Summary
Intracellular transport is essential for cytoplasm organization, but mechanisms regulating transport are mostly unknown. In Xenopus melanophores, melanosome transport is regulated by cAMP-dependent protein kinase A (PKA) [1]. Melanosome aggregation is triggered by melatonin, whereas dispersion is induced by melanocyte-stimulating hormone (MSH) [2]. The action of hormones is mediated by cAMP: high cAMP in MSH-treated cells stimulates PKA while low cAMP in melatonin-treated cells inhibits it. PKA activity is typically restricted to specific cell compartments by A-kinase anchoring proteins (AKAP) [3]. Recently, Rab32 has been implicated in protein trafficking to melanosomes [4] and shown to function as an AKAP on mitochondria [5]. Here we tested the hypothesis that Rab32 is involved in regulation of melanosome transport by PKA. We demonstrated that Rab32 is localized to the surface of melanosomes in a GTP-dependent manner and binds to the regulatory subunit RIIα of PKA. Both RIIα and Cβ subunits of PKA are required for transport regulation and are recruited to melanosomes by Rab32. Overexpression of wild type Rab32, but not mutants unable to bind PKA or melanosomes, inhibits melanosome aggregation by melatonin. Therefore, in melanophores Rab32 is a melanosome-specific AKAP that is essential for regulation of melanosome transport.
RESULTS
Identification of the PKA Isoform That Is Localized to Melanosomes and Involved in the Regulation of Melanosome Transport
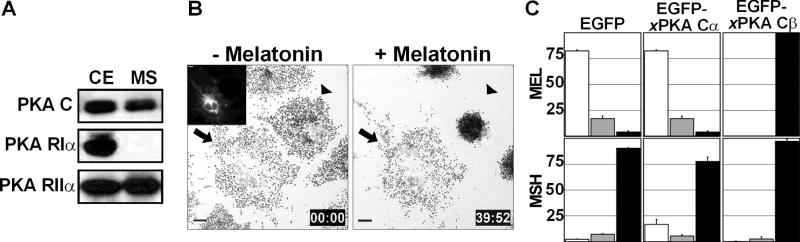
Melanosome transport in Xenopus melanophores is tightly regulated by PKA, and PKA is associated with melanosomes [1, 6]. PKA holoenzyme consists of two catalytic and two regulatory subunits [7]. In Xenopus, catalytic subunits Cα and Cβ [8] and regulatory subunits RIα and RIIα have been characterized thus far. Recently, Kashina et al. have shown that PKA RIIα is localized to melanosomes in Xenopus melanophores [6]. We first sought to determine if a catalytic subunit of PKA forms a complex with RIIα on melanosomes. Western blotting with antibodies against PKA C and PKA Rllα detected bands of molecular masses consistent to each protein in the cell extract and in the melanosome fraction (Fig. 1A). However, PKA RIα was found only in the extract (Fig. 1A), but not on melanosomes. Thus, Rllα and C are present on melanosomes, whereas RIα is not.
Figure 1. PKA Cβ/RIIα Regulates Melanosome Transport.
(A) PKA C and RIIα are localized to melanosomes. Xenopus melanophore extract (CE) and purified melanosome fraction (MS) were probed with antibodies against PKA subunit isoforms. PKA C and RIIα, but not RIα, are present in melanosome fraction. (B) PKA Cβ overexpression blocks melanosome aggregation by melatonin. The left panel is a bright-field image showing melanosome distribution before melatonin stimulation. The black arrow indicates a cell expressing EGFP-xPKA Cβ (inset) and the black arrowhead indicates a control cell. The right panel shows the distribution of melanosomes after 40 minutes of melatonin stimulation. EGFP-xPKA Cβ overexpression in a transfected cell (black arrow) completely blocks pigment aggregation. Bars, 10 μm. This figure represents two frames from Supplementary Movie 1.
(C) Xenopus melanophores were transiently transfected with EGFP, EGFP-xPKA Cα, or EGFP-xPKA Cβ. Transfected cells were treated with melatonin or MSH and scored into three groups (aggregated, partially dispersed, and dispersed). In each experiment, aggregated, partially dispersed, and dispersed cells are shown as white, gray, and black bars, respectively. n=100 for each condition. The experiment was repeated three times.
Available PKA C antibodies do not discriminate between the isoforms of the catalytic subunits, Cα and Cβ. We therefore sought to distinguish between isoforms using a functional assay. We overexpressed pEGFP-tagged Cα or Cβ in melanophores and treated cells with melatonin or MSH. Overexpression of the EGFP-Cα did not affect the ability of cells to aggregate or disperse melanosomes (Fig. 1C). However, EGFP-Cβ overexpression resulted in a complete block of aggregation (Fig. 1B, 1C and Movie 1). Thus, the overexpression studies demonstrate that xPKA Cβ is the catalytic subunit that regulates melanosome transport. Collectively, the biochemical and overexpression data demonstrate that the Cβ/RIIα complex is localized to melanosomes and regulates their transport.
Colocalization of Rab32 with Melanosomes
Localization of PKA to specific compartments is typically mediated by AKAPs. In a search for AKAPs that mediate melanosomal targeting of PKA, we noticed that one of the Rab family of Ras-like GTPases (Rab32) was reported to function as an AKAP in human fibroblasts [5]. Although in fibroblasts Rab32 is localized to mitochondria, Rab32 is involved in the biogenesis of melanosomes in human melanocytes [4] and is highly expressed in melanocytes [9]. In Xenopus, Rab32 is highly expressed in the pigment epithelium of the retina and a clone for Xenopus Rab32 (xRab32) has been isolated in a functional screen, based on its ability to cause abnormal pigmentation under conditions of overexpression in the embryonic ectoderm [10]. Based on these reports, we wanted to determine if xRab32 functions as a melanosomal AKAP and determine its role in the regulation of melanosome transport.
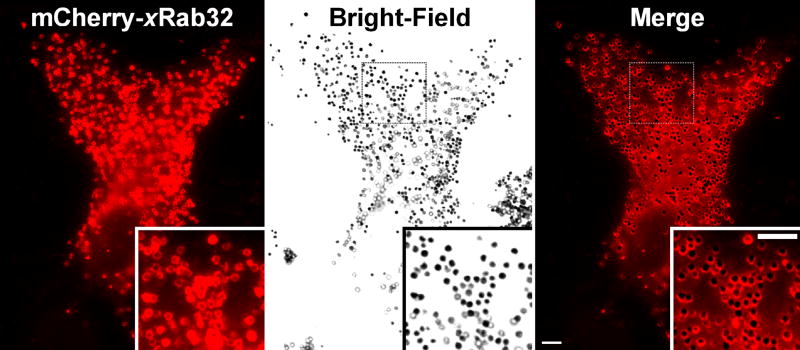
We first examined the subcellular localization of xRab32 in Xenopus melanophores using fluorescent microscopy and biochemical techniques. xRab32 was tagged with mCherry or EGFP and transiently expressed in melanophores. Both mCherry- and EGFP-xRab32 form a distinct punctate pattern in the cytoplasm (Fig. 2, left panel). At higher magnification, most of the puncta that are localized in the focal plane of the microscope are composed of a fluorescent ring encircling a dark inner core (Fig. 2, left panel inset). Comparison of the fluorescence pattern with the distribution of melanosomes, as seen using bright-field microscopy, demonstrates (Fig. 2, middle panel) that the majority of the punctate structures correspond to melanosomes (Fig. 2, right panel). In addition, mCherry-xRab32 is localized on the surface of smaller vesicles that neither have a dark inner core nor contain melanin (Fig. 2 and Movie 2). Time-lapse microscopy of the dynamics of mCherry-xRab32 demonstrates that most of the time xRab32 remains associated with melanosomes (Movie 2). Occasionally, round or elongated vesicular structures budding and fusing with melanosomes were observed in the cytoplasm (Movie 2).
Figure 2. Rab32 is Localized to Melanosomes.
mCherry-xRab32 is localized to melanosomes. Xenopus melanophores were transfected with mCherry-xRab32 for 24 hr. The left panel shows the distribution of the mCherry-xRab32 fusion protein, the middle panel shows in bright-field distribution of melanosomes, and the right panel shows the bright field image merged with the distribution of mCherry-xRab32 fusion proteins. Melanosomes are decorated with mCherry-xRab32 (right panel inset), indicating that the mCherry-xRab32 fusion protein is localized to melanosomes. Bars, 5 μm.
To demonstrate that endogenous Rab32 is expressed in Xenopus melanophores and is bound to melanosomes, we raised an antibody specific to xRab32. This antibody detects a single band with a molecular weight of 27 kD on Western blots of melanophore extracts (Fig. S1). The same 27 kD band is detected in the purified melanosome fraction (Fig. S1). It is interesting to note, that the majority of Rab32 in melanophores is associated with melanosomes and that these cells have very little soluble Rab32. Therefore, the distribution of mCherry-xRab32 faithfully reproduces the distribution of the endogenous protein.
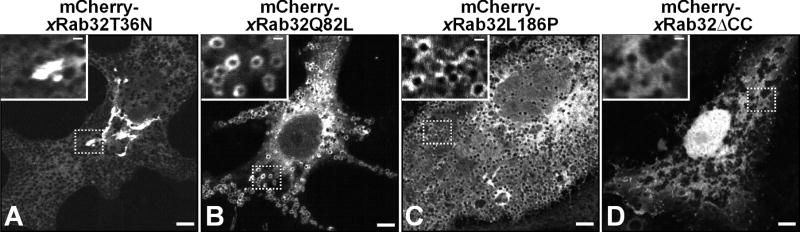
In addition to the wild-type protein, we examined the subcellular localization of Q82L and T36N mutants of xRab32, which in human Rab32 have been reported to have defects in GTP hydrolysis and GTP binding, respectively [5]. mCherry-xRab32T36N, mimicking the GDP-bound state of the protein, is not localized to melanosomes. Instead, it was predominantly concentrated in the perinuclear area and in the cytoplasm (Fig 3A). In contrast, a constitutively active mCherry-xRab32Q82L was localized to melanosomes (Fig 3B). We also generated xRab32 lacking two COOH-terminal cysteine residues (xRab32ΔCC) that are normally prenylated and required for the targeting of Rabs to the membrane [11]. This mutant did not localize to melanosomes (Fig 3D). Together these results suggest that xRab32 protein is localized to the membrane of melanosomes in a GTP-dependent manner using the COOH-terminal cysteines.
Figure 3. Localization of xRab32 mutants.
Xenopus melanophores were transfected with mCherry-xRab32T36N (mimicking the GDP-bound state of the protein) (A), mCherry-xRab32Q82L (mimicking the GTP-bound state of the protein) (B), mCherry-xRab32L186P (a mutant defective in PKA binding) (C), and mCherry-xRab32ΔCC (lacking C-terminal cysteines) (D) for 24 hr. mCherry-xRab32Q82L and mCherry-xRab32L186P are localized to melanosomes (B and C insets, respectively), whereas, mCherry-xRab32T36N and mCherry-xRab32ΔCC are not (A and D inset, respectively). Bars, 5 μm in main images and 1 μm in insets.
Xenopus Rab32 Is an A-Kinase Anchoring Protein
Human Rab32 is known to bind PKA [5]. In Xenopus melanophores, pigment movement is regulated by PKA [1], and both PKA [6] and xRab32 (see above) are localized to melanosomes. Therefore, it is logical to suggest that xRab32 is involved in targeting PKA to melanosomes. To demonstrate that xRab32 is an AKAP, we examined the binding of PKA RIIα to xRab32 using a cAMP agarose pull-down assay and immunoprecipitation. As expected, the regulatory subunit RIIα of PKA bind to the cAMP-agarose and this binding is abolished by the addition of free cAMP. The same binding pattern is detected for Rab32 (Fig. 4A). This result indicates that the two proteins probably form a complex and that xRab32 could bind to the column via RIIα. To show that Rab32 indeed binds PKA RIIα, we performed a coimmunoprecipitation assay. We cotransfected mCherry-xRab32 and EGFP-xPKA RIIα into Xenopus melanophores and pulled down mCherry-xRab32 using anti-Rab32 antibody. Precipitates were probed with a PKA RIIα antibody and HRP-Protein A [12] to avoid detection of the Rab32 antibody used in the pull-down). Fig. 4B shows that the Rab32-PKA RIIα complex is present only in Rab32 precipitates and not in preimmune precipitates (Fig. 4B).
Figure 4. Xenopus Rab32 is an A-Kinase Anchoring Protein.
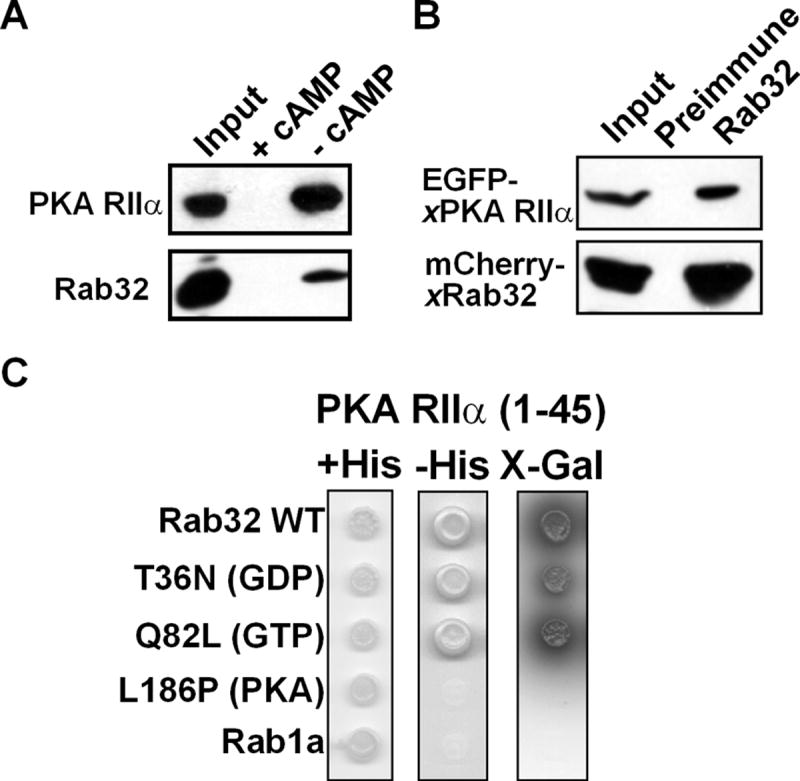
(A) PKA RIIα binds Xenopus Rab32. Xenopus melanophore extracts were incubated with cAMP-agarose resin in the presence of 75 mM cAMP (+ cAMP) or in the absence cAMP (- cAMP). cAMP-agarose resin was washed and eluted with 75mM cAMP. The Western blot was performed using PKA RIIα and xRab32 antibodies. The cAMP agarose resin binds RIIα and also pulls down xRab32. This interaction is abolished in the presence of 75 mM cAMP.
(B) Rab32 binds to PKA RIIα in vivo. Extracts from cells coexpressing EGFP-xPKA RIIα and mCherry-xRab32 were immunoprecipitated with an anti-Rab32 antibody or preimmune IgG. Precipitates were probed using anti-PKA RIIα antibody or anti-Rab32 and developed using HRP-protein A. Note that Rab32 antibody but not the preimmune IgG pulls down PKA RIIα. Inputs are 5% of cell extracts from sample.
(C) Two-hybrid analysis of Rab32-RIIα binding. xRab32 bait constructs (lacking C-terminal cysteins to prevent membrane binding) were tested against the indicated prey constructs in the yeast two-hybrid system for the ability to grow on minimal media in the presence (+His), or absence (-His) of histidine. The Rab32- PKA RIIα interaction was also tested with high-stringency (SD/-Trp/-Leu/-His/-Ade/X-α-Gal) plates. Growth on minimal media in the absence of histidine or α-galactosidase activity represents a positive interaction.
An additional test for xRab32-RIIα was performed using a yeast two-hybrid assay. To avoid membrane binding by xRab32 expressed in yeast, the assay was performed using xRab32 lacking two COOH-terminal cysteine residues required for prenylation and membrane localization. Fig. 4C demonstrates that Xenopus Rab32 binds to Xenopus RIIα, whereas Rab1a, used as a control, does not. Furthermore, both Q82L and T36N mutants of xRab32 that mimic the GTP- and GDP-bound states of the protein [5], were able to interact with xPKA RIIα (Fig. 4C). On the other hand, mutation L186P, known to inhibit PKA binding in mammalian cells, prevented the interaction of xRab32 with RIIα (Fig. 4C).
Rab32 is Required for Regulation of Melanosome Transport
To test the role of xRab32 in melanosome transport, we overexpressed pEGFP-xRab32 and induced melanosome aggregation or dispersion by melatonin or MSH, respectively. As a control, we transfected cells with pEGFP-Rab9, a Rab protein that is localized to late endosomes [13]. Strikingly, overexpression of the pEGFP-xRab32 completely prevented melanosome aggregation by melatonin, while Rab9 overexpression had no effect (Fig. 5A, 5B, and Movie 3). Time-lapse analysis shows that overexpression of xRab32 does not stop melanosome movement, but abolishes the bias of movement toward the cell center. This demonstrates that Rab32 plays a role in regulation of transport, rather than on movement itself.
Figure 5. xRab32 is Involved in Regulation of Melanosome Transport.
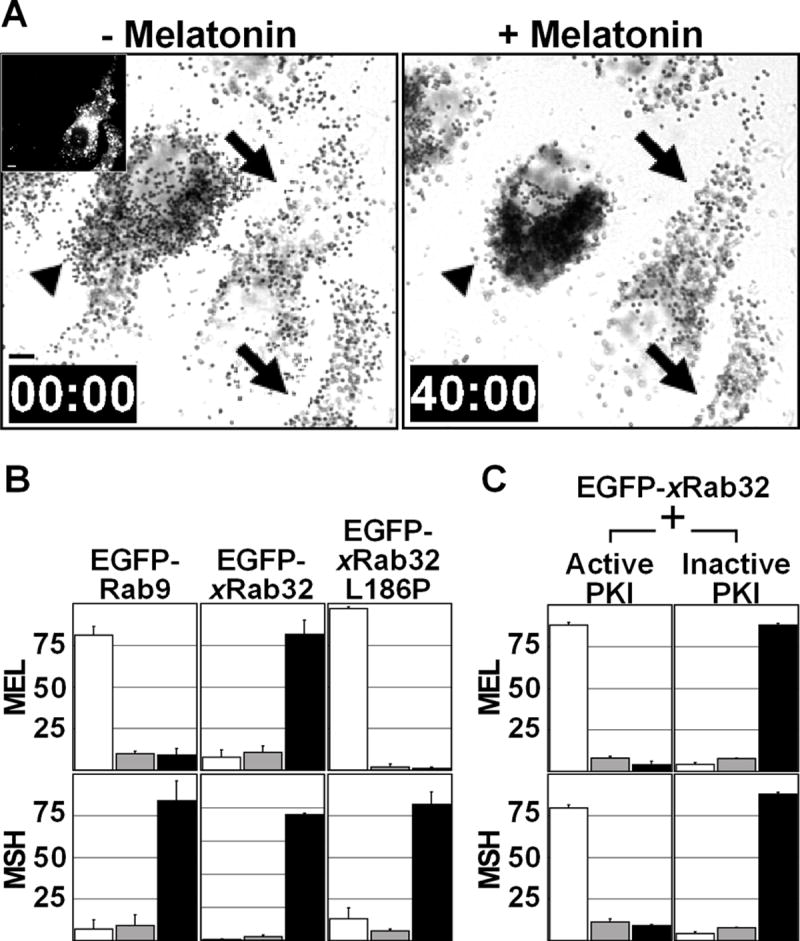
(A) xRab32 blocks melanosome aggregation by melatonin. The left panel is a bright-field image showing melanosome distribution before melatonin stimulation. The black arrows indicate EGFP-xRab32 transfected cells (inset) and the black arrowhead indicates a control cell. The right panel shows the distribution of melanosomes after 40 minutes of melatonin stimulation. EGFP-xRab32 overexpression in transfected cells (black arrows) completely blocks pigment aggregation by melatonin. Bars, 10 μm. This figure represents frames from Supplementary Movie 3.
(B) Recruitment of PKA by xRab32 to melanosomes is essential for inhibition. Xenopus melanophores were transiently transfected with EGFP-Rab9, EGFP-xRab32, or EGFP xRab32L186P. Transfected cells were treated with melatonin or MSH and scored into three groups (aggregated, partially dispersed, and dispersed). In each experiment, aggregated, partially dispersed, and dispersed cells are shown as white, gray, and black bars, respectively. n=100 for each condition. The experiment was repeated three times.
(C) xRab32 regulates melanosome movement upstream of PKA.
Xenopus melanophores were cotransfected with EGFP-xRab32 and constructs pNP210 or pNP211 encoding HA-epitope tagged active or inactive PKA inhibitor, PKI, respectively. Transfected cells were treated with melatonin or MSH, fixed, immunostained for HA and scored into three groups (aggregated, partially dispersed, and dispersed). In each experiment, aggregated, partially dispersed, and dispersed cells are shown as white, gray, and black bars, respectively. n=100 for each condition. The experiment was repeated three times.
To elucidate whether xRab32 localization is important to its effect on regulation, we tested the effect of the mutant EGFP-xRab32ΔCC that is unable to bind to melanosomes (Fig. 3D) but capable of PKA binding (Fig. 4B). Overexpression of this mutant did not affect the cell response to melatonin or MSH (Fig. S2). To determine, if Rab32-PKA binding is essential for its effects on melanosome behavior, we overexpressed a mutant xRab32L186P that does not interact with PKA (Fig. 5B). Fluorescent microscopy shows, that similarly to the wild-type protein, mCherry-xRab32L186P binds to melanosomes forming a fluorescent halo around a dark melanin core (Fig. 3C). As expected, this mutant does not inhibit melanosome aggregation Finally, if the impact of Rab32 on regulation is explained by PKA recruitment, inhibition of PKA should induce pigment aggregation even in cells overexpressing Rab32 because PKA functions downstream of Rab32. Indeed, an inhibitor of PKA, PKI, overexpressed in melanophores together with xRab32 induced pigment aggregation (Fig. 5C). Thus, overexpression experiments demonstrate that xRab32 is involved in the regulation of melanosome transport, and that its localization to melanosomes and binding to PKA are required for this function.
Conclusion
Melanosome transport in melanophores is regulated by PKA. PKA also regulates many other cellular functions and therefore PKA activation is restricted to specific areas of the cell. This is typically accomplished by AKAPs [3, 14]. In this report, we showed that Xenopus Rab32 is associated with melanosomes and links PKA to these organelles.
In other organisms, orthologues of xRab32 are localized to pigment organelles and function in their biogenesis. Human and mouse Rab32 to localize to melanosomes, and the level of Rab32 correlats to pigment production [4, 9]. A Drosophila Rab32 homolog, Rab-RP1, is localized to pigment granules in the eye and its mutation causes eye color defects [15, 16].
Although in many cell types Rab32 (like many other Rabs) functions in protein trafficking, Xenopus Rab32 has a second important function – to link PKA to the surface of melanosomes, organelles that are regulated by PKA, ensuring the spatial specificity of PKA signaling. Recent studies exploring the mechanism of PKA in melanosome transport demonstrated that a regulatory subunit of PKA, PKA-RIIα, is present on melanosomes [6]. Additionally, other signaling molecules, involved in the regulation of melanosome movement downstream of PKA are present on the surface of melanosomes [17] but the molecular mechanisms of such spatial restriction are not known. We demonstrated here that xRab32 is a key component of the signaling cascade regulating melanosome transport by linking PKA to the melanosome surface. These data agree well with the results by Scott and colleagues demonstrating that human Rab32 functions as an AKAP on mitochondria [5].
Rab proteins are known to recruit motor proteins to cargo either directly or through adapter proteins [18]. Therefore, in order to better understand the role of Rab32, it will be interesting to identify other interacting partners for xRab32 and to test if it can interact with molecular motors that move melanosomes.
Supplementary Material
Acknowledgments
We would like to thank A. Nebreda (EMBL) for PKA Cα and Cβ clones. We are grateful for J. Bartles, S. Kojima, and T. McGarry (Northwestern University) for critical reading of the manuscript. We also like to thank T. Shimi (Northwestern University) for assisting with taking confocal images. This work was supported by grant GM-52111 from NIGMS to V.I.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reilein AR, Tint IS, Peunova NI, Enikolopov GN, Gelfand VI. Regulation of organelle movement in melanophores by protein kinase A (PKA), protein kinase C (PKC), and protein phosphatase 2A (PP2A) The Journal of cell biology. 1998;142:803–813. doi: 10.1083/jcb.142.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniolos A, Lerner AB, Lerner MR. Action of light on frog pigment cells in culture. Pigment cell research. 1990;3:38–43. doi: 10.1111/j.1600-0749.1990.tb00260.x. sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. [DOI] [PubMed] [Google Scholar]
- 3.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nature reviews. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 4.Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. The Journal of cell biology. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alto NM, Soderling J, Scott JD. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. The Journal of cell biology. 2002;158:659–668. doi: 10.1083/jcb.200204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashina AS, Semenova IV, Ivanov PA, Potekhina ES, Zaliapin I, Rodionov VI. Protein kinase A, which regulates intracellular transport, forms complexes with molecular motors on organelles. Curr Biol. 2004;14:1877–1881. doi: 10.1016/j.cub.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Taylor SS, Yang J, Wu J, Haste NM, Radzio-Andzelm E, Anand G. PKA: a portrait of protein kinase dynamics. Biochimica et biophysica acta. 2004;1697:259–269. doi: 10.1016/j.bbapap.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt A, Nebreda AR. Inhibition of Xenopus oocyte meiotic maturation by catalytically inactive protein kinase A. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4361–4366. doi: 10.1073/pnas.022056399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen-Solal KA, Sood R, Marin Y, Crespo-Carbone SM, Sinsimer D, Martino JJ, Robbins C, Makalowska I, Trent J, Chen S. Identification and characterization of mouse Rab32 by mRNA and protein expression analysis. Biochimica et biophysica acta. 2003;1651:68–75. doi: 10.1016/s1570-9639(03)00236-x. [DOI] [PubMed] [Google Scholar]
- 10.Voigt J, Chen JA, Gilchrist M, Amaya E, Papalopulu N. Expression cloning screening of a unique and full-length set of cDNA clones is an efficient method for identifying genes involved in Xenopus neurogenesis. Mechanisms of development. 2005;122:289–306. doi: 10.1016/j.mod.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiological reviews. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 12.Lal A, Haynes SR, Gorospe M. Clean Western blot signals from immunoprecipitated samples. Molecular and cellular probes. 2005;19:385–388. doi: 10.1016/j.mcp.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, Pfeffer SR. Rab9 functions in transport between late endosomes and the trans Golgi network. The EMBO journal. 1993;12:677–682. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colledge M, Scott JD. AKAPs: from structure to function. Trends in cell biology. 1999;9:216–221. doi: 10.1016/s0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- 15.Fujikawa K, Satoh AK, Kawamura S, Ozaki K. Molecular and functional characterization of a unique Rab protein, RABRP1, containing the WDIAGQE sequence in a GTPase motif. Zoological science. 2002;19:981–993. doi: 10.2108/zsj.19.981. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Plesken H, Treisman JE, Edelman-Novemsky I, Ren M. Lightoid and Claret: a rab GTPase and its putative guanine nucleotide exchange factor in biogenesis of Drosophila eye pigment granules. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11652–11657. doi: 10.1073/pnas.0401926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deacon SW, Nascimento A, Serpinskaya AS, Gelfand VI. Regulation of bidirectional melanosome transport by organelle bound MAP kinase. Curr Biol. 2005;15:459–463. doi: 10.1016/j.cub.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 18.Jordens I, Marsman M, Kuijl C, Neefjes J. Traffic. Vol. 6. Copenhagen, Denmark: 2005. Rab proteins, connecting transport and vesicle fusion; pp. 1070–1077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.