Abstract
Auxin and nitric oxide (NO) play fundamental roles throughout plant life. NO is a second messenger in auxin signal transduction leading to root developmental processes. The mechanisms triggered by auxin and NO that direct adventitious root (AR) formation are beginning to be unraveled. The goal of this work was to study phospholipid (PL) signaling during the auxin- and NO-induced AR formation in cucumber (Cucumis sativus) explants. Explants were labeled with 32P-inorganic phosphate and treated with the auxins indole-3-acetic acid or 1-naphthylacetic acid, or the NO donor S-nitroso N-acetyl penicillamine, in the presence or absence of the specific NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide. PLs were separated by thin-layer chromatography and quantified. We report that the signaling PLs phosphatidic acid (PA), phosphatidylinositol phosphate, and phosphatidylinositol bisphosphate accumulated within 1 min after auxin or NO treatment. Both auxin and NO evoked similar and transient time course responses, since signaling PLs returned to control levels after 20 or 30 min of treatment. The results indicate that auxin relies on NO in inducing PA, phosphatidylinositol phosphate, and phosphatidylinositol bisphosphate accumulation. Furthermore, we demonstrate that auxin and NO trigger PA formation via phospholipase D (PLD) activity. Explants treated for 10 min with auxin or NO displayed a 200% increase in AR number compared with control explants. In addition, PLD activity was required for the auxin- and NO-induced AR formation. Finally, exogenously applied PA increased up to 300% the number of ARs. Altogether, our data support the idea that PLD-derived PA is an early signaling event during AR formation induced by auxin and NO in cucumber explants.
Auxin is a plant hormone that acts not only on morphogenesis as a coordinating signal across the whole plant but also as a local patterning signal. At the cellular level, the mechanisms underlying the auxin response seem to be very complex. Auxin is involved in root growth and developmental processes, such as root elongation, root gravitropism, root hair development, and lateral root and adventitious root (AR) formation (Davies, 1995; Berleth and Sachs, 2001; Quint and Gray, 2006; Tanaka et al., 2006).
The second messenger nitric oxide (NO) is also a highly ubiquitous molecule in plants. NO is an intermediate in the auxin-regulated signaling cascades determining root morphology and physiology, this function being one of the best described in plant NO biology (Correa-Aragunde et al., 2007). Gouvea et al. (1997) reported the first evidence for the participation of NO in the auxin-induced root elongation process. Afterward, NO was shown to be involved in the promotion of ARs (Pagnussat et al., 2002) and lateral roots (Correa-Aragunde et al., 2004), in the root gravitropic response (Hu et al., 2005), and in the initiation and elongation of root hairs (Lombardo et al., 2006). Regarding signal transduction pathways leading to AR formation in cucumber (Cucumis sativus) explants, it has been proposed that NO induces cGMP production by regulating the activity of the enzyme guanylate cyclase (Pagnussat et al., 2003). Subsequently, NO was demonstrated to activate a mitogen-activated protein kinase (MAPK) cascade in a cGMP-independent pathway (Pagnussat et al., 2004). More recent experiments have established that Ca2+ and Ca2+-dependent protein kinase (CDPK) activities mediate the auxin response leading to AR formation (Lanteri et al., 2006).
The enzyme phospholipase C (PLC; EC 3.1.4.11) hydrolyzes the signaling phospholipids (PLs) phosphatidylinositol phosphate (PIP) or phosphatidylinositol 4,5-bisphosphate (PIP2) to generate the second messengers diacylglycerol (DAG) and inositol 1,4-bisphosphate (IP2) or inositol 1,4,5-trisphosphate (IP3), respectively. IP2 can be subsequently phosphorylated to IP3, and IP3 releases Ca2+ from intracellular compartments into the cytosol (Bootman et al., 2001). Pharmacological data showed that the IP3-regulated Ca2+ channel inhibitors lithium chloride and neomycin sulfate suppressed AR formation induced by auxin or NO in cucumber explants (Lanteri et al., 2006). These results suggest the participation of PLC activity by regulating cytosolic Ca2+ concentration via increases in IP3 during AR formation. It has been demonstrated that auxin generates a rapid and transient decrease in both PIP and PIP2 levels concomitant with an increase in IP3 level in suspension-cultured Catharanthus roseus cells (Ettlinger and Lehle, 1988; Grabowski et al., 1991). In accordance, auxin has also been shown to trigger a rise in IP3 level followed by the activation of Ca2+ channels located in intracellular compartments in isolated membranes of carrot (Daucus carota; Zbell and Walter-Back, 1989; Zbell et al., 1989). The other product of PLC activity, DAG, can be phosphorylated to the signaling PL phosphatidic acid (PA) through the action of the enzyme DAG kinase (DGK); therefore, this is referred to as the PLC/DGK pathway (Munnik et al., 1998). Although evidence regarding the auxin stimulation of PLC activity has been obtained by monitoring the level of IP3, there are still no data about the involvement of the PLC/DGK pathway in auxin signaling.
Another enzymatic source of PA in plants is phospholipase D (PLD; EC 3.1.4.4). PLD hydrolyzes structural PLs such as phosphatidylcholine (PC) or phosphatidylethanolamine (PE) at the terminal phosphodiester bond to produce PA and a free head group (Wang, 2000). Early studies showed that auxin has no effect on PLD activity in isolated membranes prepared from zucchini (Cucurbita pepo) hypocotyls or in suspension-cultured parsley (Petroselinum crispum) and soybean (Glycine max) cells (André and Scherer, 1991; Paul et al., 1998). Recently, genetic evidence demonstrated that auxin responses are reduced in Arabidopsis (Arabidopsis thaliana) PLDξ2 (a member of the ξ-class PLD family) knockout mutants, probably due to an altered auxin transport and distribution (Li and Xue, 2007). However, it still remains to be determined whether PLD participates in the auxin signal transduction pathway.
In this context, the goal of this work was to study PL signaling during the auxin- and NO-induced AR formation in cucumber explants. We report that the signaling PLs PA, PIP, and PIP2 accumulate within 1 min of auxin treatment and that the auxin action is dependent on endogenous NO. Accordingly, treatment of cucumber explants with the NO donor S-nitroso N-acetyl penicillamine (SNAP) resulted in similar PA, PIP, and PIP2 accumulation. We also found that auxin and NO triggered PA formation via PLD activation. We show that 10-min treatments of cucumber explants with auxin or NO were sufficient to induce AR formation and that PLD activity was required for that induction. Finally, exogenously applied PA significantly induced AR formation. Altogether, our data firmly support the idea that PLD-derived PA is an early signaling event during AR formation induced by auxin and NO in cucumber explants.
RESULTS
Auxin and NO Trigger the Accumulation of Signaling PLs
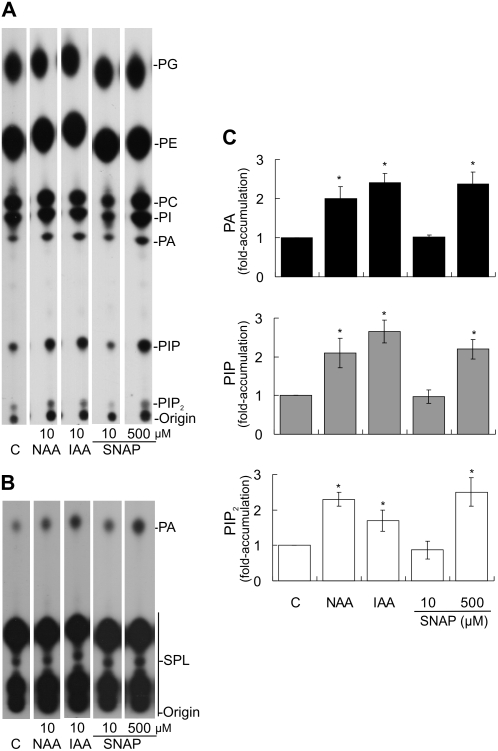
To investigate whether auxin and NO induce PL signaling, cucumber explants were labeled with inorganic phosphate (32Pi) for 16 h and subsequently treated for 10 min with buffer (C = control), auxins, or NO donors. In vivo levels of PLs were measured in hypocotyl segments 5 mm long at the hypocotyl base, where ARs develop (Fig. 1). Figure 2A shows a typical pattern of the radiolabeled PLs extracted from cucumber hypocotyls and separated by thin-layer chromatography (TLC) with an alkaline solvent system. The results indicate that this system enables the detection of (1) structural PLs: phosphatidylglycerol (PG), PE, PC, and PI; and (2) signaling PLs: PA, PIP, and PIP2. Identification of all lipids was established by cochromatography and comparison of their RF with lipid extracts of known composition and commercial standards. For a better visualization of PA, the autoradiograph corresponding to PLs separated by TLC with an ethyl acetate (EtAc) solvent system is also shown (Fig. 2B). As visualized in Figure 2, A and B, most 32Pi was incorporated into structural PLs and no significant changes in the levels of these lipids could be detected in any of the treatments performed. The radioactivity of the signaling lipids was quantified as a ratio against the radioactivity in total PLs. Values were subsequently expressed as fold accumulation with respect to the control (Fig. 2C).
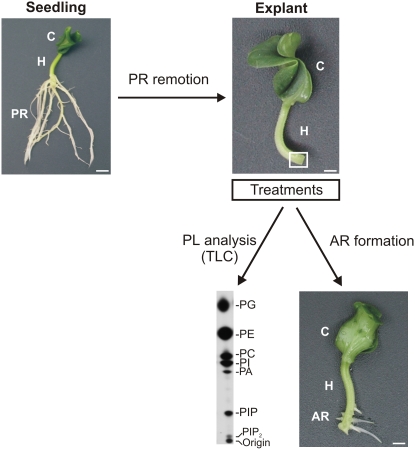
Figure 1.
Scheme showing the experimental design to analyze PLs and AR formation in cucumber explants. Primary root (PR) systems of 7-d-old cucumber seedlings were removed, and explants were treated as follows. To study PL signaling, explants were subjected to in vivo labeling with 32Pi followed by short treatments. PLs were extracted from hypocotyl (H) segments 5 mm long from the base of the hypocotyl (white square), where ARs develop. Radiolabeled PLs were then separated by TLC. To analyze AR formation, cucumber explants were treated for different times and subsequently transferred to water for 5 d. The photograph shows seven ARs formed at the hypocotyl base. C, Cotyledon. Bars, 5 mm.
Figure 2.
Auxin and NO induce the accumulation of PA, PIP, and PIP2. Cucumber explants were labeled with 15 μCi per explant for 16 h and subsequently treated for 10 min with buffer (C = control), the auxins NAA or IAA, or the NO donor SNAP as indicated. PLs were extracted and separated by TLC using an alkaline (A) or an EtAc (B) solvent system. Radioactivity was visualized by autoradiograph and quantified from nonoverexposed autoradiographs (Supplemental Fig. S1) using ImageJ software. C, Standardization was carried out by expressing the results as a ratio: labeled PA, PIP, or PIP2 to total label in PLs. Values were subsequently expressed as fold accumulation ± se by dividing the ratio in a treatment by that in the corresponding control, taking the level of the control as 1. Data represent five independent experiments. Bars with asterisks are significantly different (t test, P < 0.05) from the control treatment.
Because our aim was to analyze the effect of auxin on PL signaling pathways, two well-known auxins, 1-naphthylacetic acid (NAA) and indole-3-acetic acid (IAA), were assayed. Because 10 μm IAA induces signal transduction pathways leading to AR formation in cucumber explants (Pagnussat et al., 2002, 2003, 2004; Lanteri et al., 2006), 10 μm of either NAA or IAA was tested to analyze the in vivo levels of PLs. Figure 2A shows that 10-min treatments with 10 μm NAA or IAA triggered the accumulation of the signaling PLs PA, PIP, and PIP2. Both auxin treatments resulted in similar PL fold accumulation values, which were around 2- to 3-fold (Fig. 2B). We decided to use 10 μm IAA to further test the effect of auxin on PL signaling.
Because auxin induces NO formation in cucumber explants (Pagnussat et al., 2002), we studied whether the NO donor SNAP could mimic the effect of auxin in inducing the accumulation of signaling PLs. SNAP is known to induce AR formation in cucumber explants when assayed at 10 μm for 5 d (Pagnussat et al., 2002). Because the effect of NO depends on its concentration, and commonly applied NO donor concentrations range from 10 to 500 μm (Floryszak-Wieczorek et al., 2006), we analyzed the effect of 10 and 500 μm SNAP. Figure 2B shows that the NO released by 10 μm SNAP was not able to trigger the accumulation of PA, PIP, and PIP2 within 10 min of treatment. However, 500 μm SNAP was effective at inducing the accumulation of those signaling PLs (Fig. 2B). The NO donor sodium nitroprusside (SNP) did not induce the accumulation of PA, PIP, and PIP2 in the same experiments that SNAP did (data not shown).
The solvents ethanol and dimethyl sulfoxide (DMSO) were used to dissolve the auxins (NAA and IAA) and SNAP, respectively. No significant changes in the accumulation of the signaling PLs could be observed when explants were treated with 0.002% (v/v) ethanol and 0.5% (v/v) DMSO, which are the final concentrations used in 10 μm IAA and 500 μm SNAP treatments, respectively (data not shown).
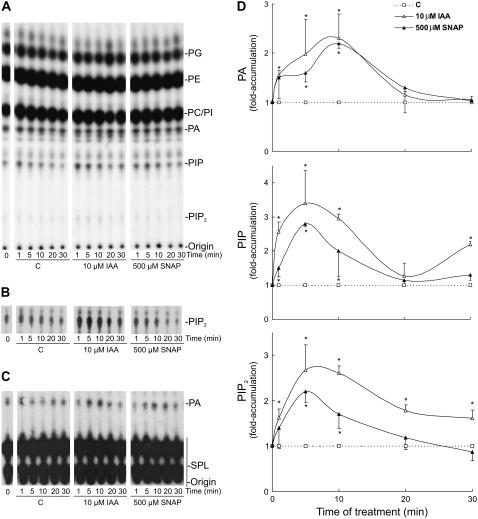
Signaling PLs Are Rapidly and Transiently Accumulated upon Auxin and NO Treatments
To further characterize the auxin- and NO-induced PL signaling pathway, we performed a time course experiment. Cucumber explants were labeled with 32Pi for 16 h and then treated for 1, 5, 10, 20, and 30 min with buffer (C = control), 10 μm IAA, or 500 μm SNAP. PLs were separated by TLC with an alkaline solvent system (Fig. 3, A and B) and an EtAc solvent system (Fig. 3C) and quantified as described previously. Figure 3D shows that the generation of PA, PIP, and PIP2 is a very rapid response to auxin and NO treatments (around 1.5-fold within 1 min after stimulation). As shown in Figure 3, PIP and PIP2 maximum levels were detected earlier (peak at 5 min) than the PA maximum level (peak at 10 min). Furthermore, both auxin and NO evoked similar and transient time course responses, since signaling PLs returned to control levels after 20 or 30 min of treatments. In conclusion, the effect of auxin and NO in inducing the accumulation of signaling PLs is rapid and transient.
Figure 3.
PA, PIP, and PIP2 accumulate rapidly and transiently upon treatment of cucumber explants with auxin and NO. Explants labeled as in Figure 2 were incubated with buffer (C = control), 10 μm IAA, or 500 μm SNAP for different times as indicated. PLs were extracted and separated by TLC using an alkaline (A) or an EtAc (C) solvent system. Radioactivity was visualized by autoradiograph and quantified as described in Figure 2. B, Autoradiograph from a longer exposure of the chromatograph shown in A demonstrating the increase in PIP2. D, Results are expressed as fold accumulation ± se taking the control values as 1. Data represent three independent experiments. Values with asterisks are significantly different (t test, P < 0.05) from the control treatment.
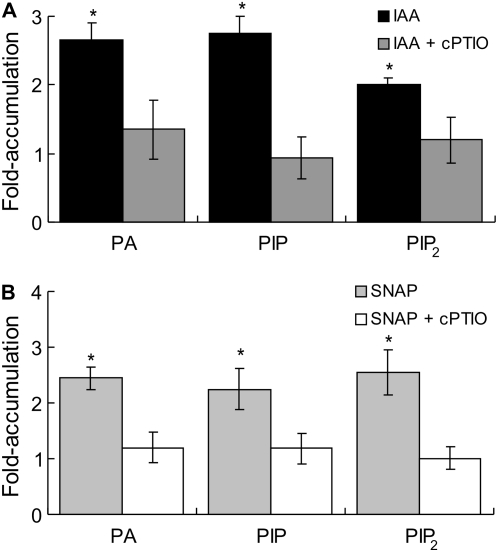
Auxin-Induced Accumulation of Signaling PLs Depends on NO
To determine whether auxin requires NO to induce the accumulation of signaling PLs, we evaluated the effect of diminishing the endogenous NO level with the specific NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO). Thus, cucumber explants were labeled with 32Pi for 16 h and then treated for 10 min with buffer (C = control) or 10 μm IAA in the presence or absence of 200 μm cPTIO. When explants were treated with cPTIO alone, the fold accumulation values for PA, PIP, and PIP2 were similar to control values (data not shown). Figure 4A shows that cPTIO reversed the stimulatory effect of auxin in triggering the accumulation of PA, PIP, and PIP2. These results firmly support the idea that NO acts downstream of auxin to trigger PA, PIP, and PIP2 accumulation.
Figure 4.
Auxin-induced accumulation of signaling PLs depends on NO. Explants were labeled as in Figure 2 and subsequently treated for 10 min with buffer (control), 10 μm IAA (A), or 500 μm SNAP (B) in the presence or absence of 200 μm of the specific NO scavenger cPTIO. Results from three independent experiments are expressed as fold accumulation ± se taking the control values as 1. Bars with asterisks are significantly different (t test, P < 0.05) from the control treatment.
Figure 4B shows that the application of cPTIO prevented the effect of 500 μm SNAP on the accumulation of PA, PIP, and PIP2, indicating that the effect of SNAP relies on NO release. These results were further confirmed by using 500 μm of 15-d-old, light-inactivated SNAP solution (old SNAP), which resulted in no accumulation of the signaling PLs (data not shown).
PLD Activity Is Responsible for the Auxin- and NO-Induced PA Accumulation
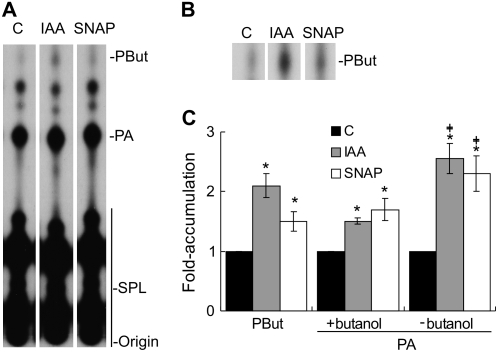
We were next interested in elucidating the enzymatic source(s) of PA production during auxin treatment in cucumber explants. At the moment, we disregard the mechanism by which auxin triggers PIP and PIP2 accumulation. Experiments were conducted to study the involvement of both PLD and the PLC/DGK pathway in auxin-induced PA accumulation. PLD has the ability to transfer the phosphatidyl moiety from its substrate to a primary alcohol (called phosphatidyltransferase activity). The subsequent formation of the product, a phosphatidylalcohol, is a measure of PLD activity (Munnik et al., 1995). PLD activation was tested in vivo in cucumber explants labeled with 32Pi for 16 h and treated for 10 min with buffer (C = control) or 10 μm IAA in the presence or absence of 0.5% (v/v) 1-butanol. Figure 5A shows a representative autoradiograph of the PLs extracted and separated by TLC using the EtAc solvent system that separates phosphatidylbutanol (PBut) and PA from all other PLs (Munnik et al., 1998). The increase in PBut is clearly visualized in Figure 5B, which corresponds to an autoradiograph from a longer exposure of the chromatograph shown in Figure 5A. The level of PBut was quantified and expressed as fold accumulation with respect to the control. Because the amount of PBut reflects the activity of PLD, the results presented in Figure 5C support the idea that auxin triggers PLD activation. Because NO acts downstream of auxin, 500 μm SNAP was assayed for its capacity to mimic the auxin effect. As expected, the NO donor was able to induce PLD activity (Fig. 5). The data indicate that PLD is activated during auxin and NO treatments generating PA.
Figure 5.
PLD activation is involved in the auxin- and NO-induced PA accumulation. Cucumber explants were labeled as in Figure 2, and in vivo PLD activity was measured by treating them for 10 min with buffer (C = control), 10 μm IAA, or 500 μm SNAP in the presence of 0.5% (v/v) 1-butanol. A, Lipids were separated by TLC using an EtAc solvent system to separate PBut and PA from the other PLs. B, Autoradiograph from a longer exposure of the chromatograph shown in A demonstrating the increase in PBut. C, Results from three independent experiments are expressed as fold accumulation ± se taking the control values as 1. Bars with asterisks are significantly different (t test, P < 0.05) from the control treatment. Values are different from PA fold accumulation in the presence of butanol.
In the presence of 1-butanol, PLD produces PBut at the expense of PA. Consequently, 1-butanol treatment reduces PA formation via PLD activity. For that reason, PA levels were quantified in explants treated with auxin or NO in the presence or absence of 1-butanol. Figure 5C shows that the effect of auxin and NO on PA fold accumulation values was smaller, but still significant (t test, P < 0.05), in the presence of 1-butanol than in the absence of the alcohol. Finally, the addition of 0.5% (v/v) 2-butanol, a secondary alcohol that is not a PLD substrate, resulted in neither PBut formation nor any effect on PA levels (data not shown). In summary, the presence of 1-butanol caused the appearance of PBut and a reduction in the amount of PA.
To test whether the activity of DGK(s) also contributes to the auxin-induced PA formation, a short radiolabeling strategy was applied (Munnik et al., 1998). The method is based on the fact that 32Pi is quickly incorporated into the ATP pool, which is then used by DGK to phosphorylate DAG to produce PA. Accordingly, cucumber explants were stabilized in buffer for 16 h and then subjected to 32Pi labeling in the presence of buffer or 10 μm IAA for 1, 5, 10, and 20 min. The percentage of radiolabeled PA compared with all radiolabeled PLs was calculated for both control and IAA treatments and was similar at any time point (data not shown). These preliminary results suggest that auxin would not produce PA via the activation of the PLC/DGK pathway.
PLD Activation Is Required for the Auxin- and NO-Induced AR Formation
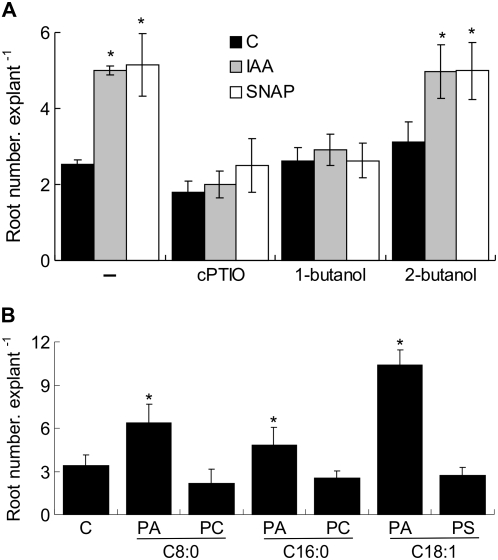
We previously showed that treatments for 5 d with auxin and NO induce AR formation in cucumber explants (Pagnussat et al., 2002). In this report, we demonstrate that PA, PIP, and PIP2 transiently accumulate within 10 min of auxin and NO treatments, returning to control levels after 20 or 30 min of treatment (Fig. 3). In an attempt to find a correlation between the PL signaling pathway induced by auxin and NO and the process of AR formation, we carried out an experiment consisting of 10-min treatments of cucumber explants with auxin or NO followed by 5 d of incubation in water. Hence, explants were treated with buffer (C = control), 10 μm IAA, or 500 μm SNAP in the presence or absence of 200 μm cPTIO. As shown in Figure 6A, 10-min treatments with auxin or NO were sufficient to induce AR formation. Explants treated with auxin or NO displayed a 200% increase of AR number in comparison with control explants (Fig. 6A), which is similar to the increase obtained in 5-d treatments (Pagnussat et al., 2002). Figure 6A shows that the effect of SNAP is due to the NO release, since cPTIO was able to block it. Figure 6A also provides evidence that auxin depends on endogenous NO to trigger AR formation, since the scavenging of NO with cPTIO significantly decreased the number of ARs triggered by auxin. Control treatments with 0.002% (v/v) ethanol, 0.5% (v/v) DMSO, or 500 μm old SNAP did not provoke any detectable response in AR formation (data not shown). The effect of a 10-min treatment with 10 or 500 μm SNAP was also evaluated on AR formation. The results indicate that SNP was unable to promote this process at either concentration (data not shown).
Figure 6.
PLD activation is required for the auxin- and NO-induced AR formation. A, Cucumber explants were treated for 10 min with buffer (C = control), 10 μm IAA, or 500 μm SNAP in the presence or absence of 200 μm cPTIO and 0.1% (v/v) 1-butanol or 2-butanol and subsequently transferred to water for 5 d. B, Explants were treated for 1 h with buffer (C = control) or with buffer plus 5 μm PA C8:0, PC C8:0, PA C16:0, PC C16:0, PA C18:1, or PS C18:1 and subsequently transferred to water for 5 d. Root number values per explant were measured from at least three independent experiments (n = 10 explants per experiment) and expressed as means ± se. Bars with asterisks are significantly different (t test, P < 0.05) from the control treatment.
To investigate whether the inhibition of PLD impedes AR formation, cucumber explants were treated for 10 min with 10 μm IAA or 500 μm SNAP in the presence or absence of 0.1% (v/v) 1-butanol or 2-butanol and subsequently transferred to water for 5 d. Figure 6A shows that while 1-butanol completely abolished both auxin and NO actions, its isomer 2-butanol had no effect on AR formation. Finally, we examined the effect of exogenous application of PA, PC, or phosphatidylserine (PS) on AR formation. Cucumber explants were treated for 1 h with buffer (C = control) or with buffer plus 5 μm PLs and subsequently transferred to water for 5 d. PLs assayed were the short saturated fatty acid chain PA C8:0 or PC C8:0, the long saturated fatty acid chain PA C16:0 or PC C16:0, or the long unsaturated fatty acid chain PA C18:1 or PS C18:1. Figure 6B shows that all of the species of PA stimulated AR formation, while treatments with PC or PS resulted in no effect on AR number. In conclusion, Figure 6 presents strong evidence supporting a role for PLD-derived PA in the auxin- and NO-induced AR formation in cucumber.
DISCUSSION
In this study, we provide evidence that the signaling PLs PA, PIP, and PIP2 accumulate in cucumber explants treated with auxin or NO. This accumulation is rapid and transient and dependent on the dose and nature of the NO donor (see below). Data obtained from biochemical, pharmacological, and physiological experiments indicate that (1) NO is required for the auxin-induced accumulation of signaling PLs, (2) PLD activation is involved in PA formation induced by auxin and NO, (3) PLD activation is required for the AR formation induced by auxin and NO, and (4) exogenously applied PA induces AR formation.
We previously showed that treatment of cucumber explants for 5 d with the NO donors SNAP and SNP induces AR formation (Pagnussat et al., 2002). The results indicated that 10-min treatment with SNP was unable to trigger the accumulation of signaling PLs and the AR formation process (data not shown). The varying effects of these two NO donors could be explained by their distinct nature. While SNAP releases mainly NO in the redox form of the free radical (NO.), SNP releases predominantly nitrosonium cation (NO+; Hou et al., 1999). The donors could also have diverse effects because of the dissimilar amounts of NO released over time. Measurements of the rates of NO release by both donors indicated that SNAP has a shorter half-life and hence a faster stimulation of the response in comparison with SNP (Floryszak-Wieczorek et al., 2006).
In this work, we demonstrate that auxin induces an NO-dependent PIP and PIP2 accumulation in cucumber hypocotyls (Fig. 4A). Three PIP isomers (PI3P, PI4P, and PI5P) and three PIP2 isomers [PI(3,5)P2, PI(4,5)P2, and PI(3,4)P2] have been identified in plants (Brearley and Hanke, 1992, 1993; Munnik et al., 1994; Meijer et al., 2001). The existence of isomers of PIP and PIP2 create specificity, determining different downstream signaling events. It has been shown that the relative abundance of PIP and PIP2 isomers is quite variable, depending on the cell types and/or growth conditions of the plants (Westergren et al., 2001). We do not know yet which isomer(s) is induced upon auxin and NO treatments. The identification of the isomer(s) goes beyond the scope of this work and will be a part of future investigations.
Our previous results suggested the participation of PLC activity during the auxin- and NO-induced AR formation in cucumber, by regulating cytosolic Ca2+ concentration via increases in IP3 (Lanteri et al., 2006). Here, we present evidence that auxin and NO trigger PA formation via PLD activation in cucumber explants (Fig. 5). This is in accordance with a recent work reporting that the expression of a human PIP kinase in suspension-cultured tobacco (Nicotiana tabacum) cells led to PIP2 accumulation and the subsequent production of IP3 via PLC (Im et al., 2007). The authors suggested that PA accumulation may arise from increased PLD activity and not via the activation of the PLC/DGK pathway. Additionally, pharmacological data from Arabidopsis seedlings suggested that PLC signaling upon salt stress involves IP3-regulated Ca2+ release from intracellular compartments but not PA signaling from DAG (Parre et al., 2007).
PIP and PIP2 were shown to induce in vitro activities of PLDs, PIP2 being the stronger activator than PIP (Chung et al., 1997; Pappan et al., 1997; Qin et al., 1997). Most of the 12 members of the Arabidopsis PLD family require Ca2+ for their in vitro activities, and only some of them are PIP2 dependent (Bargmann and Munnik, 2006). Taking this evidence into consideration and our results showing that the accumulation of both PIP and PIP2 precedes PLD-derived PA (Figs. 3 and 5), it can be suggested that PIP and/or PIP2 may directly activate cucumber PLD(s) and/or fulfill its requirement of Ca2+ via PLC activity. To date, cucumber PLD activity has only been measured in vitro in the fruit mesocarp, which proved to be stimulated by Ca2+ (Mao et al., 2007). However, the effect of PIP and/or PIP2 had not yet been studied on cucumber PLD activity. Once the isomers of PIP and PIP2 are identified, different experiments could be designed to test the hypothesis presented above. These may include studying the effects of the exogenous application of specific isomers of PIP and PIP2 on PA accumulation and AR formation and the effects of diminishing the endogenous PIP and PIP2 levels on the auxin and NO-induced PA accumulation and AR formation. In addition to the positive effect of PIP and/or PIP2 on PLD activity, it can also be suggested that NO may trigger posttranslational modifications to activate PLD, such as S-nitrosylation of Cys residues or nitration of Tyr residues. Future analysis will have to be directed at the characterization of the molecular events that link NO with PLD activation.
In this report, we demonstrate that PLD is activated in vivo during auxin and NO treatments generating PA (Fig. 5). Our experiments have been performed using cucumber explants to evoke a natural response. The effect of both auxin and NO on PLD activity has been analyzed previously in other experimental conditions and systems. No apparent stimulation of in vitro PLD activity was found upon auxin treatment in isolated membranes prepared from zucchini hypocotyls labeled with [14C]PC or [14C]PE (André and Scherer, 1991). In vivo PLD activity in response to auxin treatment was measured in suspension-cultured parsley and soybean cells using the fluorescent analog of PC bis-BODIPY-PC as substrate (Paul et al., 1998). The authors did not find an accumulation of fluorescent PA from the hydrolysis of bis-BODIPY-PC. Therefore, they excluded PLD from auxin signal transduction. It should be noted, however, that PLD-derived PA may have been sufficiently low and so undetectable and/or may have been converted to nonfluorescent PA. Additionally, it has been proposed that the metabolism of artificial lipids may not be an exact copy of the metabolism of natural lipids (Paul et al., 1998). On the other hand, the effect of NO on the in vivo phosphatidyltransferase activity of PLD was evaluated in suspension-cultured tomato (Solanum lycopersicum) cells treated with 1 mm SNAP and 0.5% (v/v) 1-butanol. This treatment did not result in a detectable increase of PLD activity (Laxalt et al., 2007). Altogether, these results pointed out the different responses obtained when working with distinct plant material (e.g. isolated membranes, suspension-cultured cells, and explants) and distinct physiological and growth conditions.
Scherer and André (1989) provided evidence that the enzyme phospholipase A2 (PLA2) is stimulated by auxin. PLA2 hydrolyzes PLs generating free fatty acids and lysoPLs. A rapid accumulation of [14C]lysoPC or [14C]lysoPE was detected in vitro upon auxin treatment in isolated membranes labeled with [14C]PC or [14C]PE, respectively. A higher auxin concentration (above 100 μm 2,4-dichlorophenoxyacetic acid) was required to measure the stimulatory effect of auxin on in vivo PLA2 activity in both suspension-cultured cells and hypocotyl segments (Scherer and André, 1989, 1993; André and Scherer, 1991; Scherer, 1995; Paul et al., 1998). In this study, we did not detect any other increase in PLs than those already discussed for PA, PIP, and PIP2. Moreover, we identified all PLs from the alkaline TLC (Fig. 2A), suggesting that there is no lysoPL present in lipids extracted from cucumber hypocotyls. This evidence indicates that PLA2 activity was not influenced by auxin treatment under our experimental conditions. The differences between the results obtained by Scherer's group and our findings could be explained by the distinct auxin concentration used for the experiments.
In this study, PLD-derived PA was shown to be involved in auxin- and NO-induced AR formation. While 1-butanol completely blocked the effects of auxin and NO, exogenously applied PA significantly stimulated AR formation. However, PA did not exert the same magnitude of response compared with auxin and NO treatments (Fig. 6, A and B). Because auxin induces several signal transduction events other than PLD activity, like cGMP-dependent and -independent pathways (Pagnussat et al., 2003, 2004), exogenous application of PA should not be expected to fully mimic the auxin action. Our results are in accordance with that reported in a recent paper in which the effects of 1-butanol, 2-butanol, and PA were analyzed in roots of Arabidopsis (Li and Xue, 2007). PA enhanced the root gravity response, whereas inhibiting PLD-derived PA with 1-butanol resulted in both decreased root gravitropism and suppressed lateral root formation. Treatment with 2-butanol displayed no effects on those processes. Additionally, it was demonstrated that Arabidopsis PLDξ2 knockout mutants were less sensitive to auxin, had reduced gravitropism, and suppressed hypocotyl elongation, whereas transgenic seedlings overexpressing PLDξ2 showed opposite phenotypes. The authors suggested that PLDξ2 positively mediates auxin responses, possibly through the regulation of vesicle trafficking and auxin transport. They also argued that there is no evidence that PLDξ2 and PA are involved directly in auxin signaling (Li and Xue, 2007). Furthermore, it was shown that the inhibition of PLD with 1-butanol affects root hair elongation in Arabidopsis (Gardiner et al., 2003). Interestingly, all of these auxin-regulated developmental processes (gravitropism, lateral root formation, hypocotyl elongation, and root hair elongation) have been shown to be tightly dependent on NO action (Beligni and Lamattina, 2000; Correa-Aragunde et al., 2004; Hu et al., 2005; Lombardo et al., 2006). Finally, it was demonstrated that the blockage of PLD activity with 1-butanol resulted in a reduced NO induction of vacuolar H+-ATPase and H+-PPase, key enzymes in salt stress responses. Accordingly, exogenously applied PA stimulated those enzyme activities (Zhang et al., 2006). The use of exogenous PLs as regulators of plant growth is in its infancy (Cowan, 2006). Exogenous PA can couple to intracellular agents to initiate signaling systems. The understanding of the effect of exogenous PL on endogenous PL homeostasis and coupling to endogenous lipid-signaling networks needs further investigation (Cowan, 2006). Because PA applied directly to the medium may be metabolized in the medium or during entry into cells (Wang et al., 2006), we checked the quality of the exogenously applied PA after treating cucumber explants for 1 h at 25°C and found that it remained unaltered throughout the experiment (Supplemental Fig. S2).
Further research is required to ascertain which PLD(s) is involved in auxin and NO signaling leading to AR formation. To date, no cucumber PLD gene has been cloned, making it impossible to correlate the enzymatic activities measured in our study to a specific gene product. Therefore, a useful strategy would be classical reverse genetics screening of PLD mutants in Arabidopsis involving the quantification of ARs and the formation of PA in response to auxin and NO. The PLDξ2 knockout mutant is a good candidate to begin with, considering recent findings reported by Li and Xue (2007). Inhibiting PA formation with 1-butanol treatment repressed auxin-induced lateral root formation and root gravitropic responses more strongly than those of the PLDξ2 knockout mutant, indicating that other PLD members or PLC may be involved in auxin responses (Li and Xue, 2007). It will be interesting to analyze in the PLDξ2 knockout mutant the PA level upon auxin treatment.
It is well documented that protein kinases belonging to diverse families act downstream of PA. In carrot, a CDPK was found to be activated by PA and localized in the membrane, consistent with a role in signal transduction pathways involving PLD (Farmer and Choi, 1999). In soybean, PA has been shown to induce a wound-activated MAPK, which is an important mediator in stress and ethylene signaling (Lee et al., 2001). In Arabidopsis, PLD-derived PA was reported to bind to 3-phosphoinositide-dependent protein kinase-1, which acts upstream of the protein kinase AGC2-1 promoting root hair elongation (Anthony et al., 2004). Additionally, 3-phosphoinositide-dependent protein kinase-1 phosphorylates and activates the protein kinase PINOID, which is another member of the AGC family and has been implicated in the asymmetrical localization of the PIN family of auxin transport facilitators (Zegzouti et al., 2006). All of these data suggest that PA could possibly activate the CDPK and MAPK pathways, which have been proposed to be involved in the auxin- and NO-induced AR formation in cucumber explants (Pagnussat et al., 2004; Lanteri et al., 2006).
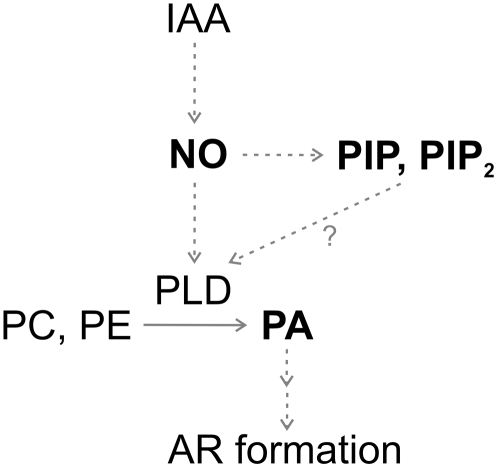
Figure 7 illustrates a schematic model integrating the signaling steps and molecules described in this study that are involved in the auxin-induced AR formation in cucumber: (1) NO acts downstream of auxin to trigger PIP, PIP2, and PA accumulation; (2) PLD is involved in PA formation induced by NO; (3) PLD could be activated by PIP and PIP2 increases and/or other NO-mediated pathways; and (4) PLD-derived PA induces AR formation.
Figure 7.
Schematic model of the PL signaling pathway induced by auxin during AR formation in cucumber. NO acts downstream of auxin (IAA) to trigger PIP, PIP2, and PA accumulation. PLD is involved in PA formation induced by NO. PLD could be activated by PIP and PIP2 increases and/or by other NO-mediated pathways (see “Discussion” for details). PLD-derived PA induces AR formation. The solid arrow denotes the enzymatic pathway. Dashed arrows denote regulatory pathways. The question mark denotes a yet undescribed pathway for PLD activation in cucumber.
In summary, our findings contribute to the identification of new components in the auxin signal transduction pathway and highlight the remarkable complexity of the mechanisms controlling root growth and developmental processes. Further progress in unraveling the precise cross talk between components will certainly improve our understanding of auxin, NO, and lipid signaling in plants.
MATERIALS AND METHODS
Plant Material and Treatments
Cucumber (Cucumis sativus ‘Poinsett 76’) seeds were germinated into petri dishes on filter papers imbibed in distilled water and maintained for 7 d at 25°C with a 14-h/10-h photoperiod at 200 μmol s−1 m−2. Primary root systems of 7-d-old seedlings were removed and cucumber explants (Fig. 1) were treated for different times with buffer (5 mm MES-KOH, pH 6, and 1 mm KCl) plus 0.02% (v/v) 1,1,1,3,5,5,5-heptamethyltrisiloxanyl propylmethoxy-poly[ethylene oxide] (Silwet L-77; Lehle Seeds; control) in the presence or absence of different compounds as mentioned below. Silwet L-77 was added to enhance the penetration of chemicals into cells, except for treatments with the lipids PA, PC, or PS. Control treatments did not cause any measurable effect in any experiment performed. Cucumber explants were treated with 10 μm of the auxins NAA (Sigma) or IAA (Fluka), or with 10 or 500 μm of the NO donors SNAP (Molecular Probes) or SNP (Merck). Equivalent volumes of solvents were added to control explants to ensure that they did not interfere with the experiments. In particular, 0.002% (v/v) ethanol and 0.5% (v/v) DMSO were equivalent to the final solvent concentrations achieved in 10 μm IAA and 500 μm SNAP treatments, respectively. Additionally, 200 μm of the specific NO scavenger cPTIO (Molecular Probes) and 0.1% or 0.5% (v/v) 1-butanol or 2-butanol were added alone or together with 10 μm IAA or 500 μm SNAP. Where indicated, 500 μm of 15-d-old, light-inactivated SNAP solution was used as a negative control (half-life for SNAP under continuous illumination conditions is 3 h; Floryszak-Wieczorek et al., 2006).
PL Labeling, Extraction, and Analysis
Cucumber explants were in vivo labeled for 16 h in 0.5-mL Eppendorf tubes containing buffer plus 15 μCi per explant carrier-free H332PO4 (32Pi; Amersham Biosciences). Explants were transferred to 0.5-mL Eppendorf tubes and treated as mentioned above. Incubations were stopped by adding 5% (v/v) perchloric acid for 30 min. In vivo levels of PLs were measured in hypocotyl segments of 5 mm of the hypocotyl base, where ARs develop (Fig. 1). To assay PLD activity in vivo (Fig. 5), the production of PBut was measured in explants treated with buffer, 10 μm IAA, or 500 μm SNAP in the presence of 0.5% (v/v) 1-butanol or 2-butanol (Munnik et al., 1995).
Reagents for lipid extraction and analysis on Silica-60 TLC plates (20 × 20 cm) were purchased from Merck. Total lipid extraction was performed as described previously (Munnik et al., 1996), with some minor modifications. Briefly, 750 μL of CHCl3:methanol:HCl (50:100:1, v/v) was added to safe lock 2-mL Eppendorf tubes containing hypocotyl segments, vigorously shaken for 15 min, and centrifuged for 2 min. A two-phase system was induced by the addition of 200 μL of 0.9% (w/v) NaCl and 750 μL of CHCl3, and their upper phases were removed. The organic phases were washed once with 750 μL of CHCl3:methanol:1 m HCl (3:45:47, v/v) for 7 min. Samples were dried by vacuum centrifugation for 45 min at 50°C and dissolved in 20 μL of CHCl3.
Lipids were chromatographed on heat-activated TLC plates employing an alkaline solvent system (CHCl3:methanol:25% [w/v] NH4OH:water [90:70:4:15, v/v]; Munnik et al., 1994) or an EtAc solvent system (EtAc:iso-octane:HCOOH:water [12:2:3:10, v/v]; Munnik et al., 1998). For the alkaline solvent system, the plates were impregnated on 1.2% (w/v) K2C2O4 and 2 mm EDTA in methanol:water (2:3, v/v) and then heat activated. The TLC alkaline solvent system was used to separate all PLs, while the TLC EtAc solvent system was specifically used to separate PBut and PA from the other PLs. Lipids were identified by cochromatography and comparison of their RF with lipid extracts of known composition and commercially purchased standards. Radiolabeled PLs were visualized using AGFA Ortho CP-G Plus films and quantified from two different nonoverexposed autoradiographs (one for structural PLs and the other for signaling PLs; Supplemental Fig. S1) using ImageJ software (1.33u; National Institutes of Health). The autoradiographs shown represent general phenomena, representative of three to five independent experiments. The same amount of radioactivity per treatment was loaded on a TLC plate. The radioactivity of the signaling lipids was quantified as a ratio against the radioactivity in total PLs. Values were subsequently expressed as fold accumulation by dividing the ratio in a treatment by that in the corresponding control, taking the level of the control as 1. For statistical analysis, the t test was used as appropriate. A value of P < 0.05 was considered significant for mean differences.
Analysis of AR Formation
Cucumber explants were treated in 0.5-mL Eppendorf tubes and subsequently transferred to petri dishes imbibed in water. Root number values per explant were measured 5 d later (Fig. 1). For statistical analysis, the t test was used as appropriate. A value of P < 0.05 was considered significant for mean differences. For the experiments shown in Figure 6A, explants were treated for 10 min with different compounds as indicated. Because application of alcohol to cells alters various cellular processes (Dhonukshe et al., 2003), the concentration of 0.1% (v/v) 1-butanol was chosen as the minor one that was effective at blocking AR formation. For the experiments shown in Figure 6B, explants were treated for 1 h with buffer or with buffer plus 5 μm of the short saturated fatty acid chain PA C8:0 (1,2-dioctanoyl-sn-glycerol 3-P sodium salt) or PC C8:0 (1,2-dioctanoyl-sn-glycerol 3-phosphocholine sodium salt), the long saturated fatty acid chain PA C16:0 (1,2-dipalmitoyl-sn-glycerol 3-P sodium salt) or PC C16:0 (1,2-dipalmitoyl-sn-glycerol 3-phosphocholine), or the unsaturated fatty acid chain PA C18:1 (1,2-dioleoyl-sn-glycerol 3-P sodium salt) or PS C18:1 (1,2-dioleoyl-sn-glycerol 3-phospho-Sers sodium salt). All of the lipids were purchased from Avanti Polar Lipids, except for PA C8:0 and PC C8:0, which were purchased from Sigma. Stock solutions of lipids were prepared as follows. Lipids were first dissolved in CHCl3 and dried by vacuum centrifugation. The appropriate amount of 10 mm Tris-HCl, pH 7.5, was added to lipids to yield stock concentrations, then hydrated for 30 min at room temperature and sonicated before being used.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Whole autoradiographs of the alkaline (A and B) and EtAc (C and D) TLCs presented in Figure 2 showing two different exposures of structural PLs (SPL; B and D) and signaling PLs (A and C).
Supplemental Figure S2. Evaluation of the quality of the exogenously applied PA and PS recovered from the medium of cucumber explants after 1 h of treatment at 25°C.
Supplementary Material
Acknowledgments
We thank Natalia Correa-Aragunde, Ayelen Distéfano, and Gabriela Gonorazky for exciting ideas, constructive comments and suggestions, and critical reading of the manuscript.
This work was supported by grants to L.L. and A.M.L. from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica, Universidad Nacional de Mar del Plata, and by a grant to A.M.L. from the Third World Academy of Sciences. L.L. and A.M.L. are members of the Permanent Research Staff, and M.L.L. is a Postgraduate Fellow, from CONICET, Argentina.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Lorenzo Lamattina (lolama@mdp.edu.ar).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- André B, Scherer GF (1991) Stimulation by auxin of phospholipase A in membrane vesicles from an auxin-sensitive tissue is mediated by an auxin receptor. Planta 185 209–214 [DOI] [PubMed] [Google Scholar]
- Anthony RG, Henriques R, Helfer A, Meszaros T, Rios G, Testerink C, Munnik T, Deak M, Koncz C, Bogre L (2004) A protein kinase target of a PDK1 signalling pathway is involved in root hair growth in Arabidopsis. EMBO J 23 572–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann BO, Munnik T (2006) The role of phospholipase D in plant stress responses. Curr Opin Plant Biol 9 515–522 [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210 215–221 [DOI] [PubMed] [Google Scholar]
- Berleth T, Sachs T (2001) Plant morphogenesis: long-distance coordination and local patterning. Curr Opin Plant Biol 4 57–62 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Peppiat CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, et al (2001) Calcium signaling: an overview. Semin Cell Dev Biol 12 3–10 [DOI] [PubMed] [Google Scholar]
- Brearley CA, Hanke DE (1992) 3- and 4-phosphorylated phosphatidylinositols in the aquatic plant Spirodela polyrhiza L. Biochem J 283 255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brearley CA, Hanke DE (1993) Pathway of synthesis of 3,4- and 4,5-phosphorylated phosphatidylinositols in the duckweed Spirodela polyrhiza L. Biochem J 290 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JK, Sekiya F, Kang HS, Lee C, Han JS, Kim SR, Bae YS, Morris AJ, Rhee SG (1997) Synaptojanin inhibition of phospholipase D activity by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J Biol Chem 272 15980–15985 [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218 900–905 [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde N, Lanteri ML, García-Mata C, ten Have A, Laxalt AM, Graziano M, Lamattina L (2007) Nitric oxide functions as intermediate in auxin, abscisic acid and lipid signaling pathways. In L Lamattina, J Polacco, eds, Nitric Oxide in Plant Growth, Development and Stress Physiology, Series Plant Cell Monographs, Vol 6. Springer, Berlin, pp 113–130
- Cowan AK (2006) Phospholipids as plant growth regulators. Plant Growth Regul 48 97–109 [Google Scholar]
- Davies PJ (1995) The plant hormones: their nature, occurrence and functions. In PJ Davies, ed, Plant Hormones. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 1–12
- Dhonukshe P, Laxalt AM, Goedhart J, Gadella TW, Munnik T (2003) Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell 15 2666–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettlinger C, Lehle L (1988) Auxin induces rapid changes in phosphatidylinositol metabolites. Nature 331 176–178 [DOI] [PubMed] [Google Scholar]
- Farmer PK, Choi JH (1999) Calcium and phospholipid activation of a recombinant calcium-dependent protein kinase (DcCPK1) from carrot (Daucus carota L.). Biochim Biophys Acta 1434 6–17 [DOI] [PubMed] [Google Scholar]
- Floryszak-Wieczorek J, Milczarek G, Arasimowicz M, Ciszewski A (2006) Do nitric oxide donors mimic endogenous NO-related response in plants? Planta 224 1363–1372 [DOI] [PubMed] [Google Scholar]
- Gardiner J, Collings DA, Harper JD, Marc J (2003) The effects of the phospholipase D-antagonist 1-butanol on seedling development and microtubule organisation in Arabidopsis. Plant Cell Physiol 44 687–696 [DOI] [PubMed] [Google Scholar]
- Gouvea CMCP, Souza JF, Magalhaes ACN, Martins IS (1997) NO releasing substances that induce growth elongation in maize root segments. Plant Growth Regul 21 183–187 [Google Scholar]
- Grabowski L, Heim S, Wagner KG (1991) Rapid changes in the enzyme activities and metabolites of the phosphatidylinositol-cycle upon induction by growth substances of auxin-starved suspension-cultured Catharanthus roseus cells. Plant Sci 75 33–38 [Google Scholar]
- Hou YC, Janczuk A, Wang PG (1999) Current trends in the development of nitric oxide donors. Curr Pharm Des 5 417–441 [PubMed] [Google Scholar]
- Hu X, Neill S, Tang Z, Cai W (2005) Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol 137 663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im YJ, Perera IY, Brglez I, Davis AJ, Stevenson-Paulik J, Phillippy BQ, Johannes E, Allen NS, Boss WF (2007) Increasing plasma membrane phosphatidylinositol(4,5)bisphosphate biosynthesis increases phosphoinositide metabolism in Nicotiana tabacum. Plant Cell 19 1603–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteri ML, Pagnussat GC, Lamattina L (2006) Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. J Exp Bot 57 1341–1351 [DOI] [PubMed] [Google Scholar]
- Laxalt AM, Raho N, Ten Have A, Lamattina L (2007) Nitric oxide is critical for inducing phosphatidic acid accumulation in xylanase-elicited tomato cells. J Biol Chem 282 21160–21168 [DOI] [PubMed] [Google Scholar]
- Lee S, Hirt H, Lee Y (2001) Phosphatidic acid activates a wound-activated MAPK in Glycine max. Plant J 26 479–486 [DOI] [PubMed] [Google Scholar]
- Li G, Xue HW (2007) Arabidopsis PLDξ2 regulates vesicle trafficking and is required for auxin response. Plant Cell 19 281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MC, Graziano M, Polacco J, Lamattina L (2006) Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav 1 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LC, Wang GZ, Zhu CG, Pang HQ (2007) Involvement of phospholipase D and lipoxygenase in response to chilling stress in postharvest cucumber fruits. Plant Sci 172 400–405 [Google Scholar]
- Meijer HJ, Berrie CP, Iurisci C, Divecha N, Musgrave A, Munnik T (2001) Identification of a new polyphosphoinositide in plants, phosphatidylinositol 5-monophosphate (PtdIns5P), and its accumulation upon osmotic stress. Biochem J 360 491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, Arisz SA, De Vrije T, Musgrave A (1995) G protein activation stimulates phospholipase D signaling in plants. Plant Cell 7 2197–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, de Vrije T, Irvine RF, Musgrave A (1996) Identification of diacylglycerol pyrophosphate as a novel metabolic product of phosphatidic acid during G-protein activation in plants. J Biol Chem 271 15708–15715 [DOI] [PubMed] [Google Scholar]
- Munnik T, Irvine RF, Musgrave A (1994) Rapid turnover of phosphatidylinositol 3-phosphate in the green alga Chlamydomonas eugametos: signs of a phosphatidylinositide 3-kinase signalling pathway in lower plants? Biochem J 298 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, Irvine RF, Musgrave A (1998) Phospholipid signalling in plants. Biochim Biophys Acta 1389 222–272 [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 132 1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L (2004) Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol 135 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129 954–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappan K, Qin W, Dyer JH, Zheng L, Wang X (1997) Molecular cloning and functional analysis of polyphosphoinositide-dependent phospholipase D, PLDβ, from Arabidopsis. J Biol Chem 272 7055–7061 [DOI] [PubMed] [Google Scholar]
- Parre E, Ghars MA, Leprince AS, Thiery L, Lefebvre D, Bordenave M, Richard L, Mazars C, Abdelly C, Savouré A (2007) Calcium signaling via phospholipase C is essential for proline accumulation upon ionic but not nonionic hyperosmotic stresses in Arabidopsis. Plant Physiol 144 503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RU, Holk A, Scherer GF (1998) Fatty acids and lysophospholipids as potential second messengers in auxin action. Rapid activation of phospholipase A2 activity by auxin in suspension-cultured parsley and soybean cells. Plant J 16 601–611 [Google Scholar]
- Qin W, Pappan K, Wang X (1997) Molecular heterogeneity of phospholipase D (PLD). Cloning of PLDgamma and regulation of plant PLDgamma, -beta, and -alpha by polyphosphoinositides and calcium. J Biol Chem 272 28267–28273 [DOI] [PubMed] [Google Scholar]
- Quint M, Gray WM (2006) Auxin signaling. Curr Opin Plant Biol 9 448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer GF (1995) Activation of phospholipase A2 by auxin and mastoparan in hypocotyl segments from zucchini and sunflower. J Plant Physiol 145 483–490 [Google Scholar]
- Scherer GF, André B (1989) A rapid response to a plant hormone: auxin stimulates phospholipase A2 in vivo and in vitro. Biochem Biophys Res Commun 163 111–117 [DOI] [PubMed] [Google Scholar]
- Scherer GF, André B (1993) Stimulation of phospholipase A2 by auxin in microsomes from suspension-cultured soybean cells is receptor-mediated and influenced by nucleotides. Planta 191 515–523 [Google Scholar]
- Tanaka H, Dhonukshe P, Brewer PB, Friml J (2006) Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell Mol Life Sci 63 2738–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X (2000) Multiple forms of phospholipase D in plants: the gene family, catalytic and regulatory properties, and cellular functions. Prog Lipid Res 39 109–149 [DOI] [PubMed] [Google Scholar]
- Wang X, Devaiah SP, Zhang W, Welti R (2006) Signaling functions of phosphatidic acid. Prog Lipid Res 45 250–278 [DOI] [PubMed] [Google Scholar]
- Westergren T, Dove SK, Sommarin M, Pical C (2001) AtPIP5K1, an Arabidopsis thaliana phosphatidylinositol phosphate kinase, synthesizes PtdIns(3,4)P(2) and PtdIns(4,5)P(2) in vitro and is inhibited by phosphorylation. Biochem J 359 583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbell B, Walter-Back C (1989) Signal transduction of auxin on isolated plant cell membranes: indications for a rapid polyphosphoinositide response stimulated by indoleacetic acid. J Plant Physiol 133 353–360 [Google Scholar]
- Zbell BA, Walter-Back C, Bucher H (1989) Evidence of an auxin-mediated phosphoinositide turnover and an inositol (1,4,5)trisphosphate effect on isolated membranes of Daucus carota L. J Cell Biochem 40 331–340 [DOI] [PubMed] [Google Scholar]
- Zegzouti H, Anthony RG, Jahchan N, Bogre L, Christensen SK (2006) Phosphorylation and activation of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in Arabidopsis. Proc Natl Acad Sci USA 103 6404–6409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang L, Liu Y, Zhang Q, Wei Q, Zhang W (2006) Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta 224 545–555 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.