Scholar]
- Garra BS, Cespedes EI, Ophir J, Spratt SR, Zuurbier RA, Magnant CM, Pennanen MF. Elastography of breast lesions: initial clinical results. Radiology. 1997;202:79–86. doi: 10.1148/radiology.202.1.8988195. [DOI] [PubMed] [Google Scholar]
- Hall TJ, Zhu Y, Spalding CS. In vivo real-time freehand palpation imaging. Ultrasound Med Biol. 2003;29:427–435. doi: 10.1016/s0301-5629(02)00733-0. [DOI] [PubMed] [Google Scholar]
- Harper P. Ultrasound mammography. Baltimore: University Park Press; 1985. [Google Scholar]
- Helvie MA, Bude RO, Joynt L, Naylor B, Rubin JM. In Vitro Sonographic Evaluation of Aspirated Hypoechoic and Anechoic Breast Cyst Fluid. Breast Dis. 1996;9:15. [Google Scholar]
- Hilton SV, Leopold GR, Olson LK, Willson SA. Real-time breast sonography: application in 300 consecutive patients. AJR Am J Roentgenol. 1986;147:479–486. doi: 10.2214/ajr.147.3.479. [DOI] [PubMed] [Google Scholar]
- Jackson VP. The role of US in breast imaging. Radiology. 1990;177:305–311. doi: 10.1148/radiology.177.2.2217759. [DOI] [PubMed] [Google Scholar]
- Jackson VP. The current role of US in breast imaging. Radiol Clin North Am. 1995;33:1167–1170. [PubMed] [Google Scholar]
- Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointestinal Endoscopy. 2007;65:971–978. doi: 10.1016/j.gie.2006.12.057. [DOI] [PubMed] [Google Scholar]
- Kaluzynski K, Chen X, Emelianov SY, Skovoroda AR, O’Donnell M. Strain rate imaging using two-dimensional speckle tracking. IEEE Trans Ultrason Ferroelectr Freq Control. 2001;48:1111–1123. doi: 10.1109/58.935730. [DOI] [PubMed] [Google Scholar]
- Kapur A, Carson PL, Eberhard J, Goodsitt MM, Thomenius K, Lokhandwalla M, Buckley D, Roubidoux MA, Helvie MA, Booi RC, LeCarpentier GL, Erkamp RQ, Chan HP, Fowlkes JB, Thomas JA, Landberg CE. Combination of digital mammography with semi-automated 3D breast ultrasound. Technol Cancer Res Treat. 2004;3:325–334. doi: 10.1177/153303460400300402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie L, Velez N, Earnest D, Staren ED. Management of nonpalpable ultrasound-indeterminate breast lesions. Surgery. 2003;134:667–673. doi: 10.1016/s0039-6060(03)00318-0. [DOI] [PubMed] [Google Scholar]
- Lubinski M, Emelianov S, O’Donnell M. Speckle Tracking Methods for Ultrasonic Elasticity Imaging Using Short-Time Correlation. IEEE Trans Ultrason Ferroelectr Freq Control. 1999;46:82–96. doi: 10.1109/58.741427. [DOI] [PubMed] [Google Scholar]
- Mendelson EB, Berg WA, Merritt CR. Toward a standardized breast ultrasound lexicon, BI-RADS: ultrasound. Semin Roentgenol. 2001;36:217–225. doi: 10.1053/sroe.2001.25125. [DOI] [PubMed] [Google Scholar]
- Merritt C, Piccoli C, Forsberg F, Wilkes A, Cavanaugh B, Lee S. Real-time spatial compound imaging of the breast: clinical evaluation of masses (abst) Radiology. 2000;217:491–492. [Google Scholar]
- National Comprehensive Cancer Network (NCCN) Breast cancer screening and diagnosis. 2007 [Google Scholar]
- Nightingale KR, Kornguth PJ, Trahey GE. The use of acoustic streaming in breast lesion diagnosis: a clinical study. Ultrasound Med Biol. 1999;25:75–87. doi: 10.1016/s0301-5629(98)00152-5. [DOI] [PubMed] [Google Scholar]
- O’Donnell M, Skovoroda AR, Shapo BM, Emelianov SY. Internal displacement and strain imaging using ultrasonic speckle tracking. IEEE Trans Ultrason Ferroelectr Freq Control. 1994;41:314–325. [Google Scholar]
- Parker SH, Jobe WE. Percutaneous breast biopsy. 1. New York: Raven; 1993. pp. 111–114. [Google Scholar]
- Rubin JM, Xie H, Kim K, Weitzel WF, Emelianov SY, Aglyamov SR, Wakefield TW, Urquhart AG, O'Donnell M. Sonographic elasticity imaging of acute and chronic deep venous thrombosis in human. J Ultrasound Med. 2006;25:1179–1186. doi: 10.7863/jum.2006.25.9.1179. [DOI] [PubMed] [Google Scholar]
- Sickles EA, Filly RA, Callen PW. Benign breast lesions: ultrasound detection and diagnosis. Radiology. 1984;151:467–470. doi: 10.1148/radiology.151.2.6709920. [DOI] [PubMed] [Google Scholar]
- Sinha SP, Goodsitt MM, Roubidoux MA, Booi RC, LeCarpentier GL, Lashbrook CR, Thomenius K, Chalek CL, Carson PL. Automated ultrasound scanning on a dual modality breast imaging system: coverage and motion issues and solutions. J Ultras Med. doi: 10.7863/jum.2007.26.5.645. in press. [DOI] [PubMed] [Google Scholar]
- Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123–134. doi: 10.1148/radiology.196.1.7784555. [DOI] [PubMed] [Google Scholar]
- Stavros AT. Breast Ultrasound. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. Sonographic evaluation of breast cysts; pp. 276–350. [Google Scholar]
- Thomas A, Kummel S, Gemeinhardt O, Fischer T. Real-time sonoelastography of the cervix: tissue elasticity of the normal and abnormal cervix. Acad Radiol. 2007;14:193–200. doi: 10.1016/j.acra.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Venta LA, Kim JP, Pelloski CE, Morrow M. Management of complex breast cysts. AJR Am J Roentgenol. 1999;173:1331–1336. doi: 10.2214/ajr.173.5.10541113. [DOI] [PubMed] [Google Scholar]


