Abstract
Members of the NF-κB/Rel and inhibitor of apoptosis (IAP) protein families have been implicated in signal transduction programs that prevent cell death elicited by the cytokine tumor necrosis factor α (TNF). Although NF-κB appears to stimulate the expression of specific protective genes, neither the identities of these genes nor the precise role of IAP proteins in this anti-apoptotic process are known. We demonstrate here that NF-κB is required for TNF-mediated induction of the gene encoding human c-IAP2. When overexpressed in mammalian cells, c-IAP2 activates NF-κB and suppresses TNF cytotoxicity. Both of these c-IAP2 activities are blocked in vivo by coexpressing a dominant form of IκB that is resistant to TNF-induced degradation. In contrast to wild-type c-IAP2, a mutant lacking the C-terminal RING domain inhibits NF-κB induction by TNF and enhances TNF killing. These findings suggest that c-IAP2 is critically involved in TNF signaling and exerts positive feedback control on NF-κB via an IκB targeting mechanism. Functional coupling of NF-κB and c-IAP2 during the TNF response may provide a signal amplification loop that promotes cell survival rather than death.
The pro-inflammatory cytokine tumor necrosis factor α (TNF) can induce a wide spectrum of biologic responses via its interaction with two cell-surface receptors, termed TNF-R1 and TNF-R2 (1, 2). In many cell types, ligand binding to TNF-R1 triggers programmed cell death (1, 3). This process is dependent on the presence of an ≈80 amino acid “death domain” in the cytoplasmic region of TNF-R1, which is absent in TNF-R2 (1, 4). Although TNF-induced cell death is mediated primarily via TNF-R1 (3), both TNF-R1 and TNF-R2 can transduce intracellular signals that stimulate the proteolytic breakdown of IκBα, a cytoplasmic inhibitor of transcription factor NF-κB (5–8). In turn, NF-κB is rapidly translocated to the nucleus, where it regulates the expression of many TNF-responsive genes (7, 8). None of these transcription units are required for TNF-induced killing, because this death response occurs in the absence of either protein synthesis (9, 10) or NF-κB signaling (11). Indeed, disruption of the NF-κB pathway enhances the cytolytic effects of TNF, suggesting that one or more downstream genes mediate protective functions (12–15). Despite these findings, the precise mechanisms that determine a cell’s decision to survive or die in response to TNF are largely undefined.
In addition to NF-κB, cellular proteins homologous to baculovirus inhibitors of apoptosis (IAPs) have been identified that interfere with the transmission of intracellular death signals (16–21). For example, members of this family defined in Drosophila suppress normal cell death when expressed in the developing eye (17). In humans, mutations in the gene encoding the structurally related neuronal apoptosis inhibitory protein correlate with excessive motor neuron death (16, 19). More recent studies have revealed two novel mammalian IAP homologs, designated c-IAP1 and c-IAP2 (18, 19, 21), which appear to suppress apoptosis induced by serum withdrawal (19). With respect to TNF signaling, Rothe et al. (18) have found that c-IAP1 and c-IAP2 form cytoplasmic complexes with TNF receptor-associated factor 2 (TRAF2). Importantly, TRAF2 associates with TNF receptors (22, 23) and is essential for triggering NF-κB activation by TNF (11, 24). However, the significance of c-IAP1 and c-IAP2 in the regulation of either NF-κB activation or TNF-induced death has not been defined.
In this report, we present evidence for the involvement of c-IAP2 in protecting cells from TNF-induced death. After TNF stimulation, steady-state levels of c-IAP2 transcripts are markedly elevated, whereas expression of c-IAP1 transcripts is unaffected. Stimulus-dependent expansion of the c-IAP2 mRNA pool proceeds in the absence of new protein synthesis, but requires the presence of an intact NF-κB signaling pathway. Functional studies revealed that overexpressed c-IAP2 stimulates NF-κB-directed transcription in the absence of TNF and interferes with TNF-induced cell death. In contrast, deletion of the C-terminal RING domain of c-IAP2 yields a trans-dominant inhibitor of NF-κB that enhances the cytolytic effects of TNF. These findings suggest an important functional interplay between c-IAP2 and NF-κB that facilitates a protective cellular response.
MATERIALS AND METHODS
Expression Vectors.
Epitope-tagged derivatives of human c-IAP2 (clone MIHC; kindly provided by David Vaux, The Walter and Eliza Hall Institute of Medical Research, Victoria, Australia) (21) were constructed by PCR-assisted amplification with a 5′ primer (5′-CCCGGTACCACCATGGACTACAAAGACGATGACGATAAAATGAACATAGTAG- AAAACAGC-3′) that fused sequences encoding the FLAG epitope (25) in frame with N-terminal coding sequences of c-IAP2. To construct the RING-deleted form of c-IAP2 (c-IAP2ΔC; amino acids 1–382), a premature termination codon was introduced into the amplified product by site-directed mutagenesis (Muta-Gene kit; Bio-Rad). These modified cDNAs were inserted into the polylinker of pCMV4 downstream of the cytomegalovirus immediate-early promoter (26). Expression vectors encoding FLAG-tagged wild-type IκBα (IκBαWT), FLAG-tagged IκBαΔN (amino acids 37–317), and CrmA (kindly provided by Tom Gilmore, Boston University) have been described (27, 28). The chloramphenicol acetyltransferase (CAT) reporter contained two NF-κB binding sites from the HIV-1 enhancer linked to a heterologous TATA box (HIV-κB-CAT) (29). A reporter plasmid encoding β-galactosidase (pZeoSVLacZ) and the pHook-1 expression vector were purchased from Invitrogen.
Generation of Jurkat T Cell Lines Stably Expressing Ectopic IκB.
Coding sequences for FLAG-tagged IκBαWT and IκBαΔN were inserted into the polylinker of the murine leukemia virus-based retroviral vector LH-M, a derivative of LN-M that confers resistance to hygromycin B (30). Retroviral stocks were generated by transient cotransfection of a A293T cell monolayer with either of these two vectors in the presence of plasmids pHIT60 and pHIT456 (31). After retroviral infection of Jurkat T cells (2.5 × 106 cells), stably transduced bulk cultures were selected in RPMI 1640 medium containing 10% fetal bovine serum and hygromycin B (300 μg/ml). Cell lines expressing comparable levels of wild-type and mutant IκBα were cloned by limiting dilution and identified by immunoblotting with monoclonal anti-FLAG antibodies (M2; IBI-Kodak).
Subtractive Hybridization and RNA Analysis.
Jurkat cells stably expressing either IκBαWT or IκBαΔN were stimulated for 4 hr with combinations of phorbol 12-myristate 13-acetate (50 ng/ml; Calbiochem) and ionomycin (1 μM; Calbiochem) (P+I). Total RNA was prepared using an Ultraspec RNA isolation kit (Biotecx) according to the manufacturer’s instructions. Poly(A)+ RNA was purified from total RNA (≈2 mg) by chromatography on oligo(dT) cellulose (GIBCO/BRL). Complementary DNAs were synthesized from 2 μg of poly(A)+ RNA, and subtractive hybridization was performed using a PCR-Select cDNA subtraction kit (CLONTECH) as previously described (32). To select for NF-κB-directed transcripts, cDNAs derived from IκBαWT-expressing cells were ligated to oligonucleotide linkers (32) and hybridized with excess cDNAs (30-fold) derived from cells expressing IκBαΔN. After hybridization, differential transcripts were selectively amplified by suppression PCR as described (32). Amplified cDNAs were ligated into pT7Blue T vector (Novagen). Partial cDNA sequences were determined and compared with entries in the GenBank database using the blast homology search program.
For Northern analysis, total RNA was fractionated by electrophoresis on 6% formaldehyde/1.2% agarose gels buffered in 20 mM Hepes (pH 7.6) and 10 μM EDTA. After electrophoresis, RNA was transferred to Zeta Probe membranes (Bio-Rad) and hybridized with the indicated 32P-radiolabeled cDNA probes at 65°C in a solution containing 6 × standard saline citrate (SSC), 1% SDS, and 10% dextran sulfate (Pharmacia). Radiolabeled probes were generated by using a Random Primed DNA Labeling Kit (Boehringer-Mannheim).
Transient Transfection Assays.
Human Jurkat T lymphocytes were cultured and transfected by electroporation as described (27). Transfected cells were expanded in culture for 24 hr and then stimulated with TNF (20 ng/ml; Promega) for 16 hr. For CAT assays, whole cell extracts were prepared, normalized for protein concentration (33), and analyzed by the diffusion-based liquid scintillation counting method of Neumann et al. (34). HeLa cells were maintained in Iscove’s medium supplemented with 10% fetal bovine serum and transfected using the calcium phosphate precipitation method (35). In some experiments, transfectants were selected by coexpression with pHook-1, followed by solid-phase adsorption to Capture-Tec magnetic beads (Invitrogen) (36). To monitor for TNF cytotoxicity, HeLa cells were transfected with pZeoSVLacZ in combination with the indicated effector plasmids. After treatment with TNF, monolayer cells were washed with PBS, fixed (2% formaldehyde/0.05% glutaraldehyde in PBS) for 5 min, and stained for β-galactosidase expression with 0.1% 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) in PBS supplemented with 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2.
Subcellular Fractionation and Extract Analyses.
Cytosolic and nuclear protein fractions were prepared as described (37). Electrophoretic mobility shift and DNA/protein crosslinking assays were performed as described using a κB probe derived from the interleukin 2 receptor-alpha promoter (κB-pd; 5′-CAACGGCAGGGGAATTCCCCTCTCCTT-3′) (38). Photoreactive derivatives of the κB-pd probe containing 5-bromo-2-deoxyuridine 5′-triphosphate (BrdU) were synthesized as described (39). FLAG-tagged IκBα was isolated from cytosolic extracts by immunoprecipitation with agarose beads conjugated with monoclonal anti-FLAG antibodies (M2; IBI-Kodak). Immunoprecipitates were fractionated by SDS/PAGE and transferred to polyvinylidene difluoride membranes (DuPont). Membranes were incubated with antipeptide antisera raised against C-terminal sequences of IκBα (amino acids 289–317). Immunoreactive proteins were detected with an enhanced chemiluminescence kit (Pierce).
RESULTS
The c-IAP2 Gene Is Under NF-κB Control.
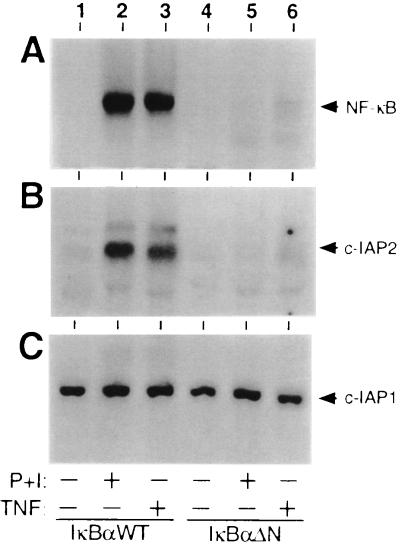
In most cell types, the biologic activity of NF-κB and other dimeric members of the Rel protein family is controlled from the cytoplasmic compartment by IκB proteins (7, 8). Recent biochemical studies have established that TNF-mediated induction of NF-κB involves site-specific phosphorylation and ubiquitination of IκBα, which is required to target this inhibitor to the 26S proteasome (7, 8, 40). To identify NF-κB-responsive genes that help protect cells from TNF-induced death, human Jurkat T cells were stably transduced with murine leukemia virus-based retroviral expression vectors for either wild-type IκBα (IκBαWT) or a truncated form of this inhibitor lacking the requisite phosphoacceptor and ubiquitin attachment sites (IκBαΔN) (27, 40). In transiently transfected T cells, IκBαΔN functions as a constitutive repressor of NF-κB that escapes from signal-dependent breakdown (27). As demonstrated in gel retardation assays with nuclear extracts from representative stable clones, NF-κB DNA binding activity was potently induced in IκBαWT-expressing cells after stimulation with TNF (Fig. 1A, compare lanes 1 and 3). Similar results were obtained with phorbol 12-myristate 13-acetate and ionomycin (P+I), a combination that mimics signals discharged from the T cell antigen receptor (Fig. 1A, lane 2). In sharp contrast, both of these agonists failed to induce the nuclear translocation of NF-κB in T lymphocytes expressing IκBαΔN (Fig. 1A, lanes 5 and 6).
Figure 1.
Regulation of the c-IAP2 gene by TNF and NF-κB. Jurkat T cells stably expressing FLAG-tagged forms of wild-type IκBα (lanes 1–3) or the IκBαΔN mutant (lanes 4–6) were stimulated for 2 hr with either combinations of phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (1 μM) (P+I, lanes 2 and 5) or TNF (20 ng/ml; lanes 3 and 6). Nuclear extracts and total RNA were prepared as described in Materials and Methods. (A) Induction of NF-κB expression. Nuclear extracts (5 μg) were incubated with a 32P-labeled κB-pd probe under standard reaction conditions (38). Resultant DNA/protein complexes were resolved on a nondenaturing 5% polyacrylamide gel and visualized by autoradiography. (B and C) Northern blot analysis of c-IAP mRNA expression. Total RNA (10 μg) was fractionated on a 1.2% agarose/formaldehyde gel, transferred to a Zeta Probe membrane, and hybridized with 32P-radiolabeled cDNA probes for either human c-IAP2 or c-IAP1 (18). After hybridization, the membrane was washed at 50°C in 2 × standard saline citrate (SSC)/0.1% SDS, and specific transcripts were detected by autoradiography.
In light of these biochemical results, mRNA was prepared from each of these stably transduced lines after cellular stimulation. Transcripts were converted to cDNAs by reverse transcription and subjected to subtractive hybridization as previously described (ref. 32; see Materials and Methods). Primary structural analysis of a partial cDNA clone isolated from the subtracted products (≈200 bp) revealed a sequence corresponding precisely to that present in the 3′ untranslated region of the human c-IAP2 gene (18, 19, 21). Subsequent RNA hybridization studies conducted with a cDNA encoding the full-length c-IAP2 protein confirmed increased steady-state levels of the c-IAP2 transcript (≈10-fold over basal) in IκBαWT-expressing cells after stimulation with either TNF or P+I (Fig. 1B, lanes 1–3). In contrast, c-IAP2 messages failed to accumulate in response to these two NF-κB-inducing agents in T lymphocytes expressing IκBαΔN (Fig. 1B, lanes 4–6). Parallel RNA blotting studies conducted with a cDNA probe for the closely related c-IAP1 protein (18) revealed that steady-state expression of the corresponding transcript was unaffected by the activation status of NF-κB (Fig. 1C). These studies with transformed human T cells suggest that NF-κB is required for TNF-mediated induction of the gene encoding c-IAP2 but not c-IAP1.
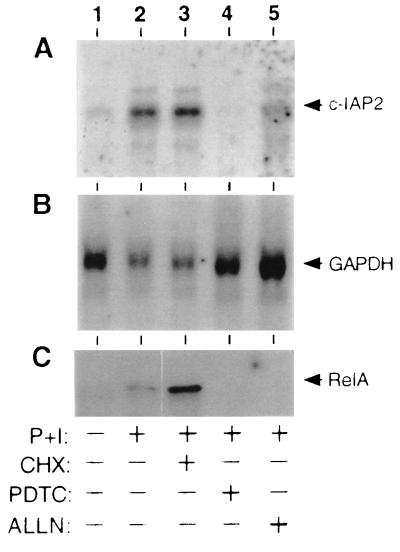
To extend these findings to a more physiologically relevant setting, we next monitored the steady-state levels of c-IAP2 mRNA in primary human T cells derived from peripheral blood. As shown in Fig. 2, stimulation of primary T cells with P+I led to a significant elevation in the c-IAP2 mRNA pool (Fig. 2A, lanes 1 and 2). This induction event correlated temporally with the appearance of the RelA transactivating subunit of NF-κB in the nuclear compartment (Fig. 2C, lane 2). Treatment of cells with the protein synthesis inhibitor cycloheximide (CHX) before exposure to P+I had no detectable inhibitory effect on these two signal-dependent events (Fig. 2 A and C, lane 3), suggesting that c-IAP2 is encoded by an immediate-early gene subject to control by preformed factors. To confirm that one of these pre-existing factors is NF-κB, primary T cells were treated with the antioxidant pyrrolidinedithiocarbamate, which prevents the signal-induced phosphorylation of IκBα (42). Alternatively, cells were exposed to the proteasome antagonist N-acetyl-Leu-Leu-norleucinal, an agent known to interfere with the signal-induced degradation of IκBα (43). Both of these agents blocked the induction of NF-κB in primary T cell cultures (Fig. 2C, lanes 4 and 5). Inhibition of NF-κB activity by either mechanism correlated with a substantial reduction in the level of c-IAP2 mRNA in P+I-treated cells (Fig. 2A, lanes 4 and 5). These findings with primary T lymphocytes are fully consistent with results obtained using transformed T cells expressing IκBαΔN (Fig. 1).
Figure 2.
NF-κB-dependent expression of c-IAP2 mRNA in primary T lymphocytes. Human peripheral blood T lymphocytes were purified by sheep erythrocyte rosetting (41) and cultured overnight in RPMI 1640 medium supplemented with 10% fetal bovine serum. Cells were stimulated for 2 hr with P+I in the presence or absence of CHX (50 μg/ml), pyrrolidinedithiocarbamate (PDTC) (200 μM), or N-acetyl-Leu-Leu-norleucinal (ALLN) (100 μg/ml) as indicated. Total RNAs (≈5 μg) were fractionated by agarose gel electrophoresis, transferred to a Zeta Probe membrane, and sequentially hybridized with 32P-radiolabeled probes for human c-IAP2 (A) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (B). (C) Nuclear extracts were prepared from a portion of these cells and incubated with a photoreactive κB-pd probe (38). After exposure to UV light, DNA/protein adducts were immunoprecipitated with RelA-specific antisera and fractionated by SDS/PAGE (38, 39). Only the major adduct detected by autoradiography (≈70 kDa) is shown.
Overexpression of c-IAP2 Inhibits the Cytolytic Activity of TNF.
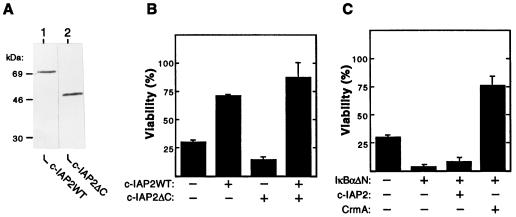
The human c-IAP2 protein shares two domains of homology with viral and mammalian IAPs, including c-IAP1 (18). The N-terminal half of c-IAP2 (amino acids 1–382) contains three tandem zinc finger motifs, termed baculovirus IAP repeats (BIRs). Mutational studies indicate that the BIR domain of c-IAP2 mediates its stable interaction with TRAF2 (18), a cytoplasmic factor that is required for the induction of NF-κB by TNF (22, 24). In addition, the C-terminal region of c-IAP2 (amino acids 383–604) contains a cysteine-rich RING-finger motif of unknown function (18). To examine the potential role of c-IAP2 in the control of TNF-induced death, we constructed expression plasmids for full-length c-IAP2 (c-IAP2WT) and a truncated derivative lacking its C-terminal RING domain (c-IAP2ΔC; amino acids 1–382). As shown in Fig. 3A, these two proteins were comparably expressed in the cytoplasmic compartment of transiently transfected HeLa cells.
Figure 3.
Human c-IAP2 protects cells from TNF-induced killing via an NF-κB-dependent mechanism. (A) HeLa cells were transfected with 10 μg of the indicated FLAG-tagged expression vectors. Cytoplasmic extracts were prepared 48 hr after transfection and fractionated by SDS/PAGE. Proteins were transferred to polyvinylidene difluoride membranes and subjected to immunoblotting with monoclonal anti-FLAG antibodies. (B) Effects of wild-type and mutant c-IAP2 on TNF-induced death. HeLa cells (3 × 105) were cotransfected with pZeoSVLacZ DNA (1 μg) and pCMV4-based expression plasmids (1 μg) encoding either c-IAP2WT or c-IAP2ΔC. Total DNA input was normalized with pCMV4. After 24 hr of growth, transfected cultures were split into 24-well plates and propagated for 24 hr in the presence or absence of TNF (15 ng/ml). Cells were stained for β-galactosidase expression and scored. Cell viability is expressed as the percentage of blue cells in TNF-treated versus unstimulated cultures. The data shown represent the mean viability (± SEM) of cells determined from triplicate transfections in two separate experiments. (C) Requirement of NF-κB for c-IAP2-mediated protection. HeLa cells were cotransfected with pZeoSVLacZ (1 μg) and the indicated combinations of expression vectors for IκBαΔN (0.1 μg), c-IAP2 (1 μg), and CrmA (1 μg). The percentage of cells (± SEM) surviving exposure to TNF (24 hr) under each experimental condition was determined as described for B. Similar results were obtained in three separate experiments.
In subsequent studies, we monitored the effect of c-IAP2 overexpression on the cytolytic activity of TNF. For these experiments, HeLa cells were cotransfected with either the c-IAP2WT or c-IAP2ΔC effector plasmid in the presence of a lacZ reporter construct and treated with TNF. Under our conditions, ≈70% of the transfected cells were killed by TNF in the absence of ectopic IAP (Fig. 3B). Overexpression of wild-type c-IAP2 led to a significant increase in cell viability (≈2.4-fold). In contrast to these protective effects, overexpression of truncated c-IAP2 potentiated cell death in response to TNF. Enhanced killing in the presence of c-IAP2ΔC could not be attributed to ectopic protein toxicity, because coexpression with wild-type c-IAP2 rescued the protective response. These opposing functional effects suggested that sequences containing the C-terminal RING domain of c-IAP2 are required for protection.
Like c-IAP2, recent studies indicate that NF-κB plays a role in protection from TNF-induced death (12–15). Our finding that the c-IAP2 gene is under NF-κB control led us to test whether c-IAP2 alone is sufficient to mediate protection under conditions in which the expression of other NF-κB-responsive genes is blocked. For these studies, HeLa cells were cotransfected with expression vectors for IκBαΔN, c-IAP2, and lacZ. In keeping with previous reports (12–15), cell killing induced by TNF was markedly amplified in cultures expressing IκBαΔN as compared with IκBαΔN-deficient cells (Fig. 3C). Coexpression of c-IAP2 in cells defective for NF-κB signaling failed to elicit a protective response. Similar results were obtained when c-IAP2 was introduced into Jurkat T cells that stably express IκBαΔN (see Fig. 1; data not shown). In contrast, IκBαΔN-expressing cells were shielded from TNF killing by coexpression of CrmA, a cowpox virus protein that prevents apoptosis via an NF-κB-independent mechanism (44). These findings demonstrate that an intact NF-κB signaling pathway is required to endow c-IAP2 with its protective function.
Overexpression of the c-IAP2 Protein Stimulates NF-κB-Directed Transcription and IκBα Degradation.
The observed loss of c-IAP2 function in cells arrested for NF-κB activation could reflect the involvement of other protective genes under NF-κB control. Alternatively, c-IAP2 may act upstream of NF-κB in the TNF signaling cascade at a step leading to IκB degradation (7, 8, 40). In this regard, previous biochemical studies have suggested that the induction of NF-κB by TNF is mediated via TRAF2 (22, 24), a cytoplasmic protein that is recruited to TNF receptors in a ligand-dependent fashion (23). Although recent experiments indicate that TRAF2 interacts with c-IAP2 (18), the precise role of c-IAP2 in this cytokine signaling pathway remains unclear.
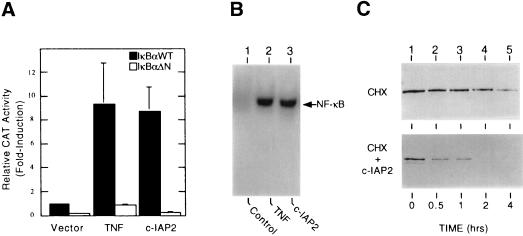
To explore the functional significance of c-IAP2 in the mechanism for NF-κB induction, Jurkat T cells expressing either IκBαWT or IκBαΔN (see Fig. 1) were cotransfected with an effector plasmid encoding full-length c-IAP2 and a CAT reporter plasmid containing two NF-κB binding sites from the 5′ long-terminal repeat of HIV-1 (HIV-κB-CAT). As shown in Fig. 4A, treatment of IκBαWT-expressing transfectants with TNF led to a significant increase in NF-κB-directed transcription relative to the magnitude of this functional response in cells containing the constitutive repressor IκBαΔN. More importantly, overexpression of c-IAP2 in the absence of TNF treatment induced reporter gene activity to similar levels. As determined by gel retardation assays, the induction of NF-κB-directed transcription by ectopic c-IAP2 correlated with its capacity to activate NF-κB DNA binding activity in transfected HeLa cells (Fig. 4B, lane 3). However, the ability of c-IAP2 to mediate NF-κB activation was completely abolished in transfectants harboring IκBαΔN, which is resistant to TNF-induced degradation (Fig. 4A) (27). These functional studies strongly suggest that overexpressed c-IAP induces NF-κB by a mechanism involving the proteolytic inactivation of IκBα.
Figure 4.
Overexpressed c-IAP2 activates NF-κB. (A) Stimulation of NF-κB-directed transcription. Jurkat cells stably expressing either IκBαWT (filled bars) or IκBαΔN (empty bars) were cotransfected with HIV-κB-CAT (5 μg) and 10 μg of either empty pCMV4 vector or the wild-type c-IAP2 expression plasmid. After 24 hr of growth, the indicated cultures were exposed to TNF (20 ng/ml) for 16 hr. Whole cell extracts were prepared at 40-hr posttransfection and assayed for CAT activity. Results from triplicate transfections are reported as the fold induction in CAT activity (mean ± SEM) relative to that measured in unstimulated Jurkat/IκBαWT cells transfected with HIV-κB-CAT alone (normalized to 1). (B) Induction of NF-κB DNA binding activity. HeLa cells (2 × 106) were cotransfected with pHook-1 (5 μg) and 15 μg of either blank pCMV4 plasmid (lanes 1 and 2) or the c-IAP2WT expression vector (lane 3). Transfected cells were selected by magnetic bead capture (36) after 24 hr of growth. Selected transfectants were propagated for 24 hr and then treated with CHX (50 μg/ml; 4 hr) in the presence (lane 2) or absence (lanes 1 and 3) of TNF (20 ng/ml). Nuclear extracts were prepared and analyzed in gel retardation assays with a radiolabeled κB probe (see Fig. 1A). (C) Stimulation of IκBα degradation. HeLa cells were cotransfected with an expression vector for wild-type IκBα (1 μg) and 10 μg of either pCMV4 (Upper) or c-IAP2WT (Lower). Cytoplasmic extracts were prepared from transfected cells after treatment with CHX (50 μg/ml) for the indicated times. Ectopic forms of IκBα were isolated by immunoprecipitation with anti-FLAG antibodies, resolved by SDS/PAGE, and immunoblotted with IκBα-specific antisera.
To test this hypothesis directly, vectors encoding FLAG epitope-tagged forms of IκBα and c-IAP2 were expressed transiently in HeLa cells. Transfected cells were exposed to CHX to prevent de novo synthesis of IκBα in this overexpression system. As shown in Fig. 4C, coexpression with full-length c-IAP2 led to a significant reduction in the cytoplasmic level of ectopic IκBα as compared with control cells transfected with the empty parental vector. In contrast, overexpressed c-IAP2 failed to accelerate the turnover of ectopic IκBαΔN, which lacks sequences required for phosphorylation, ubiquitination, and proteasome-mediated breakdown in TNF-treated cells (data not shown) (7, 8, 40).
The C-Terminal RING Domain of c-IAP2 is Required for NF-κB Induction by TNF.
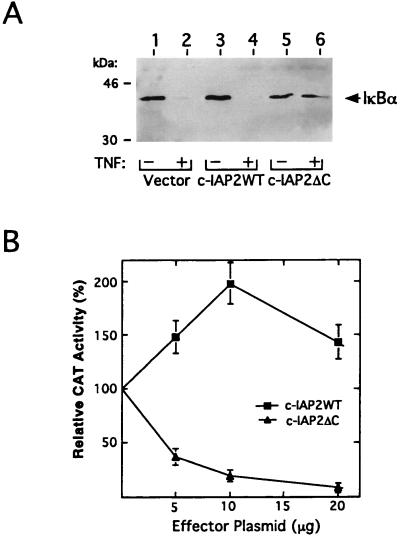
The above findings indicated that overexpressed c-IAP2 functions not only to suppress apoptosis (Fig. 3B), but also to activate NF-κB (Fig. 4). Importantly, results from cell viability assays suggested that the C-terminal RING domain of c-IAP2 is essential for its protective function (Fig. 3B). To examine the role of the RING domain in the regulation of IκBα turnover, HeLa cells were cotransfected with plasmids encoding either c-IAP2WT or c-IAP2ΔC in combination with an expression vector for FLAG-tagged IκBα. After cellular stimulation with TNF, cytoplasmic extracts were prepared and subjected to immunoprecipitation with FLAG-specific antibodies. As demonstrated by immunoblotting, steady-state levels of this inhibitor in IAP-deficient controls were dramatically reduced in response to TNF (Fig. 5A, lanes 1 and 2). Similar results were obtained using cells transfected with a full-length cDNA for c-IAP2 (Fig. 5A, lanes 3 and 4). However, overexpression of the RING-deleted form of c-IAP2 prevented cytokine-induced breakdown of IκBα (Fig. 5A, lanes 5 and 6). These findings suggest that the RING domain of c-IAP2 couples this protein to the NF-κB signaling pathway via an IκBα-dependent mechanism.
Figure 5.
Dominant-negative effects of RING-deleted c-IAP2 on TNF-mediated NF-κB activation. (A) c-IAP2ΔC inhibits TNF-induced degradation of IκBα. HeLa cells were cotransfected with a plasmid encoding FLAG-tagged IκBα (1 μg) and 10 μg of either pCMV4 (lanes 1 and 2) or the indicated c-IAP2 expression vectors (lanes 3–6). After 48 hr of growth, transfected cells were treated for 1 hr with CHX (50 μg/ml) in the presence or absence of TNF (20 ng/ml). Ectopic IκBα was purified from cytoplasmic extracts by immunoprecipitation with anti-FLAG antibodies, subjected to SDS/PAGE, and detected by immunoblotting with IκBα-specific antibodies. Molecular mass markers (in kDa) are indicated. (B) Effects of c-IAP2ΔC on NF-κB-directed transcription in TNF-treated cells. Jurkat T lymphocytes were cotransfected with HIV-κB-CAT (5 μg), a CrmA expression vector (5 μg), and graded doses of the indicated c-IAP2 expression vectors. Total DNA input in each transfection was normalized with pCMV4. After 24 hr of growth, transfectants were stimulated for 16 hr with TNF (20 ng/ml). Whole cell extracts were prepared and assayed for CAT activity. For each titration point (n = 3), results are expressed as the mean percentage (± SEM) of CAT activity relative to that induced by TNF in cells lacking ectopic IAP protein (fold-induction over basal level = 7.6 ± 1.5; normalized to 100%).
To complement these biochemical results, we next transfected Jurkat T cells with the HIV-κB-CAT plasmid and graded doses of expression vectors for either full-length or C-terminally truncated c-IAP2. Recipient cells were stimulated with TNF and then monitored for relative levels of reporter gene expression (Fig. 5B). In control cells lacking ectopic c-IAP, TNF induced the transcriptional activity of the HIV-1 enhancer 7- to 8-fold over basal levels. This activity was modestly stimulated (≈2-fold) in cells titrated with full-length c-IAP2. In contrast, the RING-deleted mutant of c-IAP2 (c-IAP2ΔC), which retains its TRAF2-binding activity (18), inhibited the TNF functional response in a dose-dependent fashion. However, c-IAP2ΔC failed to efficiently block the induction of promoter activity by interleukin 1 (data not shown), a cytokine that stimulates the expression of NF-κB by a TRAF2-independent mechanism (11). Together with our biochemical data (Fig. 5A), this trans-dominant phenotype implicates c-IAP2 as an integral component of the TNF signaling cascade that mediates IκBα inactivation and the induction of NF-κB.
DISCUSSION
In addition to its cytolytic effects on receptor-bearing target cells, TNF is a potent inducer of NF-κB (7, 8). Paradoxically, recent studies have established that NF-κB has the capacity to shield mammalian cells from TNF-induced apoptosis, presumably by activating the expression of specific protective genes (12–15). The present work indicates that one of these NF-κB-responsive genes encodes c-IAP2. Specifically, in transformed human T cells, steady-state levels of the c-IAP2 transcript are dramatically up-regulated by TNF, whereas this response is completely blocked by overexpressing a constitutive inhibitor of NF-κB. Moreover, in primary human T cells, pharmacologic agents that inhibit NF-κB activation also prevent the accumulation of c-IAP2 transcripts. Although effects of NF-κB on c-IAP2 mRNA stability cannot be completely excluded, one explanation for these results is that the c-IAP2 gene contains a functional κB enhancer element(s). In contrast to c-IAP2, we have been unable to detect significant steady-state changes in the amount of message encoding c-IAP1, a closely related protein that forms cytoplasmic complexes containing c-IAP2 (18, 23). This differential response may impact the intracellular ratio of c-IAP1 and c-IAP2, perhaps leading to distinct biologic consequences.
Recent studies suggest an evolutionarily conserved role for viral and cellular IAP proteins in the negative control of programmed cell death (17, 19–21). However, only c-IAP1 and c-IAP2 have been shown to interact with TNF signal transducers such as TRAF2 (18, 21). This experimental observation prompted us to explore the significance of c-IAP2 in the regulation of either TNF-mediated killing or NF-κB activation. We have found that human c-IAP2 suppresses TNF-induced death when this protein is overexpressed in mammalian cells. In the absence of TNF stimulation, enforced expression of c-IAP2 is sufficient to activate NF-κB. These two functions of c-IAP2 are biochemically coupled, because both are lost in cells harboring a dominant mutant of IκBα that constitutively represses NF-κB. Furthermore, RING-deleted forms of c-IAP2 block NF-κB activation and enhance cell killing by TNF. Thus, like TRAF2 (24), c-IAP2 appears to be critically involved in the regulation of NF-κB activity by TNF. This implies that one mechanism by which c-IAP2 facilitates cellular resistance to TNF cytotoxicity involves its direct interaction with the NF-κB pathway rather than with the apoptotic machinery. However, we cannot exclude the possibility that other NF-κB-inducible gene products act in concert with c-IAP2 to suppress the apoptotic response.
Taken together, our findings suggest a positive feedback loop involving NF-κB and c-IAP2 that facilitates cell survival in the presence of TNF. In this proposed model, newly synthesized c-IAP2 can potentiate the NF-κB signaling cascade via its capacity to destabilize IκBα. This mechanism of c-IAP2 action may be subject to negative regulation. For example, recent studies have identified another TNF-inducible gene, termed A20, whose expression is stimulated by multiple agents that activate NF-κB (45). Although both c-IAP2 and A20 bind TRAF2 in vitro (18, 21, 46), overexpression of the A20 protein in mammalian cells potently blocks NF-κB activation by TNF (46). As such, the precise stoichiometric balance between c-IAP2 and the appropriate antagonist may provide a key checkpoint for the regulated expression of survival factors under NF-κB control, including c-IAP2 itself.
Acknowledgments
We thank Dr. David Vaux for the human c-IAP1 and c-IAP2 cDNAs, Dr. Stanford Krantz for purified T cells, and Emily Vance for manuscript preparation. We thank Chris Aiken, Patrick Green, Jacek Hawiger, Earl Ruley, Eugene Oltz, and Luc Van Kaer for providing critical comments. This work was supported in part by a grant from the National Institute of Allergy and Infectious Diseases (to D.W.B.). T.A.M. was supported by the National Institutes of Health (Training Program in Cellular, Biochemical, and Molecular Sciences). D.W.B. and M.H.M. are investigators of the Howard Hughes Medical Institute.
ABBREVIATIONS
- IAP
inhibitor of apoptosis
- TNF
tumor necrosis factor α
- CAT
chloramphenicol acetyltransferase
- CHX
cycloheximide
- TRAF2
TNF receptor-associated factor 2
References
- 1.Smith C A, Farrah T, Goodwin R G. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 2.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 3.Tartaglia L A, Rothe M, Hu Y-F, Goeddel D V. Cell. 1993;73:213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
- 4.Tartaglia L A, Ayres T M, Wong G H W, Goeddel D V. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 5.Kruppa G, Thoma B, Machleidt T, Wiegmann K, Krönke M. J Immunol. 1992;148:3152–3157. [PubMed] [Google Scholar]
- 6.Laegreid A, Medreder A, Nonstad U, Bombara M P, Ranges G, Sundan A, Espevik T. J Biol Chem. 1994;269:7785–7791. [PubMed] [Google Scholar]
- 7.Finco T, Baldwin A S., Jr Immunity. 1995;3:263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 8.Verma I M, Stevenson J K, Schwartz E M, Van Antwerp D, Miyamoto S. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 9.Wallach D. J Immunol. 1984;132:2464–2469. [PubMed] [Google Scholar]
- 10.Wallach D. Interferon. 1986;7:89–124. [PubMed] [Google Scholar]
- 11.Hsu H, Shu H-B, Pan M-G, Goeddel D V. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 12.Beg A A, Baltimore D. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Hsu H, Goeddel D V, Karin M. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 14.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 15.Wang C-Y, Mayo M W, Baldwin A S., Jr Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 16.Roy N, Mahadevan M S, McLean M, Shutler G, Yaraghi Z, et al. Cell. 1995;80:167–178. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- 17.Hay B A, Wassarman D A, Rubin G M. Cell. 1995;83:1253–1262. doi: 10.1016/0092-8674(95)90150-7. [DOI] [PubMed] [Google Scholar]
- 18.Rothe M, Pan M-G, Henzel W J, Ayres T M, Goeddel D V. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 19.Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherto-Horvat G, Farahani R, McLean M, Ikeda J E, Mackenzie A, Korneluk R G. Nature (London) 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 20.Duckett C S, Nava V E, Gedrich R W, Clem R J, Van Dongen J L, Gilfillan M C, Shiels H, Hardwick J M, Thompson C B. EMBO J. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- 21.Uren A G, Pakusch M, Hawkins C J, Puls K L, Vaux D L. Proc Natl Acad Sci USA. 1996;93:4974–4978. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothe M, Wong S C, Henzel W J, Goeddel D V. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 23.Shu H-B, Takeuchi M, Goeddel D V. Proc Natl Acad Sci USA. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothe M, Sarma V, Dixit V M, Goeddel D V. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 25.Blanar M, Rutter W J. Science. 1992;256:1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- 26.Andersson S, Davis D L, Dahlback H, Jörnvall H, Russell D W. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- 27.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White D W, Gilmore T D. Oncogene. 1996;13:891–899. [PubMed] [Google Scholar]
- 29.Stein B, Rahmsdorf H, Steffen A, Liffin M, Herrlich P. Mol Cell Biol. 1989;9:5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon J H M, Southerling T E, Peterson J C, Meyer B E, Malim M H. J Virol. 1995;69:4166–4172. doi: 10.1128/jvi.69.7.4166-4172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon P M, Kim N, Kingsman S M, Kingsman A J. J Virol. 1996;70:8234–8240. doi: 10.1128/jvi.70.11.8234-8240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diatchenko L, Lau C Y-F, Campbell A P, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov E D, Siebert P D. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Neumann J, Morency C, Russian K. Biotechniques. 1987;5:444–447. [Google Scholar]
- 35.Cullen B R. Methods Enzymol. 1987;152:684–703. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- 36.Chestnut J D, Bayton A R, Russell M, Chang M P, Bernard A, Maxwell I H, Hoeffler J P. J Immunol Methods. 1996;193:17–27. doi: 10.1016/0022-1759(96)00032-4. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber E, Matthias P, Muller M, Schaffner W. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballard D W, Walker W H, Doerre S, Sista P, Molitor J A, Dixon E P, Peffer N J, Hannink M, Greene W C. Cell. 1990;63:803–814. doi: 10.1016/0092-8674(90)90146-6. [DOI] [PubMed] [Google Scholar]
- 39.Ballard D W, Böhnlein E, Hoffman J A, Bogerd H P, Dixon E P, Franza B R, Greene W C. New Biol. 1989;1:83–92. [PubMed] [Google Scholar]
- 40.Scherer D C, Brockman J A, Chen Z, Maniatis T, Ballard D W. Proc Natl Acad Sci USA. 1995;92:11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawada K, Krantz S B, Kans J S, Dessypris E D, Sawyer S, Glick A D, Civin C I. J Clin Invest. 1987;80:357–364. doi: 10.1172/JCI113080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traenckner E B, Wilk S, Baeuerle P A. EMBO J. 1994;13:5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 44.Miura M, Friedlander R M, Yuan J. Proc Natl Acad Sci USA. 1995;92:8318–8322. doi: 10.1073/pnas.92.18.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laherty C D, Perkins N D, Dixit V M. J Biol Chem. 1993;268:5032–5039. [PubMed] [Google Scholar]
- 46.Song H Y, Rothe M, Goeddel D V. Proc Natl Acad Sci USA. 1996;93:6721–6725. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]