Summary
Tobacco dependence is a chronic disorder that is characterized by relapse after periods of abstinence. It has been hypothesized that the activation of brain stress systems mediates stress-induced relapse to smoking. The aim of these experiments was to investigate the role of corticotropin-releasing factor (CRF) and norepinephrine in stress-induced reinstatement of extinguished nicotine-seeking behavior. Rats were allowed to self-administer nicotine under a fixed-ratio 5 schedule for 14 days and then nicotine-seeking behavior was extinguished by substituting saline for nicotine. In the first experiment, footshocks reinstated extinguished nicotine-seeking behavior. In the second experiment, there was a trend for the CRF1/2 receptor antagonist D-Phe CRF(12-41) (5, 25 μg, icv) to decrease stress-induced reinstatement of nicotine-seeking behavior. Footshock-induced reinstatement of nicotine-seeking behavior was observed only in a subset of stress-responsive rats (71%). D-Phe CRF(12-41) significantly attenuated stress-induced reinstatement of nicotine-seeking behavior in this subset of rats. In the third experiment, the α2-adrenergic receptor agonist clonidine (20, 40 μg/kg, sc) attenuated footshock-induced reinstatement of nicotine-seeking behavior. In the fourth experiment, the effects of D-Phe CRF(12-41) and clonidine on responding for chocolate-flavored food pellets was investigated in order to determine if these compounds have sedative effects. D-Phe CRF(12-41) did not affect responding for food pellets. Clonidine slightly, but significantly, decreased responding for food pellets. Clonidine decreased responding for food to a lesser degree than it decreased stress-induced reinstatement of nicotine-seeking behavior. These data provide support for the hypothesis that an increased activity of brain CRF and norepinephrine systems mediates stress-induced relapse to nicotine-seeking behavior.
Keywords: Nicotine, corticotropin-releasing factor, norepinephrine, withdrawal, rats
1. Introduction
Tobacco dependence is a chronic disorder that is characterized by loss of control over tobacco smoking, the appearance of affective withdrawal symptoms upon the cessation of tobacco smoking, and relapse after periods of abstinence (American Psychiatric Association 2000; McLellan et al. 2000; O'Brien 2003). Experimental evidence indicates that nicotine is one of the main components of tobacco smoke that leads to and maintains tobacco smoking behavior (Bardo et al. 1999; Crooks and Dwoskin 1997; Stolerman and Jarvis 1995). Nicotine mediates its postive reinforcing effects (e.g., euphoria, increased attention) at least partly via the activation of neuronal nicotinic acetylcholine receptors (nAChRs). Drugs that block nAChRs decrease nicotine self-administration in rats (Corrigall et al. 1994; Corrigall and Coen 1989; Donny et al. 1999; Picciotto et al. 1998; Watkins et al. 1999). In addition, mice that lack the β2-subunit of the nAChR self-administer less nicotine than wild-type controls (Picciotto et al., 1998). Cessation of tobacco smoking in humans precipitates an acute withdrawal syndrome that is characterized by negative affective symptoms such as depressed mood, anxiety, and difficulty concentrating (American Psychiatric Association 2000; Bruijnzeel and Gold 2005). It has been hypothesized that the acute negative affective aspects of drug withdrawal provide a powerful motivational force for the continuation of drug use (Koob et al. 1997; Markou et al. 1998).
After the acute withdrawal phase (i.e., protracted withdrawal), exposure to stressors greatly increases the likelihood of relapse to tobacco smoking (Cohen and Lichtenstein 1990; Doherty et al. 1995; Kassel et al. 2003; McKee et al. 2003; Niaura et al. 2002; Shiffman and Waters 2004; Swan et al. 1988). Patients with psychiatric disorders such as major depression and generalized anxiety are twice as likely to smoke cigarettes than healthy controls; an increased vulnerability to stressors could possibly contribute to the high relapse rates in this group of patients (Lasser et al. 2000). Exposure to footshocks has been shown to reinstate extinguished heroin, cocaine, alcohol, and nicotine-seeking behavior in rats (Buczek et al. 1999; Erb et al. 2001; Liu and Weiss 2002). It is important to note that a number of studies by independent research groups have shown that footshocks do not reinstate operant responding previously maintained by food pellets, sucrose pellets, or sucrose solutions (Ahmed and Koob 1997; Buczek et al. 1999; Le et al. 1998; Mantsch and Goeders 1999). Despite the important role of stressors in relapse to tobacco smoking behavior, preclinical studies to investigate the neuronal mechanism that mediate stress-induced relapse to nicotine-seeking behavior have not been conducted. Studies that focused on investigating the neurobiological substrates underlying footshock-induced reinstatement of cocaine, heroin, and alcohol-seeking behavior suggest that an increased release of CRF and norepinephrine at least partly mediates stress-induced reinstatement of drug-seeking behavior (Erb et al. 2000; Erb et al. 2001; Le et al. 2000; Shaham et al. 1998). Stewart and colleagues reported that systemic administration of the α2-adrenergic receptor agonists clonidine, lofexidine, or guanabenz attenuates stress, but not cue, induced reinstatement of cocaine-seeking behavior (Erb et al. 2000). The α2-adrenergic receptor agonists mediate their pharmacological effects by hyperpolarizing noradrenergic cell groups in the brain stem and thereby reducing stress-induced release of norepinephrine (Aghajanian and VanderMaelen 1982; Pudovkina et al. 2001). Stewart and colleagues also investigated the role of CRF receptors in stress-induced reinstatement of alcohol-seeking behavior. They reported that intraventricular (icv) administration of the nonspecific CRF1/2 receptor antagonist D-Phe CRF(12-41) or systemic administration of the specific CRF1 receptor antagonist CP-154,526 attenuates stress-induced reinstatement of alcohol-seeking behavior (Le et al. 2000). The results of these studies are in line with the observation that blockade of CRF receptors prevents the neurobehavioral effects of stressors (Reul and Holsboer 2002).
Taken together, the above-discussed studies suggest that increased CRF and norepinephrine transmission plays a role in stress-induced reinstatement of drug-seeking behavior. Although there is strong evidence that stressors contribute to relapse to tobacco smoking, the neurobiological substrates that mediate stress-induced reinstatement of nicotine-seeking behavior have not been investigated. It is hypothesized here that a heightened CRF and noradrenergic transmission mediates footshock-induced reinstatement of nicotine-seeking behavior. The aim of the present experiments was to investigate the effects of the non-specific CRF1/2 receptor antagonist D-Phe CRF(12-41) and the α2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior. The first experiment investigated the effects of footshocks on extinguished nicotine-seeking behavior using a rodent nicotine self-administration procedure. The second experiment investigated the effects of D-Phe CRF(12-41) on footshock-induced reinstatement of nicotine-seeking behavior. The third experiment investigated the effects of clonidine on footshock induced-reinstatement of nicotine-seeking behavior. In order to investigate if D-Phe CRF(12-41) or clonidine has sedative effect, a fourth experiment was conducted in which the effects of D-Phe CRF(12-41) or clonidine on responding for food pellets was investigated.
Experiments that investigate the neurobiological substrates underlying stress-induced reinstatement of nicotine-seeking behavior may contribute to the development of pharmacotherapies that prevent stress-induced relapse to tobacco smoking behavior and thereby increase the percentage of people that is able to quit smoking permanently.
2. Materials and Methods
2.1. Subjects
Male Wistar rats (Charles River, Raleigh, NC) weighing 250–300 g at the beginning of the experiments were used. Animals were single-housed in a temperature and humidity-controlled vivarium and maintained on a 12 hr light-dark cycle (lights off at 6 p.m.). All testing occurred at the end of the light cycle. Food and water were available ad libitum in the home cages. All subjects were treated in accordance with the National Institutes of Health guidelines regarding the principles of animal care. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) and approved by the University of Florida Institutional Animal Care and Use Committee.
2.2. Drugs
Nicotine hydrogen tartrate salt, clonidine hydrochloride, and pentobarbital sodium salt were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA) and dissolved in sterile saline (0.9% sodium chloride). The CRF antagonist [DPhe12, Nle21,38CαMe Leu37]r/hCRF(12–41) (D-Phe CRF(12-41)) was synthesized by The Clayton Foundation Laboratories for Peptide Biology and kindly provided by Dr. Jean Rivier (Salk Institute for Biological Studies, La Jolla, CA). The peptide was dissolved in distilled water and administered within one hour after being dissolved.
2.3. Surgical Procedures
2.3.1 Catherization surgery
Rats were anesthetized with an isoflurane / oxygen vapor mixture (1–3% isoflurane) and prepared with a chronic catheter in the right jugular vein. Catheters consisted of silastic tubing (length 13.5 cm, 0.51 mm inside diameter × 0.94 mm outside diameter, Dow Corning, Midland, MI) that was connected to a 22 gauge stainless steel guide cannula, which was molded onto a durable polyester fiber mesh (Plastics One, Roanoke, VA). The tubing was passed subcutaneously from the mid scapular region to the ventral thorax / lower part of the neck, inserted into the jugular vein (4.0 cm), and secured with silk suture thread. After the implantation of the catheters, the animals were allowed to recover for 7 days. During the recovery period, the catheters were flushed daily with 0.2 ml sterile heparinized saline (30 units/ml) followed by 0.2 ml of the antibiotic Timentin (7.0 mg/kg day, ticarcillin disodium and clavulanate potassium, Beecham Laboratories, Piscataway, NJ) dissolved in saline to prevent postoperative infections. To ensure long-term patency of the catheters, 0.1 ml lock solution (heparinized glycerol, 10000 unites/ml; Amresco Inc., Solon, Ohio) was infused into the catheter after the administration of Timentin. When the catheters were not being used, they were closed with a short length of Tygon tubing (Saint-Gobain Performance Plastics, Valley Forge, PA) plugged with monofilament and covered with a stainless steel cap. Catheter patency was tested with 0.1 ml Brevital (1% methohexital sodium, King Pharmaceuticals, Bristol, TE) at the end of the recovery period or when an animal displayed a sudden decrease in responding for nicotine. Animals with patent catheters exhibited pronounced loss of muscle tone within 2 s of intravenous injection of Brevital.
2.3.2. Cannula implantation
For experiments 2 and 4, the rats were prepared with cannulae above the lateral ventricle. In experiment 2, cannulae were implanted immediately after the implantation of the intravenous catheter. Rats were placed in a Kopf stereotaxic frame (David Kopf Instruments, Tujunga, CA) with the incisor bar set 3.3 mm below the interaural line (flat skull). The rats were prepared with stainless steel 23 gauge cannulae 11 mm in length located immediately above the lateral ventricle using the following flat skull coordinates: anterior posterior (AP) −0.9 mm, medial lateral (ML) ±1.4 mm, dorsal ventral (DV) −3.0 mm from skull (Paxinos and Watson 1998). At the end of the surgery, removable 30 gauge wire stylets 11 mm in length were inserted in the cannulae so that the cannulae would maintain patency. The cannulae were permanently secured to the skull using dental cement anchored with four skull screws.
2.4. Apparatus
Food training and drug self-administration sessions were conducted in eight operant conditioning chambers that were located inside sound attenuated boxes (Med Associates, St. Albans, VT). One side of the operant conditioning chambers was equipped with an active and an inactive lever, and above each lever was a cue light. Data collection and test sessions were controlled by a microcomputer. Delivery of the nicotine solution was controlled by a syringe pump (Model A, Razel Scientific Instruments, Stamford, CT). Tygon tubing (0.508 mm inside diameter × 1.524 mm outside diameter, Saint-Gobain Performance Plastics, Valley Forge, PA) connected a 10 ml syringe, which was placed in the pump, to the backmount of the rat. A protective metal spring covered the tubing. One side of the spring was connected to a stainless steel swivel (Instech Laboratories, Plymouth Meeting, PA) and the other side of the spring was connected to the backmount.
2.5. Experimental procedures
2.5.1. Food training
Prior to the onset of food training, the rats were food deprived for 48 h (5 g lab chow / day). After the onset of food training, the rats were fed 17–20 gram (80–95% of baseline ad libitum calories) of lab chow per day, at least 1 h after the end of the food training session. Standard food pellets were used for Experiment 1–3, and chocolate-flavored food pellets were used for Experiment 4 (Bio-Serv, Frenchtown, NJ). During the first training session, the rats received 45 mg of food pellets at fixed intervals (one pellet every 12 s for 5 min, then a 5 min break; this sequence was repeated during the initial 30 min session; total number of pellets delivered was 75) with no requirement to respond on the active lever. After this session, the rats had to respond on the active lever to receive food pellets. Instrumental training started on a fixed-ratio 1, time-out 1 s (FR1 TO1-s) schedule of reinforcement and the training sessions lasted 1 hr. The training schedule was progressively changed according to the following sequence: FR1 TO1, FR1 TO10, FR1 TO20, FR2 TO20, FR5 TO20-s. The rats had to reach the criterion of 100 pellets earned during a daily 1 hr session before training at the next level started. Food training continued until the subjects earned 100 food pellets in a daily 1 hr session on an FR5 TO20-s schedule of reinforcement. Food training typically required 7–9 days. After the completion of the food training sessions, all rats were fed 20 g of lab chow per day (95% of baseline ad libitum calories) 1 hour after the end of testing.
2.5.2. Nicotine self-administration, extinction, and stress-induced reinstatement
After successful completion of food training and catherization surgeries, the rats were allowed to self-administer nicotine at the 0.03 mg/kg/infusion (free base) dose by switching the delivery of a food pellet for the delivery of a nicotine infusion as described previously (Bruijnzeel and Markou 2003). The operant conditioning chambers were equipped with two retractable levers. Responding on the active lever resulted in the delivery of a nicotine infusion and responding on the inactive lever was recorded but had no scheduled consequences. The delivery of an infusion (0.1 ml / infusion over a 5.6 s time-period) was earned by responding five times on the active lever (FR5 TO20-s). The initiation of the delivery of an infusion was paired with a cue light, which remained illuminated throughout the 20-s time-out period (initiated simultaneously with the initiation of delivery of a nicotine infusion). The active lever was retracted during the time-out period. Responding for nicotine was extinguished by replacing nicotine with saline. Extinction training was considered completed when the average number of infusions was less then 2. Reinstatement sessions started one day after extinction training was completed. Nicotine-seeking behavior was reinstated by the administration of footshocks (8 shocks in a 10-min time-period, 0.8 mA, 1 s shocks, mean off period 37 s) immediately prior to the self-administration session. Shock parameters were based on reinstatement studies conducted by Stewart and Shaham (Buczek et al. 1999). The nicotine self-administration and extinction sessions were conducted 7 days per week.
2.5.3. Drug administration procedure
For icv injections, 30 gauge stainless steel injectors projecting 2.5 mm beyond the guide cannula were used. All icv injections were made by gravity induced by raising the Hamilton syringe above the animal's head. Five μl of solution was administered over a 30–60 second period, and the injector was left in place for another 30 seconds to allow diffusion from the injector tip. Immediately after finishing drug administration, the dummy stylets were re-inserted. At the end of the experiment the rats were killed using an overdose of pentobarbital (150 mg/kg, intraperitoneally [ip]), and cannulae placement were verified by administering 5 μl of a 0.5% aqueous methyl blue solution at the injections site.
2.6 Experimental Design
2.6.1. Experiment 1: Effects of footshocks on extinguished nicotine-seeking behavior
The aim of this experiment was to investigate the effects of footshocks on extinguished nicotine seeking-behavior. Naïve rats were used for this experiment. After recovery from the catheter implantations, the rats (n = 8) were allowed to self-administer nicotine (FR5 TO20-s, 0.03 mg/kg of nicotine per infusion; free base) for 14 consecutive days. Responding on the active lever was extinguished by substituting saline for nicotine. Nicotine-seeking behavior was considered extinguished when the average number of infusions was less than 2. Footshocks (8 shocks, 0.8 mA, 1 s, 10-min time period) were administered immediately prior to the self-administration session. All test conditions were exactly the same during nicotine self-administration, extinction learning, and reinstatement testing with the exception that nicotine was replaced by saline after 14 days of nicotine self-administration.
2.6.2. Experiment 2: Effects of D-Phe CRF(12-41) on stress-induced reinstatement of nicotine-seeking behavior
The aim of this experiment was to investigate the effects of the CRF receptor antagonist D-Phe CRF(12-41) on footshock-induced reinstatement of nicotine-seeking behavior. Drug naïve rats (n = 14) were allowed to self-administer nicotine (FR5 TO20-s, 0.03 mg/kg of nicotine per infusion; free base) for 14 consecutive days and nicotine-seeking behavior was extinguished by substituting saline for nicotine. Extinction training was considered complete when the average number of infusion was less than 2. Reinstatement sessions started one day after extinction training was completed. D-Phe CRF(12-41) (0, 5, 25 μg, icv) was administered according to a Latin-square design 15 minutes before the footshock session. There was at least one day between test days and on these days the rats were left undisturbed. The design of this study was based on previous studies that showed that repeated footshock sessions or repeated exposure to cues associated with nicotine delivery reliably reinstates extinguished nicotine-seeking behavior (Buczek et al. 1999; Paterson et al. 2005). In order to investigate if contextual cues associated with prior footshock sessions contributed to footshock-induced reinstatement of nicotine-seeking behavior (reinstatement sessions 2 and 3), an additional reinstatement session was conducted two days after the last footshock session in which the rats did not receive any footshocks. At the end of the experiment the rats were killed using an overdose of pentobarbital and cannulae placement were verified.
2.6.3. Experiment 3: Effects of clonidine on stress-induced reinstatement of nicotine-seeking behavior
The aim of this experiment was to investigate the effects of the α2-adrenergic receptor agonist clonidine on footshock-induced reinstatement of nicotine-seeking behavior. The design of this experiment was the same as that of experiment 2, with the exception that clonidine (0, 20, 40 μg / kg, volume 1 ml / kg, subcutaneously [sc]) was administered to the animals (n = 15) 30 minutes prior the footshock session.
2.6.4. Experiment 4: Effects of D-Phe CRF(12-41) and clonidine on responding for food pellets
The aim of this experiment was to investigate the effects of D-Phe CRF(12-41 and clonidine on responding for chocolate-flavored food pellets. Rats were prepared with a cannula in the lateral ventricle and after at least one week of recovery, the rats (n = 10) were allowed to respond for chocolate-flavored food pellets under a FR5 TO20-s schedule of reinforcement. D-Phe CRF(12-41) (0, 5, 25 μg, icv) was administered according to a Latin-square design 15 minutes before the rats were placed in the operant conditioning chambers. There were at least two off-days between the drug days. Rats were allowed to respond for chocolate-flavored food pellets on off-days. The experiment that investigated the effects of clonidine on responding for chocolate-flavored food pellets started three days after the D-Phe CRF(12-41) injections were completed. Clonidine (0, 20, 40 μg / kg, volume 1 ml / kg, sc) was administered according to a Latin-square design 30 minutes prior to the test sessions and there was at least one off-day between drug days. The rats were allowed to respond for food 7 days per week and all the sessions were 20 minutes. At the end of the experiment the rats were killed using an overdose of pentobarbital and cannulae placement were verified.
2.7. Data analyses
The dependent variables were number of responses on the active lever and number of responses on the inactive lever. For the first experiment, the number of responses on the active and inactive lever during nicotine self-administration were analyzed using one-way repeated-measures analyses of variance (ANOVA) with time as the within-subject factor. The effect of extinction training on responding on the active and inactive lever was analyzed using a one-way repeated-measures ANOVA with time as the within subjects factor. The effect of footshocks on responding on the active and inactive lever was analyzed using one-way repeated-measures ANOVA with footshocks as the within subjects factor. For the second experiment, the effect of extinction training on responding on the active and inactive lever was analyzed using one-way repeated-measures ANOVA with time as the within subjects factor. The effect of D-Phe CRF(12-41) on responding on the active and inactive lever after the administration of footshocks was analyzed using one-way repeated-measures ANOVA with the dose of D-Phe CRF(12-41) as the within subjects factor. The data from experiment 3 were analyzed with the same statistical methods as the data from experiment 2. The effect of D-Phe CRF(12-41) and clonidine on responding on the active and inactive lever during food sessions was analyzed using one-way repeated-measures ANOVA with the dose of D-Phe CRF(12-41) or clonidine as the within subjects factor. Correlation values were calculated using a Pearson correlation analyses. For all experiments, statistically significant results in the ANOVA were followed by the Newman-Keuls post-hoc test. The criterion for significance was set at 0.05. The statistical analyses were performed using SPSS for Windows software, version 15.0
3. Results
3.1. Experiment 1: Effects of footshocks on extinguished nicotine-seeking behavior
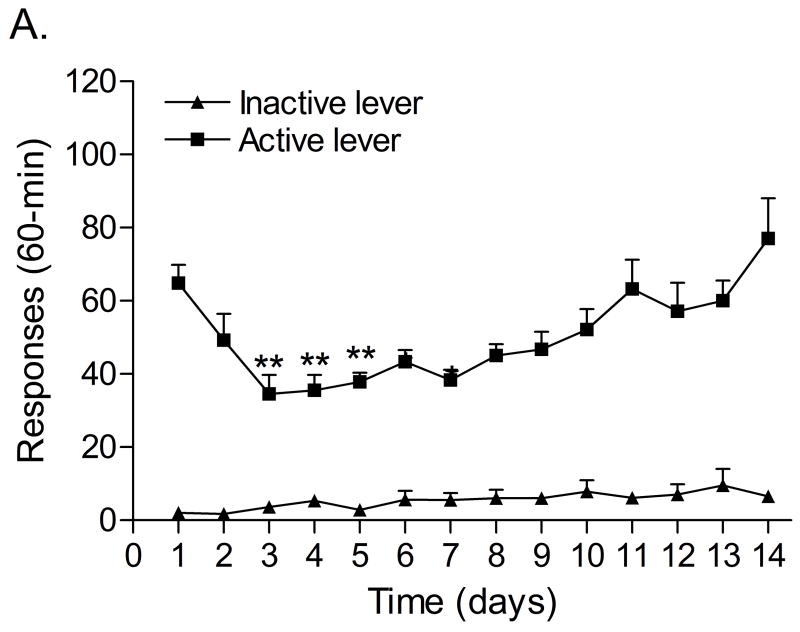
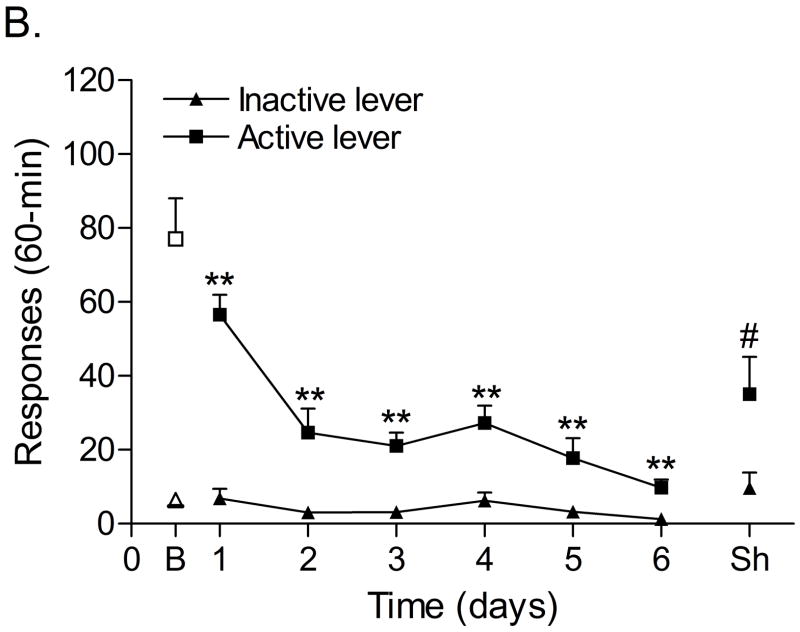
Figure 1A shows responding on the active lever and the inactive lever during the 1 hour nicotine self-administration sessions. Responding on the active lever decreased during the first days of nicotine self-administration and then gradually increased (F13,91=6.98, P<0.0001), indicating that nicotine maintained responding under a FR5 TO20-s schedule of reinforcement. Responding on the inactive lever did not change over time (F13,91=1.54, n.s.). The mean (±S.E.M.) number of infusions of 0.03 mg/kg of nicotine, responses on the active lever, and responses on the inactive lever during the last day of nicotine self-administration were 15.13 ± 2.12, 77.00 ± 11.06, 6.50 ± 1.72, respectively. Extinction training resulted in a rapid decline in the number of responses on the active lever (Figure 1B, F6,42=21.57, P<0.0001), but did not affect responding on the inactive lever (F6,42=2.24, n.s.). The extinction criterion (less than 2 saline infusions [10 responses on active lever]) was met 6 days after substituting saline for nicotine. Exposure to footshocks increased the number of responses on the active lever (F1,7=6.99, P<0.03) and did not affect the number of responses on the inactive lever (F1,7=3.27, n.s.). Pearson correlation analyses indicated that there was no correlation between the number of responses on the active lever (r = 0.37, n.s.) or inactive lever (r = − 0.37, n.s.) during the last day of extinction training and stress-induced reinstatement testing.
Figure 1.
Responding on the active and inactive lever during nicotine self-administration (A, n = 8), extinction training, and relapse (B, n = 8). In figure 1A, asterisks (** P<0.01, * P<0.05) indicate a decrease in responding on the active lever compared to the first day of nicotine self-administration. In figure 1B, asterisks (** P<0.01, * P<0.05) indicate a decrease in responding on the active lever compared to baseline responding (last day of nicotine self-administration). Pound signs (# P<0.05) indicate a footshock-induced increase in responding on the active lever compared to day 6 of extinction training. Abbreviations: B, baseline; Sh, footshocks. Data are expressed as means ± SEM.
3.2. Experiment 2: Effects of D-Phe CRF(12-41) on stress-induced reinstatement of nicotine-seeking behavior
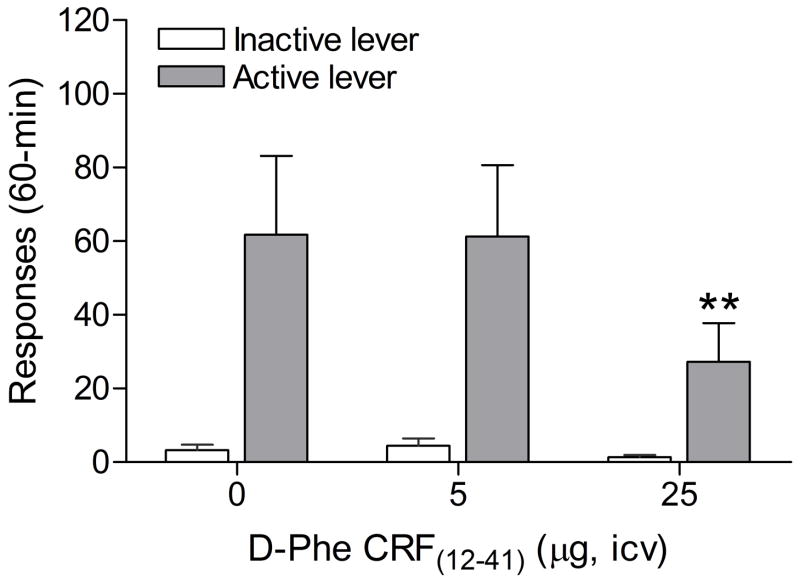
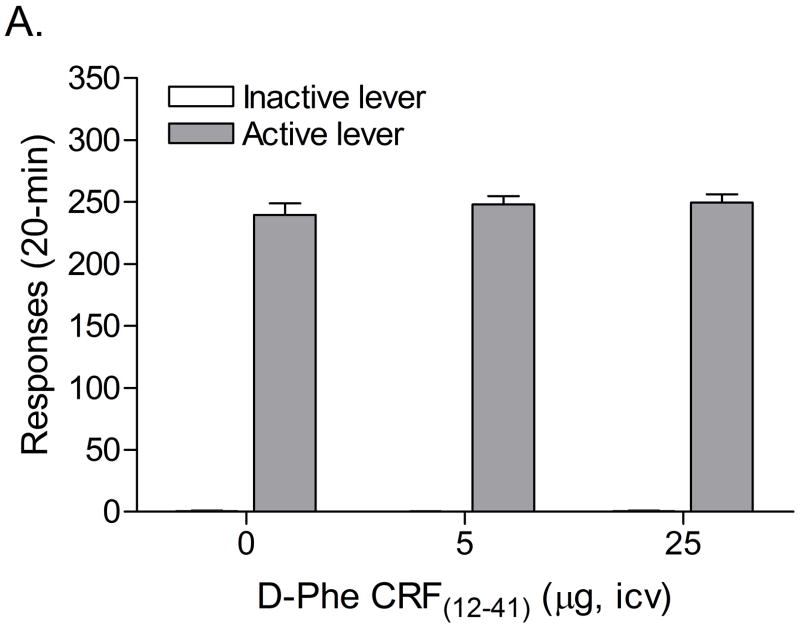
The mean (±S.E.M.) number of infusions of 0.03 mg/kg of nicotine, responses on the active lever, and responses on the inactive lever during the last day of nicotine self-administration were 10.79 ± 1.53, 54.57 ± 7.79, 2.5 ± 0.75, respectively. Substituting saline for nicotine caused a rapid decline in the number of responses on the active lever (F5,52=12.73, P<0.0001) and a decline in the number of responses on the inactive lever (F6,42=2.76, P<0.04). The extinction criterion (less than 10 responses on the active lever) was met 5 days after the onset of extinction training. There was a trend towards a decrease in footshock-induced responding on the active lever after the administration of the CRF1/2 receptor antagonist D-Phe CRF(12-41) (F2,26=3.24, P<0.055). D-Phe CRF(12-41) did not affect responding on the inactive lever (F2,26=1.13, n.s.). A close look at the data revealed that a subgroup of rats did not respond on the active lever after the administration of footshocks. These non-responders (average number of responses on the active lever fewer than 5 [1 infusion] during 3 reinstatement sessions) were excluded and the data were re-analyzed. Four out of 14 rats met the exclusion criterion (28.6% stress-unresponsive and 71.4% stress-responsive rats). Statistical analyses indicated that pretreatment with D-Phe CRF(12-41) attenuated footshock-induced responding on the active lever in the stress-responsive rats (Figure 2; F2,18=3.87, P<0.04) and did not affect responding on the inactive lever (F2,18=1.06, n.s.). Posthoc analyses indicated that the highest dose of D-Phe CRF(12-41), 25 μg icv, decreased responding on the active lever. D-Phe CRF(12-41) did not affect responding on the active (Table 1; F2,6=0.826, n.s.) or inactive lever (F2,6=0.429, n.s.) in the stress-resistant rats. Pearson correlation analyses indicated that there was no correlation between the number of responses on the active lever (r = 0.36, n.s.) or inactive lever (r = 0.05, n.s.) during the last day of extinction training and stress-induced reinstatement testing (vehicle condition). In order to investigate if contextual stimuli associated with prior footshock-stress contributed to responding on the active lever, rats were placed in the operant conditioning chambers two days after the last footshock session and lever pressing was recorded. Statistical analysis indicated that there was no difference in the number of responses on the active lever during the last extinction training session and the test session that was conducted two days after the last footshock session (F1,13=0.81, n.s.). This suggests that contextual stimuli did not contribute to footshock-induced reinstatement of nicotine-seeking behavior.
Figure 2.
Effects of CRF receptor antagonist D-Phe CRF(12-41) on footshock-induced reinstatement of nicotine-seeking behavior (n = 10) in a subset of stress-responsive rats. Asterisks (P<0.05) indicate a decrease in responding on the active lever compared to rats that were exposed to footshocks and pretreated with vehicle. Data are expressed as means ± SEM.
Table 1.
Effects of D-Phe CRF(12-41) on stress-induced reinstatement of nicotine-seeking behavior in stress-resistant rats.
| Dose of D-Phe CRF(12-41) (μg, icv) | Responses inactive lever | Responses active lever |
|---|---|---|
| 0 | 0.25 ± 0.25 | 1.00 ± 0.71 |
| 5 | 0.25 ± 0.25 | 0.25 ± 0.25 |
| 25 | 0.00 ± 0.00 | 3.50 ± 3.18 |
3.3. Experiment 3: Effects of clonidine on stress-induced reinstatement of nicotine-seeking behavior
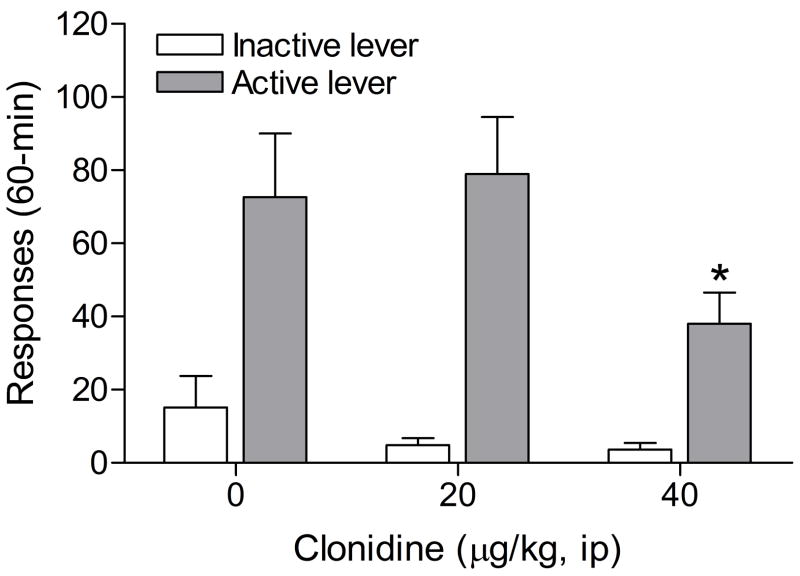
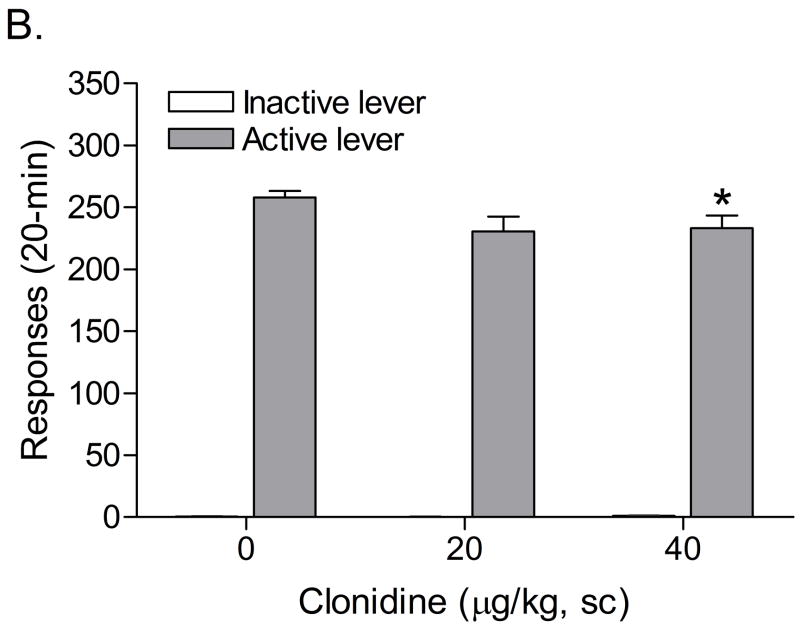
The mean (±S.E.M.) number of infusions of 0.03 mg/kg of nicotine, responses on the active lever, and responses on the inactive lever during the last day of nicotine self-administration were 13.20 ± 1.43, 66.47 ± 7.19, 8.87 ± 2.25, respectively. Substituting saline for nicotine caused a decline in the number of responses on the active lever (F5,70=8.09, P<0.0001), but did not affect responding on the inactive lever (F5,70=2.22, n.s.). The extinction criterion (less than 10 responses on the active lever) was met 6 days after the onset of extinction training. Responding on the active lever after the administration of footshocks was higher in experiment 3 than in experiment 2. In the second experiment, 28.6% (4 out of 14) of the rats met the exclusion criterion; average number of responses on the active lever fewer than 5 [1 infusion] during 3 reinstatement sessions. In the third experiment, none of the rats (0 out of 15) met the exclusion criterion. As none of the rats met the exclusion criterion the rats were not divided into subgroups. The administration of clonidine prior to footshocks attenuated stress-induced responding on the active lever (Figure 3; F2,28=3.63, P<0.4). Posthoc analyses indicated that the highest dose of clonidine, 40 μg/kg sc, decreased responding on the active lever. Clonidine did not have an effect on responding on the inactive lever (F2,28=1.53, n.s.). Pearson correlation analyses indicated that there was no correlation between the number of responses on the active lever (r = 0.36, n.s.) or inactive lever (r = 0.05, n.s.) during the last day of extinction training and stress-induced reinstatement testing (vehicle condition). Statistical analysis indicated that there was no difference in the number of responses on the active lever during the last extinction training session and a test session that was conducted two days after the last footshock session (F1,14=4.60, n.s.). Therefore, it is unlikely that contextual stimuli contributed to footshock-induced reinstatement of nicotine-seeking behavior.
Figure 3.
Effects of the α2-adrenergic receptor agonist clonidine on footshock-induced reinstatement of nicotine-seeking behavior (n = 15). Asterisks (P<0.05) indicate a decrease in responding on the active lever compared to rats that were exposed to footshocks and pretreated with vehicle. Data are expressed as means ± SEM.
3.4 Experiment 4: Effects of D-Phe CRF(12-41) and clonidine on responding for food pellets
Figure 4A shows the effects of D-Phe CRF(12-41) on responding for chocolate-flavored food pellets under a FR5 TO20-s schedule of reinforcement. D-Phe CRF(12-41) did not affect responding on the active (F2,18=0.608, n.s.) or the inactive lever (F2,18=0.367, n.s.). Figure 4B shows the effects of clonidine on responding for chocolate-flavored food pellets. Clonidine slightly, but significantly, decreased responding on the active lever (F2,18=3.631, P<0.047) and did not affect responding on the inactive lever (F2,18=1.519, n.s.). Posthoc analyses indicated that the highest dose of clonidine, 40 μg/kg sc, slightly decreased responding for chocolate-flavored food pellets.
Figure 4.
Effects of the CRF receptor antagonist D-Phe CRF(12-41) (A) and the α2-adrenergic receptor agonist clonidine (B) on responding for chocolate-flavored food pellets (n = 10). Asterisks (P<0.05) indicate a decrease in responding on the active lever compared to rats that were pretreated with vehicle. Data are expressed as means ± SEM.
4. Discussion
The present results showed that intermittent footshocks reinstate extinguished nicotine-seeking behavior. There was a trend towards a decrease in stress-induced reinstatement of nicotine-seeking behavior after the administration of the CRF1/2 receptor antagonist D-Phe CRF(12-41). D-Phe CRF(12-41) attenuated footshock-induced reinstatement of nicotine-seeking behavior in a subset (10 out of 14) of stress-responsive rats. The CRF1/2 receptor antagonist did not affect the behavior of rats that did not respond to the footshocks (4 out of 10). Responding in this subgroup of rats was so low (average number of responses on active lever during 3 reinstatement sessions was 1.6) that an effect of D-Phe CRF(12-41) could not have been detected. Similar to the CRF1/2 receptor antagonist, the α2-adrenergic receptor agonist clonidine attenuated footshock-induced reinstatement of nicotine-seeking behavior.
The results presented here are in line with a previous study that showed that footshocks reinstate extinguished nicotine-seeking behavior (Buczek et al. 1999). Our findings extend and corroborate previous research by demonstrating that enhanced CRF or norepinephrine transmission at least partly mediates footshock-induced reinstatement of extinguished nicotine-seeking behavior. The results presented here indicate that antagonism of CRF receptors attenuates footshock-induced reinstatement of extinguished nicotine-seeking behavior. To our knowledge, this is the first study to show that CRF receptor antagonism could be an efficacious treatment for stress-induced relapse to tobacco smoking behavior. Previous research suggest that a hyperactivity of brain CRF systems plays a role stress-induced reinstatement of extinguished cocaine, heroin, and alcohol-seeking behavior (Erb et al. 2001; Le et al. 2000; Liu and Weiss 2002; Shaham et al. 1998). The effective dose of D-Phe CRF(12-41) was higher in the present study than in other studies that investigated the effects of icv D-Phe CRF(12-41) on footshock-induced reinstatement of drug-seeking behavior. Shaham and colleagues reported that 1 μg of D-Phe CRF(12-41), but not 0.3 μg, attenuated footshock-induced reinstatement of alcohol-seeking behavior (Le et al. 2000). Liu and Weiss reported that 10 μg of D-Phe CRF(12-41), but not 1 μg, attenuated footshock-induced reinstatement of alcohol-seeking behavior (Liu and Weiss 2002). Ten μg of D-Phe CRF(12-41) was not tested in the present study, therefore it cannot be ruled out that this dose might have attenuated footshock-induced reinstatement of nicotine-seeking behavior. In the present study, 25 μg of D-Phe CRF(12-41) attenuated footshock-induced reinstatement of nicotine-seeking behavior, but lower doses, 5 μg or 1 μg (pilot study, unpublished observation), were ineffective. The difference in the effectiveness of D-Phe CRF(12-41) between the present study and the Shaham study can not have been caused by a difference in rat strain; male Wistar rats were used in both studies. The footshock procedures were also very similar. In the present study, reinstatement of nicotine-seeking behavior was established by the administration of 0.8 mA shocks that lasted 1 s with a mean off period of 37 s and in the Shaham study drug-seeking behavior was reinstated by the administration 0.8 mA shocks that lasted 0.5 s with a mean off period of 40 s. However, a difference between the present study and the Shaham study was the number of responses on the active lever after the administration of footshocks (present study recorded 62 responses / 60 minutes and the Shaham study recorded ~ 50 responses / 60 minutes). This indicates that our subset of rats displayed a slightly more pronounced behavioral response to the stressor than the rats used by Shaham and therefore it might be possible that a higher dose of D-Phe CRF(12-41) was required to counteract the neurobehavioral effects of the stressor. The observation that only a relatively high dose of D-Phe CRF(12-41) attenuated stress-induced reinstatement of nicotine-seeking behavior is in line with a previous study conducted by our research group in which the effect of D-Phe CRF(12-41) on the deficit in brain reward function (elevations in brain reward thresholds as assessed with an intracranial self-stimulation procedure) associated with nicotine withdrawal was investigated (Bruijnzeel et al. 2007). A high dose of D-Phe CRF(12-41), 20 μg icv, prevented the dysphoria associated with nicotine withdrawal, but lower doses, 1, 5, or 10 μg icv, were ineffective.
The results of the third experiment indicated that the α2-adrenergic receptor agonist clonidine attenuates footshock-induced reinstatement of extinguished nicotine-seeking behavior. The lowest dose of clonidine, 20 μg sc, was ineffective, but the highest dose of clonidine, 40 μg sc, decreased responding on the active lever by almost 50%. This pattern of results is similar to the results of a previous study that investigated the effects of clonidine on stress-induced reinstatement of cocaine-seeking behavior (Erb et al. 2000). It was reported that 40 μg of clonidine ip, but not 20 μg ip, attenuates footshock-induced reinstatement of cocaine-seeking behavior. This suggests that the results of studies that investigated the neurobiological substrates that mediate stress-induced reinstatement of cocaine-seeking behavior can be generalized to other abused psychostimulants such as nicotine.
In the second and third experiment, rats were exposed to repeated footshock sessions. In order to investigate if contextual cues contributed to footshock-induced reinstatement of nicotine-seeking behavior during the second and third session, rats were placed in the operant conditioning chamber two days after the last footshock session and operant responding was recorded. There was no difference in responding on the active lever between the last day of extinction training and the test session that was conducted two days after the last footshock session. This indicates that exposure to the operant conditioning chamber, in which the rats previously received footshocks, did not mediate reinstatement of nicotine-seeking behavior. This suggests that contextual cues did not contribute to footshock-induced reinstatement of nicotine-seeking behavior. It is likely that prolonged exposure to the operant conditioning chambers prior to the administration of footshocks prevented the formation of an association between the operant conditioning chamber and footshocks (i.e., latent inhibition) (Lubow and Moore, 1959).
A close look at the outcome of the three experiments indicates that there was some between-experiment variation in the percentage of rats that responded on the active lever after exposure to footshocks. The percentage of rats that met the exclusion criterion (average number of responses on the active lever is fewer than 5 [1 infusion] during the 3 reinstatement sessions) in the first, second, and third experiment was, 0% (0 out of 8), 28.6% (4 out of 14), and 0% (0 out of 15), respectively. At this point in time, we can only speculate about what caused the between experiment variation. We suggest that genetic variability might have contributed to the variation in response rates as environmental conditions were the same in all three experiments.
A final experiment was conducted to determine if D-Phe CRF(12-41) and clonidine may have sedative effects. It was shown that D-Phe CRF(12-41) does not affect responding for chocolate-flavored food pellets. This suggest that D-Phe CRF(12-41), at least within the investigated dose range, does not have sedative effects. Clonidine had a small, but significant, effect on responding on the active lever and did not affect responding on the inactive lever. Responding on the inactive lever was extremely low, which might explain the fact that clonidine did not affect responding on the inactive lever. The observation that clonidine decreases responding for chocolate-flavored food pellets is in agreement with a previous study that reported that clonidine decreases responding for non-flavored / standard food pellets (Schulteis et al. 1998). It should be noted that in the present study the effect of clonidine on food responding was much smaller than the effect of clonidine on stress-induced reinstatement of nicotine-seeking behavior. The highest dose of clonidine reduced responding for chocolate-flavored food pellets by 9.7% and decreased stress-induced reinstatement of nicotine-seeking behavior by 47.7%. This suggest that clonidine may have some sedative effects under baseline conditions but it is unlikely that this accounted for the large decrease in responding on the active lever after the administration of footshocks. These findings suggest that at the doses used in the present study, D-Phe CRF(12-41) and clonidine attenuate stress-induced reinstatement of nicotine-seeking behavior and have no or minimal sedative effects.
At this point in time, it can only be speculated which brain sites mediated the effects of D-Phe CRF(12-41) and clonidine on footshock-induced reinstatement of extinguished nicotine-seeking behavior. Preclinical studies that have investigated the neurobiological substrates underlying stress-induced relapse to cocaine and morphine-seeking behavior may provide information about which neuronal system could possibly contribute to stress-induced relapse of nicotine-seeking behavior. Erb and Stewart showed that the administration of D-Phe CRF(12-41) into the bed nucleus of the stria terminalis (BNST), but not in the central nucleus of the amygdala (CeA), blocks footshock-induced reinstatement of cocaine-seeking behavior (Erb and Stewart 1999). In addition, it was shown that the administration of CRF into the BNST, but not in the CeA, induces reinstatement of cocaine-seeking behavior. Experimental evidence suggest that noradrenergic transmission in both the CeA and the BNST plays a role in stress-induced relapse to drug-seeking behavior. The administration of a cocktail of the ß1-adrenergic receptor antagonist betaxolol and the ß2-adrenergic receptor antagonists ICI-118,551 into either the CeA or the BNST blocks footshock-induced reinstatement of cocaine-seeking behavior (Leri et al. 2002). The administration of the α2-adrenergic receptor agonist clonidine into the BNST has also be shown to prevent footshock-induced reinstatement of morphine conditioned place preference (Wang et al. 2001). Taken together, these studies suggest that CRF transmission in the BNST and noradrenergic transmission in the CeA and the BNST play a role in stress-induced relapse to drug-seeking behavior. Additional studies are warranted to investigate if the same neurobiological substrates play a role in stress-induced reinstatement of nicotine-seeking behavior.
Taken together, the present findings indicate that blockade of CRF receptors or stimulation of α2-adrenergic receptors attenuates footshock-induced reinstatement of extinguished nicotine-seeking behavior . Experiments that investigate the role of brain stress systems in relapse to nicotine-seeking behavior may contribute to the development of novel, non-addictive, treatments that prevent relapse to tobacco smoking behavior.
Acknowledgments
This research was funded by developmental funds from the Department of Psychiatry at the University of Florida, a National Institute on Drug Abuse grant (1R03DA020504-01), and a Flight Attendant Medical Research Institute (FAMRI) Young Clinical Scientist Award to AWB. The authors would like to thank Dr. Jean Rivier (The Clayton Foundation Laboratories for Peptide Biology, The Salk Institute for Biological Studies, San Diego, CA) for generously providing D-Phe CRF(12-41).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, VanderMaelen CP. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl) 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50:20–28. doi: 10.1002/syn.10242. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Buczek Y, Le AD, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Cohen S, Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychol. 1990;9:466–478. doi: 10.1037//0278-6133.9.4.466. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997;54:743–753. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology (Berl) 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19(20):RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: the effect of nonreinforced pre-exposure to the conditional stimulus. J Comp Physiol Psychol. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology (Berl) 1999;142:399–407. doi: 10.1007/s002130050905. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McKee SA, Maciejewski PK, Falba T, Mazure CM. Sex differences in the effects of stressful life events on changes in smoking status. Addiction. 2003;98:847–855. doi: 10.1046/j.1360-0443.2003.00408.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Britt DM, Abrams DB. Response to social stress, urge to smoke, and smoking cessation. Addict Behav. 2002;27:241–250. doi: 10.1016/s0306-4603(00)00180-5. [DOI] [PubMed] [Google Scholar]
- O'Brien CP. Research advances in the understanding and treatment of addiction. Am J Addict. 2003;12(Suppl 2):S36–S47. [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology. 2005;30:119–128. doi: 10.1038/sj.npp.1300524. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Merlo Pich E, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pudovkina OL, Kawahara Y, de Vries J, Westerink BH. The release of noradrenaline in the locus coeruleus and prefrontal cortex studied with dual-probe microdialysis. Brain Res. 2001;906:38–45. doi: 10.1016/s0006-8993(01)02553-7. [DOI] [PubMed] [Google Scholar]
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Stinus L, Risbrough VB, Koob GF. Clonidine blocks acquisition but not expression of conditioned opiate withdrawal in rats. Neuropsychopharmacology. 1998;19:406–416. doi: 10.1016/S0893-133X(98)00036-0. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP–154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Swan GE, Denk CE, Parker SD, Carmelli D, Furze CT, Rosenman RH. Risk factors for late relapse in male and female ex-smokers. Addict Behav. 1988;13:253–266. doi: 10.1016/0306-4603(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol. 2001;432:153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]